Abstract
The effect of the glucocorticoid inducible protein annexin 1 (ANXA1) on the process of monocytic cell migration was studied using transfected U937 cells expressing variable protein levels. An antisense (AS) (36.4AS; ~50% less ANXA1) and a sense (S) clone (15S; overexpressing the bioactive 24-kDa fragment) together with the empty plasmid CMV clone were obtained and compared with wild-type U937 cells in various models of cell migration in vitro and in vivo. 15S-transfected U937 cells displayed a reduced (50%) degree of trans-endothelial migration in response to stromal cell-derived factor-1α (CXC chemokine ligand 12 (CXCL12)). In addition, the inhibitory role of endogenous ANXA1 on U937 cell migration in vitro was confirmed by the potentiating effect of a neutralizing anti-ANXA1 serum. Importantly, overexpression of ANXA1 in clone 15S inhibited the extent of cell migration into rheumatoid synovial grafts transplanted into SCID mice. ANXA1 inhibitory effects were not due to modifications in adhesion molecule or CXCL12 receptor (CXCR4) expression as shown by the similar amounts of surface molecules found in transfected and wild-type U937 cells. Likewise, an equal chemotactic response to CXCL12 in vitro excluded an intrinsic defect in cell motility in clones 15S and 36.4AS. These data strongly support the notion that ANXA1 critically interferes with a leukocyte endothelial step essential for U937 cell, and possibly monocyte, transmigration both in vitro and in vivo.
The process of leukocyte egress from the blood circulation into specific tissue sites is central to the host control of infectious and inflammatory diseases and survival. For this reason, several mechanisms and mediators are involved to finely regulate this process that entails the initial interaction of free-flowing blood cells with the activated endothelium of post-capillary venules, diapedesis, and subendothelial migration in the tissue matrix. These phases are modulated by multiple interactions involving cell adhesion molecules (CAMs),3 leukocyte activators (signaling molecules), and proteolytic enzymes (1-3). In contrast, once in the tissue, the damaging potential of blood borne leukocytes is high and if uncontrolled, the continuous influx of inflammatory cells can be responsible for tissue destruction and disease. Therefore, complex mechanisms exist in the host not only to promote leukocyte tissue extravasation, but also to down-regulate this phenomenon in a time-dependent fashion. Among the known examples of endogenous anti-inflammatory mediators capable of down-modulating leukocyte tissue localization are adenosine, lipoxins, and annexin (ANXA)1, to cite a few (4-6).
In the past 10 years, several studies have described the inhibitory actions that the glucocorticoid-regulated protein ANXA1, previously called lipocortin 1 (7), exerts on the process of neutrophil extravasation. Human rANXA1 and peptides derived from the N terminus region of the protein inhibit neutrophil migration in several distinct models of acute inflammation (8) as well as in models of arthritis (9, 10). Experiments of intravital microscopy have restricted the site of action of ANXA1 and its “mimetics” to the adherent neutrophils. Sytemic (i.v.) treatment of mice with these agents provoke detachment of adherent leukocytes from the activated postcapillary endothelium, reducing in this manner the extent of cell recruitment to the site of inflammation (11). Passive neutralization of ANXA1 greatly diminishes not only the antimigratory property of the potent synthetic glucocorticoid dexamethasone (10, 12, 13), but also the detachment effect produced by this drug in the hamster cheek pouch microcirculation (14), strongly indicating an anti-inflammatory role for this endogenous mediator (15). More recently, the receptor for formylated peptides has been proposed to be involved in mediating the effects displayed by ANXA1 and mimetics on human and rodent neutrophils, both in vitro and in vivo (16, 17).
In contrast to neutrophils, the potential inhibitory role of ANXA1 on the process of monocyte recruitment has been poorly investigated. The literature on this particular issue is scanty, despite the clear evidence of an increase in monocyte-associated ANXA1 levels, both in humans and rodents, following systemic glucocorticoid administration (18, 19). In terms of biological actions, purified preparations of ANXA1 inhibit the oxidative burst produced by human monocyte activation (20, 21). In addition, anti-ANXA1 sera abrogate the inhibitory effect of dexamethasone on monocyte recruitment in a mouse model of peritonitis (22).
In the present study, we have addressed the potential modulating role that endogenous ANXA1 may have on monocyte extravasation by examining the effects of overexpressing directly this molecule in U937 cells as a surrogate of human monocytes. This immortalized cell line has been used in many studies, and the results obtained are generally reported as being applicable to monocyte or macrophage (when using differentiated U937 cells) physiopathology (23). In addition to this, our choice fell on this cell type for three specific reasons: first, we have produced and characterized stably transfected U937 cell clones expressing different amounts of ANXA1 (24); second, using these cell lines, we have demonstrated that ANXA1 modulates U937 cell adhesive properties by interfering with α4β1 (CD49d/CD29) interaction with its endothelial counterpart in vitro (25); third, we have recently shown that wild-type (WT) U937 cell migration in vivo, in a human rheumatoid arthritis (RA) synovium/SCID mouse transplantation model (26), is efficient and modulated by cytokines and chemokines (27). We chose this model of cell migration in vivo for the importance that monocyte recruitment and differentiation into the RA synovium play in the pathogenesis of the disease (28).
The data obtained from these experiments show an association between higher levels of ANXA1 expression and inhibition of U937 cell transmigration across an endothelial cell monolayer in vitro and cell migration to human RA synovium transplanted into SCID mice in vivo.
Materials and Methods
U937 cell culture and transfection
The U937 cell line, a human monomyelocytic cell line, was cultured in RPMI 1640 medium supplemented with 10% FCS, 1% pen-strep, and 2 mM l-glutamine (all reagents from Sigma-Aldrich, Poole, U.K.). Cells were subcultured three times a week and maintained at a concentration of 0.5–1.0 × 106 cells/ml. The generation and characterization of U937 cell stable clones expressing lower or higher amounts of ANXA1 has been described in detail in recent publications (24, 29). As a further control, a clone transfected with the empty plasmid was also used (Fig. 1A). In total, we cultured four distinct sets of cells: WT U937 cells, CMV empty plasmid clone, a sense (S) clone termed 15S, and an antisense (AS) clone termed 36.4AS (Fig. 1A). Transfection of cDNA coding for the first 147 aa of ANXA1 led to the production of the S clone that expressed the bioactive ANXA1 N-terminal fragment of 24 kDa (30). It is noteworthy that transfection of full-length ANXA1 yields a cell clone that spontaneously enters into apoptosis and undergoes cell death within a week of culture (29). Transfection of an AS cDNA to the same portion of the protein produced cells with a reduced (~40%) full-length ANXA1 expression. Cells were maintained in the medium described above supplemented with 0.4 mg/ml geneticin, with the antibiotic being removed from the culture medium at least 3 wk before experimentation.
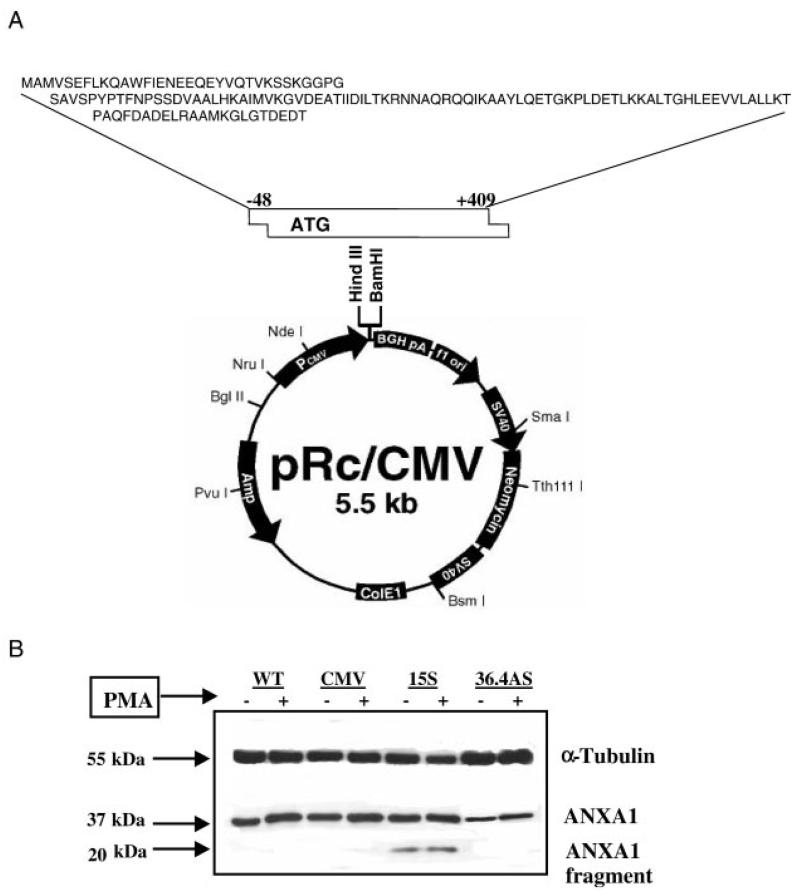
FIGURE 1.
Generation of U937 cell clones. A, Schematic representation of the plasmid produced to transfect U937 cells. The ATG box indicates the nucleotide sequence inserted to generate a peptide spanning from first methionine to aa 136 in the second repeat. B, Representative Western blotting showing ANXA1 expression in WT U937 cells and in three clones, as well as the immunoreactivity for α-tubulin (used for data normalization). Cells were used undifferentiated or following differentiation with PMA (6 ng/ml, 48 h). Clone 15S displays the 20-kDa fragment that corresponds to the sequence shown in A. In three distinct experiments, WT and CMV cells had similar amounts of ANXA1, with 60 ± 5% after PMA addition. The 36.4AS clone had ~40% less protein in both conditions, whereas the clone 15S showed the ANXA1 fragment and an increase varying between 40 and 55% after incubation with PMA.
In selected experiments, U937 cell clones were incubated with 6 ng/ml PMA for 48 h (24, 31) before further analysis of CAM expression (see below).
ANXA1 protein expression
ANXA1 protein expression was analyzed by Western blot according to Solito et al. (24). Briefly, 30 μg of total protein were separated on 10% polyacrylamide gels and electroblotted onto nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Immunostaining was performed using a rabbit polyclonal Ab directed against the whole ANXA1. The blot was then reprobed with an mAb directed against α-tubulin. Data were analyzed semiquantitatively by densitometry and normalized vs α-tubulin levels (24).
Cell surface marker expression
Quantification of CAM expression on the plasma membrane was performed as described (31). Briefly, aliquots of the cell suspension (1–3 × 105 cells in 100 μl) were added to a 96-flat well plate in triplicate and incubated with specific mAbs (all at the saturating concentration of 20 μg/ml). Nonspecific binding was minimized by the addition of human IgG (6 mg/ml). Plates were incubated at 4°C for 1 h. Cells were then washed and incubated with F(ab′)2 of FITC-conjugated goat anti-mouse IgG for 30 min before further washing and analyzed immediately after by flow cytometry. A BD Biosciences FACScan II analyzer with air-cooled 100 mW argon ion laser tuned to 488 nm and Consort 32 computer running Lysis II software (BD Biosciences, Mountain View, CA) was used. At least 5000 events were analyzed for each labeling. Data were analyzed as unit of fluorescence measured in the FL1 channel.
The following murine mAbs anti-human Ags were purchased from Serotec (Oxford, U.K.): clone B-C14 for ICAM-1, clone HC1/6 for platelet-endothelial CAM-1 (PECAM-1), clone B-B15 for CD11a, clone 44 for CD11b, clone 3.9 for CD11a, and clone 44716.111 for CXCR4. The anti-CD49d mAb was clone 2B4 (R&D Systems, Abingdon, U.K.).
U937 cell chemotaxis assay
For this assay, we have used the commercially available Neuroprobe ChemoTxplate 96-well plate (Receptor Technologies, Adderbury, U.K.) with polycarbonate membrane filters and 5-μm membrane pores. U937 cells were washed and resuspended at a concentration of 4 × 106/ml in medium (RPMI 1640 supplemented with 0.1% BSA). Human recombinant CXC chemokine ligand (rCXCL)12 (stromal cell-derived factor (SDF)-1α; 1 or 10 ng/ml; PeproTech, London, U.K.) or medium were added to the bottom wells (27 μl), the filter was placed on top, and 25 μl of the U937 cell suspension was placed above the membrane. The plate was incubated for 2 h in a humidified incubator at 37°C with 5% CO2. Cells remaining on top of the filter were absorbed off and the surface was washed with the vehicle. The plate was then centrifuged (312 × g, 5 min), the filter was removed, pelleted cells resuspended, and 20 μl removed and counted by light microscopy. In selected experiments, anti-human CXCR4 mAb (clone 44716.111; Serotec) was preincubated at the saturating concentration of 20 μg/ml with U937 cells for 30 min at 37°C, before cell addition to the plate. In all cases, data are reported as the mean ± SEM of migrated cells per well.
EA.hy926 culture conditions and transmigration assay
EA.hy926 cells were provided by Dr. C.-J. Edgell (University of North Carolina, Chapel Hill, NC). EA.hy926 cells are a hybridoma between HUVECs and the epithelioma A549, and retain most of the features of HUVEC, including the expression of endothelial adhesion molecules and human factor VIII-related Ag (32). EA.hy926 cells were cultured in DMEM-F12 supplemented with 10% FCS and antibiotics (cultured medium) and subcultured every 3 days. The transmigration assay was performed using a protocol modified from a previous study (33). After washes in PBS, EA.hy926 cells were added (5 × 104 cells in 500 μl of cultured medium) and cultured onto inserts (8 μm) pore Biocoat 24-well plates (Stratech Scientific, Oxford, U.K.) for 48 h. U937 cell clones were added at 1.5 × 106 cells per well on top of inserts, while 0–50 ng/ml SDF-1α (CXCL12) were added in the bottom compartment. After 18 h at 37°C in 95% air/5% CO2, cells which had migrated through the filters were retrieved from the lower compartment and counted using a Neubauer hema-cytometer (Sigma-Aldrich) following staining in Turk’s solution. In some experiments, anti-human CXCR4 mAb (20–50 μg/ml; clone 44716.111; Serotec) was added to cells for 30 min before cell addition into the Transwell. In other cases, the effect of a neutralizing anti-ANXA1 mAb (25, 34) on WT U937 cell transmigration was determined in comparison to a similar concentration (5 μg/ml) of mouse IgG2a (Serotec). The anti-CD49d mAb was used at a final concentration of 20 μg/ml, again preincubated with the cells from 30 min before addition to the upper compartment.
Apoptosis
U937 cells that remained in the upper well of the Transwell system were collected and the degree of early apoptosis was measured following staining with FITC-labeled ANXA5 as described (35). Flow cytometry was performed with a FACScan II analyzer (BD Biosciences) with a 15-mW argon ion laser (488 nm) and a Consort 32 computer running Lysis II software. ANXA5 staining was detected in the FL1 (green) channel, whereas PI staining was monitored in the FL2 (red) channel.
U937 cell migration into human synovium transplanted onto SCID mice
Beige SCID C.B-17 mice were anesthetized and samples of human synovial tissue inserted s.c. in the dorsal area. Human synovial samples were obtained at joint replacement surgery from RA patients (diagnosed according to the American College of Rheumatology criteria) after informed consent approved by the Ethics Committee (Local Research Ethics Committee 98/11/27). Recent studies have described this methodology in details, including the >90% rate of success of human tissue transplantation, the maintenance of human adhesion molecules by the graft microvascular endothelium, and the formation of patent and functional anastomoses between the mouse subdermal circulation and human graft vessels (26, 27).
CMV or 15S U937 cell clones (20 × 106 cells) were incubated with 5 ml of PKH26 dye (1/50 diluted in diluent buffer; Sigma-Aldrich) for 2 min at room temperature. Reaction was stopped by addition of heat-inactivated FCS, and cells were then washed twice in sterile PBS (pH 7.6) at a final concentration of 50 × 106 cells/ml. Cell viability was determined via trypan blue exclusion and always found to be >95%. PKH26 labeling efficiency was confirmed before injection by examining a wet preparation of labeled U937 cells under the UV microscope.
PKH26-labeled U937 cells (CMV or 15S clone; 5 × 106 cells/100 μl) were injected into the tail vein of grafted SCID mice. At the same time, to evaluate baseline and chemokine-induced migration, 1 μg SDF-1α (PeproTech) in 100 μl of sterile saline or saline alone were injected directly into the graft. Animals were killed 48 h later, grafts extracted and immediately snap frozen. U937 cell migration into the grafts was assessed histologically by UV fluorescence microscopy. To obtain an accurate representation of the number of PKH26 positive cells present in the synovial grafts, three different sections taken from a different cutting level (i.e., from the top, bottom, and middle of the transplant) of each graft were analyzed. Sections were washed for 5 min in PBS (pH 7.6), mounted using aqueous mounting media (Immunofluor; ICN Pharmaceuticals, Costa Mesa, CA), and analyzed using a fluorescence microscope (Olympus BX-60; Olympus, Melville, NY). The results were expressed as the average number of cells identified in each section per high power field (×40 objective). On average, ~100 high power fields were counted per transplant.
Data and statistics
Data are shown as mean ± SEM, with n = 3–6 for the in vitro experiments (performed in triplicate or quadruplicate), and n = 6 mice for the experiments of U937 cell migration into the human synovium transplanted onto SCID mice. Statistical differences were analyzed with ANOVA followed by Bonferroni test, taking a p value <0.05 as significant.
Results
ANXA1 expression in WT and transfected U937 cells in resting and stimulated conditions
Four clones of U937 cells were characterized and used throughout the entire study. Fig. 1A shows the construct used to generate the 15S clone, with different levels of ANXA1. Fig. 1B illustrates the expression of ANXA1 species in the cell clones, in resting conditions as well as after cell activation with PMA (6 ng/ml for 48 h). WT U937 cells and CMV clone displayed similar amounts of the protein in resting condition and appeared to respond similarly to PMA stimulation with an approximate increase of 60% in ANXA1 immunoreactivity (Fig. 1B). In the 15S clone, the ANXA1 fragment (~20 kDa) was expressed in basal condition, and its levels were augmented after PMA stimulation (+57%).
In line with a previous study (24), U937 clone transfected with the AS cDNA (clone 36.4AS) showed ~40–60% reduction in protein expression respect WT and CMV clones, in resting conditions as well as after cell stimulation.
As expected (31, 36, 37), resting WT U937 cells had detectable levels of CD54, CD31, and CD11a on their cell surface (Table I). Cell incubation with PMA led to a marked increase in CD54 and CD11a expression as well as to up-regulation of CD31, CD11b, CD11c, and CD49d. There was no apparent difference in CAM expression among all four clones, both in resting conditions and after PMA stimulation (Table I).
Table I. Analysis of selective cell surface markers on resting and differentiated U937 cell clonesa.
| U9337 Cell Clone |
|||||
|---|---|---|---|---|---|
| Marker | Treatment | WT | CMV | 36.4AS | 15S |
| ICAM-1 | None | 400 ± 20 | 380 ± 20 | 370 ± 15 | 410 ± 20 |
| ICAM-1 | PMA | 1250 ± 50 | 1400 ± 150 | 1200 ± 200 | 1300 ± 100 |
| PECAM-1 | None | 450 ± 20 | 470 ± 30 | 380 ± 30 | 500 ± 50 |
| PECAM-1 | PMA | 600 ± 20 | 700 ± 50 | 500 ± 40 | 650 ± 40 |
| CD11a | None | 410 ± 10 | 400 ± 20 | 400 ± 10 | 400 ± 15 |
| CD11a | PMA | 1000 ± 50 | 1100 ± 50 | 1050 ± 50 | 1000 ± 30 |
| CD11b | None | 40 ± 5 | 42 ± 4 | 38 ± 5 | 45 ± 5 |
| CD11b | PMA | 200 ± 15 | 205 ± 15 | 210 ± 5 | 200 ± 5 |
| CD11c | None | 85 ± 5 | 70 ± 10 | 75 ± 5 | 88 ± 4 |
| CD11c | PMA | 215 ± 15 | 230 ± 20 | 200 ± 10 | 235 ± 25 |
| CD49d | None | 11 ± 3 | 13 ± 3 | 13 ± 2 | 20 ± 10 |
| CD49d | PMA | 90 ± 16 | 81 ± 9 | 74 ± 13 | 75 ± 5 |
| CXCR4 | None | 1250 ± 100 | 800 ± 20 | 830 ± 30 | 850 ± 50 |
| CXCR4 | PMA | 250 ± 20 | 300 ± 30 | 250 ± 15 | 280 ± 20 |
Values (mean ± SEM) are in median fluorescence intensity units of n = 4 determinations. Cell incubation with PMA (6 ng/ml, 48 h) significantly up-regulated adhesion molecule expression and down-regulated CXCR4 levels, and this occurred equally in all clones.
The CXCL12 receptor CXCR4 was expressed on the cell surface of resting U937 cells. In this case, there was a modest difference between CMV and WT clones. However, modification of ANXA1 levels in the 15S and 36.4AS clones did not alter CXCR4 expression compared with CMV control clone. Similarly, PMA activation led to a marked reduction in CXCR4 immunoreactivity, and this occurred equally in all clones (Table I).
ANXA1 transfection does not inhibit U937 cell chemotactic response to SDF-1α (CXCL12)
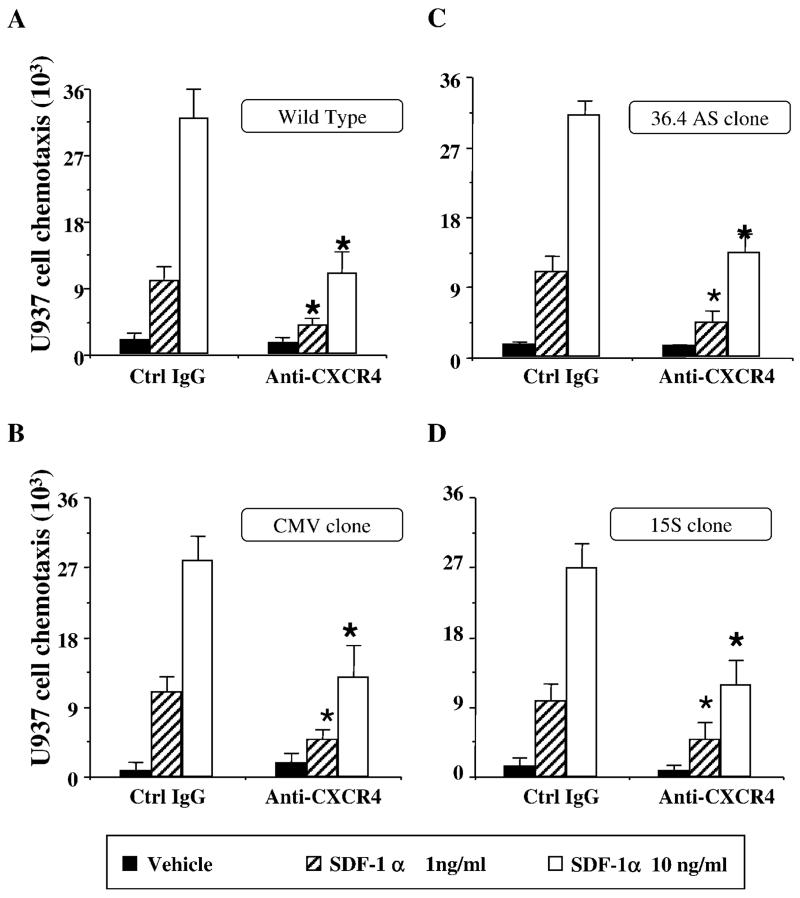
To monitor the potential effects of different ANXA1 levels on U937 cell migration, we began from the chemotaxis assay. The observed CXCR4 expression prompted the use of SDF-1α (CXCL12) as the chemotactic stimulus of choice. In fact, this cell line does not respond to other classical chemoattractant, including FMLP (because the receptor for this peptide is only expressed upon differentiation; Ref. 38). Two hours of incubation at 37°C was sufficient to produce a measurable chemotactic response of WT U937 cells to SDF-1α (CXCL12); the effect of the chemokine was concentration-dependent and was markedly attenuated by cell preincubation with an anti-CXCR4 mAb (Fig. 2A). Cell transfection with the plasmid did not modify cell locomotion to this chemotactic stimulus as seen using the CMV empty clone (Fig. 2B). Similar to the WT, CMV U937 cell clone chemotaxis was significantly reduced by the anti-CXCR4 mAb. Alteration of basal ANXA1 protein levels did not affect U937 cell locomotion to the SDF-1α (CXCL12) chemotactic gradient, because clones 15S and 36.4AS responded as well as WT and CMV cells. (Fig. 2, C and D). Likewise, and as expected from the same level of CXCR4 expression, the chemotactic response of clones 15S and 36.4AS were comparably inhibited by anti-CXCR4 mAb treatment (Fig. 2, C and D).
FIGURE 2.
ANXA1 transfection does not modify U937 cell chemotactic response to SDF-1α. Cells (105) were added to the top compartment of a Neuroprobe ChemoTxplate 96-well plate and chemotaxis in response to SDF-1α (1–10 ng/ml) was determined after a 2-h incubation at 37°C. The effect of preincubation with an anti-CXCR4 mAb (20 μg/ml) was also determined. Data are mean ± SEM of n = 3 experiments performed in quadruplicate. The anti-CXCR4 mAb produced a similar degree of inhibition in all cell clones: *, p < 0.05 vs respective control.
ANXA1 transfection inhibits U937 cell transmigration in response to SDF-1α (CXCL12) in vitro
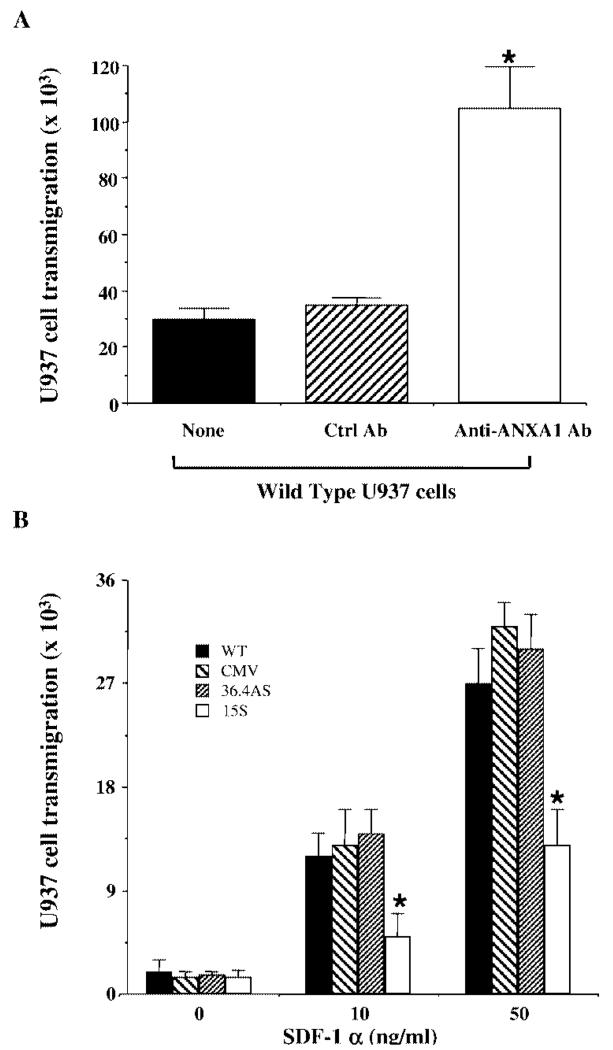
The potential effect of different endogenous ANXA1 levels on U937 cell trans-endothelial migration was addressed using the Transwell system. Initially, we studied the behavior of the WT U937 cells using an overnight assay and a monolayer of EA.hy926 cells shown to be suitable for leukocyte transmigration (15). These cells migrated in response SDF-1α (CXCL12), although in this condition, higher concentrations of the chemokine were required (Fig. 3) compared with the chemotaxis assay. To examine indirectly the effects of endogenous ANXA1 on U937 cell transmigration in this assay, we first conducted experiments in the presence and absence of a neutralizing anti-ANXA1 serum. The results shown in Fig. 3A demonstrated a significantly higher (~3-fold) degree of transmigration when WT U937 cells were coincubated with an anti-ANXA1, but not control rabbit serum. Therefore, these experiments indicate indirectly that endogenous ANXA1 expressed in U937 cells is capable of inhibiting the phenomenon of transmigration. This result was then confirmed directly using the clones described above. Fig. 3B shows that CMV and 36.4AS clones migrated in response SDF-1α (CXCL12) in a similar fashion to WT cells. In contrast, the clone 15S displayed a reduced degree of migration, with approximate inhibitions of 50–60% for both 10 and 50 ng/ml SDF-1α (CXCL12; Fig. 3B).
FIGURE 3.
ANXA1 transfection inhibits U937 cell transmigration in response to SDF-1α. A, Effect of endogenous ANXA1 as determined by WT U937 cell incubation with a neutralizing mAb (1 μg/ml) in relation to a control mAb or untreated cells, on cell transmigration promoted by 50 ng/ml SDF-1α using the Transwell system (see Materials and Methods). Data are mean ± SEM of three experiments performed in triplicate. *, p < 0.05 vs control mAb. B, Comparison of the migratory ability of U937 cell clones in the Transwell system using 0–50 ng/ml SDF-1α. Data are mean ± SEM of five experiments performed in triplicate. *, p < 0.05 vs CMV empty plasmid clone.
The effect of the anti-CXCR4 mAb in the transmigration assay was then determined. This mAb produced a similar degree of inhibition in all four cell types. Using 10 ng/ml SDF-1α and 20 μg/ml of anti-CXCR4 mAb, values of inhibition ranged from 30–40% on all cell types (p < 0.05 for the effect of mAb against control migration for each cell type, and p > 0.05 across the four cell clones; n = 3 experiments in triplicate). Against the higher concentration of SDF-1α (50 ng/ml), the mAb produced again a similar degree of inhibition (~50–60%) across the four cell types, but had to be used at 50 μg/ml final concentration (mean ± SEM of n = 3 experiments, p > 0.05 for all cell types).
In some of the experiments of transmigration, cells that had not migrated were recovered from the top well and tested for their ability to bind the FITC-labeled ANXA5 probe (29). For all cell clones, low values of apoptosis were measured with no differences between CMV and 15S or 36.4AS clones: 5 ± 2, 7 ± 3, and 10 ± 4% of positive cells, respectively (n = 4 determinations). Therefore, the reduced degree of U937 cell emigration for the clone 15S reported in Fig. 3B was not due to a higher degree of apoptosis, but it was rather genuinely linked to the process of migration itself.
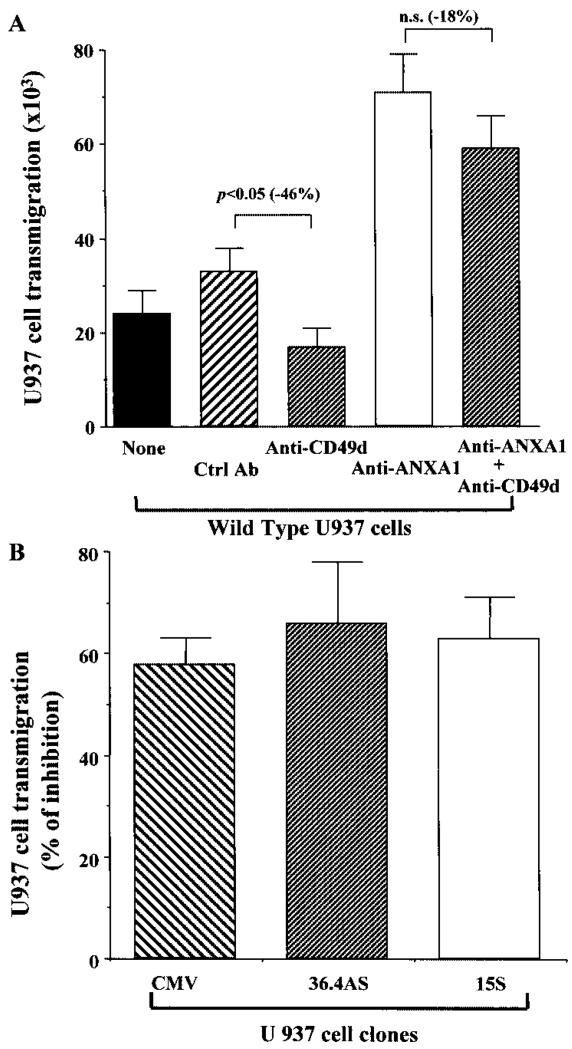
Finally, in view of the demonstrated effects of the endogenous ANXA1 in this assay, we used this experimental system to gain some information on the potential mechanisms involved. In our previous study in which U937 cell adhesion to endothelial cell monolayers was investigated, we demonstrated that a possible mechanism of action of ANXA1 was to block CD49d function (25). Therefore, the effect of an anti-CD49d mAb successfully used in adhesion assays (25) was tested in the transmigration experiments. Fig. 4A shows that WT U937 cell incubation with this mAb reduced cell transmigration by ~50%. In addition, the potentiating effect displayed by the anti-ANXA1 rabbit serum (shown also in Fig. 3A) was partially affected by the anti-CD49d mAb (<20% inhibition). The functional involvement of endogenous CD49d was similar in all clones tested, producing ~60% inhibition in CMV, 15S, and 36.4AS U937 cell clones (Fig. 4B).
FIGURE 4.
Potential functional interaction of endogenous CD49d and ANXA1. A, The effect of an anti-CD49d mAb (20 μg/ml) alone or in combination of the anti-ANXA1 mAb (described in Fig. 3A) on WT U937 cell transmigration to SDF-1α (50 ng/ml) was determined. Data are mean ± SEM of three experiments performed in triplicate. B, Anti-CD49d mAb produced a similar degree of inhibition on SDF-1α (50 ng/ml) induced transmigration of CMV empty plasmid, 36.4AS, and 15S clones (p < 0.05, n = 4 in all three cases).
ANXA1 transfection inhibits U937 cell migration in response to SDF-1α (CXCL12) in vivo
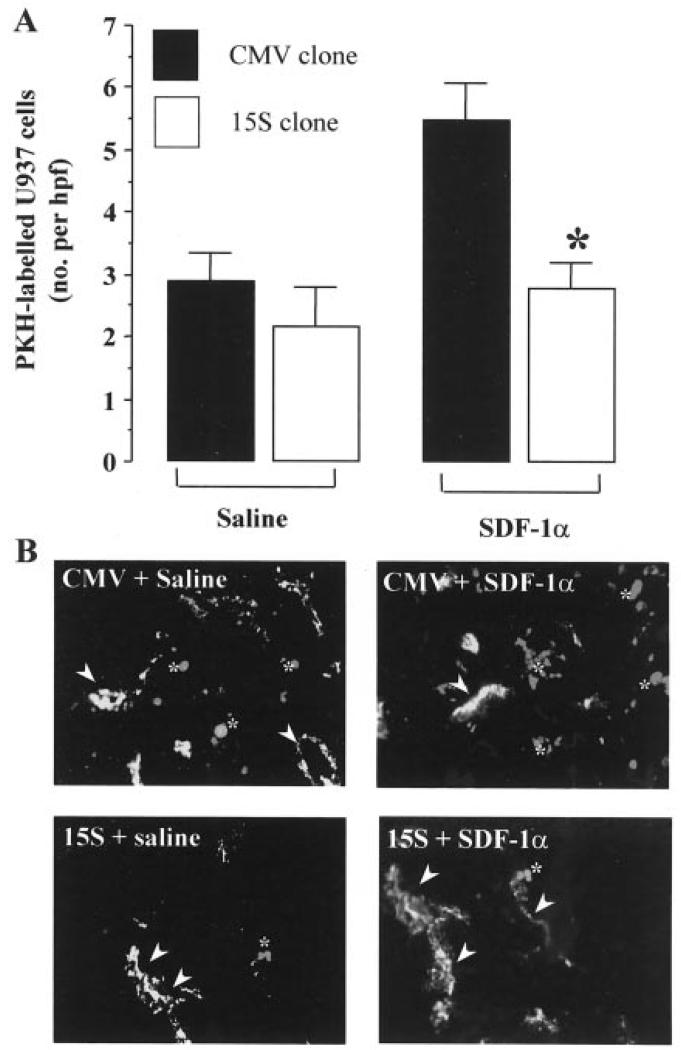
In the final experimental section of our study, we sought to give in vivo relevance to the ANXA1 effects on U937 cell transmigration described above. Carrying out these types of experiments in humans can be difficult and burdened with ethical issues. For this reason, we turn to our human-SCID transplantation model in which the migration of human cells to human tissues living in the animals can be studied under controlled experimental conditions. Recently, we have demonstrated that intragraft injection of SDF-1α caused a dose-dependent increase in the migration of WT U937 cells to human synovial grafts, an effect that was CXCR4 dependent. In addition, SDF-1α-induced U937 cell migration was more powerful than that produced by TNF-α (27). Given the results obtained in the transmigration assay in vitro (reported in Fig. 3B) and the complexity of the human-SCID transplantation model, we limited this part of the study to two U937 clones only, CMV and 15S. PKH26-labeled CMV or 15S U937 cells were injected i.v. into grafted animals and the migration into the transplants in response to SDF-1α or saline quantified as described in Materials and Methods. Clone 15S displayed a similar degree of basal extravasation as the CMV empty plasmid clone (Fig. 5A). In line with our previous study with WT U937 cells, local (intrasynovial) injection of SDF-1α (CXCL12) produced a significant increase in the extravasation of CMV U937 cells. However, clone 15S showed a marked inhibition in its ability to migrate in response to this chemokine, with values of U937 cells counted in the grafts within the range of those measured in absence of SDF-1α injection (Fig. 5A). This is also visualized in the histological microphotographs shown in Fig. 5B.
FIGURE 5.
ANXA1 transfection inhibits U937 cell migration in response to local application of SDF-1α in SCID mice. Fragments of human rheumatoid synovium were grafted on the dorsal area of SCID mice as described in Materials and Methods. A, Saline (100 μl) or SDF-1α (1 μg) was applied locally into the synovium, and the accumulation of PKH26-labeled U937 cell clones evaluated at the 48-h time point. Data are mean ± SEM of six mice per group. *, p < 0.05 vs CMV group. B, Representative microphotographs showing PKH26 dye fluorescence of U937 cells migrated into the human synovium. Upper left panel, CMV group injected with saline; upper right panel, CMV group injected with SDF-1α; lower left panel, 15S clone injected with saline; lower right panel, 15S clone injected with SDF-1α. Arrowheads indicate Factor VIII positive human microvascular endothelium, whereas asterisks indicate representative PKH26-labeled U937 cells.
Discussion
The major result of this study is the demonstration that modulation of endogenous ANXA1 levels selectively inhibits the transmigration ability of a monocytic cell line. Equally important is the relevance of this phenomenon to in vivo extravasation in an experimental model that mimics the leukocyte influx observed in RA.
Most of the studies that have addressed the pharmacological and physiopathological role of ANXA1 in the process of leukocyte migration have so far focused on the polymorphonuclear leukocyte (reviewed in Ref. 39). In this leukocyte type, endogenous ANXA1 is externalized on the cell surface selectively after adhesion to endothelium in vitro (15) and in vivo (40). At this level, cell surface ANXA1 inhibits trans-endothelial passage (19) possibly by interacting with the receptor for FMLP (16) and/or with other receptors of this family (41).
Human monocytes contain high levels of ANXA1 not only in the circulation (18) but also in monocytes/macrophages that are resident in the synovium (42). In vitro, monocytes mobilize ANXA1 during cell adhesion, although less than PMN prepared from the same donor (43). Although it is clear that exogenous application of full-length ANXA1 down-regulate monocyte functions in vitro (20, 21) and monocyte extravasation in vivo (22), no studies have yet addressed the role that the endogenous protein plays in this cell type.
To address this question, we have used the strategy of altering the levels of ANXA1 gene expression in U937 cells. The choice of this cell line was quite straightforward for the following reasons. First, several studies have used U937 cells to draw mechanistic conclusions relevant to the process of monocyte activation and migration (e.g., Refs. 44 and 45-47). Second, U937 cell clones expressing different levels of ANXA1 have recently been produced, and initially characterized with regard to eicosanoid generation (24) and susceptibility to apoptosis (29). Finally, using WT U937 cells we have demonstrated that ANXA1 translocates on the cell surface upon adhesion to endothelial cell monolayers (25), a feature that resembles the data obtained with human monocytes (43). Because transfection of full-length ANXA1 causes high incidence of cell death (29), we used the 15S and 36.4AS clones, previously shown to display modest incidence of apoptosis in resting conditions (35). Importantly, this observation was also repeated in this study with no particular difference in the basal degree of apoptotis among the four cell clones as measured following the transmigration assay. The use of clone 15S as a tool to investigate the role of endogenous ANXA1 is supported by the fact that the fragment expressed in this clone (spanning from the N-terminal down to the first portion of the second repeat; Ref. 48) retains the biological activity of full-length ANXA1 (8, 30, 49).
A further important aspect that we verified in the present study was the level of adhesion molecule surface expression in the various U937 clones used. This was necessary to exclude that some of the ANXA1 effects observed in our experimental settings might have been explained by a differential expression of surface CAMs. In line with previous studies (31, 44), we found modest or no difference at all in the basal as well as PMA-stimulated expression of CD54, CD31, CD11a to c, CD49d, and CXCR4 across the four cell lines used. Likewise, a similar chemotaxis response to SDF-1α (CXCL12) was observed in WT and the three U937 cell clones used.
In contrast to this, a striking difference was seen in the capacity of various U937 clones to bind and migrate across an endothelial barrier. Overexpression of the ANXA1 bioactive long fragment greatly reduced the extent of U937 cell transmigration (clone 15S). This effect is highly unlikely to be due to a different degree of cell susceptibility to the stimulus used, because as mentioned above, CXCR4 expression was homogenous among the three clones (with a modest decrease vs WT U937 cells). In addition and more importantly, there were no differences in the extent of SDF-1α-induced chemotaxis and inhibition by the anti-CXCR4 mAb among all cell types. Combined analysis of the data obtained in these two assays also indicates that it is very unlikely that endogenous ANXA1, including the transgenic fragment, can directly alter SDF-1α (CXCL12) binding to CXCR4. In addition, we have already shown that ANXA1 and CXCR4 did not colocalize after CXCL12-induced U937 cell activation and chemotaxis (25).
Thus, the different level of ANXA1 expression appears directly responsible for the lower degree of trans-endothelial migration. This is in line with that observed in human neutrophils, where the endogenous ANXA1 pathway becomes operative selectively following interaction with endothelial cells (15). We propose that the engagement of leukocyte adhesion receptors with the endothelial counterpart produces the signal necessary to externalize ANXA1, as visualized for human neutrophils (50) and U937 cells (25). This hypothesis is further supported by the notion that cell interaction with the porous filter occurring during chemotaxis does not provide the appropriate signal for ANXA1 externalization. The situation is clearly different when leukocytes interact with endothelial monolayers. We have shown that human neutrophil adhesion to molecules expressed by endothelial cells such as CD54 or CD31 is sufficient to trigger “controlled exocytosis” and export ANXA1 from the cytosol onto the plasma membrane (50). Importantly, resting WT and transfected U937 cells express both CD31 that can form homologous interaction with endothelial CD31 (2), and CD11a that can interact with endothelial CD54 (51). Reciprocally, the endothelial cell line used in our experiment expresses high levels of both CD31 and CD54 (32, 33). Thus, we propose that similar adhesive interactions are responsible for ANXA1 externalization when human neutrophils, U937 cells, and, by extension, human monocytes engage endothelial ligands during the process of extravasation.
The last series of experiments addressed probably the most important question of the present investigation, providing relevance for ANXA1 modulatory action in human chronic inflammatory pathologies. To this end, we have used a model of cell migration into human rheumatoid synovium grafted onto SCID mice in which mouse subdermal vessels form anastomoses with human vessels that maintain the expression of human CAMs (26). The anastomoses are patent and functional as shown by the capacity to deliver Abs and human cells to the grafts via the murine systemic circulation (26). More recently, this model has been validated for WT U937 cells (27). In that paper, we showed that SDF-1α (CXCL12) induced a dose-dependent increase in the migration of WT U937 cells to human synovial grafts. In addition, we demonstrated that the effects of the chemokines were CXCR4-dependent and more powerful than TNF-α (27). In this current paper, in addition to confirming those findings, we show that expression of the bioactive fragment of ANXA1 inhibits U937 cell (clone 15S) migration to the synovial transplants in response to intragraft injection of SDF-1α (CXCL12). ANXA1 inhibitory action is unlikely to be restricted to a specific stimulus, because preliminary experiments have indicated that clone 15S displays reduced accumulation also in response to TNF-α injection into the grafts (M. C. Blades and C. Pitzales, unpublished observations). Thus, the mechanisms responsible for ANXA1 efficacy in transmigration assay in vitro are likely to be also applicable to the in vivo situation.
Finally, despite the reasonable caution for the use of a monocyte surrogate, these findings may have implications for the anti-inflammatory efficacy of glucocorticoids. These drugs are widely used to control RA and other chronic inflammatory pathologies characterized by mononuclear cell accumulation into specific tissue sites, a phenomenon they potently affect (52). ANXA1 synthesis was originally shown to be under glucocorticoid controls, not only in U937 cells vitro (53), but also following single steroid administration to humans (18). The in vivo data generated in this study together with our previous study on U937 cell adhesion (25), also in relation to the large literature linking steroids and ANXA1 expression (e.g., Refs. 54 and 55), suggest that at least part of the antimigratory action displayed by these potent anti-inflammatory drugs could be via an increase of leukocyte-associated ANXA1.
In summary, we have demonstrated that endogenous ANXA1 modulates the transmigration process of a monomyelocytic cell line both in vitro and in vivo. Because the accumulation of cells of the monocyte/macrophage lineage represents a critical component in chronic inflammation, these findings contribute to explain the powerful therapeutic effects of glucocorticoids. In addition, it is likely that the same mechanisms are involved in the endogenous homeostatic machinery responsible for down-modulating cell migration during resolution of an inflammatory response.
Footnotes
This work was supported by the Arthritis Research Campaign U.K., Fellowship P0567 (to M.P.) and Project Grant P0547 (to M.C.B.), and by the Wellcome Trust Fellowship 04783/Z/96/Z (to C.P.).
Abbreviations used in this paper: CAM, cell adhesion molecule; ANXA, annexin; AS, antisense; SDF, stromal cell-derived factor; WT, wild type; RA, rheumatoid arthritis; S, sense; CXCL, CXC chemokine ligand; PECAM-1, platelet endothelial CAM-1.
References
- 1.Panés J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br. J. Pharmacol. 1999;126:537. doi: 10.1038/sj.bjp.0702328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller WA. Migration of leukocytes across vascular intima: molecules and mechanisms. Trends Cardiovasc. Med. 1995;5:15. doi: 10.1016/1050-1738(94)00028-T. [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu K, Harris N, Granger DN. Modulation of leukocyte migration in mesenteric interstitium. Am. J. Physiol. 1994;267:H1573. doi: 10.1152/ajpheart.1994.267.4.H1573. [DOI] [PubMed] [Google Scholar]
- 4.Cronstein BN, Weissmann G. Targets for antiinflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 1995;35:449. doi: 10.1146/annurev.pa.35.040195.002313. [DOI] [PubMed] [Google Scholar]
- 5.Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol. Sci. 1997;18:418. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- 6.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 7.Flower RJ, Rothwell NJ. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 1994;15:71. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 8.Perretti M, Ahluwalia A, Harris JG, Goulding NJ, Flower RJ. Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse: a qualitative comparison with an anti-CD11b monoclonal antibody. J. Immunol. 1993;151:4306. [PubMed] [Google Scholar]
- 9.Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation. 1997;21:583. doi: 10.1023/a:1027330021479. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Hutchinson P, Morand EF. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999;42:1538. doi: 10.1002/1529-0131(199907)42:7<1538::AID-ANR29>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. USA. 1998;95:14535. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J. Immunol. 1993;150:992. [PubMed] [Google Scholar]
- 13.Teixeira MM, Das AM, Miotla JM, Perretti M, Hellewell PG. The role of lipocortin 1 in the inhibitory action of dexamethasone on eosinophil trafficking in cutaneous inflammatory reactions in the mouse. Br. J. Pharmacol. 1998;123:538. doi: 10.1038/sj.bjp.0701625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso F, Flower RJ, Perretti M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule: involvement of endogenous lipocortin 1. J. Immunol. 1995;155:377. [PubMed] [Google Scholar]
- 15.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 1996;22:1259. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 16.Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell. 2000;5:831. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 17.Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 2001;158:1969. doi: 10.1016/S0002-9440(10)64667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulding NJ, Godolphin JL, Sharland PR, Peers SH, Sampson M, Maddison PJ, Flower RJ. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990;335:1416. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- 19.Perretti M, Flower RJ. Measurement of lipocortin 1 levels in murine peripheral blood leukocytes by flow cytometry: modulation by glucocorticoids and inflammation. Br. J. Pharmacol. 1996;118:605. doi: 10.1111/j.1476-5381.1996.tb15444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maridonneau-Parini I, Errasfa M, Russo-Marie F. Inhibition of O2− generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Invest. 1989;83:1936. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Euzger HS, Flower RJ, Goulding NJ, Perretti M. Differential modulation of annexin I binding sites on monocytes and neutrophils. Mediators Inflamm. 1999;8:53. doi: 10.1080/09629359990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997;120:1075. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hass R, Bartels H, Topley N, Hadam M, Kohler L, Goppelt-Strube M, Resch K. TPA-induced differentiation and adhesion of U937 cells: changes in ultrastructure, cytoskeletal organization and expression of cell surface antigens. Eur. J. Cell Biol. 1989;48:282. [PubMed] [Google Scholar]
- 24.Solito E, Raguenes-Nicol C, de Coupade C, Bisagni-Faure A, Russo-Marie F. U937 cells deprived of annexin 1 demonstrate an increased PLA2 activity. Br. J. Pharmacol. 1998;124:1675. doi: 10.1038/sj.bjp.0701991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solito E, Romero IA, Marullo S, Russo-Marie F, Weksler BB. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the α4β1 integrin. J. Immunol. 2000;165:1573. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- 26.Wahid S, Blades MC, De Lord D, Brown I, Blake G, Yanni G, Haskard DO, Panayi GS, Pitzalis C. Tumour necrosis factor-α (TNF-α) enhances lymphocyte migration into rheumatoid synovial tissue transplanted into severe combined immunodeficient (SCID) mice. Clin. Exp. Immunol. 2000;122:133. doi: 10.1046/j.1365-2249.2000.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, Pitzalis C. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID mice. Arthritis Rheum. 2002;46:824. doi: 10.1002/art.10102. [DOI] [PubMed] [Google Scholar]
- 28.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Annu. Rheum. Dis. 1994;53:39. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solito E, de Coupade C, Canaider S, Goulding NJ, Perretti M. Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation. Br. J. Pharmacol. 2001;133:217. doi: 10.1038/sj.bjp.0704054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Relton JK, Strijbos PJLM, O’Shaughnessy CT, Carey F, Forder RA, Tilders FJ, Rothwell NJ. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J. Exp. Med. 1991;174:305. doi: 10.1084/jem.174.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perretti M, Wheller SK, Harris JG, Flower RJ. Modulation of ICAM-1 levels on U-937 cells and mouse macrophages by interleukin-1β and dexamethasone. Biochem. Biophys. Res. Comm. 1996;223:112. doi: 10.1006/bbrc.1996.0854. [DOI] [PubMed] [Google Scholar]
- 32.Edgell C-J, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA. 1983;80:3734. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheller SK, Perretti M. Dexamethasone inhibits cytokine-induced intercellular adhesion molecule-1 up-regulation on endothelial cell lines. Eur. J. Pharmacol. 1997;331:65. doi: 10.1016/s0014-2999(97)01015-7. [DOI] [PubMed] [Google Scholar]
- 34.Pepinsky RB, Sinclair LK, Dougas I, Liang C-M, Lawton P, Browning JL. Monoclonal antibodies to lipocortin-1 as probes for biological functions. FEBS Lett. 1990;261:247. doi: 10.1016/0014-5793(90)80564-y. [DOI] [PubMed] [Google Scholar]
- 35.Canaider S, Solito E, de Coupade C, Flower RJ, Russo-Marie F, Goulding NJ, Perretti M. Increased apoptosis in U937 cells over-expressing lipocortin 1 (annexin I) Life Sci. 2000;66:L265. doi: 10.1016/s0024-3205(00)00500-2. [DOI] [PubMed] [Google Scholar]
- 36.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell. Immunol. 1994;156:191. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 37.Hoff T, Spencker T, Emmendoerffer A, Goppelt-Struebe M. Effects of glucocorticoids on TPA-induced monocytic differentiation. J. Leukocyte Biol. 1992;52:173. doi: 10.1002/jlb.52.2.173. [DOI] [PubMed] [Google Scholar]
- 38.Kew RR, Peng T, DiMartino SJ, Madhavan D, Weinman SJ, Cheng D, Prossnitz ER. Undifferenziated U937 cells transfected with chemoattractant receptors: a model system to investigate chemotactic mechanisms and receptor structure/function relationships. J. Leukocyte Biol. 1997;61:329. doi: 10.1002/jlb.61.3.329. [DOI] [PubMed] [Google Scholar]
- 39.Perretti M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen. Pharmacol. 1998;31:545. doi: 10.1016/s0306-3623(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 40.Oliani SM, Paul-Clark MJ, Christian HC, Flower RJ, Perretti M. Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am. J. Pathol. 2001;158:603. doi: 10.1016/S0002-9440(10)64002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La M, D’Amico M, Bandiera S, Di Filippo C, Oliani SM, Gavins FN, Flower RJ, Perretti M. Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: analysis of their mechanism of action. FASEB J. 2001;15:2247. doi: 10.1096/fj.01-0196com. [DOI] [PubMed] [Google Scholar]
- 42.Goulding NJ, Dixey J, Morand EF, Dodds RA, Pitsillides AA, Edwards JCW. Differential distribution of annexins-I, -II, -IV, and -VI in synovium. Annu. Rheum. Dis. 1996;54:841. doi: 10.1136/ard.54.10.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perretti M, Wheller SK, Flower RJ, Wahid S, Pitzalis C. Modulation of cellular annexin I in human leukocytes infiltrating DTH skin reactions. J. Leukocyte Biol. 1999;65:583. doi: 10.1002/jlb.65.5.583. [DOI] [PubMed] [Google Scholar]
- 44.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte α4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J. Exp. Med. 2001;193:1149. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lastres P, Almendro N, Bellón T, López-Guerrero JA, Eritja R, Bernabéu C. Functional regulation of platelet/endothelial cell adhesion molecule-1 by TGF-β1 in promonocytic U-937 cells. J. Immunol. 1994;153:4206. [PubMed] [Google Scholar]
- 46.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J. Biol. Chem. 1997;272:6972. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 47.Cavender DE, Edelbaum D, Welkovich L. Effects of inflammatory cytokines and phorbol esters on the adhesion of U937 cells, a human monocyte-like cell line, to endothelial cell monolayers and extracellular matrix proteins. J. Leukocyte Biol. 1991;49:566. doi: 10.1002/jlb.49.6.566. [DOI] [PubMed] [Google Scholar]
- 48.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta. 1994;1197:63. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 49.Taylor AD, Cowell A-M, Flower RJ, Buckingham JC. Lipocortin 1 mediates an early inhibitory action of glucocorticoids on the secretion of ACTH by the rat anterior pituitary gland in vitro. Neuroendocrinology. 1993;58:430. doi: 10.1159/000126572. [DOI] [PubMed] [Google Scholar]
- 50.Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin I is stored within gelatinase granules of human neutrophils and mobilised on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 2000;24:163. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- 51.Panés J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 52.Pitzalis C, Koch A. The vascular endothelial system in the pathogenesis of inflammation and systemic rheumatic diseases: relation to the neuroendocrine system. Rheum. Dis. Clin. North Am. 2000;26:765. doi: 10.1016/s0889-857x(05)70168-x. [DOI] [PubMed] [Google Scholar]
- 53.Solito E, Raugei G, Melli M, Parente L. Dexamethasone induces the expression of the mRNA of lipocortin 1 and 2 and the release of lipocortin 1 and 5 in differentiated, but not undifferentiated U-937 cells. FEBS Lett. 1991;291:238. doi: 10.1016/0014-5793(91)81293-h. [DOI] [PubMed] [Google Scholar]
- 54.Flower RJ. Lipocortin and the mechanism of action of the glucocorticoids. Br. J. Pharmacol. 1988;94:987. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckingham JC. Stress and the neuroendocrine-immune axis: the pivotal role of glucocorticoids and lipocortin 1. Br. J. Pharmacol. 1996;118:1. doi: 10.1111/j.1476-5381.1996.tb15360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]