Abstract
Dengue hemorrhagic fever can occur in primary dengue virus (DENV) infection of infants. The decay of maternally derived DENV immunoglobulin (Ig) G and the incidence of DENV infection were determined in a prospectively studied cohort of 1244 Vietnamese infants. Higher concentrations of total IgG and DENV-reactive IgG were found in cord plasma relative to maternal plasma. Maternally derived DENV-neutralizing and E protein–reactive IgG titers declined to below measurable levels in >90% of infants by 6 months of age. In contrast, IgG reactive with whole DENV virions persisted until 12 months of age in 20% of infants. Serological surveillance identified 10 infants with asymptomatic DENV infection for an incidence of 1.7 cases per 100 person-years. DENV-neutralizing antibodies remained measurable for ≥1 year after infection. These results suggest that whereas DENV infection in infants is frequently subclinical, there is a window between 4 and 12 months of age where virion-binding but nonneutralizing IgG could facilitate antibody-dependent enhancement.
Dengue is a major public health problem in many tropical and subtropical countries. World Health Organization data suggest that the disease burden of dengue has risen exponentially in the last 20 years, and the number of countries affected has increased 10-fold [1]. This striking emergence of dengue is associated with substantial costs to both health care systems and patients [2], and it is highly likely that the true disease burden is underestimated by data based only on hospitalized cases [3]
Dengue virus (DENV) belongs to the Flaviviridae family and includes 4 serotypes. Each serotype is capable of causing severe disease, called dengue hemorrhagic fever (DHF). A well-defined risk factor for DHF is sequential infections by 2 different serotypes [4–7]. Although most of the DHF disease burden is the result of secondary infections in children and young adults, DHF also occurs in primary DENV infection of infants <1 year of age [8, 9]. When DHF occurs in infants, it can be clinically challenging to manage, and the mortality rate is higher than in older children [10].
Maternally derived immunoglobulin (Ig) G is likely to play a central role in immunity and pathogenesis of dengue in infancy. The presence of maternally derived neutralizing antibody is presumed to explain the low prevalence of symptomatic dengue in infants <3–4 months of age [9]. Thereafter, subneutralizing levels of maternally derived anti-DENV IgG might enhance DENV infection in Fc receptor–bearing cells, an event that could contribute to DHF [9]. In support of this hypothesis, neat plasma from healthy infants born to dengue-immune mothers has been shown to enhance virus infection in a manner that correlates with the age-related case burden of dengue in infants [11].
The epidemiology of DENV infection in infants is not well defined. The only previous prospective cohort study of infants <1 year of age demonstrated an infection incidence rate of ~2 cases per 100 person-years [12]. In southern Vietnam the annual exposure risk in children is ~10% per annum [13, 14], but the exposure risk in infants is unknown. A better understanding of the epidemiology of dengue in infants and the incidence of symptomatic illness will help guide decisions as to when dengue vaccines should be introduced to endemic regions. In addition, prospective cohort studies of infants at risk of dengue can address important questions about the quantitative and qualitative features of maternally derived antibody and its role in immunity or pathogenesis.
This study aimed to determine the incidence of dengue exposure and disease in a cohort of infants followed up prospectively from birth and to establish the kinetics of decay of maternally derived dengue-reactive antibody during the first year of life. The main findings are that maternally derived DENV virion–binding IgG persists for longer in infants than neutralizing antibody and that the kinetics of this decay are consistent with an association between nonneutralizing maternal antibody and the age-related burden of dengue in infants. Furthermore, the incidence of DENV infection in a cohort of infants was determined to be 1.7 cases per 100 person-years, with all infections asymptomatic.
MATERIALS AND METHODS
Study population
This was a prospective birth cohort study. Healthy pregnant women (n = 1244) in their last trimester were enrolled at Hung Vuong Obstetric Hospital in Ho Chi Minh City, Vietnam, between September 2006 and August 2007. Women were eligible to enroll in the study if they were human immunodeficiency virus negative, had singleton pregnancies at >37 weeks gestation, and lived in districts adjacent to Hung Vuong Obstetric Hospital and Children’s Hospital 1. Demographic information was recorded at enrollment, and cord and maternal blood samples were collected at birth. The infant and mother were then invited to return to the Hung Vuong Obstetric Hospital every 3 months until the infant reached 1 year of age and then again at 18 months and 2 years of age. At each study visit, information on health care–seeking behavior was recorded, and a capillary or venous blood sample was collected from the infant. In line with national guidelines, no infant was vaccinated against Japanese encephalitis virus before his or her first birthday. The study was approved by the ethics committees of the Hospital for Tropical Diseases and Hung Vuong Obstetric Hospital and the Oxford Tropical Research Ethics Committee.
Surveillance for febrile illnesses in the study population
Study participants were encouraged to visit Children’s Hospital 1 if their infant developed a febrile illness. At the time of presentation, information on clinical symptoms and history was recorded in case record forms. If dengue was a possible diagnosis, a laboratory investigation (nonstructural protein 1 [NS1] and IgM testing) was performed. In addition, a passive surveillance system was used to detect febrile illnesses in the study population. At each follow-up visit, the mother was asked whether her infant had experienced a fever since the last visit, and, if so, what health care had been sought and what diagnosis had been given. If the mother and infant did not attend follow-up, this information was obtained by telephone interview.
Dengue serological testing
Dengue antigen-capture IgM and IgG enzyme-linked immunosorbent assays (ELISAs) were performed as described elsewhere, using antigens to DENV and Japanese encephalitis virus [15]. Dengue indirect IgG ELISAs were performed using a pool of recombinant DENV E (recE) proteins from each serotype (kindly provided by Hawaii Biotech). Briefly, ELISA plates (Maxisorb; Nunc) were coated with a pool of recE proteins from DENV-1, -2, -3, and -4 (final concentration, 1 μg/mL) overnight, washed and then blocked by 2% bovine serum albumin. After washing, 2-fold serial dilutions of plasma, starting at 1:100, were added for 1 h, plates were washed again, and secondary antibody (horseradish per-oxidase [HRP]–labeled anti-human IgG) was added for 1 h. After another washing, plates were developed using tetramethylbenzidine (TMB) substrate, and the absorbance was read at 490 nm. End point titers were defined as the reciprocal of the dilution that yielded an optical density of 0.3 after subtraction of the absorbance obtained for the blank control and uncoated wells.
In the Panbio Dengue IgG Indirect ELISA (Panbio), purified DENV-1-4 virions are coated directly onto the microwells of the plate. To measure end-point titers of IgG against these virions, serial 3-fold dilutions of plasma were added to each well for 1 h. After 5 washings, DENV-reactive IgG molecules were detected by incubation with HRP-conjugated anti-human IgG for 1 h, and after another washing, they were detected by TMB substrate. End point titers were defined as the reciprocal of the dilution that yielded an optical density of 0.3 after subtraction of the absorbance obtained for the blank control and uncoated wells.
Definition of laboratory-confirmed DENV infection
A laboratory-confirmed DENV infection was defined as a positive IgM or IgG antigen-capture ELISA result or a 4-fold increase in titer between serial samples in the recE protein indirect ELISA.
Plaque-reduction neutralization test
Neutralizing antibody titers were determined by a complement-enhanced, plaque-reduction neutralization test (PRNT). Briefly, test plasma was heat inactivated (56°C for 30 min), and serial 2-fold dilutions beginning at 1:10 were prepared in serum-free medium containing 0.25% human serum albumin. Test virus, diluted to 500 plaque-forming units/mL in medium containing guinea pig complement (Cambrex) at a complement fixation titer of 1:10, was added to equal volumes of diluted test serum and incubated at 37°C for 30 min. Medium was removed from monolayer cultures of Vero cells on 24-well plates, and 0.1 mL of virus-serum mixture was transferred into duplicate wells. After incubation for 60 min at 37°C, the wells were overlaid with medium containing 1% methylcellulose and 2% fetal bovine serum. Samples were incubated at 37°C for 4–5 days, and plaques were visualized by immunoperoxidase staining, and a 50% PRNT (PRNT50) titer was calculated. We used the following viruses: DEN1 Puerto Rico/94, DEN2 NGC prototype, DEN3 Sleman/78, DEN4 814669. The limit of detection was a titer of 10.
Statistical analysis
Geometric mean titers were calculated to summarize DENV-reactive IgG populations in maternal and cord plasma, and a paired t test was used to test the null hypothesis of no difference in log titer between paired cord and maternal samples. The incidence of DENV infection was calculated as the number of seroconversions per 100 person-years of follow-up, and confidence intervals (CIs) were determined based on the Poisson distribution. For each child, we estimated the time required for IgG levels to reach half the birth (cord) value, based on a linear regression of the child’s detectable log-transformed measurements, which depended on follow-up time. Children with undetectable anti-DENV IgG values at their first follow-up visit (age 3 months) were treated as having a half-life shorter than all other children. A CI for the median population half-life was based on the Kaplan-Meier estimator of the half-life distribution and its CI. The proportion of children with detectable IgG values over time was also graphically displayed. All analyses were performed with R software (version 2.8.1; R Foundation for Statistical Computing). All reported CIs are 2-sided 95% intervals, and tests were performed at a 2-sided significance level of 5%.
RESULTS
Study population
In this prospective birth cohort study, 1244 infants were enrolled at Hung Vuong Obstetric Hospital, Ho Chi Minh City, Vietnam, between September 2006 and August 2007. The majority of participants (96%) were resident in Ho Chi Minh City districts 5, 6, 8, 10, 11, Binh Chanh, Binh Tan, Tan Binh, and Tan Phu, and the average maternal age was 27 years (range, 17–45 years). The newborn male-female ratio was 1.06:1. The median gestational age was 39 weeks (range, 30–43 weeks), and the mean birth weight was 3.1 kg (range, 2.5–4.5 kg). Overall, 66% of all enrolled infants (819 of 1244) returned for ≥1 scheduled follow-up visit; 226 of (28%) 819 infants returned for 1 visit only, 143 (17%) of 819 for 2 visits, 166 (20%) of 819 for 3 visits, and 284 (35%) of 819 for all 4 follow-up visits. The reported use of bed nets was high in the cohort, with >95% of infants reportedly sleeping under bed nets at 3, 6, 9, and 12 months of age. There were no apparent demographic or socioeconomic differences between infants who did not return for any scheduled follow-up (n = 425) and those who returned at least once (n = 819) (data not shown).
Maternal antibody transfer
To better understand the characteristics of transplacental IgG transfer and the decay of different populations of anti-DENV antibody, we randomly selected 50 infants with plasma samples for all 4 scheduled follow-up visits (ages 3, 6, 9 and 12 months) and no serological evidence of DENV exposure. The average maternal age of this group was 27.5 years (range, 20–35 years). The median gestational age was 39 weeks (range, 35–41.5 weeks). In maternal plasma, 36 of 50 mothers (72%) had neutralizing antibody (PRNT50) against all 4 DENV serotypes, and 98% had neutralizing antibody to least 1 serotype (Table 1). Total IgG concentrations in cord blood were significantly higher than the corresponding maternal sample, which probably explains the observation of significantly higher anti–DENV recE protein IgG titers in cord blood than in maternal blood. PRNT50 titers for each DENV serotype were also higher on average in cord blood than in maternal blood, and this difference was significant for DENV-2 and DENV-3 (Table 1). This finding suggests active transport of maternal IgG (irrespective of specificity) across the placental barrier.
Table 1. Anti–Dengue Virus (DENV) Antibody Titers in Maternal and Cord Plasma in 50 Infants.
| Maternal plasma samples (n = 50) |
Cord plasma (n = 50) |
|||||
|---|---|---|---|---|---|---|
| Assay | No. with measurable levels | Antibody titer, GMT (range) | No. with measurable levels | Antibody titer, GMT (range) | Cord-maternal plasma ratio, median (range) | P a |
| Neutralizing antibody (PRNT50) | ||||||
|
| ||||||
| DENV-1 | 46 | 53 (<10 to 805) | 38 | 58 (21–1902) | 1.2 (0.3–11.1) | .7 |
|
| ||||||
| DENV-2 | 44 | 30 (<10 to 408) | 48 | 58 (11–940) | 1.7 (0.5–4.6) | <.001 |
|
| ||||||
| DENV-3 | 42 | 42 (<10 to 4616) | 46 | 80 (11–2014) | 1.5 (0.7–9.2) | <.001 |
|
| ||||||
| DENV-4 | 36 | 26 (<10 to 460) | 36 | 30 (10–3650) | 1.3 (0.2–30.4) | .5 |
|
| ||||||
| Anti–recE protein IgG | 48 | 2365 (100–13,183) | 50 | 3991 (100–20,105) | 1.3 (0.5–3.8) | <.001 |
|
| ||||||
| Total human IgG | 50 | 913 (582–1297)b | 50 | 1025 (600–1429)b | 1.1 (0.6–1.7) | .002 |
NOTE. The limit of detection for the 50% plaque-reduction neutralization test (PRNT50) was 10; for comparisons between cord and maternal samples, all titers were included, and samples with titers <10 were given a log10 value of 0.5. For anti–recombinant E (recE) protein immunoglobulin (Ig) G, the end-point limit of detection for indirect enzyme-linked immunosorbent assay was 100. GMT, geometric mean titer.
P values determined with paired-sample t test of log10-transformed values.
Values for total human IgG concentrations are given in milligrams per deciliter.
Decay of maternally derived DENV-reactive IgG
The levels of maternally derived antibodies against purified DENV virions and pooled recE proteins and neutralizing antibodies (PRNT50) were assessed in cord plasma and in each plasma sample collected at 3, 6, 9, and 12 months of age. Figure 1 shows the fractions of infants having measurable levels of these maternal antibody populations at different times during the first year of life. By 6 months of age almost all infants had lost IgG capable of binding DENV-1–4 recE proteins in an indirect ELISA. At the same age, neutralizing antibodies to DENV-1 were undetectable, and neutralizing antibodies to DENV-2, -3, and -4 were measurable in only 10% of infants (Figure 1). Interestingly, levels of IgG to DENV-1–4 virions (indirect ELISA) were measurable for longer, being detectable at 12 months of age in ~20% of infants (Figure 1). The estimated median half-life of anti–DENV virion IgG was 5.2 weeks (95% CI, 4.8–6.5 weeks), but this finding should be interpreted with caution, because it is based on 3-monthly sampling only. The important finding here is that neutralizing antibody (at least as measured by PRNT50 assay) does not persist for as long as antibodies that can bind the surface of DENV virions.
Figure 1.
Dynamics of anti–dengue virus (DENV) maternal antibody decay in infants. Plots show the fraction of infants at each follow-up time point with measurable anti–DENV recombinant E (recE) protein immunoglobulin (Ig) G titers, 50% plaque-reduction neutralization test (PRNT50) titers, and anti–DENV virion IgG titers. This analysis includes only those infants whose cord blood contained a measurable level of antibody. Lines showing the decay of anti–DENV recE protein IgG titers (in 49 infants) and anti–DENV virion IgG titers (in 49 infants) are identical in each panel; the third line in each panel shows the fraction of infants with measurable PRNT50 titers for DENV-1 (n = 38; A), DENV-2 (n = 48; B), DENV-3 (n = 46; C), or DENV-4 (n = 36; D). Ab, antibody; Nt Ab, neutralizing antibody.
Serological detection of DENV infection during the first year of life
Because maternal IgM does not cross the placental barrier, the presence of DENV-reactive IgM in infant plasma reflects recent infection. DENV IgM seroconversions occurred in 9 of 819 infants (2111 unique samples). To complement detection of DENV IgM, an indirect IgG ELISA employing pooled recE proteins from DENV-1–4 was used to screen cord and infant plasma samples. With this approach, it was possible to detect a >4-fold increase in the end point titer of IgG to recE proteins in 1 additional infant. A summary of the findings from the serological investigations is shown in Table 2. Assuming that the indirect DENV-1–4 recE protein IgG ELISA provided serological surveillance for DENV infection from birth until the last blood sample was collected from each infant, then the 10 DENV infections detected in 572.5 person-years of serological surveillance yielded an infection incidence of 1.7 cases per 100 person-years (95% CI, 0.8–3.2 cases per 100 person years). Most of the DENV infections (9 of 10) were documented in the rainy season of May to November 2007. The timing of DENV infection in case 812 could not be defined accurately because of the 9-month gap between follow-up samples. One-half of the infections occurred before the age of 6 months but none before age 3 months.
Table 2. Results of Dengue Virus (DENV) Diagnostic Serological Tests in 10 Infants with Documented DENV Seroconversions.
| Age at infection, range, months | Cord plasma |
Preinfection infant plasma |
Postinfection infant plasma |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Study code | IgMa | IgGb titer | IgMa | IgGa | IgGb titer | IgMa | IgGa | IgGb titer | |
| 38 | 9–12 | − | 8828 | − | − | 655 | + | − | 5041 |
|
| |||||||||
| 128 | 9–12 | − | <100 | − | − | <100 | + | + | 9736 |
|
| |||||||||
| 277 | 9–12 | − | 4286 | − | − | <100 | + | + | 8129 |
|
| |||||||||
| 382 | 3–6 | − | 2945 | − | − | 200 | + | − | 1497 |
|
| |||||||||
| 387 | 9–12 | − | 825 | − | − | <100 | + | + | 2763 |
|
| |||||||||
| 880 | 3–6 | − | 25000 | NA | NA | NA | + | − | 1440 |
|
| |||||||||
| 812 | 3–12 | − | 4145 | − | − | 235 | − | − | 2332 |
|
| |||||||||
| 886 | 3–6 | − | 2383 | − | − | 380 | + | − | 1262 |
|
| |||||||||
| 953 | 3–6 | − | 955 | − | − | <100 | + | + | 5146 |
|
| |||||||||
| 1006 | 3–6 | − | 5906 | − | − | 436 | + | − | 2907 |
NOTE. Ig, immunoglobulin; NA, sample not available; +, positive; −, negative.
IgM and IgG were identified with IgM and IgG antigen-capture enzyme-linked immunosorbent assay.
End-point titers of IgG against pooled DENV-1–4 recombinant E proteins were measured with indirect enzyme-linked immunosorbent assay The limit of detection was 100.
Surveillance for febrile events in the cohort of 1244 infants
There were 252 fever episodes in 206 infants. Fever without a clinical diagnosis was reported in 192 of 252 febrile infants (76%). No febrile infant in the cohort had laboratory-confirmed dengue. Among the 10 infants with serological evidence of infection, in only 1 was there a report of a fever (undiagnosed) in the 3 months preceding the detection of DENV IgM. These data suggest that the large majority of DENV infections, and possibly all, were not clinically apparent.
Antibody responses after DENV infection
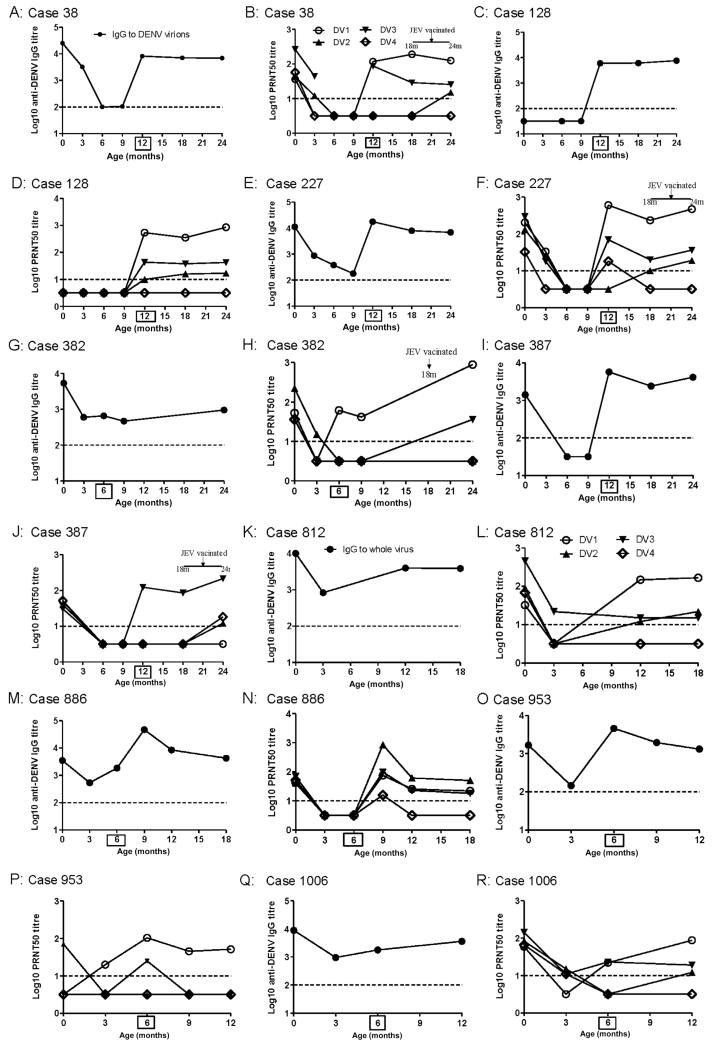
IgG to DENV virions (indirect ELISA) and neutralizing antibody (PRNT50) were measured in serial plasma samples from 9 of the 10 infants with DENV infections. Samples were obtained from infants with DENV infections until they were 2 years of age. One infant had insufficient samples for evaluation. In the plasma sample preceding infection, 7 (78%) of 9 infants still had detectable levels of IgG to DENV virions, and 5 (56%) of 9 had IgG to recE proteins (Table 2). Neutralizing antibody to any serotype was below the limit of detection in preinfection samples from 5 of 9 infants (Figure 2). Because all infections were asymptomatic, it was not possible to determine the precise age of infants at infection and therefore not possible to extrapolate the titer of DENV-reactive IgG populations at that time.
Figure 2.
Antibody profiles in 9 infants with asymptomatic dengue virus (DENV) infections. A, C, E, G, I, K, M, O, and Q, Titers of immunoglobulin (Ig) G to DENV virions in serial plasma samples before and after infection. B, D, F, H, J, L, N, P, and R, 50% plaque-reduction neutralization test (PRNT50) titers for neutralizing antibodies to DENV-1–4 in the same serial plasma samples. Samples at time 0 are cord plasma. Boxed numbers on x axes indicate ages at which DENV IgM was first, and dashed lines represent assay limits of detection. JEV, Japanese encephalitis virus.
Postinfection PRNT50 patterns were often multitypic, though in most cases there was clearly 1 serotype that dominated the response (Figure 2). DENV-1 was inferred to be the infecting serotype in 7 (78%) of 9 infants on the basis of the PRNT50 pattern after infection, consistent with the widespread prevalence of DENV-1 in southern Vietnam during 2006–2007 (unpublished results). DENV-2 and DENV-3 were inferred to be the infecting serotypes in cases 886 and 387, respectively (Figure 2J and 2N). Despite their immunological immaturity, all infants, including those <6 months of age, had robust neutralizing antibody responses that were long-lived, being measurable >1 year after infection in all 7 infants with available follow-up samples (Figure 2). Four infants were reportedly vaccinated against Japanese encephalitis virus between the ages of 18 and 24 months, but this did not appear to significantly affect either neutralizing antibody or IgG titers to DENV virions (Figure 2). Collectively, these results imply that asymptomatic DENV infections elicit robust and durable neutralizing antibody responses in infants and that these response are independent of age at exposure.
DISCUSSION
Understanding the disease burden and epidemiology of dengue in infants is important in guiding how and when dengue vaccines are best used, if and when they become available. The incidence of DENV infection in this cohort was 1.7 cases per 100 person-years (95% CI, 0.8–3.2 cases per 100 person-years), a substantially lower exposure rate than that reported for older children in Vietnam [13, 14]. Furthermore, our results suggest the vast majority of DENV infections in infants are likely to be asymptomatic. Despite a low infection incidence and an even lower (unmeasured) incidence of symptomatic infection, we nonetheless demonstrated that decay of maternally derived anti-DENV IgG occurs with kinetics that create conditions potentially conducive for antibody-dependent enhancement (ADE). The loss of neutralizing antibody several months before the loss of IgG that can bind intact DENV virions might facilitate enhanced viral infection and predispose some infants to symptomatic or possibly severe disease.
Among the population of women we studied, almost all (98%) had serological evidence of previous DENV infection, consistent with high seroprevalence in southern Vietnam [13, 14]. As in other studies [12, 16, 17], we found that total IgG and anti-DENV antibody titers were higher in cord blood than corresponding maternal titers. The important wider relevance of this finding is that studies of infants with dengue that use maternal anti-DENV titers as a surrogate of cord blood titers [8, 18] will sometimes underestimate the infant’s real titer at birth.
The decay of maternally derived DENV-reactive IgG, measured by PRNT50 and hemagglutination inhibition assay (HIA), has been described elsewhere in separate cohorts of healthy Thai infants [12, 16]. These studies have shown incremental decay of antibody at PRNT50 or HIA, with neutralizing antibody declining below measurable levels in 80% of infants by 9 months of age [12]. Our results are in agreement with the findings of these studies and extend them by showing that the kinetics of decay of recE-specific IgG correlate with levels of neutralizing antibody but that IgG reactive with whole virions is measurable for much longer. This could reflect the binding of specific IgG to other antigens exposed on the viral surface (eg, pre M/M) or the binding of antibodies to epitopes that are conformationally not present in recE proteins. We excluded the possibility that these findings reflected binding to NS1 proteins by demonstrating that NS1 was not detectable on the virion-coated wells of the assay plate.
The presence of IgG that can bind the surface of the virion, but not neutralize it, may provide the environment for ADE to occur in infants 4–12 months of age. Consistent with this possibility, findings from our other studies have indicated that plasma from 6-month-old infants mediates better DENV infection enhancement in vitro than plasma from younger or older infants [11]. Collectively, the results described here and elsewhere are consistent with a relationship between sub- or nonneutralizing, maternally derived, virion-binding IgG and the age-related hospitalized case burden of dengue in infants, with ADE an obvious candidate to explain this relationship.
The incidence of DENV infection in the cohort of infants described here was 1.7 cases per 100 person-years. A similar infection rate (~2 cases per 100 person-years) was observed in a cohort of Thai infants <1 year of age [12] in whom almost all infections were asymptomatic, as in the current study. The incidence of infections in infants described here is much lower than the incidence of dengue in older Vietnamese children suggested by epidemiological surveillance in southern Vietnam for 2007 (~700 hospitalizations for dengue per 100,000 children <15 years old; estimate based on data from [19, 20]) and by other seroprevalence studies, the findings of which have suggested an infection rate of ~10% per annum. The basis for a lower DENV infection incidence in infants may be related to parental behavior that reduces exposure risk in infants. For example, Vietnamese infants are typically swaddled in clothes and/or blankets for the first months of life, and, as this study showed, most typically sleep under bed nets. Collectively these factors will serve to limit exposure to biting Aedes aegypti. It is also conceivable, but difficult to demonstrate, that maternal antibody can prevent infection outright so that infants do not undergo seroconversion and are therefore not detected as case patients by serological surveillance.
The DENV infections documented here were associated with the induction of long-lived neutralizing antibodies that were often multitypic but occasionally monotypic. Responses in infants <6 months old were as robust as those in older infants, suggesting that their inherent immunological immaturity in the B cell compartment was not a barrier to establishing a pool of memory B cells, of which a subset maintain relatively constant antibody levels [21].
Our results and those of others [12] revealing the relatively low incidence of dengue in infants suggest that implementation of dengue vaccines would be better directed at older age groups, in which the incidence and prevalence of disease are much greater. A vaccine that reduces DENV transmission in the community will benefit infants by reducing the risk of infection.
Acknowledgments
We thank the families of study participants and physicians and nurses at Hung Vuong Hospital and Children’s Hospital 1 for their contribution to the study. Ms Hoang Thi Sen is thanked for her tireless efforts in follow-up activities.
Financial support: Wellcome Trust.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.World Health Organization . Dengue and dengue haemorrhagic fever. World Health Organization; Geneva: 2008. [Google Scholar]
- 2.Suaya JA, Shepard DS, Siqueira JB, et al. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009;80:846–55. [PubMed] [Google Scholar]
- 3.Anderson KB, Chunsuttiwat S, Nisalak A, et al. Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. Lancet. 2007;369:1452–9. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 4.Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. Studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–9. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 5.Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 6.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 7.Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 8.Simmons CP, Chau TN, Thuy TT, et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–24. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstead SB, Lan NT, Myint TT, et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–9. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TH, Nguyen TL, Lei HY, et al. Volume replacement in infants with dengue hemorrhagic fever/dengue shock syndrome. Am J Trop Med Hyg. 2006;74:684–91. [PubMed] [Google Scholar]
- 11.Chau TN, Quyen NT, Thuy TT, et al. Dengue in Vietnamese infants: results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–24. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pengsaa K, Luxemburger C, Sabchareon A, et al. Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in Thai children. J Infect Dis. 2006;194:1570–6. doi: 10.1086/508492. [DOI] [PubMed] [Google Scholar]
- 13.Bartley LM, Carabin H, Vinh Chau N, et al. Assessment of the factors associated with flavivirus seroprevalence in a population in Southern Vietnam. Epidemiol Infect. 2002;128:213–20. doi: 10.1017/s0950268801006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thai KT, Binh TQ, Giao PT, et al. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10:379–86. doi: 10.1111/j.1365-3156.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- 15.Hang VT, Nguyet NM, Trung DT, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3:e360. doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanaveeradej V, Endy TP, Samakoses R, et al. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg. 2003;69:123–8. [PubMed] [Google Scholar]
- 17.Ventura AK, Ehrenkranz NJ, Rosenthal D. Placental passage of antibodies to Dengue virus in persons living in a region of hyperendemic dengue virus infection. J Infect Dis. 1975;131(Suppl):S62–8. doi: 10.1093/infdis/131.supplement.s62. [DOI] [PubMed] [Google Scholar]
- 18.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–9. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 19.Household living standards survey, 2006. General Statistics Office of Vietnam; Hanoi, Vietnam: 2006. [Google Scholar]
- 20.Pasteur Institute . Annual report of the 2007 activities and 2008 plan for the dengue control program in Southern Vietnam. Pasteur Institute; Ho Chi Minh City, Vietnam: 2008. [Google Scholar]
- 21.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]