Abstract
Background: Because of its reported similarities to Sydenham chorea, therapeutic plasma apheresis (TPA) has been proposed as a potential treatment of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). To date, support for the use of TPA has been limited to a few anecdotal reports and a small placebo-controlled trial demonstrating dramatic symptom improvements at 1 month and 1 year follow-up. To evaluate the safety and efficacy of TPA further, we undertook a retrospective review of all PANDAS patients treated with TPA at Georgetown University Hospital between August 2009 and October 2013.
Methods: Forty patients were identified, and sufficient information was available from medical records and telephone interview for 35 cases (88%). All 35 (23 boys; 12 girls) met diagnostic criteria for PANDAS (Swedo et al. 1998) and had severe symptoms. The TPA procedures were performed at Georgetown University Hospital using a protocol that processes a total of 4.5 blood volumes over 3–5 days (three treatments of 1.5 volumes each). Overall symptom improvements at 6 months post-TPA and long-term follow-up were estimated by parents, who also rated changes in individual symptoms to provide information about patterns of improvement.
Results: All patients were reported to have received at least some benefit from TPA, with average improvement of 65% at 6 months post-TPA and 78% at longer-term follow-up. A decrease in the number of reported symptoms also occurred, with particular improvements in obsessive-compulsive disorder (OCD), anxiety, tics, and somatic symptoms, including dysgraphia, sleep difficulties, and urinary urgency or frequency. Contrary to expectations, preceding duration of illness was not correlated with degree of improvement following TPA, suggesting that acuity of illness is not a factor affecting response. Only two adverse events were reported: both involved reopening of the site where the central line had been placed and resolved immediately following application of pressure and re-dressing of the puncture site.
Conclusions: Therapeutic plasma apheresis is an invasive medical intervention that should be reserved for treatment of children and adolescents who are severely affected by PANDAS. In such patients, it appears to be a safe, well-tolerated, and beneficial treatment option.
Introduction
Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS) has been reported to resemble Sydenham chorea (SC) in its etiology, pathophysiology, and response to treatment (Garvey et al. 1998; Swedo et al. 1998, 2012.) In both disorders, symptoms begin abruptly several weeks to months following prolonged infections with Group A streptococci (GAS), and are hypothesized to result from postinfectious autoimmunity mediated through cross-reactive antibodies, produced against molecular “mimics” or epitopes on the GAS cell wall that resemble host antigens (Swedo 1994; Kirvan et al. 2006a,b; Brimberg et al. 2012.) If the postulated disease mechanism is correct, then removal of the offending autoantibodies via therapeutic plasma apheresis (TPA) should produce symptomatic improvements (Perlmutter et al. 1999; Dale 2005). However, it is worth noting that TPA has a broad range of actions, and that the mechanism of therapeutic benefit is not known for any disorder, including PANDAS and SC (Weinstein 2008). For SC, a randomized comparison of TPA against oral prednisone or intravenous immunoglobulin (IVIG) demonstrated that TPA produced greater, longer-lasting improvements than the other two immunomodulatory therapies; however, the sample size was small (n=8 for the TPA group) (Garvey et al. 2005.) For PANDAS, two anecdotal case reports and a single clinical trial suggest that TPA is beneficial in reducing obsessive-compulsive disorder (OCD), tics, and associated neuropsychiatric symptoms (Tucker et al. 1996; Perlmutter et al. 1999; Elia et al. 2005).
A double-blind, randomized entry clinical trial compared five single-volume apheresis treatments (n=10) against 2 g/kg IVIG (n=9) or sham IVIG placebo (n=10), and demonstrated that obsessive-compulsive symptoms were reduced by an average of 65% in the TPA group (compared with 42% in the IVIG group and no response in the placebo group) (Perlmutter et al. 1999). Treatment gains were maintained at 12 month follow-up, suggesting that a single course of TPA might be effective in producing long-lasting remission of symptoms (Perlmutter et al. 1999.) Five patients with non-PANDAS OCD had no improvement following open-label treatment with TPA, providing indirect support for its postulated mechanism of action in PANDAS (Nicolson et al. 2000.) Further evidence is provided by investigations that showed reduced titers of cross-reactive antibodies following TPA (Kirvan et al. 2006b).
The American Society for Apheresis considers the controlled trials and published case reports sufficient to recommend TPA as first-line treatment for PANDAS (and SC) (Szczepiorkowski et al. 2007a,b; Weinstein 2008) However, when members of the American Academy of Neurology (AAN) examined the same literature, they concluded that “there is insufficient evidence to support or refute the use of plasmapheresis for PANDAS” (and SC) (Cortese et al. 2011). The conflicting treatment guidelines not only caused confusion for clinicians, but also created barriers to care for children and adolescentswith PANDAS. In an attempt to obtain additional controlled-trial data, a sham-apheresis trial was designed, received institutional review board (IRB) approval, and was initiated at the National Institute of Mental Health (NIMH) in the early 2000s. Only three subjects were enrolled before the principal investigator (S.E.S.) stopped the protocol because of ethical concerns related to central line placement and hospitalization of children for nontherapeutic (placebo) interventions. Consequently, questions about the utility of TPA must be answered by additional clinical experience, such as this report of 35 children and adolescents receiving TPA for PANDAS.
Methods
Between January 2009 and October 2013, 40 patients (25 boys, 15 girls) were referred by M.E.L. to Georgetown University Hospital for treatment with TPA. Follow-up information was available for 23 boys and 12 girls (n=35, 88%). All patients met diagnostic criteria for PANDAS (Swedo et al. 1998, 2004) and had severe symptoms, with marked impairments of functioning at home, at school, and with peers. Additional indications for TPA included aggressive, violent behavior or danger to the patient or others; severe restrictions of food and/or fluid intake; or minimal or no response to prior treatment with oral steroids (n=5; 14%) or IVIG (n=17; 48%). All subjects had received therapeutic doses of antibiotics without relief of symptoms, but all were maintained on antibiotic prophylaxis throughout the treatment period. Ten of the 35 patients (29%) described here are also included in a report of clinical characteristics of a larger sample (n=40) of children and adolescents with PANDAS (Swedo et al., 2015).
The TPA procedures were performed at Georgetown University Hospital Department of Pediatrics, Division of Hematology and Oncology, using the Georgetown TPA protocol for PANDAS/pediatric acute-onset neuropsychiatric syndrome (PANS). In brief, this protocol involves insertion of a central line (usually in the femoral vein) and administration of three 1.5 volume therapeutic exchanges over the course of 3–5 days.
Data for this retrospective report were collected from several sources including the child's pediatric records, consultation notes, and medical records from the child's admission to Georgetown University Hospital. Additional clinical information was provided by parents' responses on a questionnaire assessing symptoms of PANS, which had been administered at initial evaluation, and by a follow-up telephone call conducted by a bachelor's level research assistant (N.L., J.S.) under the supervision of the treating physician (M.E.L.). The information gathered was used to confirm and expand upon the medical records, and included specific queries about adverse events, presence and severity of symptoms at baseline and post-TPA treatment, and estimates of improvement at 6 months post-TPA and long-term follow-up. The duration of the long-term follow-up varied from 6 months to 5.4 years (mean=3 years±1.5).
Results
The mean age at TPA was 11.5 years (±3.6; range 4–18.75); mean age at symptom onset was 7.6 years (±3.5; range 2–14.5); and average duration of illness was 4.2 years (±3.7). At baseline, all patients were described by their parents as “severely” or “extremely” ill and had OCD, tics, separation anxiety, sleep difficulties and other neuropsychiatric symptoms (see Table 1). Six months after TPA, parents reported that their child's symptoms had improved by 65% on average (±28; range 5–100%). Two subjects appeared to have only minimal response to TPA (5% and 20%, respectively); however, this appears to have been the result of an infection-triggered relapse (GAS and mycoplasma), rather than a lack of response to the intervention. At long-term follow-up, all subjects were reported to be improved from baseline, with average reduction in symptom severity of 78% (±23.2; range 20–100%) and seven patients reported to be in complete symptom remission. Three illustrative cases are described.
Table 1.
Response of Individual Symptoms to Therapeutic Plasma Apheresis (TPA)
| Symptom | Baseline (n=35) n (%) | ≤6 Months Post-TPA (n=35) n (%) |
|---|---|---|
| Obsessive-compulsive disorder | 34 (97) | 8 (23) |
| Tics | 22 (63) | 6 (17) |
| Separation anxiety | 27 (77) | 2 (6) |
| Frequent urination | 17 (49) | 2 (6) |
| Irritability and aggression | 24 (69) | 2 (6) |
| Psychotic features | 8 (23) | 1 (3) |
| Anorexia | 7 (20) | 1 (3) |
| Dysgraphia | 19 (54) | 3 (9) |
| Suicidal thoughts | 8 (23) | 0 (0) |
| Initial insomnia, interrupted sleep | 20 (57) | 3 (9) |
| Anxiety | 20 (57) | 4 (11) |
| Choreiform movements | 12 (34) | 5 (14) |
| Depressed mood | 11 (31) | 1 (3) |
| Behavioral regression | 14 (40) | 1 (3) |
Case 1
A 10.5-year-old girl developed emotional lability, irritability, and sensory issues a few weeks after receiving antibiotics for GAS pharyngitis. Within 48–72 hours, her symptoms had escalated to include OCD, separation anxiety, initial insomnia, interrupted sleep, and rage episodes. She returned to the pediatrician and again tested positive for GAS, which was treated with a second course of antibiotics. She also received oral steroids, which relieved her symptoms for a few months.
Without obvious reinfection, the child experienced an abrupt recurrence of OCD, depression (crying in her room every night), and separation anxiety. She refused to shower, left her room with greatly reluctance, and developed an obsession with matches, lighting them repeatedly as she stood over the kitchen sink in the evenings. Her parents were concerned that she would start a fire, as she had had significant sleep interruptions and often woke in the night to light and relight matches (sometimes in her room).
The patient received three 1.5 volume TPA procedures over 4 days, and began to improve within 1 week. By the end of 4 weeks, she was completely symptom free. The patient did well for 4 months and then had an abrupt onset of food restriction. This time, she was treated with 2 g/kg IVIG (over 2 days) and a course of cognitive-behavior therapy. Symptoms remitted once again, and she has remained symptom free for the past 3 years.
Case 2
This 7-year-old boy developed sudden onset of severe inattention and impulsivity, and received a diagnosis of attention-deficit/hyperactivity disorder (ADHD), despite an early developmental history that was devoid of behavioral problems. He was started on a stimulant and quickly developed episodes of uncontrollable rage; therefore, the medication was discontinued. Over the next few days, he developed OCD (fears that a man would kill his family), severe separation anxiety, generalized anxiety, emotional lability, initial insomnia, sleep interruptions, and bed wetting. The severity of the irritability, temper tantrums and “out of control” behavior prompted a diagnosis of bipolar disorder, rather than PANDAS. However, a throat culture revealed GAS; therefore, he received a 10 day course of antibiotics. He had developed dysgraphia, and an infectious diseases specialist recommended continued treatment with antibiotics. A trial of oral steroids produced an increase in symptoms, but this may have been the result of a recurrent GAS infection, as a throat culture was positive during this interval (while the patient was on antibiotics). Because of the recurrent GAS infections, he was referred for a tonsillectomy, which neither improved nor exacerbated his symptoms. Similarly, treatment with 2 g/kg IVIG had no discernible benefits. The patient continued to deteriorate with worsening OCD, rage episodes, and separation anxiety.
He was admitted for TPA with 4.5 total volumes of blood processed. He felt better during the course of TPA, and parents reported that he was “much better” in the days following discharge. All of his neuropsychiatric symptoms, including the sleep difficulties, enuresis, and dysgraphia, had improved, and he was functioning at his pre-illness level.
One week later, he developed a red bump on his leg, which tested positive for methicillin-resistant Staphylococcus aureus (MRSA). He had a major escalation in his behavioral problems, which were manifested by constant screaming, raging, and rolling about on the floor. He became completely nonfunctional, despite antibiotic treatment of the MRSA infection. He was readmitted for a second course of TPA. He improved quickly once again, and his parents report that he is “95% better” at long-term follow-up.
Case 3
A 10-year-old girl in the “gifted and talented” 5th grade class (her hobby was writing chapter books) developed sudden onset of OCD, separation anxiety, emotional lability, regressive behavior, generalized anxiety, sleep disorder, and urinary frequency. Her handwriting deteriorated to the point where she refused to write, and she was unable to attend school. A throat culture was negative, but she had been exposed to GAS; therefore, a course of antibiotics was prescribed. It produced no meaningful improvements, and a subsequent course of steroids was similarly ineffective. She received 2 g/kg IVIG and began to improve after a few weeks, returning to her normal level of functioning by 6 months.
The patient did well for 2 years and then developed severe eating restrictions. IVIG was administered again; however, this time there was no improvement in symptoms. The patient received TPA and behavior therapy for anorexia, with slow improvement, and, eventually, complete remission of symptoms. At age 16, she is reported to be doing well.
Interestingly, the index patient (X) has a sister who is 3 years younger. Two years after the onset of X's symptoms, her younger sibling presented with an abrupt onset of separation anxiety, OCD (contamination fears), impulsivity, irritability, aggression, sleep disorder, frequent urination, dysgraphia, and behavioral regression. The rages/impulsive episodes were so severe that the child had broken all of the family's dishes, put her fist through all of the artwork in the house, and attempted to jump from a moving car. Her father's throat culture was positive for GAS, but the patient's culture was negative. A course of antibiotics produced no improvement, and steroids were not a viable option because of the behavioral symptoms. She received 2 g/kg IVIG, which produced 70% reduction in symptom severity, but she failed to return to her premorbid baseline. Two years later, this patient developed eating restrictions, which led to weight loss>20% of her body weight, and extreme malnutrition, as evidenced by bradycardia (resting heart rate of 40). The patient received TPA (1.5 volumes/procedure×3) and slowly improved. At long-term follow-up, she also was reported to be doing well.
Summary of cases
All individual symptoms had decreased in frequency by 6 months, as shown in Table 1. Importantly, some of the most worrisome symptoms had dropped to negligible levels, including a reduction in psychosis (from 23% to 3% of patients); eating restrictions (from 20% to 3%), and suicidality (from 23% to 0%).
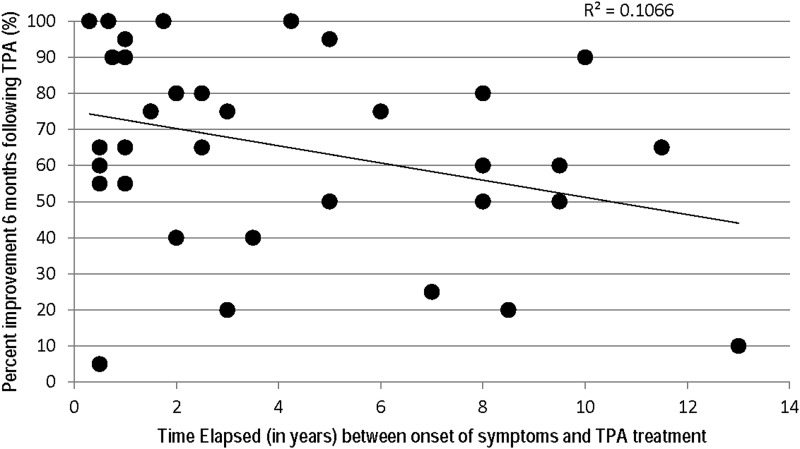
Based on TPA's theorized mechanisms of action, we expected that children and adolescents with chronic symptoms would have a less robust response to the immunomodulatory treatment. However, there was no correlation between symptom duration and degree of improvement following TPA, as is shown in Figure 1.
FIG. 1.
Comparison of symptomatic improvement and duration of illness prior to therapeutic plasma apheresis (TPA) (n=35).
A number of transient adverse events can occur during TPA, ranging from tingling of the lips caused by calcium fluxes, to more serious vasovagal reactions (e.g., Perlmutter et al. 1999). None of these was severe enough to be noted in the TPA procedure records. The only notation was for a midprocedure line change in one patient. Similarly, post-TPA complications were rare, and clinically nonsignificant. Two subjects experienced bleeding at the site of central line insertion shortly after being discharged from the hospital (possibly because of failure to apply pressure at the site for the instructed period of time). One subject returned to Georgetown, and the other reported to an urgent care center for application of pressure and a new dressing. No further bleeding occurred, and the puncture wound healed in both subjects without further incident.
Discussion
This case series demonstrates that TPA is both safe and effective for treatment of severely ill children and adolescents with PANDAS. Data were available for 35 of 40 consecutively treated subjects, and revealed that TPA administration was associated with an average decrease in symptoms of 65% at 6 months and 78% at long-term evaluation. Comparable improvements were reported for the number and severity of individual PANDAS symptoms. Complications of TPA were limited to two minor episodes of postdischarge bleeding at the central line insertion site.
Although these cases represent the largest treatment series reported to date for PANDAS, the study was limited by its retrospective design. The information that could be gleaned from the medical records was limited to recorded, clinically relevant data, and the parent reports may have been subject to recall bias. In addition, there was significant variation in the duration of the “long-term” outcomes, as the telephone interviews were conducted from May through August 2014 and subjects had been treated between January 2009 and October 2013. However, these potential limitations are mitigated by the fact that the retrospective review included 35 of 40 consecutively treated patients (88%) and multiple sources of information. Further, the average improvement observed in this case series is equal to that demonstrated in the placebo-controlled trial of TPA conducted by Perlmutter and colleagues (1999), with symptoms reduced by 65% in both the research and clinical settings. Importantly, these striking short-term improvements were maintained for extended periods of time when subjects received adequate prophylaxis against symptom-triggering infections.
It is worth noting that all patients in this series were severely ill at the time of treatment, despite treatment with standard psychiatric interventions, as well as antibiotics and steroids or IVIG. Further, the average duration of illness prior to TPA was greater than 4 years, indicating that this was the “treatment of last resort” for many patients. Fortunately, duration of illness was not correlated with degree of symptom improvement, which allows clinicians to employ a systematic, graduated approach to treatment of PANDAS. It is generally accepted that less invasive treatments such as antibiotics (Murphy et al., 2014), and possibly oral steroids, should be the initial consideration for all PANDAS patients. TPA and IVIG should be reserved for subjects who remain severely symptomatic after treatment with the less-invasive therapies. There are two potential exceptions to this graduated treatment approach: Subjects with life-threatening suicidality or aggressive behaviors, and those with severe restrictions of food and/or fluid intake. Examples of violent behaviors in this series included a child who was found in his younger sibling's bedroom with a knife poised over the sleeping child's chest; two children who attempted to jump from a moving vehicle; and a child who was rescued from a rooftop where he intended to jump to his death, because he “didn't deserve to live.” Eating restrictions that might prompt use of TPA include those leading to a weight loss of>10–15% of the subject's starting body weight, and those requiring tube feedings to maintain adequate nutrition and hydration.
It is also important to note that the patients in this series were not treated with TPA alone. All received psychiatric treatment, including supportive therapy, anti-obsessional medications and/or cognitive-behavior therapy to address obsessive-compulsive symptoms, and a variety of psychotropic medications for relief of comorbid neuropsychiatric symptoms. All subjects also received antibiotics; initially at therapeutic doses (Murphy et al. 2002; Murphy et al., 2014), and then at dosages that would provide prophylaxis against GAS infections (Garvey et al. 1999; Snider et al. 2005). Despite these interventions, these patients remained severely ill until receiving TPA, when they experienced dramatic, long-lasting reductions in the number and severity of symptoms. Importantly, none of the subjects experienced a worsening of symptoms following TPA administration, and the treatments were particularly beneficial for the most concerning symptoms of suicidality, violent/aggressive behaviors, and eating restrictions. Although a retrospective case series cannot provide definitive proof that the symptomatic improvements were directly related to TPA, these cases clearly demonstrate that inclusion of TPA in a multifaceted treatment approach has potential benefits for children and adolescents with severe symptoms of PANDAS.
Conclusion
In this series of 35 youths with severe symptoms of PANDAS, TPA was found to produce dramatic clinical benefits, with reported improvements averaging 65% and 78% at six months and long-term, respectively. Only two patients failed to respond to TPA and both had intercurrent infections that may have militated against response. Adverse effects of TPA were minor and limited to temporary discomforts associated with central line placement and the apheresis procedures. Thus, it appears that TPA provides a safe, efficacious treatment option for severely ill pediatric patents.
Clinical Significance
This large case series confirms and extends the findings of Perlmutter et al. (1999) by demonstrating that TPA is safe and efficacious in the treatment of PANDAS symptoms. Both studies found that TPA produced dramatic, long-lasting benefits, with an average reduction of 65% in the severity of obsessions, compulsions, anxiety, and other symptoms. It is worth noting the current series utilized three 1.5-volume apheresis procedures over 4–5 days, rather than five single-volume exchanges over 8–10 days. Since therapeutic benefits appear to be comparable for the two regimens, the shorter treatment protocol is preferable, as it requires fewer hospitalization days, decreases the risks of infection and other complications, and (for larger youths) permits use of peripheral intravenous catheters rather than a central line.
The case series also emphasizes the importance of preventing future infection-triggered recurrences through the use of appropriate hygienic controls and prophylactic antibiotics. For patients who remained infection free, a single course of TPA was sufficient to produce long-lasting remissions of symptoms, while those suffering from intercurrent infections were noted to have post-infectious relapses. Thus prevention of post-TPA infections should be a high priority in the treatment of children with PANDAS.
Acknowledgment
The authors acknowledge and thank Andrea Dorsett for her assistance with data management.
Disclosures
No competing financial interests exist.
References
- Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D: Behavioral, pharmacological, and immunological abnormalities after streptoccal exposure: A novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 37:2076–87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A: Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 76:294–300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC: Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol 47:785–91, 2005 [DOI] [PubMed] [Google Scholar]
- Elia J, Dell ML, Friedman DF, Zimmerman RA, Balamuth N, Ahmed AA, Pati S: PANDAS with catatonia: A case report. Therapeutic response to lorazepam and plasmapheresis. J Am Acad Child Adolesc Psychiatry 44:1145–1150, 2005 [DOI] [PubMed] [Google Scholar]
- Garvey MA, Giedd J, Swedo SE: PANDAS: The search for environmental triggers of pediatric neuropsychiatric disorders. Lessons from rheumatic fever. J Child Neurol 13:413–423, 1998 [DOI] [PubMed] [Google Scholar]
- Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, Witowski ME, Dubbert B, Swedo SE: A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry 45:1465–1571, 1999 [DOI] [PubMed] [Google Scholar]
- Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE: Treatment of Sydenham's chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J Child Neurol 5:424–429, 2005 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW: Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham chorea Autoimmunity 39:21–29, 2006a [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW: Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 179:173–179, 2006b [DOI] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME: Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med 156:356–361, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Parker-Athill EC, Lewin AB, Storch EA, Mutch PJ: Cefdinir for recent onset pediatric neuropsychiatric disorders: A pilot randomized trial. J Child Adolesc Psychopharmacol 2014October9 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Swedo SE, Lenane M, Bedwell J, Wudarsky M, Gochman P, Hamburger SD, Rapoport JL: An open trial of plasma exchange in childhood-onset obsessive-compulsive disorder without poststreptococcal exacerbations. J Am Acad Child Adolesc Psychiatry 39:1313–1315, 2000 [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE: Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 354:1153–1158, 1999 [DOI] [PubMed] [Google Scholar]
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE: Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry 57:788–792, 2005 [DOI] [PubMed] [Google Scholar]
- Swedo SE: Sydenham's chorea. A model for childhood autoimmune neuropsychiatric disorders. JAMA 272:1788–1791, 1994 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR: From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (pediatric Acute-onset neuropsychiatric syndrome. Pediatr Therapeut 2:113, 2012. http://dx.doi.org/10.4172/2161-0665.1000113
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998. Erratum in Am J Psychiatry 155: 578, 1998 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Rapoport JL: The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: Separating fact from fiction. Pediatrics 113:907–911, 2004 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Seidlitz J, Kovacevic M, Latimer ME, Hommer R, Lougee L, Grant P.: Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) in research and community settings. J Child Adolesc Psychopharmacol 25:xxx—xxx, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepiorkowski ZM, Bandarenko N, Kim HC, Linenberger ML, Marques MB, Sarode R, Schwartz J, Weinstein R, Shaz BH, Apheresis Applications Committee of theAmerican Society for Apheresis: Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 22:106–175, 2007a [DOI] [PubMed] [Google Scholar]
- Szczepiorkowski ZM, Shaz BH, Bandarenko N, Winters JL: The new approach to assignment of AFA categories – introduction to the fourth special issue: Clinical applications of therapeutic apheresis. J Clin Apher 22:96–105, 2007b [DOI] [PubMed] [Google Scholar]
- Tucker DM, Leckman JF, Scahill L, Wilf GE, LaCamera R, Cardona L, Cohen P, Heidmann S, Goldstein J, Judge J, Snyder E, Bult A, Peterson BS, King R, Lombroso P: A putative poststreptococcal case of OCD with chronic tic disorder, not otherwise specified. J Am Acad Child Adolesc Psychiatry 35:1684–1691, 1996 [DOI] [PubMed] [Google Scholar]
- Weinstein R: Therapeutic apheresis in neurological disorders: A survey of the evidence in support of current category I and II indications for therapeutic plasma exchange. J Clin Apher 23:196–201, 2008 [DOI] [PubMed] [Google Scholar]