Abstract
The mutualistic associations of tall fescue (Festuca arundinacea) with seed-borne fungal symbionts (endophytes) are important for fitness of the grass host and its survival under biotic and abiotic stress. The tall fescue endophytes are asexual relatives of biological species (mating populations) of genus Epichloë (Clavicipitaceae), sexual fungi that cause grass choke disease. Isozyme studies have suggested considerable genetic diversity among endophytes of tall fescue. Phylogenetic relationships among seven isolates from tall fescue, three from meadow fescue (a probable ancestor of tall fescue), and nine Epichloë isolates from other host species were investigated by comparing sequences of noncoding segments of the beta-tubulin (tub2) and rRNA (rrn) genes. Whereas each Epichloë isolate and meadow fescue endophyte had only a single tub2 gene, most tall fescue endophytes had two or three distinct tub2 copies. Phylogenetic analysis of tub2 sequences indicated that the presence of multiple copies in the tall fescue endophytes was a consequence of hybridization with Epichloë species. At least three hybridization events account for the distribution and relationships of tub2 genes. These results suggest that interspecific hybridization is the major cause of genetic diversification of the tall fescue endophytes.
Full text
PDF


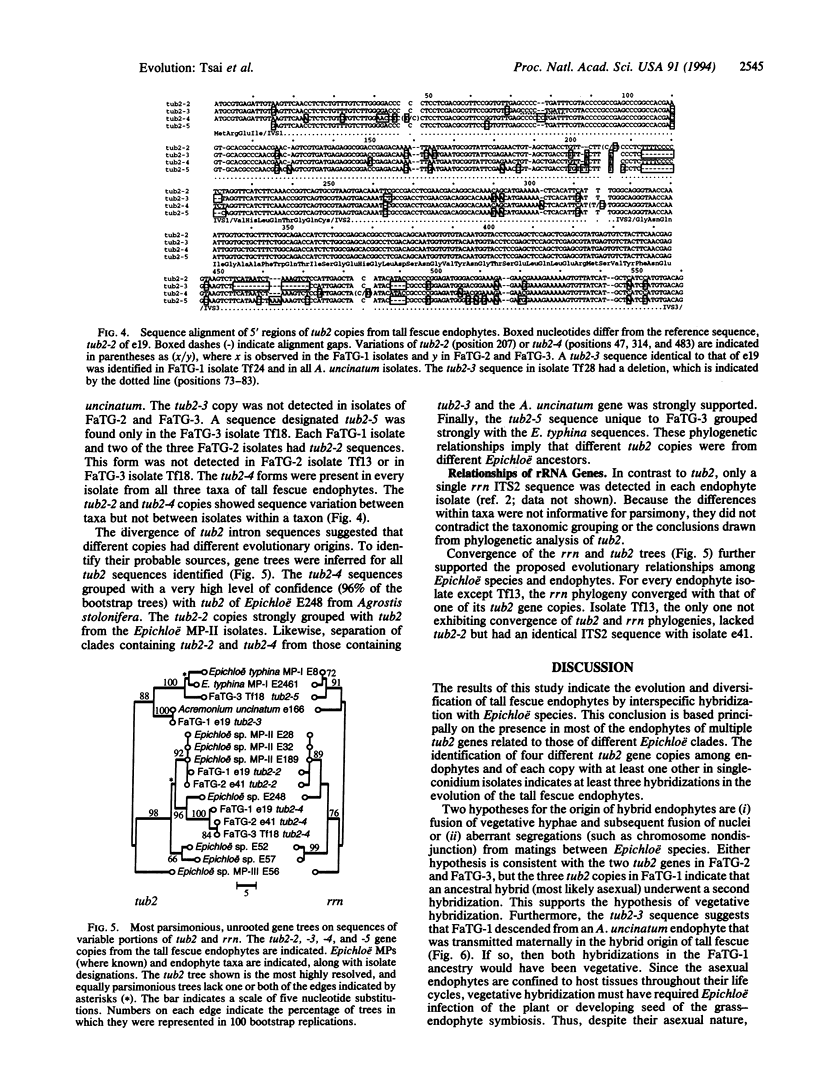

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrd A. D., Schardl C. L., Songlin P. J., Mogen K. L., Siegel M. R. The beta-tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr Genet. 1990 Nov;18(4):347–354. doi: 10.1007/BF00318216. [DOI] [PubMed] [Google Scholar]
- Lion T., Haas O. A. Nonradioactive labeling of probe with digoxigenin by polymerase chain reaction. Anal Biochem. 1990 Aug 1;188(2):335–337. doi: 10.1016/0003-2697(90)90616-h. [DOI] [PubMed] [Google Scholar]
- MULLER H. J. THE RELATION OF RECOMBINATION TO MUTATIONAL ADVANCE. Mutat Res. 1964 May;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Tsai H. F. Molecular biology and evolution of the grass endophytes. Nat Toxins. 1992;1(3):171–184. doi: 10.1002/nt.2620010305. [DOI] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Genome walking by single-specific-primer polymerase chain reaction: SSP-PCR. Gene. 1989 Dec 7;84(1):1–8. doi: 10.1016/0378-1119(89)90132-7. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Tsai H. F., Siegel M. R., Schardl C. L. Transformation of Acremonium coenophialum, a protective fungal symbiont of the grass Festuca arundinacea. Curr Genet. 1992 Nov;22(5):399–406. doi: 10.1007/BF00352441. [DOI] [PubMed] [Google Scholar]