Abstract
Background
Incretin hormones, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), play an important role in meal-related insulin secretion. We previously demonstrated that glutamine is a potent stimulus of GLP-1 secretion in vitro.
Objective
To determine whether glutamine increases circulating GLP-1 and GIP levels in vivo and, if so, whether this is associated with an increase in plasma insulin.
Design
We recruited 8 healthy, normal-weight volunteers (LEAN), 8 obese individuals with type 2 diabetes or impaired glucose tolerance (OB-DIAB) and 8 obese non-diabetic controls (OB-CON). Oral glucose (75g), glutamine (30g) and water were administered on three separate days in random order and plasma concentrations of GLP-1, GIP, insulin, glucagon and glucose were measured over 120 minutes.
Results
Oral glucose led to increases in circulating GLP-1 levels, peaking at 30 min in LEAN (31.9±5.7 pmol/L) and OB-CON (24.3±2.1 pmol/L) subjects and at 45 min in OB-DIAB subjects (19.5±1.8 pmol/L). Circulating GLP-1 levels increased in all study groups following glutamine ingestion, with peak levels at 30 min of 22.5±3.4 pmol/L, 17.9±1.1 pmol/L and 17.3±3.4 pmol/L in LEAN, OB-CON and OB-DIAB subjects, respectively. Glutamine also increased plasma GIP levels, but less effectively than glucose. Consistent with the increases in GLP-1 and GIP, glutamine significantly increased circulating plasma insulin levels. Glutamine stimulated glucagon secretion in all three study groups.
Conclusion
Glutamine effectively increases circulating GLP-1, GIP and insulin levels in vivo and may represent a novel therapeutic approach to stimulating insulin secretion in obesity and type 2 diabetes.
Keywords: GLP-1, GIP, glucagon, insulin secretion, glutamine, diabetes
INTRODUCTION
Nutritional therapy is a key intervention for reducing weight and improving glycaemic control in type 2 diabetes. In addition to insulin resistance, impaired insulin secretion is a key pathogenic factor in the development of type 2 diabetes (1). Incretin hormones, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), play a major role in determining the physiological profile of insulin release following a meal (2, 3). The ability of GLP-1 to enhance glucose-dependent insulin release has led to the development of several therapeutic strategies to harness the GLP-1 system for the treatment of diabetes. Indeed, the GLP-1 receptor agonist, exenatide, and orally active dipeptidyl peptidase-IV (DPP-IV) inhibitors have recently entered clinical practice. An alternative therapeutic approach would be to stimulate the release of endogenous GLP-1. However, it remains unclear whether this would be feasible in individuals with diabetes, as diabetes itself has been associated with impaired GLP-1 responses to nutrient ingestion (4-7), albeit inconsistently (8, 9), in part due to the use of different assays. This approach would have other potential metabolic benefits, as the L-cells that produce GLP-1 also secrete GLP-2, which stimulates regeneration and repair of the intestinal epithelium (10), peptide YY, which may suppress appetite (11, 12) and oxyntomodulin, which is thought to reduce food intake in part via the GLP-1 receptor (13).
Understanding the pathways involved in GLP-1 release from L-cells is clearly key to developing agents that enhance GLP-1 secretion in vivo. Experiments in humans and isolated perfused gut preparations in animals have demonstrated that carbohydrate, fat and protein are all involved in meal-stimulated GLP-1 release (14, 15). Extensive use has been made of the GLP-1-secreting murine cell line, GLUTag, which is responsive to a range of sugars, amino acids and fats. As in other endocrine cells, GLP-1 release is triggered by an elevation of intracellular calcium, either following membrane depolarisation and the opening of voltage-gated calcium channels, or by the release of calcium from intracellular stores (15). By screening GLUTag cells for GLP-1 release in response to a range of amino acids and sugars, we previously identified glutamine as a highly effective stimulus (16). Glutamine was effective at physiological concentrations (EC50~0.5mM); the underlying mechanism involved both a triggering step associated with electrogenic amino acid uptake and an enhancing step downstream of the calcium signal (16).
Glutamine is widely available as a nutritional supplement. There is accumulating evidence that its addition to enteral and parenteral nutrition is beneficial for the maintenance of intestinal integrity (17-19). Glutamine is also administered orally at high doses to protect the gut during chemo- and radiotherapy (20, 21). The finding that glutamine triggers GLP-1 release from GLUTag cells raises the possibility that stimulation of GLP-1 and GLP-2 release from L-cells might contribute to its known physiological effects in vivo.
The aim of the present study was to investigate whether glutamine increases GLP-1, GIP, glucagon and insulin levels in human subjects.
SUBJECTS AND METHODS
Subjects
Twenty four subjects were recruited via advertisements placed around the hospital campus. All non-diabetic obese subjects (Body Mass Index [BMI] >30 kg/m2) had a screening 75g oral glucose tolerance test (OGTT) performed prior to inclusion in the study. Subjects were recruited into three groups of 8 subjects: healthy, lean (BMI <25 kg/m2) volunteers (LEAN), obese subjects with type 2 diabetes (n = 6) or impaired glucose tolerance (n = 2) (OB-DIAB) and obese control subjects with normal glucose tolerance (OB-CON), age- and BMI-matched to the OB-DIAB group. This sample size is consistent with that used previously in studies comparing nutrient-stimulated GLP-1 release in type 2 diabetic subjects and healthy controls (8, 9). No subject in the OB-DIAB group was taking oral hypoglycaemic agents; five were diagnosed with impaired glucose tolerance or type 2 diabetes at the screening OGTT. Diabetes was defined as fasting plasma glucose of 7.0 mmol/L or greater and/or a plasma glucose of 11.1 mmol/L or greater 120 min after a 75g glucose load. Impaired glucose tolerance was diagnosed as a plasma glucose level 7.8 – 11.0 mmol/L 120 min after a 75g glucose load. Patients with mild degrees of hyperglycaemia were specifically recruited to avoid any potentially confounding effects of ‘glucotoxicity’ and oral hypoglycaemic agents on GLP-1 release. Exclusion criteria were: excessive alcohol intake, liver, kidney or thyroid disease, anaemia, treatment with weight loss or lipid-lowering agents, previous bowel surgery and documented malabsorption. All studies were approved by the Local Regional Ethics Committee in Cambridge and were conducted in accordance with the principles of the Declaration of Helsinki. All subjects provided written informed consent.
Study design
Subjects attended the Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital between 0745 and 0800 on three separate occasions over a period of <1 month, having fasted from 2200 the night before the study. Subjects were instructed not to walk or cycle to study visits. Studies were performed in a random order on separate days. A large-bore intravenous indwelling cannula was inserted into an antecubital vein on each visit for blood sampling. At the first visit, weight and height were measured with the subject in light street clothing. BMI was calculated as weight divided by height squared (kg/m2). Fasting blood was drawn for HbA1c, full blood count and renal, liver and thyroid function tests. On each study day, three fasting baseline blood samples were taken for glucose, insulin and GLP-1 at t = −10, −5 and −1 min. Following collection of the last baseline sample, subjects ingested glucose (75g, 0.42 moles, in 300ml of water), glutamine (30g, 0.21 moles, in 300ml of water) or water (300ml) over approximately 2 minutes (last mouthful at t = 0). We examined the effect of a maximum dose of glutamine known to be safely tolerated in humans (22) and compared this with the effect of water (negative control) and of a dose of glucose previously reported to be a reliable stimulus of GLP-1 secretion (positive control) (23). Blood samples were collected at t = 15, 30, 45, 60, 90 and 120 min for plasma glucose and GLP-1. Plasma insulin was assayed at t = 15, 30, 60 and 120 min. Plasma GIP was measured at t = −10, −5, 15, 30, 60 and 90 min in a subset of 11 subjects from the OB-CON and OB-DIAB groups, covering a range of BMI values (reduced numbers due to limited plasma availability). Plasma glucagon was assayed at t = −10, −5, 15, 30, 60 and 90 min in 18 subjects from all three groups. The updated Homeostasis Assessment Model (HOMA2) was used to estimate values of insulin sensitivity (HOMA2-%S) and β-cell function (HOMA2-%β) as previously described (HOMA values not calculated in one OB-DIAB subject with fasting plasma insulin >400 pmol/L) (24). Subjects were provided with breakfast at the end of the study.
To determine the time course of the glutamine response and to ensure adequate absorption of oral glutamine, plasma glutamine levels were measured at all time-points following glutamine ingestion in one LEAN subject. Plasma glutamine increased from 728 μmol/L at baseline to 1260 μmol/L at 15 min, 2052 μmol/L at 30 min, 2559 μmol/L at 60 min and 1863 μmol/L at 120 min. In all remaining subjects, plasma glutamine was assayed at the 60 min time point only.
Analytical methods
Blood for plasma glucose was collected in a fluoride oxalate tube; plasma glucose was assayed on the same day by the glucose oxidase method. Insulin and glutamine were assayed in plasma collected in a lithium-heparin-containing tube; plasma was frozen and stored at −80 degrees until analysis. Insulin was quantified using a commercially available immunoassay (AutoDELFIA ® Insulin Kit, Perkin Elmer, Wellesley, MA), which has a cross reactivity with intact proinsulin of <0.1% and a cross reactivity with 32-33 split proinsulin of <0.44%; intra-assay coefficient of variation (CV) was 3.5 – 4.5%. Glutamine was measured by cation exchange chromatography with post-column ninhydrin derivitisation (Biochrom 30 amino acid analyser), with an intra-assay CV of 3.2% and an inter-assay CV of 4%. Blood for GLP-1, GIP and glucagon was collected into chilled EDTA-coated tubes, which were immediately centrifuged for 7 minutes at 3000 rpm. The plasma sample was snap frozen and stored at −80 degrees until analysis. Plasma total GLP-1 concentrations were measured by radioimmunoassay after extraction of plasma with 70 % ethanol (vol/vol, final concentration). Carboxy-terminal GLP-1 immunoreactivity was determined using antiserum 89390 which has an absolute requirement for the intact amidated carboxy-terminus of GLP-1 7-36 amide and cross-reacts less than 0.01% with carboxy-terminally truncated fragments and 89% with GLP-1 9-36 amide, the primary metabolite of DPP-IV mediated degradation. The sum of the two components (total GLP-1 concentration) reflects the rate of secretion of the L-cell. Sensitivity was <1 pmol/l, and intra-assay CV <5 % at 20 pmol/l (25). Total GIP was measured using a human GIP (total) ELISA kit (LINCO Research, St Charles, MO), which cross-reacts 100% with human GIP(1-42) and GIP(3-42), has a sensitivity of <2 pmol/l and an intra-assay CV of 7 % at 4 pmol/l. Plasma glucagon concentrations were measured after extraction of plasma with 70 % ethanol (vol/vol, final concentration). The glucagon assay is directed against the COOH-terminus of the glucagon molecule (antibody code no. 4305) and therefore measures glucagon of mainly pancreatic origin. The detection limit of the assay was 1 pmol/l and intra-assay coefficient <6%.
Statistical methods
Normally distributed data are expressed as mean ± standard deviation (SD) or standard error (SE). Due to their skewed distribution, plasma insulin and HOMA values were analysed after log10-transformation and are presented as geometric mean (1 SD or SE range) after back-transformation. SE is presented except for the baseline data in Table 1. Incremental area under the curve was calculated using the trapezoidal rule, subtracting the baseline values extrapolated over 120 min from the total area under the curve. Results from different study days on the same subject were compared using paired t-tests. Comparisons between study groups were made by analysis of variance (ANOVA) or regression; post-hoc unpaired t-tests were performed as appropriate. Correlations between variables were examined using Pearson correlation. Data were analysed using Statview 5.0.1 ® (SAS Institute Inc., Cary, North Carolina) or Microsoft Excel. All P values were two-sided. P < 0.05 was considered statistically significant.
Table 1.
Demographic, anthropometric and fasting metabolic characteristics of study participants.
| LEAN subjects | OB-CON subjects | OB-DIAB subjects | ANOVA P value | |
|---|---|---|---|---|
| Gender (M/F) | 6/2 | 7/1 | 8/0 | - |
| Age (years) | 30 ± 5.8 | 39 ± 9.8 | 38.5 ± 8 | 0.06 |
| Weight (kg) | 70.3 ± 8.6 | 106 ± 14.8† | 120.6 ± 24.2† | <0.0001 |
| Body Mass Index (kg/m2) | 21.9 ± 2.2 | 34.5 ± 4.4† | 38.5 ± 6.5† | <0.0001 |
| Fasting plasma glucose (mmol/L)* | 4.6 ± 0.2 | 4.9 ± 0.2‡ | 6.2 ± 0.9†§ | <0.0001 |
| Fasting plasma insulin (pmol/L)* | 34 (25 – 47) | 103 (74 – 144)† | 147 (82 – 266)† | <0.0001 |
| HOMA2-%S | 160 (116 – 220) | 53 (38 – 73)† | 42 (33 – 55)† | <0.0001 |
| HOMA2-%β | 85 (68 – 105) | 153 (118 – 198)† | 115 (79 – 167) | 0.002 |
| HbA1c (%) | Not done | 5.2 ± 0.3 | 6.3 ± 0.5∥ | - |
| Fasting total GLP-1 (pmol/L)* | 14.4 ± 1.5 | 13.0 ± 2.0 | 9.4 ± 2.9†¶ | 0.006 |
Data are mean ± SD or geometric mean (1 SD range).
Average of baseline samples from glucose, glutamine and water study days.
P<0.001 vs LEAN subjects (unpaired t-test).
P<0.01 vs LEAN subjects (unpaired t-test).
P<0.01, vs OB-CON subjects (unpaired t-test).
P<0.001 vs OB-CON subjects (unpaired t-test).
P<0.05 vs OB-CON subjects (unpaired t-test).
RESULTS
Cohort characteristics
Demographic, anthropometric and fasting metabolic parameters of the study participants are shown in Table 1. OB-CON and OB-DIAB subjects were matched for gender, age, weight and BMI. As expected, OB-DIAB subjects had higher fasting plasma glucose levels than non-diabetic subjects. OB-CON and OB-DIAB subjects had higher fasting insulin levels than LEAN subjects. Although LEAN subjects were more insulin-sensitive than OB-DIAB and OB-CON subjects, only the latter exhibited higher HOMA2-%β relative to LEAN controls (Table 1).
Fasting GLP-1 levels in OB-DIAB subjects were significantly lower when compared to subjects without diabetes (Table 1). However, when compared across the whole study cohort by regression analysis, fasting GLP-1 levels were found to be inversely related to BMI (P = 0.003), but not associated with fasting plasma glucose per se.
Plasma glucose responses
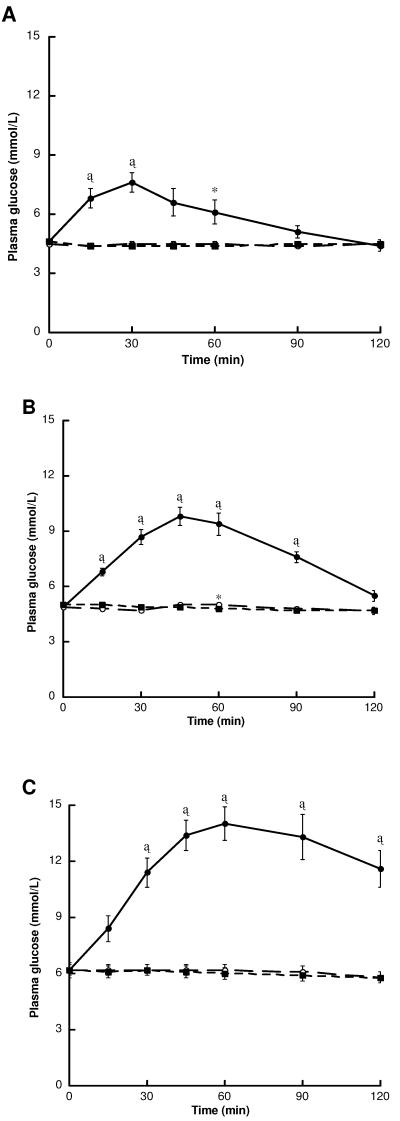
Plasma glucose responses following glucose, glutamine and water are shown in Figure 1. As expected, mean plasma glucose levels increased rapidly above baseline following the consumption of oral glucose, peaking at 30 min in LEAN subjects (7.6 ± 0.5 mmol/L), 45 min in OB-CON subjects (9.8 ± 0.5 mmol/L) and 60 min in OB-DIAB subjects (14 ± 0.9 mmol/L). Plasma glucose levels following glutamine ingestion were comparable to those following water alone in all study groups.
Figure 1.
Plasma glucose levels following the ingestion of glucose (black circles), glutamine (white circles) and water (black squares) in (A) LEAN (n = 8) (B) OB-CON (n = 8) and (C) OB-DIAB (n = 8) subjects. Data are mean ± SE. *P < 0.05, †P < 0.01 and ‡P < 0.001 vs water (paired t-test). There were no differences in baseline glucose levels within each group between visits.
Plasma GLP-1 responses
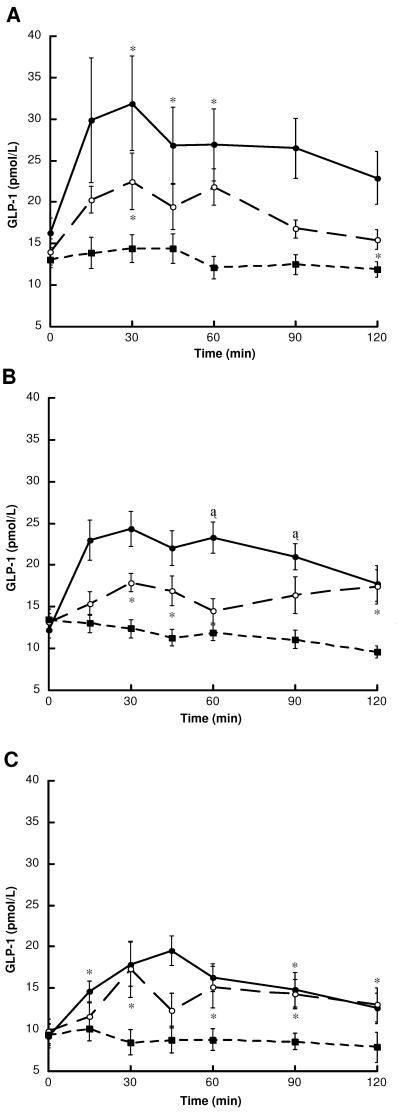
GLP-1 levels and total and incremental GLP-1 area under the curve following the ingestion of glucose, glutamine and water are shown in Figure 2 and Table 2. Following the ingestion of water alone, GLP-1 levels remained at or below baseline throughout the 120 min sampling period in all study groups. In contrast, glucose induced a rapid increase in GLP-1 release, with mean peak levels observed at 30 min in LEAN subjects (31.9 ± 5.7 pmol/L) and OB-CON subjects (24.3 ± 2.1 pmol/L) and at 45 min in OB-DIAB subjects (19.5 ± 1.8 pmol/L) (Figure 2). Consistent with lower fasting GLP-1 levels in OB-DIAB subjects, the glucose-induced GLP-1 response (area under the curve) was significantly impaired in the OB-DIAB group compared to non-diabetic subjects (Table 2).
Figure 2.
Plasma GLP-1 levels following the ingestion of glucose (black circles), glutamine (white circles) and water (black squares) in (A) LEAN (n = 8) (B) OB-CON (n = 8) and (C) OB-DIAB (n = 8) subjects. Data are mean ± SE. *P < 0.05, †P < 0.01 and ‡P < 0.001 vs water (paired t-test). There were no differences in baseline GLP-1 levels within each group between visits.
Table 2.
Total (AUC) and incremental (iAUC) areas under the curve for GLP-1 (pmol/L·120 min) following glucose, glutamine and water ingestion in LEAN (n = 8), OB-CON (n = 8) and OB-DIAB (n = 8) subjects.
| AUC | LEAN Subjects | OB-CON subjects | OB-DIAB subjects | ANOVA P value |
|---|---|---|---|---|
| Glucose | 3192 ± 431 (0.01) | 2550 ± 145 (<0.001) | 1846 ± 185 (0.001)*† | 0.01 |
| Glutamine | 2245 ± 187 (<0.001) | 1929 ± 169 (0.05) | 1653 ± 207 (0.004) | 0.11 |
| Water | 1548 ± 146 | 1396 ± 97 | 1054 ± 137* | 0.04 |
| iAUC | LEAN Subjects | OB-CON subjects | OB-DIAB subjects | ANOVA P value |
|---|---|---|---|---|
| Glucose | 1242 ± 385 (0.008) | 1082 ± 102 (<0.001) | 746 ± 159 (0.005) | 0.37 |
| Glutamine | 560 ± 185 (0.01) | 344 ± 199 (0.05) | 480 ± 170 (0.04) | 0.71 |
| Water | −17 ± 89 | −219 ± 82 | −73 ± 86 | 0.25 |
Data are mean ± SE (P vs water, paired t-test). ANOVA P value for comparison between subject groups.
P<0.05 vs LEAN subjects (unpaired t-test).
P<0.01 vs OB-CON subjects (unpaired t-test).
Thirty grams of oral glutamine was well-tolerated by all subjects. Mean 60 min plasma glutamine levels were 1782 ± 191 μmol/L (range 1287 – 2675 μmol/L) in LEAN subjects, 1584 ± 206 μmol/L (range 1022 – 2866 μmol/L) in OB-CON and 1854 ± 241 μmol/L (range 931 – 2723 μmol/L) in OB-DIAB subjects. Ingestion of glutamine resulted in elevation of circulating GLP-1 levels, with the increase above baseline detectable as early as 15 min (Figure 2). In all groups, there was the suggestion of a biphasic response, with an initial peak at 30 min (22.5 ± 3.4 pmol/L, 17.9 ± 1.1 pmol/L and 17.3 ± 3.4 pmol/L in LEAN, OB-CON and OB-DIAB subjects, respectively) and a second peak at 60 – 90 min. In the whole group, both total and incremental GLP-1 responses to glutamine were significantly greater than those observed following the ingestion of water alone (P < 0.0001). Of note, the magnitude of the glutamine-induced incremental GLP-1 response in OB-DIAB subjects was not significantly different from that observed in LEAN and OB-CON subjects (Table 2). It was also unrelated to the subject’s age (r = −0.04, P = 0.85), weight (r = −0.19, P = 0.36), BMI (r = −0.09, P = 0.67) and fasting plasma glucose concentration (r = −0.30, P = 0.15).
Plasma GIP responses
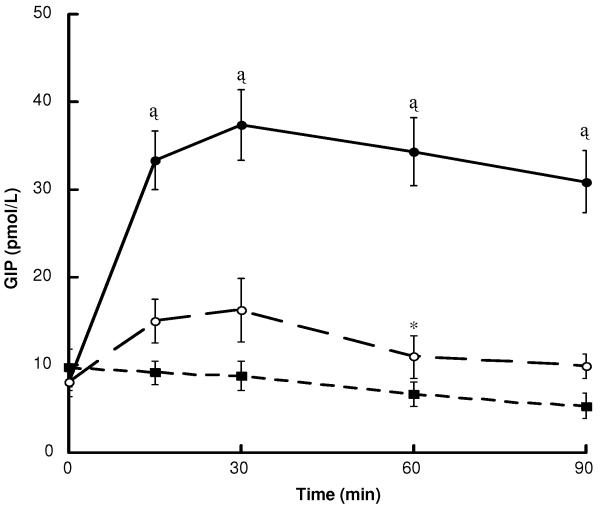
Plasma GIP concentrations were measured only in subjects from the LEAN and OB-CON groups, as GIP levels are not significantly affected by diabetes (26). Fasting GIP concentration (8.6 ± 1.4 pmol/L) were not correlated with BMI. Glucose was a potent stimulus of GIP secretion (area under the curve: 2894 ± 276 vs 686 ± 133 pmol/L·90 min, glucose vs water, respectively, P < 0.001), with elevated levels detected as early as 15 min and peak levels of 37.4 ± 4.0 pmol/L at 30 min (Figure 3). Glutamine also triggered GIP secretion (peak 16.3 ± 3.6 pmol/L at 30 min), but was markedly less effective than glucose (area under the curve: 1127 ± 216 vs 686 ± 133 pmol/L·90 min, glutamine vs water, respectively, P = 0.001).
Figure 3.
Plasma GIP levels following the ingestion of glucose (black circles), glutamine (white circles) and water (black squares) in LEAN (n = 3) and OB-CON (n = 8) subjects. Data are mean ± SE. *P < 0.05, †P < 0.01 and ‡P < 0.001 vs water (paired t-test).
Plasma insulin responses
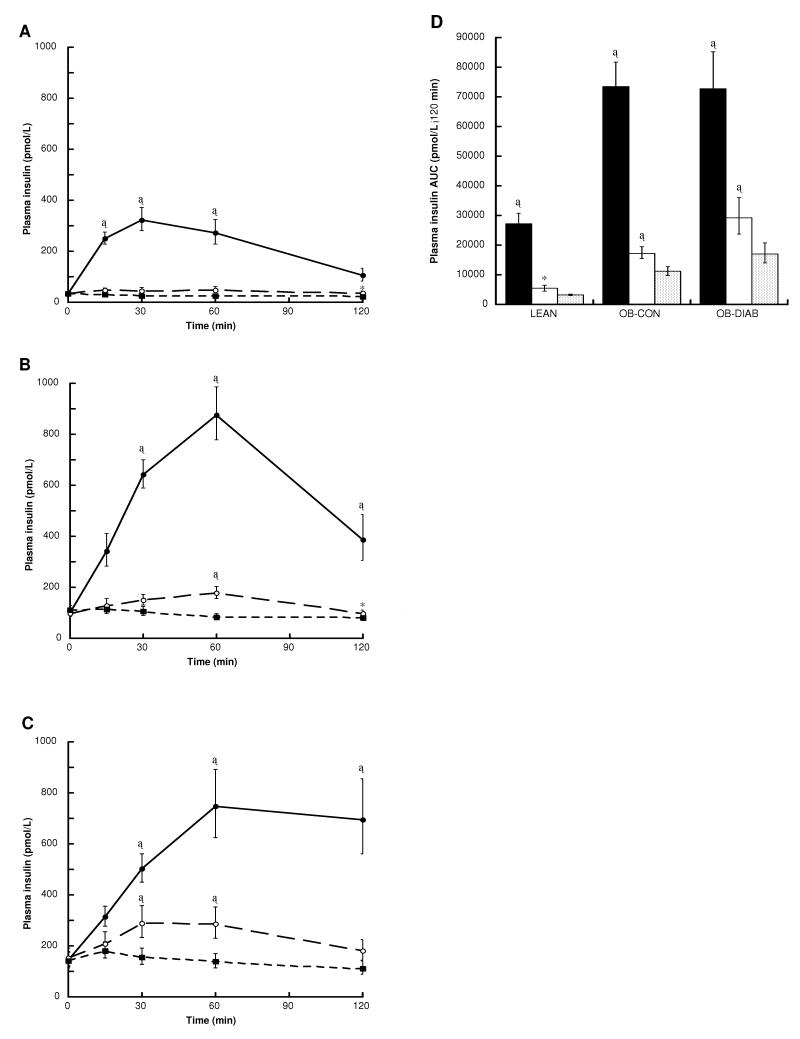
Insulin responses to glucose, glutamine and water are shown in Figure 4. As expected, insulin levels increased markedly in all groups following the glucose load. Ingestion of glutamine was also associated with a significant increase in plasma insulin levels, particularly in the OB-CON and OB-DIAB groups. The absolute glutamine-induced insulin response was greatest in OB-DIAB subjects, intermediate in OB-CON subjects and lowest in LEAN subjects (ANOVA P < 0.0001 for comparison of log10insulin area under the curve across the groups). A similar result was found when comparing the incremental area under the curve for insulin (ANOVA P = 0.0013). Differences between the glutamine-induced and water-induced insulin responses (area under the curve) were significant when all subjects were considered together (P < 0.0001) and when each group of subjects was analysed separately (Figure 4). Consistent with these results, the incremental area under the curve for insulin was greater following ingestion of glutamine than of water in all groups (LEAN, P = 0.01; OB-CON, P = 0.0002; OB-DIAB, P = 0.007).
Figure 4.
Plasma insulin levels following the ingestion of glucose (black circles), glutamine (white circles) and water (black squares) in (A) LEAN (n = 8) (B) OB-CON (n = 8) and (C) OB-DIAB (n = 8) subjects. (D) Insulin area under the curve (AUC) following the ingestion of glucose (black bars), glutamine (white bars) and water (grey bars) in the three study groups. Data are geometric mean (1 SE range). *P < 0.05, †P < 0.01 and ‡P < 0.001 vs water (paired t-test). There were no differences in baseline insulin levels within each group between visits.
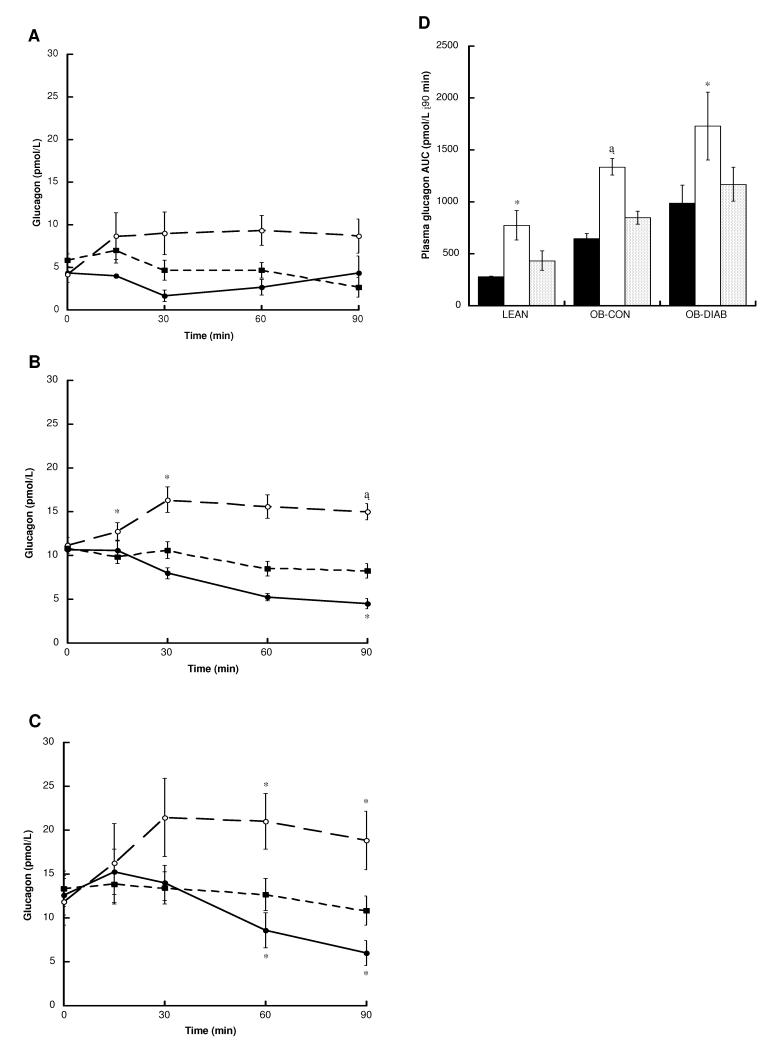
Plasma glucagon responses
Fasting plasma glucagon levels were positively associated with BMI (P = 0.016), but unrelated to the fasting plasma glucose concentration. Changes in glucagon following the ingestion of glucose, glutamine and water are shown in Figure 5. Glutamine led to a marked early stimulation of glucagon release, with significant differences in area under the curve between the glutamine and water visits observed in all three groups.
Figure 5.
Plasma glucagon levels following the ingestion of glucose (black circles), glutamine (white circles) and water (black squares) in (A) LEAN (n = 3) (B) OB-CON (n = 8) and (C) OB-DIAB (n = 7) subjects. (D) Glucagon area under the curve (AUC) following the ingestion of glucose (black bars), glutamine (white bars) and water (grey bars) in the three study groups. Data are mean ± SE. *P < 0.05, †P < 0.01 and ‡P < 0.001 vs water (paired t-test). There were no differences in baseline glucagon levels within each group between visits.
DISCUSSION
Consistent with our previous findings on the GLP-1-secreting cell line GLUTag, that glutamine is an effective stimulus of GLP-1 secretion at physiological concentrations (16), we show here that oral glutamine increases circulating GLP-1 levels in human subjects. Glutamine-triggered GLP-1 release was observed in lean, obese and type 2 diabetic subjects, with no significant difference in the magnitude of the response between the three study groups. By contrast, glutamine was a relatively weak stimulus of GIP secretion, consistent with previous observations that GIP release is primarily triggered by the ingestion of carbohydrate and fat (26).
There are a number of reports that diabetes is associated with impaired GLP-1 release in response to glucose and, particularly, mixed meals (3-7). However, in one study, the incremental GLP-1 response to oral glucose was greater in patients with type 2 diabetes (9). In a more recent report, small intestinal administration of glucose led to comparable increases in plasma GLP-1 in patients with type 2 diabetes and controls (8). In our diabetic study group, we observed a lower GLP-1 response to glucose compared with the other groups. In part, this may be related to the finding of lower fasting GLP-1 levels in subjects with diabetes, resulting from a negative association between fasting GLP-1 and BMI.
Interestingly, the GLP-1 response to glutamine was not significantly different in the diabetic group compared with the lean and obese controls, suggesting that it may be possible to circumvent the diabetes-associated GLP-1 secretory defect with agents that target alternative pathways in the L-cells. The GLP-1 responses to glucose and glutamine could be divided into two phases, the first occurring with a peak at around 30 min, and a second extending for up to 2 hours. Similar separation of GLP-1 release into early and late phases has been reported previously in response to glucose or meal ingestion (4, 5, 14).
The mechanism by which glutamine stimulates GLP-1 release in vivo remains uncertain. In GLUTag cells, we previously showed that sodium-dependent glutamine uptake could itself act as a trigger for GLP-1 release (16). However, glutamine is also an important energy source for the gut and is metabolised via a range of pathways culminating in the production of CO2, lactate, proline, citrulline, alanine, ornithine and glucose (27).
Glutamine is used extensively as a supplement to enteral and parenteral nutrition, as it is believed to maintain the integrity of the intestinal mucosa (17-19). It has also been shown to protect the gut from the toxic effects of radio- and chemotherapy, where doses used are in the range of 0.3 – 0.65 g/kg body weight (21, 22), similar to those used in the current study (~0.25 – 0.4 g/kg). In agreement with previous dose-evaluation studies (21), mean glutamine concentrations 60 min after ingestion were 1584 – 1854 μmol/L in our study. Time course measurements have shown that oral glutamine doses of 0.35 – 0.65 g/kg result in peak levels at 30 to 60 min, with a subsequent fall to baseline over the following 180 min (21). These high doses, although somewhat unpalatable because of the limited solubility of glutamine, are well tolerated and do not cause dangerous levels of hyperammonaemia (21). As GLP-2 is co-produced with GLP-1 at a 1:1 molar ratio, it is tempting to speculate that part of the reported protective effect of glutamine on the gut mucosa is attributable to the release of GLP-2, the principal action of which is to stimulate the proliferation and repair of the intestinal epithelium (10).
Following glutamine ingestion, insulin levels rose in parallel with GLP-1 in all study groups. Whilst this could reflect a direct effect of glutamine on pancreatic β-cells, previous studies have shown that glutamine is not a classical trigger of insulin release (28), but instead enhances secretion in the presence of other stimuli. However, further studies in humans comparing insulin levels in response to oral and intravenous glutamine administration with similar raised plasma glutamine levels will be required to rule out a direct effect of circulating glutamine on the pancreatic β-cell.
Glutamine was a potent stimulus for glucagon secretion in all groups. This is likely to explain the observation that plasma glucose concentrations were similar in the glutamine and water (control) arms of the study, despite stimulation of GLP-1 and insulin release following glutamine ingestion. Future studies are required to determine whether glutamine reduces post-prandial glucose levels when ingested with a glucose load, conditions expecteded to inhibit glucagon release. The absence of an effect of raised insulin concentrations on plasma glucose may also be due to glutamine itself acting as a precursor for gluconeogenesis. Further studies will be required to ascertain whether it is possible to stimulate similar GLP-1 and insulin release with lower doses of glutamine that provide a smaller load of gluconeogenic precursors and do not stimulate glucagon release. Finally, the relatively small increase in insulin levels in LEAN subjects and prevailing insulin resistance in OB-CON and OB-DIAB subjects may also partly explain the absence of an effect on glucose concentrations.
Our study has limitations. Firstly, the lack of an amino acid comparator means that we are unable to definitively conclude that the GLP-1 response to glutamine is specific to this amino acid. Although it is possible that this is a generalised amino acid effect, the in vitro data reported by Reimann et al (16) would argue against this suggestion. Secondly, as we specifically selected subjects with impaired glucose tolerance or mild type 2 diabetes, who had relatively intact β-cell function, we are unable to comment on whether the effect of glutamine on post-prandial insulinaemia would be more or less pronounced in patients with a longer duration of diabetes and a greater impairment of insulin secretion.
In conclusion, together with the in vitro data on GLP-1 release from GLUTag cells, our results raise the possibility that nutritional supplementation with agents such as glutamine may increase GLP-1 release, enhance insulin secretion and, possibly, improve glycaemic control in type 2 diabetic subjects. As patients are often poorly adherent with the large number of medications required to adequately treat chronic diseases such as type 2 diabetes, but are positively disposed towards nutritional therapies, this potential therapeutic approach to enhance insulin secretion deserves further exploration.
Acknowledgements
The authors would like to thank Eamonn O’Driscoll who performed the glutamine assays, Fiona Tulloch and Keith Burling who performed the insulin assays and Lone Bagger who performed the GLP-1 assays.
Dr Greenfield was supported by the National Health & Medical Research Council of Australia, The Royal Australasian College of Physicians and St. Vincent’s Clinic Foundation, Sydney, Australia. Drs Farooqi and Gribble are funded by the Wellcome Trust.
Footnotes
None of the authors reported a conflict of interest.
References
- 1.Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–80. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ, Deacon CF. Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol. 2004;4:589–96. doi: 10.1016/j.coph.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–66. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 4.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 5.Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 6.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 8.O’Donovan DG, Doran S, Feinle-Bisset C, et al. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3431–5. doi: 10.1210/jc.2004-0334. [DOI] [PubMed] [Google Scholar]
- 9.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–23. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallis K, Walters JR, Forbes A. Review article: glucagon-like peptide 2--current applications and future directions. Aliment Pharmacol Ther. 2007;25:365–72. doi: 10.1111/j.1365-2036.2006.03193.x. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 12.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 13.Stanley S, Wynne K, Bloom S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 2004;286:G693–7. doi: 10.1152/ajpgi.00536.2003. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–26. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 15.Reimann F, Ward PS, Gribble FM. Signaling Mechanisms Underlying the Release of Glucagon-Like Peptide 1. Diabetes. 2006;55:S78–S85. [Google Scholar]
- 16.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 17.van der Hulst RR, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363–5. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]
- 18.Tremel H, Kienle B, Weilemann LS, Stehle P, Furst P. Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology. 1994;107:1595–601. doi: 10.1016/0016-5085(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 19.Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. 2001;131:2505S–8S. doi: 10.1093/jn/131.9.2505S. discussion 23S-4S. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433–9. doi: 10.1002/(sici)1097-0142(19981001)83:7<1433::aid-cncr22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Ward E, Picton S, Reid U, et al. Oral glutamine in paediatric oncology patients: a dose finding study. Eur J Clin Nutr. 2003;57:31–6. doi: 10.1038/sj.ejcn.1601517. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler TR, Benfell K, Smith RJ, et al. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990;14:137S–46S. doi: 10.1177/0148607190014004201. [DOI] [PubMed] [Google Scholar]
- 23.Ahren B, Larsson H, Holst JJ. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137:127–31. doi: 10.1530/eje.0.1370127. [DOI] [PubMed] [Google Scholar]
- 24.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 25.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 26.Meier JJ, Nauck MA. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide. Best Practice & Research. Clinical Endocrinology & Metabolism. 2004;18:587–606. doi: 10.1016/j.beem.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- 28.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–7. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]