Abstract
As intra-thyroidal iodine stores should be maximised before conception to facilitate the increased thyroid-hormone production of pregnancy, women who may become pregnant should ideally consume 150 μg iodine/day [US Recommended Dietary Allowance (RDA)]. As few UK data exist in this population group, our aim was to assess iodine intake and status in women of childbearing age in a cross-sectional study at the University of Surrey. Total iodine excretion was measured from 24-h urine samples in 57 women; iodine intake was estimated by assuming that 90% of ingested iodine was excreted. Average iodine intake was also estimated from 48-h food diaries that the women completed. The median urinary-iodine concentration (63.1 μg/L) classified the group as mildly iodine deficient by WHO criteria. By contrast, the median 24-h iodine excretion (149.8 μg/24-h), suggested a relatively low risk of iodine deficiency. Median estimated iodine intake, extrapolated from urinary excretion, was 167 μg/day, whereas it was lower, at 123 μg/day, when estimated from the 48-h food-diaries. Iodine intake from food diaries and 24-h iodine excretion were strongly correlated (r=0.75, p<0.001). Intake of milk, eggs and dairy products was positively associated with iodine status. The iodine status of this UK cohort is probably a best-case scenario as the women were mostly nutrition students and were recruited in the winter when milk-iodine content is at its highest; further study in more representative cohorts of UK women is required. Our study highlights a need for revised cut-offs for iodine deficiency that are method- and age-group specific.
Keywords: iodine, iodine deficiency, milk, UK, public health, pregnancy, diet
Introduction
Iodine is required for the production of thyroid hormones (thyroxine and tri-iodothyronine), which in turn are required for normal fetal brain and neurological development(1). A sufficient intake of iodine during pregnancy is needed to avoid the potential adverse consequences of deficiency on the developing brain that can persist throughout life. Severe iodine deficiency during pregnancy is well-known to result in cretinism, a disorder associated with mental retardation, deafness and motor dysfunction in the child. Even mild-to-moderate iodine deficiency is associated with lower IQ, reading(2) and spelling ability(3) up to the age of 9 years.
While is it important for pregnant women to have a sufficient intake of iodine, it is arguably more important that women of childbearing age, particularly those planning a pregnancy, should consume enough; emerging data suggest that pregnant women who have had a regular adequate intake of iodine have a better thyroid hormone profile than those who only begin iodine supplementation when they become pregnant(4-6). This is probably because the thyroid can store iodine that can be drawn on during the course of pregnancy(5). As many pregnancies are unplanned and because pregnancy may not be confirmed until several weeks into the first trimester – a critical period for thyroid hormone need – it is essential that women of childbearing age consume an adequate amount of iodine on a regular basis and meet the Recommended Dietary Allowance (US RDA) of 150 μg/day(7).
For many years, the UK was considered to be an iodine-sufficient country, despite reports of endemic goitre in the past(8). Iodine deficiency was eradicated in the UK through changes in the dairy-farming industry in the 1930s (i.e. through increased use of iodine-fortified cattle feed and iodine-containing disinfectants) and the concurrent increase in milk consumption in the post-war years(8); from the 1960s, iodine sufficiency was assumed in the UK and there was a dearth of data on the status of the population. In fact, at the time that this study was conducted, there were no national UK data on population iodine status(9) and just two studies on iodine status in pregnant women(10;11). There are now UK-wide data that suggest that teenage schoolgirls are mildly iodine deficient(12) and regional studies that show iodine deficiency in UK pregnant women(2;10;13;14). However, data are still lacking on the iodine status of UK women of childbearing age and few studies have measures of both dietary iodine intake and status. Urinary iodine excretion is a widely accepted method of measuring iodine status; approximately 90% of recently ingested iodine is ultimately excreted in the urine(1). For the assessment of iodine status in an individual, total iodine excretion in a 24-h urine collection is considered to be preferable to iodine concentration measured in spot-urine samples(15;16).
The aim of our study was to assess iodine status from 24-h urine collections in UK women of childbearing age, i.e. in a cohort of women who could potentially become pregnant in the short-to-medium term. In addition, through the use of food diaries, we investigated which iodine-rich food groups had the most influence on iodine status. We also compared two methods of estimating iodine intake:- (i) extrapolation from 24-h urinary iodine excretion, and, (ii) estimation of intake by dietary assessment. This is the first study to be conducted on the iodine status of women of childbearing age in the UK using these methods and the first to report the comparison of two methods of estimating iodine intake.
Experimental Methods
Recruitment and protocol
The study was conducted at the University of Surrey, Guildford, UK. Women of childbearing age (defined as still menstruating) were recruited during January and February, 2007, and again in January and February, 2008. Women were recruited by word of mouth in the University through friends and colleagues and through responses to an email advertisement. Exclusion criteria included current or recent pregnancy (in the last six months), breastfeeding, known thyroid disease or use of medication for thyroid disease – thyroxine, amiodarone, carbimazole or propylthiouracil.
For assessment of the iodine status of a population, the WHO recommends that the median iodine concentration from spot-urine samples is compared to their published cut-offs for adequacy(17). However, urinary iodine concentration is not suitable for assessment of individual iodine status; for this purpose multiple spot urine samples or 24-h urine collections (for measurement of total iodine excretion) is required(18). Though the 24-h iodine excretion from a single 24-h urine collection does not account for the day-to-day variability in iodine intake, it does overcome the variability associated with urine volume that affects the interpretation of iodine concentration in a spot-urine sample and for that reason can be considered preferable to a single-spot urine sample(15;16). Subjects who volunteered for the study were required to collect all the urine passed in a 24-h time period (completeness of the sample was self-reported). Participants were provided with clear instructions, a wide-necked jug and a leak-proof 5-litre container (both had been acid-washed and rinsed prior to use). Women were advised not to wash the jug between urine collections and to ensure that the container lid was well sealed. The total urine volume of each participant was measured; 20 ml aliquots were taken and stored at −20°C until analysis.
Subjects completed a questionnaire to provide basic information including date of birth, use of nutritional supplements, medication and any dietary exclusion practised. Participants were required to keep a detailed food diary both for the 24 hours before they collected urine and during the 24 hours of urine collection.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Surrey Ethics Committee. Written informed consent was obtained from all subjects.
Analysis of urinary iodine
Iodine concentration was measured on a ThermoElemental X-Series ICP-MS (ThermoFisher Scientific, Hemel Hempstead, UK) in the trace element laboratory at the University of Surrey. In order to produce samples in the analytical range for ICP-MS, the samples were first diluted with an alkaline diluent. The diluent used was prepared by dissolving 3.32 g NH4H2PO4, and 1.16 g (NH4)2H2EDTA (Analar grade, Sigma-Aldrich, Dorset, UK) in deionised water, adding 10.0 ml ammonia solution (s.g. 0.88), and making up to 1000 ml with deionised water. The final concentrations in the diluent solution were 0.14M ammonia, 0.003M (NH4)2H2EDTA, and 0.029M NH4H2PO4. 400 μL of each participant’s urine sample was made up to 10 ml with the alkaline diluent. Standard iodine solutions were prepared with potassium iodide (Analar grade, Fisher Scientific, Loughborough, UK) for the construction of calibration curves. 400 μL of control urine (taken from laboratory stock with a median urinary iodine concentration of 80.6 μg/L and 46.5 μg/L in 2007 and 2008 respectively) was added to 400 μL of each standard in order to obtain matrix-matched standards. An internal standard was added to all samples: Rhodium (103Rh) and Iridium (192Ir) (both SPEX Certiprep Ltd, Middlesex, UK) were made up in a working standard solution of 1 mg/L (ppm) in 1% v/v nitric acid (Trace analysis grade, Fisher Scientific). This was made up 1 in 10 with the diluent and 150 μL was added to every tube.
To evaluate the accuracy of the method, a number of certified reference materials (CRM) were used [Seronorm urine (Bio-Stat House, Cheshire, UK) in 2007 and EQUIP (Ensuring the Quality of Urinary Iodine Procedures)(19) samples in 2008]. Our observed mean values (μg/L) for the EQUIP CRMs were: 28.9 (SD 0.1, n=2) for U02 (certified mean 28.7 μg/L, range 20.1-37.3); 45.9 (SD 0.0, n=2) for U05 (certified mean 45.0 μg/L, range 31.5-58.5); 298.0 (SD 0.0, n=2) for U09 (certified mean 296.3 μg/L, range 251.9-340.7), 11.4 (SD 0.6, n=2) for U10 (certified mean 12.2 μg/L, range 8.5-15.9) and: 264 (SD 1.0, n=3) for Seronorm Urine (certified mean 282 μg/L, range 264-300).
Derivation of total urinary iodine excretion and extrapolation to give estimated iodine intake
Total 24-h iodine excretion was calculated for each subject by multiplying iodine concentration (μg/L) by total urine volume (L) collected. It has been estimated that approximately 90%(1) of ingested iodine is eventually excreted in the urine and this assumption allows dietary iodine intake to be estimated by dividing total urinary iodine excretion (μg/24 h) by 0.90.
Dietary analysis
The information recorded in the 48-h food diaries was entered into the Windiets Research programme (Robert Gordon, 2005) to estimate iodine intake both on the day before, and the day of, urine collection. Where portion weights were not recorded in the diaries, medium portion sizes were entered(20). The iodine content of reported multivitamin and mineral supplements was added to the total iodine intake estimated from the food diaries on each day and an average iodine intake across the two days was calculated. The quantity of iodine-rich foods (milk, meat, fish and eggs) and soya milk (as a potential replacement for iodine-rich cows’ milk) was extracted from the subject’s diary from each 24-h period and an average was calculated; the average figure for each food item was then used to examine the relationship with urinary-iodine excretion.
Classification of iodine status and estimation of prevalence of iodine deficiency
The median urinary iodine concentration of the group was compared to the WHO criteria for risk of iodine deficiency (Table 1). To calculate the risk in individuals, total urinary iodine excretion in 24 hours was compared to values from other studies(15;21) and to the thresholds (i.e. μg per day) proposed for population-based studies (Table 1)(22).
Table 1.
Classification of iodine deficiency using measures of iodine concentration and total iodine excretion in a 24-h period.
For the purposes of evaluating iodine intake (either extrapolated from urinary iodine excretion or estimated from food diaries), the Dietary Reference Intakes published by the Institute of Medicine (IOM)(7) were used; neither the UK Dietary Reference Values(23) nor the WHO recommendations(17) has a value for the Estimated Average Requirement (EAR) which is required for prevalence estimates of nutrient deficiencies in a population(24). The percentage with an iodine intake below the adult EAR (95 μg/day) (7) was used to describe the prevalence of deficiency in the cohort.
Statistical Analysis
Variables were not normally distributed and therefore median and interquartile ranges are reported; variables were transformed using the natural logarithm to allow parametric testing where possible. Intake of food groups (e.g. fish and milk) was not normally distributed, even after transformation and therefore non-parametric tests were used.
Independent t-tests or one-way ANOVA were used to compare (log-transformed) 24-h iodine excretion between groups. A paired t-test was used to compare intake over the two days of dietary records and the two methods of iodine-intake estimation. Analysis of the correlation between two continuous variables was conducted using Pearson correlation when both variables were normally distributed or Spearman Rank when variables were not normally distributed. Forward stepwise linear regression (using log-transformed 24-h iodine excretion) was performed to evaluate the most important dietary predictors of iodine status; all dietary variables and dose of iodine in a multivitamin and mineral supplement were entered as independent variables.
Bland-Altman plots were used to compare the two methods of iodine intake (i.e. extrapolation from 24-h urinary iodine excretion or from estimation from food diaries). The mean difference between the two methods was plotted against the mean of the methods.
Statistical significance was set at p<0.05 and analysis was performed with the Statistical Package for Social Sciences (Version 21.0; SPSS Inc. Chicago, IL).
Results
Twenty-six women volunteered to participate in 2007 and 31 in 2008, giving a total of 57 women of childbearing age. Approximately 90% of the women were studying for a degree in nutrition or nutrition/dietetics. The median age of the women was 23 years (range 19-45 years). Five participants (8.8%) were lacto-ovo vegetarians and three (5.3%) were pescatarian (excluded meat and poultry but included fish); there were no vegans in the study.
Iodine excretion and estimated iodine intake
Table 2 shows results for urinary iodine concentration, 24-h iodine excretion and estimated iodine intake (extrapolated from urinary iodine excretion). The median UIC value (63.1 μg/L) and the fact that 31.6% (n=18) subjects had a urinary iodine concentration below 50 μg/L, classifies the group as mildly iodine deficient by WHO criteria(17). However, the median 24-h iodine excretion (149.8 μg/24 h) classifies that same group as having adequate iodine status according to the criteria in Table 1(15;21).
Table 2.
Summary table of urinary iodine concentration, 24-h urine volume, 24-h iodine excretion and extrapolated daily iodine intake for all 57 participants
| Median | 25th-75th percentile | |
|---|---|---|
| Urinary iodine concentration (μg/L) | 63.1 | 40.8, 95.0 |
| Urine volume (L/24 h) | 2.52 | 1.76, 3.02 |
| Total iodine excretion (μg/24 h) | 149.8 | 102.4, 220.5 |
| Iodine intake† (μg/day) | 167 | 114, 245 |
estimated by extrapolation from urinary excretion (dividing by 0.90)
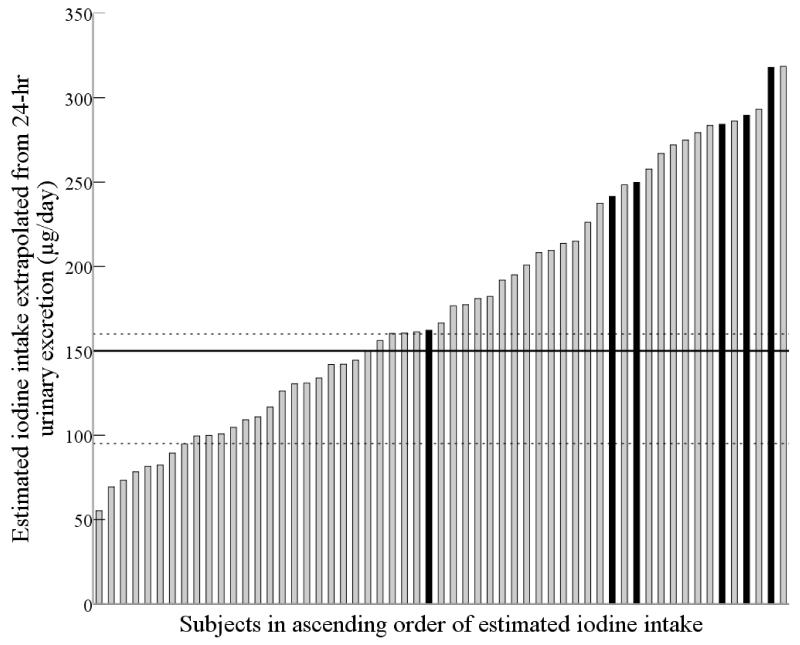
When 24-h urinary iodine excretion was extrapolated to estimate daily intake (on the basis that 90% is excreted), the median (167 μg/day) was above the adult RDA of 150 μg/day(17). Estimated iodine intake for the 57 subjects is shown in Figure 1; the dotted lines denote the RDA and EAR for adults(7) and the EAR for pregnant women(7). Fourteen percent (n=8) had an estimated intake below the adult EAR of 95 μg/day(7) and 40.3% (n=23) had an estimated iodine intake below the adult RDA (150 μg/day). Forty-two percent of women (n=24) had an iodine intake below the EAR for pregnancy (160 μg/day)(7), 11% of whom (n=3) were taking a multivitamin and mineral supplement containing iodine.
Figure 1.
Subjects in ascending order of iodine intake extrapolated from 24-h urinary iodine excretion. Black bars indicate subjects who took a supplement containing iodine. The middle solid line shows the RDA for adults (150 μg/day)(7). The dashed lines represent the IOM EAR values: the lower dashed line represents the EAR for adults (95 μg/day) and the upper dashed line represents the EAR for pregnant women (160 μg/day)(7).
The median iodine intake estimated from the food diaries was above the adult EAR (95 μg/day) but below the RDA (150 μg/day) for both days of dietary record as was the average of the two days (Table 3). A total of 16 women (28.1%) had an iodine intake (averaged over the two 24-h periods) that was below the EAR, a higher figure than that estimated from extrapolation of 24-h iodine excretion (n=8, 14%). Thirty-four (59.6%) women had an average iodine intake below the EAR for pregnancy.
Table 3.
Iodine intake estimated from food diaries plus supplements (μg/day)
| Median | 25th-75th percentile | Correlation with 24-h iodine excretion (μg/24-h) | |
|---|---|---|---|
| 24-h before urine collection | 148† | 82, 228 | r=0.70, p<0.0001 |
| 24-h of urine collection | 116 | 74, 213 | r=0.66, p<0.0001 |
| Average of the two 24-h records | 123 | 87, 211 | r=0.75, p<0.0001 |
No significant difference in iodine intake between the 24 h before urine collection and the 24 h of urine collection; paired t-test: p=0.23
The results from the 48-h food diaries show that there was no significant difference in iodine intake between the two 24-h periods (paired t-test: p=0.23). This suggests little variation in iodine intake over two consecutive days in this population.
Relationship between two methods for estimating iodine intake
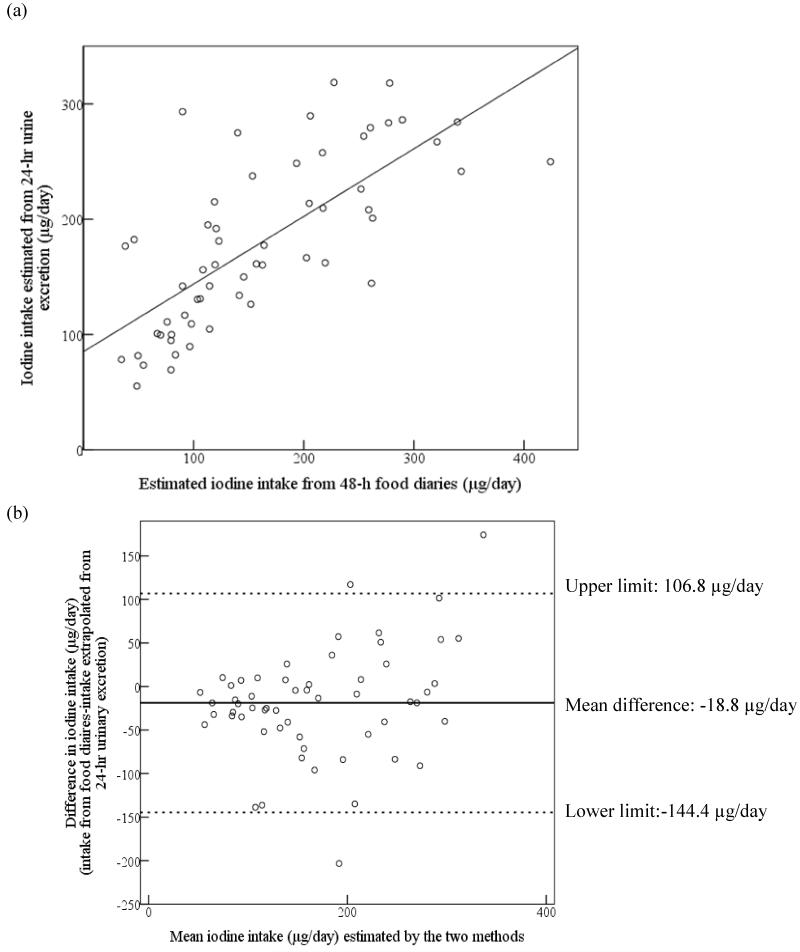
The estimated iodine intake from the diaries was strongly correlated with 24-h urinary iodine excretion (Table 3; Figure 2A). Figure 2 shows the correlation and agreement between the two methods of estimating iodine intake:- (i) estimated from the 48-h food diaries and supplements, or, (ii) from extrapolation of the 24-h urinary iodine excretion. There was a significant difference in estimated iodine intake between the two methods (paired t-test: p=0.001). The Bland Altman plot shows that there was a considerable lack of agreement between the methods as, on average, iodine intake estimated from food diaries was lower than that estimated from urinary excretion (mean difference −18.8 μg/day) and, on an individual basis, the difference ranged from −144.4 to 106.8 μg/day (Figure 2B); this suggests that the methods cannot be used interchangeably.
Figure 2.
(a) Correlation of estimated iodine intake from food diaries and supplements (average of two 24-h periods) vs. extrapolation from 24-h urinary iodine excretion; r=0.71 (r2=0.50). (b) Bland–Altman plot showing differences between the two methods; solid black line represents the mean difference between the two methods and the dotted lines represent the limits of agreement corresponding to ± 2SD.
Dietary exclusions, use of iodine-containing supplements and intake of iodine-rich food items
There was no significant difference in 24-h iodine excretion between omnivores, vegetarians or pescatarians (p = 0.17). Six subjects (10.5%) reported use of an iodine-containing multivitamin and mineral supplement in which the dose of iodine ranged from 75 to 200 μg/day. The women who used an iodine-containing supplement excreted significantly more iodine in 24 h than non-supplement users [240 vs. 144 μg/24 h; p=0.01].
In the 24 h before urine collection and/or during the 24-h urine collection, cows’ milk was consumed by 84.3% of women (n=48), fish was consumed by 28.1%, (n=16) and eggs by 21.1% (n=12). Soya products were consumed by five women (8.8%) and there was a negative correlation between intake of soya products and cows’ milk (r=−0.43, p=0.001), suggesting that women were using soya products as an alternative to cows’ milk.
Intake of milk, dairy products, fish and eggs was positively correlated with 24-h iodine excretion whereas intake of soya products and meat was negatively correlated (Table 4). The strongest correlation was for milk, followed by eggs (Table 4). When the variables in Table 4 were entered into a linear regression model along with dose of iodine in any supplement used (with log-transformed 24-h iodine excretion as the dependent variable), milk intake (p<0.0005), egg consumption (p=0.004) and intake of other dairy products (p=0.013) were all significant predictors of iodine status; soya products, fish and meat intake were not significant predictors in the final model, which explained 49.3% of the variation in 24-h iodine excretion (r2 =0.493).
Table 4.
Correlation between dietary components and 24-h iodine excretion
| Correlation with 24-h iodine excretion | |||
|---|---|---|---|
| Food group (g/day)† | Correlation (r) | P value | r2 |
| Milk | 0.67 | P<0.0001 | 44.9 |
| Other dairy products | 0.29 | 0.03 | 8.4 |
| Fish | 0.24 | 0.08 | 5.8 |
| Eggs | 0.36 | 0.006 | 13.0 |
| Meat | −0.26 | 0.05 | 6.8 |
| Soya products | −0.33 | 0.01 | 10.9 |
average over the 48-h food diary (24-h before and during the 24-h urine collection)
Discussion
Iodine intake and status
The median urinary iodine concentration (63.1 μg/L) is suggestive of mild iodine deficiency when using the current WHO cut-offs for adequacy(17), echoing the finding of mild iodine deficiency in UK schoolgirls(12). However, if using the more recently proposed cut-off for adults of 60-70 μg/L(22), these women would be classified as having adequate iodine status. Indeed, based on the 24-h iodine excretion, the risk of deficiency within the group was low, i.e. after accounting for total urine volume. The median intake based on urinary iodine excretion was above both the EAR and RDA, whereas the value estimated from the food diaries was above the EAR but below the RDA. The proportion with iodine intake below the EAR (14% and 28% for intake extrapolated from urine and food diary estimates, respectively) suggested a degree of deficiency within the cohort. However, it is important to acknowledge that because of day-to-day variability in iodine intake, this does not necessarily mean that those individuals were iodine deficient.
Our results highlight the fact that the degree of iodine deficiency in the cohort varies according to the method used for classification. It is important to highlight that the WHO cut-off for iodine adequacy in adults was based on the fact that goitre risk was low when the median urinary iodine excretion was above 100 μg/day, a figure that was later used for the cut-off in a spot-urine sample on the basis that the units (i.e. μg/day and μg/L) were interchangeable(22). If average urine volume is one L/day, as it is likely to be in children, the units can be used interchangeably but in adults this is probably not appropriate. Indeed, a lower cut-off for iodine adequacy in adults has recently been proposed on the basis that the average urine volume for adults is more likely to be 1.5 L/day and thus the cut-off should be lowered to 60-70 μg/L(22). In fact, in our study mean urine volume was close to 2.5 litres and this explains why the risk of deficiency is over-estimated when using the urinary iodine concentration rather than the 24-h iodine excretion. The food diaries indicated that the women (mostly nutrition students) drank water throughout the day and this accounted for the high urine volume seen in this study. Our results support the need for method- (24-h vs spot collection) and age-specific (adults vs schoolchildren/teenagers) criteria for iodine deficiency(21;22).
On balance, it is likely that this group had a minimal risk of iodine deficiency. Though the median urinary iodine concentration was suggestive of mild deficiency, this is likely to be a result of the high urine volume in the group and therefore dilute urine samples. It is important to point out that these women are by no means representative of UK women of childbearing age as they were highly educated women (mostly science degree students/graduates) in an affluent area of the UK (Surrey). Furthermore as over 90% were studying for a degree in nutrition or nutrition/dietetics, it is likely that their knowledge of good nutrition may have skewed the results (see limitations for further explanation).
Although this was a study in women of reproductive age and not in pregnancy, there are implications for the pregnant state as iodine intake recommendations are higher in pregnancy than for non-pregnant adults(7). When intake is extrapolated from the measured 24-h iodine excretion, 42% of the women (or 60% if using data from the food diaries), failed to reach the EAR for pregnancy (i.e. 160 μg/day)(7), suggesting that UK women may be unable to meet the increased iodine needs of pregnancy, as previously found in other UK studies(10;13;14) and in recent European studies(5;25-27). Bearing in mind the fact that pregnant women are not given advice on iodine intake(28), they are unlikely to modify their diet to increase consumption of iodine-rich foods when they become pregnant. Indeed, results from the Southampton Women’s Survey, where dietary intake was estimated before and after pregnancy in the same women, show that dietary patterns do not change considerably when women become pregnant(29); in terms of iodine-rich foods, intake of fish and milk does not appear to change in early pregnancy, a time-point that is critical for iodine supply for brain development(1;2). Our results suggest that advice to women planning a pregnancy and those who are pregnant should include specific mention of iodine.
Relationship between the two methods used for estimating iodine intake
Our study provides the first opportunity to evaluate how iodine intake, as estimated from food diaries, compares with iodine intake estimated by extrapolation from 24-h urinary iodine excretion; the results show a strong correlation between the two methods, both for intake in the 24-h before the urine collection and during the 24-h collection. This suggests that intake over at least 48-h contributes to iodine excreted in the 24-h urine sample, a finding that echoes that of an earlier study in Denmark(30). However, this finding may also be a result of the fact that there was no significant difference in iodine intake between the two 24-h periods. Despite strong correlations, the Bland-Altman plots show that iodine intake from the food diaries is lower than that extrapolated from 24-h iodine excretion (Figure 2B) by approximately 19 μg/day on average; this explains why a higher percentage of women had iodine intakes below the EAR when using the food diaries than when estimating intake from 24-h excretion (28.1% vs. 14%). This finding is in contrast to data from Denmark where 24-h iodine excretion was lower than estimated intake from either an FFQ or weighed food diary(31). Food-diary analysis has been suggested to be an inaccurate method of estimating iodine intake, in part attributed to the fact that it is difficult to capture the amount of iodine ingested from iodised salt(22;32); however, this criticism is less relevant in the UK where use of iodised salt is low(33;34). The lower iodine-intake estimation from food diary analysis in our study may at least partly be explained by under-reporting – a known problem with this methodology(35). Furthermore, the food-table values for iodine in the Windiets programme may be inaccurate [as a result of poor-quality or out-of-date iodine data in food composition tables(22)] and values are missing for certain foods which may result in an estimate that is lower than actual intake.
Effect of food consumption on iodine intake and status
Milk, eggs and dairy products were positively associated with iodine status in the regression analysis. Iodine excretion correlated more strongly with consumption of milk than with other dietary components, reflecting the importance of milk and milk products as a source of dietary iodine in the UK(36) and supporting previous associations between milk intake and urinary iodine status in UK women(12;13). Milk has also been found to be an important source of iodine for adults in other European countries(37-39). Interestingly, there was a negative correlation between soya product intake and iodine excretion but soya milk was not a significant predictor of iodine status in a regression model when other dietary sources of iodine were included. This probably reflects the negative correlation between soya product and cows’ milk intake, suggesting that the negative correlation in univariate analyses was a result of the displacement of iodine-rich cows’ milk from the diet. Although the number of soya consumers was relatively low in our study, our findings warrant further investigation in view of the increasing use of alternatives to cows’ milk in UK women; for example the volume of soya drinks sold in the UK increased by 10.1% between Jan 2009 and Jan 2013(40). Very few of these milk-alternatives are fortified with iodine and therefore women who rely on these products are likely to be considerably more at risk of iodine deficiency than those who regularly consume cows’ milk.
Fish intake was positively correlated with iodine excretion but the correlation failed to reach significance and was not a significant predictor of iodine status in the regression analysis, perhaps because of the relatively small number of fish consumers in the study. Other UK(12;13) and European studies(41;42) have failed to find significant associations between iodine status and fish consumption. Egg consumption was positively associated with iodine status as has been found in previous studies in children(43), though not in the study of UK teenage girls(12). Results of the latter study were derived from ambiguous questions on egg consumption which likely explains the disparity(44).
Study limitations
Our study is limited by the small number of participants involved; caution should therefore be used when interpreting the results of the sub-group analysis of dietary intake (e.g. of soya products). Although we have detailed information on our subjects (e.g. 48-h recorded dietary intake), accuracy would have been improved if we had had a repeated urine collection, even if only for a sub-sample of the cohort(22;45); we could then have corrected for intra-individual variation in iodine intake. This might have resulted in an improved estimate of intake for individuals falling below the EAR; our estimate of individual intake on the basis of urine excretion may have resulted in misclassification of the percentage with estimated intake below the EAR. However, at the time that this study was designed (2006), the use of multiple 24-h urine collections (as opposed to a single 24-hr collection) was not considered as important as it is now. Completeness of the 24-hr urine sample was self-reported and thus incomplete samples may have been measured; we consider that this is fairly unlikely as the subjects were motivated individuals who understood the implications of incomplete urine collections. Finally, the food-diary analysis is limited by the inherent limitations of dietary assessment, including under-reporting of intake and use of inaccurate food-table values for iodine(31;35). We tried to reduce inaccuracies as far as possible, for example by using the same researcher to code all diaries and enter the data into Windiets.
Other limiting factors are that this study was carried out under circumstances likely to have maximised iodine intake and status. Firstly, the sampling was conducted during the winter months and it is known that winter milk has a higher iodine concentration than summer milk due to an increased use of supplemented cattle feed(46-49). Were this study to be repeated in the summer months, the percentage of women classified as iodine deficient would likely be higher. Secondly, the majority of the subjects were students on a nutrition/dietetics degree programme and likely to have had a greater understanding of a healthy diet; indeed, the food diaries demonstrated that the group ate regular meals, with healthy food choices (such as fruit and vegetables) and were perhaps not typical of a population of young UK women. This may have resulted in a relatively high intake of iodine-rich foods such as fish and milk; indeed the average milk consumption was higher than that of adult women in the recent NDNS (150 vs 124 g/day)(50).
Conclusion
For many years, the UK has been assumed to be iodine sufficient but our study adds to the growing evidence-base that this may not be the case in women of childbearing age. Women entering pregnancy need to have adequate iodine status to ensure optimal fetal neurological development and pregnancy outcome. Our results suggest that a proportion of UK women may be entering pregnancy with low iodine stores, particularly in view of the fact that our study design probably resulted in a best-case scenario. Further study in UK women of childbearing age is required; from 2015, results will be available on iodine concentration measured in spot-urine samples in the National Diet and Nutrition Survey (NDNS) which will provide important data on these women. On the basis of our results, we suggest that the urine samples should be corrected for urine dilution (i.e. by measurement of urinary creatinine concentration). Finally our study has highlighted the need for revised cut-offs for iodine adequacy in adults given that we found that classification of iodine status differed depending on whether UIC values or 24-h iodine excretion measures were used.
Acknowledgments
We are grateful to all the participants of the study and to Dr Christine Sieniawska, at the Trace Element Laboratory in Southampton Hospital, for advice on urinary iodine analysis.
Financial support
The costs of laboratory analysis and consumables were covered by the University of Surrey. SCB is in receipt of an MRC Population Health Scientist Fellowship, which supported the data analysis and the writing of the manuscript.
Footnotes
Conflict of interest
None.
Disclosure statement: The authors have nothing to disclose
References
- 1.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 2.Bath SC, Steer CD, Golding J, et al. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC) Lancet. 2013;382:331–337. doi: 10.1016/S0140-6736(13)60436-5. [DOI] [PubMed] [Google Scholar]
- 3.Hynes KL, Otahal P, Hay I, et al. Mild Iodine Deficiency During Pregnancy Is Associated With Reduced Educational Outcomes in the Offspring: 9-Year Follow-up of the Gestational Iodine Cohort. J Clin Endocrinol Metab. 2013;98:1954–1962. doi: 10.1210/jc.2012-4249. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutr. 2007;10:1542–1546. doi: 10.1017/S1368980007360886. [DOI] [PubMed] [Google Scholar]
- 5.Moleti M, Di Bella B, Giorgianni G, et al. Maternal thyroid function in different conditions of iodine nutrition in pregnant women exposed to mild-moderate iodine deficiency: an observational study. Clin Endocrinol (Oxf) 2011;74:762–768. doi: 10.1111/j.1365-2265.2011.04007.x. [DOI] [PubMed] [Google Scholar]
- 6.Moleti M, Lo Presti VP, Campolo MC, et al. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93:2616–2621. doi: 10.1210/jc.2008-0352. [DOI] [PubMed] [Google Scholar]
- 7.Food and Nutrition Board Institute of Medicine . Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, manganese, molybdenum, nickel, silicon, vanadium and zinc. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 8.Phillips DI. Iodine, milk, and the elimination of endemic goitre in Britain: the story of an accidental public health triumph. J Epidemiol Community Health. 1997;51:391–393. doi: 10.1136/jech.51.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Benoist B, McLean E, Andersson M, et al. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008;29:195–202. doi: 10.1177/156482650802900305. [DOI] [PubMed] [Google Scholar]
- 10.Kibirige MS, Hutchison S, Owen CJ, et al. Prevalence of maternal dietary iodine insufficiency in the north east of England: implications for the fetus. Arch Dis Child Fetal Neonatal Ed. 2004;89:F436–439. doi: 10.1136/adc.2003.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett C, Visser T, Williams F, et al. Inadequate iodine intake of 40% of pregnant women from a region in Scotland. J Endocrinol. Invest. 2002;25(Supp. No. 7):90, P110. [Google Scholar]
- 12.Vanderpump MP, Lazarus JH, Smyth PP, et al. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet. 2011;377:2007–2012. doi: 10.1016/S0140-6736(11)60693-4. [DOI] [PubMed] [Google Scholar]
- 13.Bath SC, Walter A, Taylor A, et al. Iodine deficiency in pregnant women living in the South East of the UK: the influence of diet and nutritional supplements on iodine status. Br J Nutr. 2014;111:1622–1631. doi: 10.1017/S0007114513004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce EN, Lazarus JH, Smyth PP, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–3215. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- 15.Thomson CD, Colls AJ, Conaglen JV, et al. Iodine status of New Zealand residents as assessed by urinary iodide excretion and thyroid hormones. Br J Nutr. 1997;78:901–912. doi: 10.1079/bjn19970208. [DOI] [PubMed] [Google Scholar]
- 16.Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid. 2009;19:1281–1286. doi: 10.1089/thy.2009.0094. [DOI] [PubMed] [Google Scholar]
- 17.WHO. UNICEF. ICCIDD . Assessment of iodine deficiency disorders and monitoring their elimination. 3rd edition World Health Organisation; Geneva: 2007. [Google Scholar]
- 18.Konig F, Andersson M, Hotz K, et al. Ten Repeat Collections for Urinary Iodine from Spot Samples or 24-Hour Samples Are Needed to Reliably Estimate Individual Iodine Status in Women. J Nutr. 2011;141:2049–2054. doi: 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell KL, Makhmudov A, Jones RL, et al. EQUIP: A worldwide program to ensure the quality of urinary iodine procedures. Accred Qual Assur. 2005;10:356–361. [Google Scholar]
- 20.Food Standards Agency . Food Portion Sizes. 3rd ed. The Stationary Office; London: 2002. [Google Scholar]
- 21.Als C, Minder C, Willems D, et al. Quantification of urinary iodine: a need for revised thresholds. Eur J Clin Nutr. 2003;57:1181–1188. doi: 10.1038/sj.ejcn.1601740. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70:553–570. doi: 10.1111/j.1753-4887.2012.00528.x. [DOI] [PubMed] [Google Scholar]
- 23.Department of Health . Report on Health and Social Subjects: 41. Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom. The Stationery Office; London: 1991. [PubMed] [Google Scholar]
- 24.Beaton GH. When is an individual an individual versus a member of a group? An issue in the application of the dietary reference intakes. Nutr Rev. 2006;64:211–225. doi: 10.1301/nr.2006.may.211-225. [DOI] [PubMed] [Google Scholar]
- 25.Vandevijvere S, Amsalkhir S, Mourri AB, et al. Iodine deficiency among Belgian pregnant women not fully corrected by iodine-containing multivitamins: a national cross-sectional survey. Br J Nutr. 2013;109:2276–2284. doi: 10.1017/S0007114512004473. [DOI] [PubMed] [Google Scholar]
- 26.Aguayo A, Grau G, Vela A, et al. Urinary iodine and thyroid function in a population of healthy pregnant women in the North of Spain. J Trace Elem Med Biol. 2013;27:302–306. doi: 10.1016/j.jtemb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Raverot V, Bournaud C, Sassolas G, et al. Pregnant French women in the Lyon area are iodine deficient and have elevated serum thyroglobulin concentrations. Thyroid. 2012;22:522–528. doi: 10.1089/thy.2011.0184. [DOI] [PubMed] [Google Scholar]
- 28.NHS Choices [Accessed: 19 July 2011];Your health during pregnancy. Vitamins, minerals and special diets. The pregnancy care planner. 2009 Available at: http://www.nhs.uk/Planners/pregnancycareplanner/pages/Vitaminsmineralsdiets.aspx.
- 29.Crozier SR, Robinson SM, Godfrey KM, et al. Women’s dietary patterns change little from before to during pregnancy. J Nutr. 2009;139:1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53:401–407. doi: 10.1038/sj.ejcn.1600762. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen LB, Ovesen L, Bulow I, et al. Dietary iodine intake and urinary iodine excretion in a Danish population: effect of geography, supplements and food choice. Br J Nutr. 2002;87:61–69. doi: 10.1079/bjn2001474. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen LB, Ovesen L, Bulow I, et al. Evaluation of a semi-quantitative food frequency questionnaire to estimate iodine intake. Eur J Clin Nutr. 2001;55:287–292. doi: 10.1038/sj.ejcn.1601156. [DOI] [PubMed] [Google Scholar]
- 33.Bath S, Button S, Rayman MP. Availability of iodised table salt in the UK – is it likely to influence population iodine intake? Public Health Nutr. 2014;17:450–454. doi: 10.1017/S1368980012005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus JH, Smyth PP. Iodine deficiency in the UK and Ireland. Lancet. 2008;372:888. doi: 10.1016/S0140-6736(08)61390-2. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone MB, Prentice AM, Strain JJ, et al. Accuracy of weighed dietary records in studies of diet and health. Bmj. 1990;300:708–712. doi: 10.1136/bmj.300.6726.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson L, Irving K, Gregory J, et al. The National Diet & Nutrition Survey: adults aged 19 to 64 years. Volume 3: Vitamin and mineral intake and urinary analytes. HMSO; London: 2003. [Google Scholar]
- 37.Gunnarsdottir I, Gustavsdottir AG, Steingrimsdottir L, et al. Iodine status of pregnant women in a population changing from high to lower fish and milk consumption. Public Health Nutr. 2013;16:325–329. doi: 10.1017/S1368980012001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen LB, Carle A, Jorgensen T, et al. Iodine intake before and after mandatory iodization in Denmark: results from the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr) study. Br J Nutr. 2008;100:166–173. doi: 10.1017/S0007114507886387. [DOI] [PubMed] [Google Scholar]
- 39.Soriguer F, Garcia-Fuentes E, Gutierrez-Repiso C, et al. Iodine intake in the adult population. Di@bet.es study. Clin Nutr. 2012;31:882–888. doi: 10.1016/j.clnu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Datum DC. [accessed 10 March 2014];Kantar Worldpanel Liquid – Soya. 2014 Available at: http://www.dairyco.org.uk/resources-library/market-information/dairy-sales-consumption/kantar-worldpanel-liquid-milk-market/
- 41.Brantsaeter AL, Haugen M, Thomassen Y, et al. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13:54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- 42.Johner SA, Thamm M, Nothlings U, et al. Iodine status in preschool children and evaluation of major dietary iodine sources: a German experience. Eur J Nutr. 2013;52:1711–1719. doi: 10.1007/s00394-012-0474-6. [DOI] [PubMed] [Google Scholar]
- 43.Remer T, Fonteyn N, Alexy U, et al. Longitudinal examination of 24-h urinary iodine excretion in schoolchildren as a sensitive, hydration status-independent research tool for studying iodine status. Am J Clin Nutr. 2006;83:639–646. doi: 10.1093/ajcn.83.3.639. [DOI] [PubMed] [Google Scholar]
- 44.Bath S, Rayman MP. Iodine deficiency in UK schoolgirls. Lancet. 2011;378:1623. doi: 10.1016/S0140-6736(11)61690-5. author reply 1624. [DOI] [PubMed] [Google Scholar]
- 45.Charlton KE, Batterham MJ, Buchanan LM, et al. Intraindividual variation in urinary iodine concentrations: effect of adjustment on population distribution using two and three repeated spot urine collections. BMJ Open. 2014;4:e003799. doi: 10.1136/bmjopen-2013-003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips DI, Nelson M, Barker DJ, et al. Iodine in milk and the incidence of thyrotoxicosis in England. Clin Endocrinol (Oxf) 1988;28:61–66. doi: 10.1111/j.1365-2265.1988.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee SM, Lewis J, Buss DH, et al. Iodine in British foods and diets. Br J Nutr. 1994;72:435–446. doi: 10.1079/bjn19940045. [DOI] [PubMed] [Google Scholar]
- 48.Wenlock RW, Buss DH, Moxon RE, et al. Trace nutrients. 4. Iodine in British food. Br J Nutr. 1982;47:381–390. doi: 10.1079/bjn19820049. [DOI] [PubMed] [Google Scholar]
- 49.Food Standards Agency . Retail Survey of Iodine in UK produced dairy foods. FSIS; [Accessed: 11 October 2010]. 2008. 02/08. Available at: http://www.food.gov.uk/multimedia/pdfs/fsis0208.pdf. [Google Scholar]
- 50.Bates B, Lennox A, Prentice A, et al. NDNS Headline Results from Years 1, 2 and 3 (combined) Department of Health. Food Standards Agency; [accessed 29th May 2013]. 2012. Available at: http://media.dh.gov.uk/network/261/files/2012/07/NDNS-Y3-report_All-TEXT-docs-combined.pdf. [Google Scholar]