Abstract
Band 3, the major anion transport protein of human erythrocytes forms the core of a multiprotein complex in the erythrocyte membrane. Here we studied the spatio-temporal mechanisms of band 3 multiprotein complex assembly during erythropoiesis. Significant pools of intracellular band 3 and RhAG were found in the basophilic erythroblast. These intracellular pools decreased in the polychromatic erythroblast whilst surface expression increased, and were lowest in the orthochromatic erythroblast and reticulocytes. Protease treatment of intact cells to remove extracellular epitopes recognized by antibodies to band 3 and RhAG was used to study surface delivery kinetics and intracellular complex composition from the proerythroblast stage to the enucleated reticulocyte. Newly synthesized band 3 and protein 4.2 interact initially in the early stages of the secretory pathway and are found associated at the plasma membrane from the basophilic stage of erythropoiesis. Although we could successfully co-immunoprecipitate Rh with RhAG from plasma membrane pools at a similar stage, no intracellular interaction between these proteins was detectable. Knockdown of RhAG during early erythropoiesis was accompanied by a concomitant drop in membrane expression of Rh polypeptides. These data are consistent with assembly of major components of the band 3 macrocomplex at an early stage during erythropoiesis.
Introduction
During erythropoiesis erythroblasts mature, exhibit a progressive decrease in cell volume, an increase in hemoglobin synthesis and a loss of intracellular organelles culminating in the extrusion of the nucleus from the cell to form an enucleated reticulocyte. The reticulocyte exits the bone marrow niche and enters the circulation where additional membrane protein and skeletal remodeling occurs to form the recognizable biconcave erythrocyte 1. Whilst undergoing this unique process each progenitor cell must synthesize, deliver and assemble a variety of membrane protein complexes into the plasma membrane. A proportion of these membrane complexes become attached to the cell cytoskeleton where they contribute to the specialized structure-function properties of the erythrocyte membrane. Whilst a variety of studies in a range of model systems have broadly established the temporal expression of individual membrane complex components 2-9, there remains a paucity of information regarding the timing and subcellular localization at which the key interactions and associations within multiprotein membrane complexes establish. One of the highest density multiprotein complexes in the erythrocyte membrane is the band 3 macrocomplex 10-11. This complex is itself composed of two well described sub-complexes, the band 3 complex and the Rh complex (for review see 12).
The band 3 complex consists of a tetramer of band 3 associated with glycophorin A and is connected to the erythrocyte spectrin skeleton via ankyrin and protein 4.2. The absence of band 3 results in the complete secondary absence of protein 4.2 10,13-14, whilst severe band 3 deficiency results in a proportional reduction in protein 4.2 highlighting the dependency of protein 4.2 expression on band 3 11. We recently showed that protein 4.2 associates with band 3 at the basophilic stage of erythropoiesis and that the characteristic membrane alterations associated with protein 4.2 deficiency are evident from this point suggesting that assembly of proteins into complexes begins at this early stage 9. The location within the cell at which this initial association occurs is unknown.
The Rh complex comprises a group of associated membrane proteins that are missing or severely reduced in erythrocytes of individuals with the Rh null phenotype. These are the Rh polypeptides (RhCe, RhD), RhAG, CD47, LW, and GPB 15. The Rh polypeptides and RhAG form the core of this complex, which were originally thought to form a tetramer 16-17 but now seem more likely to be heterotrimeric 18. RhAG can transport NH3 and CO2 19-21 but the contribution of Rh polypeptides to erythrocyte function is unclear 22. The majority of reported Rh null phenotypes are due to mutations in RhAG 23-26 and since these erythrocytes also lack Rh polypeptides, a role for RhAG in regulating Rh expression is likely. A similar dependence was observed upon in vitro expression of RhD with RhAG in K562 and HEK cells leading to the suggestion that the presence of RhAG post-translationally regulates expression of Rh 27. The expression of RhAG is reported to occur prior to Rh during in vitro differentiation 6-7 although we recently showed in a different in vitro culture system that RhAG and Rh follow parallel expression patterns 9. Interestingly, whilst expressed at low levels earlier during erythropoiesis, the bulk of Rh/RhAG surface expression appeared delayed relative to band 3, raising the possibility that these proteins may form a complex at a later stage. Establishing when and where RhAG and Rh associate during erythropoiesis and determining the stage of differentiation at which the dependency of Rh on RhAG actually arises will provide insight into complex formation in both normal and aberrant erythropoiesis.
In this study we have focused on the establishment of two critical associations within the band 3 macrocomplex: the interaction between band 3 and protein 4.2 and that between RhAG and Rh. We use an in vitro culture system to expand and differentiate erythroblasts alongside confocal imaging and biochemical techniques to provide a better understanding of the trafficking dynamics and interactions of band 3, protein 4.2, RhAG and Rh throughout normal terminal differentiation. This has allowed us to dissect both the temporal and spatial organization of this developing multiprotein complex and of its key interactions as it assembles during erythropoiesis.
Materials and Methods Erythroblast Expansion and Differentiation
Peripheral blood mononuclear cells (PBMCs) were isolated by density purification (ρ=1.077; Percoll; GE Healthcare) from healthy donors with informed consent given in accordance with the Declaration of Helsinki. Erythroblasts were expanded and differentiated as described previously 28. To remove free nuclei and nucleated precursors, cells were filtered after 10 days in differentiation medium, using a PALL RCM3 leucocyte filter as described by Douay and Giarratana 29
Antibodies
Monoclonal antibodies (IBGRL, Bristol) were BRIC6, BRAC66 and BRIC170 (band 3); LA1818 (RhAG); BRIC69, R6A and BRIC207 (Rh). Rabbit polyclonal antibodies to protein 4.2, band 3, RhAG, Rh were previously described 9-11,30, and beta-actin was purchased from Santa Cruz. Secondary antibodies used were PE-conjugated rabbit anti-mouse, rabbit anti-mouse IgG1, HRP-conjugated rabbit-anti mouse and swine-anti-rabbit (DAKO) and goat anti-mouse-Alexa™488 (Invitrogen).
Staining of Cytospins
5×105 cells were cytospun onto glass slides, fixed in methanol and stained with May Grünwald/Giemsa stains according to manufacturers protocol. Images were taken with a Leica DM750 microscope coupled to a Pixera Penguin 600CL camera using a 40× lens and processed using Adobe Photoshop 9.0 (Adobe).
Immunofluorescence
Cells (6 × 105) were fixed in suspension in 0.5% acrolein in PBS (Sigma) for 5 minutes at room temperature and washed three times in PBS-0.1M glycine at 400g for 5 minutes before being cytospun onto coverslips coated with Cell Tak (BD Biosciences) according to manufacturer’s instructions and permeabilized with 0.05% Triton X-100 for 5 minutes at room temperature. Cells were blocked in PBS-4% BSA for 45 minutes, incubated with primary antibodies in PBS-4% BSA for 1 hour, washed with PBS and incubated for 1 hour with goat anti-mouse Alexa™-488 conjugated (Invitrogen) secondary antibodies and DAPI (4′,6-diamidino-2-phenylindole, Invitrogen). Coverslips were washed and mounted on microscope slides using Mowiol (Calbiochem) containing 2.5% (w/v) Dabco anti-fade reagent (Sigma). Confocal images were taken using using a Leica AOBS SP2 confocal microscope (63× oil immersion lens 1.4 NA), with settings maintained throughout (PMT gain was set at 500) and processed using Adobe Photoshop 9.0 (Adobe).
Immunoprecipitations and total cell lysates
Immunoprecipitations were performed on erythrocytes (3×107 cells) and differentiating erythroblasts (1.5×107 or 3×107 cells). For total cell immunoprecipitations, cells were lysed and immunoprecipitation of band 3, Rh or RhAG performed as previously described 9. For plasma membrane complex immunoprecipitations antibodies recognizing extracellular epitopes (BRIC6, BRIC69 and LA1818) were incubated with intact cells at 4°C for 1 hour in the presence of PBS-4% BSA with constant mixing by rotation. Cells were washed twice in ice cold PBS, lysed (as described 9) and incubated for 1 hour at 4°C whilst rotating with protein G beads pre-blocked with 4% BSA. Beads were washed 5 times in lysis buffer and bound material eluted using Laemmli sample buffer at 95°C. For internal compartment immunoprecipitations, cells were treated with pronase (500μg/ml Sigma) in PBS at 37°C for 30min. If required, cells were pre-incubated at 37°C for 3 hours in media containing 2.5μg/ml brefeldin A (BFA) (Sigma) or 100ng/ml cycloheximide (CHX) (Sigma). BFA or CHX presence was maintained during pronase treatment and subsequent washes. After pronase treatment, cells were washed at least three times (ice cold PBS), lysed and immunoprecipitations performed as described for total cell immunoprecipitations using antibodies recognizing extracellular epitopes of band 3, RhAG and Rh. Immunoprecipitates were separated by SDS-PAGE and immunoblotted as described 9.
Deglycosylation of Immunoprecipitates
For deglycosylation reactions 2 volumes of 2.5% NP40 (w/v) in 125mM sodium phosphate buffer (pH 7.5) were added to immunoprecipitates eluted in sample buffer or cell lysates. Equal aliquots of the sample were not digested or digested with 500U endoglycosidase H (EndoH) or N-glycanase F (PNGase F) (New England Biolabs).
Flow cytometry
Cells (3×105) were washed twice in PBS then either treated or not treated with pronase (500μg/ml) in PBS at 37°C for 30 minutes followed by 3 washes in ice cold PBS. Cells were incubated with antibodies recognizing extracellular epitopes for 1 hour on ice, washed in ice cold PBS and incubated with PE-conjugated anti-mouse secondary antibodies. Fluorescent signals were measured using a FACS CantoII-F60 machine (BD Biosciences). Data was analyzed using Flowjo 7.2.5 software (Flowjo, Ashland, OR). For re-appearance experiments, pronase treated cells (30min, 37°C, in PBS) were incubated for 0, 30, 60 or 120 minutes at 37°C in pre-warmed differentiation medium. At the indicated times, cells were taken, washed in ice cold PBS-1% BSA and incubated with antibodies for flow cytometry as above. If required, cells were pre-treated for 30 minutes with 100ng/ml cycloheximide or 3 hours with 2.5μg/ml brefeldin A, which were maintained during successive steps.
siRNA knockdown
1.5×106 erythroblasts were transfected with 1μM pool of 3 siRNAs - sequences–GGGCAUAUUCUUUGAGUUA, GCACUAUUGUACAGGGAAU, CCAUUUGGUUCUAUGAUUA directed against RhAG mRNA or 1μM control non targeting siRNA (Santa Cruz) using the Amaxa Nucleofector System (Lonza; setting X-001; end volume 100μl) according to the manufacturer’s protocol. Transfected cells were transferred to differentiation medium.
HEK293T Culture and Transfection
Human embryonic kidney cells (HEK293T) were cultured in DMEM supplemented with 10% FBS, 100U/ml penicillin, and 100μg/ml streptomycin under 5% CO2 at 37°C. Cells were transfected with 5μg pBUDCE4.1RhAGV5-His together with 30μg pBUDCE4.1-RhCemyc-His or pBUDCE4.1-RhDmyc-His or control vector by the calcium phosphate precipitation method and allowed to express for 48 hours before use for immunoprecipitations.
Results
Intracellular pools of band 3 and RhAG are observed early in erythropoiesis
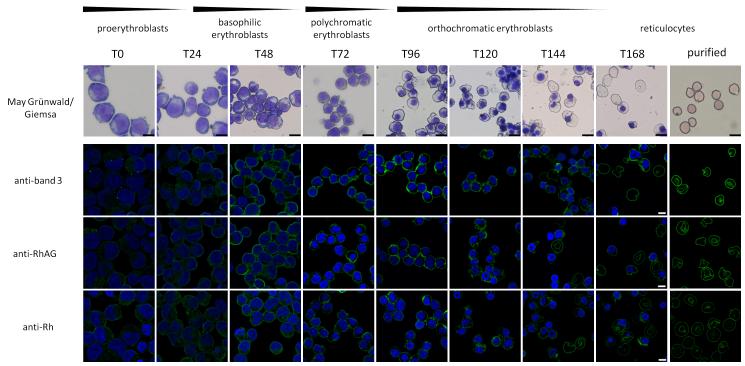
We investigated the localization of proteins of the band 3 multiprotein complex and their assembly during erythropoiesis using human erythroblasts expanded and differentiated from peripheral blood mononuclear cells (PBMCs) according to van den Akker et al28. Using confocal microscopy significant amounts of intracellular band 3, RhAG and Rh were observed at the basophilic stage of erythropoiesis (Figure 1 and Supplemental Figure 1; T24-T72 in differentiation). These proteins appear at the plasma membrane within 48h of differentiation and the intracellular protein pools are markedly reduced at later stages of terminal differentiation (T96-T168). This observation suggested to us the possibility that interactions within the band 3 multiprotein complex may establish initially within intracellular compartments before being delivered to the plasma membrane.
Figure 1. Intracellular pools of band 3, RhAG and Rh are visible early in erythropoiesis.
The top panel represents cells removed from cultures at the indicated timepoints during differentiation, cytospun and stained with May Grünwald/Giemsa. Scale bar represents 10μm. For immunofluorescence, subsequent panels, cells were fixed in 0.5% acrolein, cytospun onto Cell Tak coated coverslips and permeabilized using 0.05% Triton X-100. Cells were stained with antibodies against band 3 (BRIC170), RhAG (LA1818) and Rh (BRIC69) as indicated and visualized using goat anti-mouse-Alexa™488. Nuclei were stained with DAPI. Scale bar represents 5μm.
The interactions of band 3 with protein 4.2 and RhAG with Rh are protease resistant
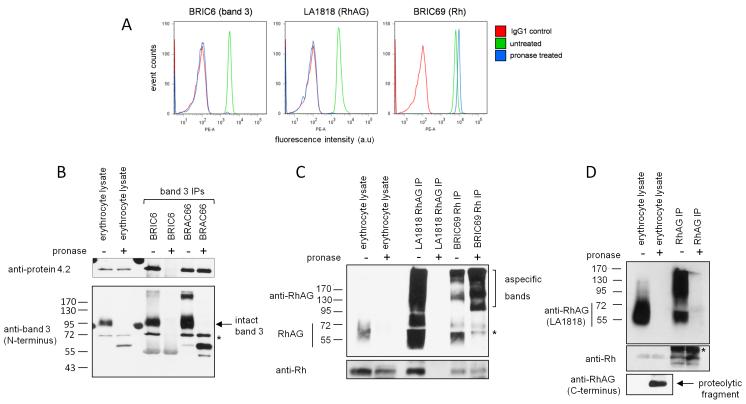
To study the subcellular localization of band 3 and RhAG complex assembly during erythropoiesis, we used proteolytic cleavage of extracellular epitopes and subsequent immunoprecipitation of intracellular protein pools. Erythrocytes were first used to evaluate the efficiency of protease epitope disruption because no intracellular reservoirs of band 3 or RhAG exist in these cells and expression of the majority of erythroid membrane proteins is at its highest level. As expected, pronase treatment of intact erythrocytes completely removed the extracellular epitopes of specific antibodies against band 3 (BRIC6) and RhAG (LA1818) but not for Rh (BRIC69) (Figure 2A), R6A or BRIC207 (data not shown). Pronase treatment increased the fluorescence intensity with BRIC69 (Rh), likely due to increased epitope accessibility caused by removal of extracellular domains from proteins proximate to Rh (e.g. the extracellular domains of RhAG). Other proteases tested (trypsin, bromelain, papain, subtilisin or combinations of proteases) also did not result in Rh epitope destruction for the antibodies tested (data not shown).
Figure 2. Band 3 and RhAG extracellular epitopes are disrupted by cell impermeable pronase treatment.
A) Flow cytometry histograms show the destruction of BRIC6 (band 3) and LA1818 (RhAG) but not BRIC69 (Rh) epitopes upon treatment with 500μg/ml pronase. B) 3×107 untreated erythrocytes or erythrocytes treated with 500μg/ml pronase were lysed and used for each immunoprecipitation with anti-band 3 antibodies BRIC6 and BRAC66. Immunoprecipitates were subjected to SDS-PAGE, western blotting and immunoblotted with anti-protein 4.2 and anti-band 3 N-terminal specific antibodies sequentially without stripping. Lane 1 and 2 represent 1/30th of the immunoprecipitation input. * indicates original protein 4.2 staining C) RhAG IP (LA1818) and Rh IP (BRIC69) immunoprecipitations from pronase treated or untreated intact erythrocytes. Lane 1 and 2 represent 1/30th of the immunoprecipitation input. Proteins were detected with antibodies raised against the C-termini of Rh and RhAG. D) RhAG Immunoprecipitation using a rabbit polyclonal antibody raised against the C-terminus of RhAG. Proteins were detected with a C-terminal specific Rh antibody or with LA1818 for RhAG. * indicates cross reactivity with IgG. Erythrocyte lysates represent 1/30th of the immunoprecipitation input.
Lysates from pronase treated erythrocytes immunoblotted with BRIC170 (band 3, intracellular N-terminal epitope) or polyclonal anti-RhAG (C-terminal intracellular epitope) demonstrate the complete absence of full length band 3 and RhAG and the presence of multiple cleavage products (Figure 2B, C). There was no cleavage of protein 4.2 (Figure 2B), or other intracellular proteins (protein 4.1, actin - data not shown), indicating that pronase is strictly cell impermeable. Immunoprecipitates from intact erythrocytes also confirmed the inability of BRIC6 (band 3) and LA1818 (RhAG) to bind to pronase treated cells (Figure 2B-D). However, immunoprecipitation of band 3 using the intracellular N-terminal specific antibody BRAC66 showed that protein 4.2 remained associated with band 3 even after cleavage of extracellular loops.
The fact that the epitope of the Rh extracellular-specific antibody BRIC69 was unaffected by pronase treatment (see Figure 2C) allowed us to assess the interaction between Rh and RhAG in the absence of intact extracellular domains of RhAG. Immunoprecipitation using BRIC69 demonstrated an interaction between Rh and the ~28kDa cleavage product of RhAG detectable with an antibody raised against the intracellular C-terminus of RhAG as previously reported 16 (Figure 2C and 2D). Similarly Rh was also co-immunoprecipitated using this C-terminal RhAG antibody from both untreated and pronase treated cells (Figure 2D).
The bulk of band 3 and RhAG is delivered to the plasma membrane early during differentiation
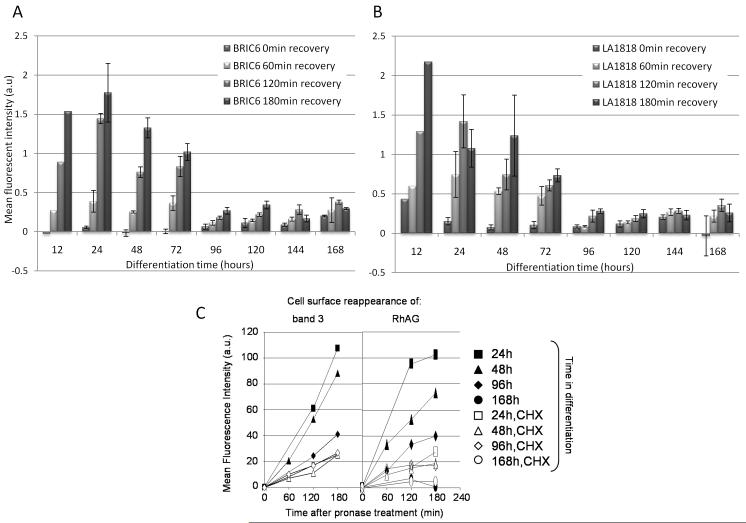
To gain a more detailed picture of band 3 and RhAG delivery to the plasma membrane during erythropoiesis, all detectable epitopes of LA1818 (RhAG) and BRIC6 (band 3) were cleared from the cell surface by pronase treatment and the reappearance of BRIC6 and LA1818 epitopes as a measurement of band 3 and RhAG plasma-membrane delivery at 37°C was monitored using flow cytometry. This approach, when used in conjunction with the protein synthesis inhibitor cycloheximide, allows us to discriminate between de novo synthesis and delivery of already synthesized proteins from intracellular compartments to the plasma-membrane at different times during erythropoiesis. Figure 3 shows that during the first 72 hours of differentiation, band 3 (A) and RhAG (B) extracellular epitopes steadily reappear, consistent with continuous delivery of both proteins to the plasma membrane. This delivery is severely attenuated at the later stages of erythropoiesis (96h, 120h) and was not detectable in the late erythroblast and enucleated reticulocyte stage (144h-168h). Cycloheximide treatment of differentiating erythroblasts at these early stages prior to pronase recovery experiments attenuates but does not negate the reappearance of both band 3 and RhAG epitopes consistent with the existence of a significant mobile intracellular pool of band 3 and RhAG early during differentiation (Figure 3C). Thus the gross reappearance shown in Figures 3A and 3B is comprised both of newly synthesized proteins and proteins trafficked from intracellular compartments and occurs mainly between 0 and 96 hrs. Supplemental Figure 2 shows that 100ng/ml cycloheximide treatment halted all band 3 synthesis indicating that protein synthesis is blocked.
Figure 3. Reappearance of BRIC6 (band 3) and LA1818 (RhAG) epitopes after pronase treatment occurs most rapidly at the early stages of erythropoiesis.
A-B) Assessment of recovery of BRIC6 (band 3, A) and LA1818 (RhAG, B) epitopes on cells pretreated with 500μg/ml pronase for 0, 60, 120 and 180 min, as measured by flow cytometry (mean fluorescent intensity (arbitrary units) in differentiation medium at 37°C at indicated periods of time during differentiation (hours). Means and standard deviations represent three experiments. C) Cells were pretreated with 100ng/ml cycloheximide (white symbols) or not (black symbols) prior to pronase treatment. Recovery of BRIC6 (band 3) and LA1818 (RhAG) epitopes was monitored at 0, 60, 120 and 180 min after pronase treatment at specific time points during differentiation. Data expressed as mean fluorescence intensity (arbitrary units) as a function of recovery time from two independent experiments.
Protein 4.2 associates with band 3 in an intracellular compartment
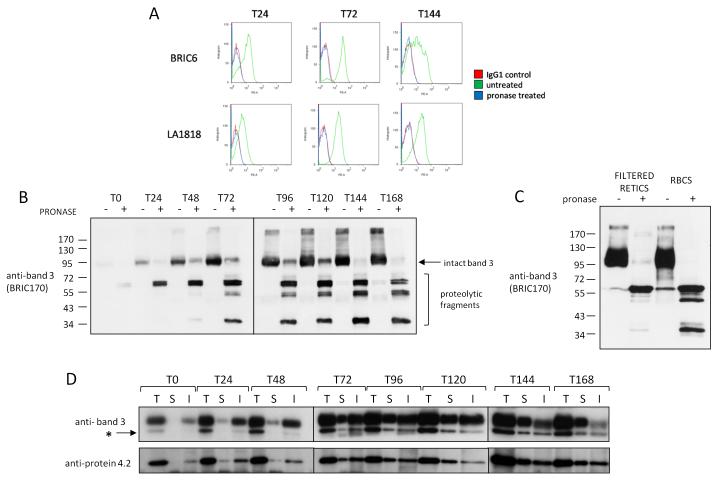
To dissect the subcellular location and timing of key band 3 and RhAG interactions during erythropoiesis we used pronase treatment in combination with immunoprecipitation. Aliquots of differentiating cells at 24 hour intervals were taken from the culture. Figure 4A confirms that pronase treatment of progenitors results in the complete removal of extracellular epitopes of BRIC6 (band 3) and LA1818 (RhAG). Total cell lysates from pronase treated or untreated cells were immunoblotted with BRIC170 and revealed cleavage of band 3 and the existence of an intact pronase resistant pool of band 3 (Figure 4B). Taken together with the fact that all BRIC6 epitopes were removed from the plasma membrane (Figure 4A); it is concluded that the pronase resistant pool in the Figure 4B represents intracellular band 3.
Figure 4. Band 3 and protein 4.2 interact initially in an intracellular compartment.
A) Example of flow cytometry histograms confirming destruction of BRIC6 (band 3) and LA1818 (RhAG) epitopes on differentiating erythroblasts at 24, 72 and 144 hours of differentiation B) 1×106 cells from indicated stages of differentiation were either treated (+) or not (−) with 500μg/ml pronase before lysis (T= time in hours). Proteins were immunoblotted with the N terminal intracellular epitope antibody BRIC170 (band 3). Intact uncleaved band 3 and N terminal cleavage products are indicated C) Total cell lysate from equal numbers (1×106) of a purified reticulocyte population or erythrocytes treated or not with 500μg/ml pronase were immunoblotted with anti-band 3 antibody BRIC170. Note the existence of a pool of internal band 3 (~1% by densitometry) in pronase treated reticulocytes that is absent in pronase treated erythrocytes. D) 1.5×107 cells at the indicated stages of differentiation were used for total (T), internal (I) or surface (S) immunoprecipitations respectively using the band 3 antibody BRIC6 as described in Materials and Methods. Proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C-terminus of band 3 sequentially without stripping. The asterisk indicates the original protein 4.2 staining prior to detection of band 3
Densitometry analysis on BRIC170 untreated and treated lysates shows that the internal pool of band 3 in basophilic erythroblasts after 48 hours of differentiation is 32.4+/−1.71% (n=3). This is reduced to <5% in late orthochromatic erythroblasts. It is notable that at the orthochromatic and reticulocyte stage of differentiation, an internal pool of band 3 remains detectable by western blot despite the negligible reappearance of BRIC6 epitopes in our reappearance assay and absence of staining by immunofluorescence. To exclude the possibility that a minority of remaining nucleated cells were contributing to this internal pool at T168, we pronase treated cells matured for a further 48 hours that were passed through a leukofilter to remove any remaining nucleated cells. This more mature, purified reticulocyte population showed a reduced but still detectable internal pool of band 3 representing approximately <1% of total band 3 (Figure 4C), indicating that a pool of internal band 3 does exist in reticulocytes but not erythrocytes. This could be slowly incorporated during further membrane remodeling to form the biconcave erythrocyte or degraded during the maturation process via unknown mechanisms, similar to the degradation of cytosolic actin and tubulin at this stage as previously reported 31.
BRIC6, which recognizes an extracellular epitope, was used to immunoprecipitate band 3 from total cell lysates, from the cell plasma membrane and from the internal pool (i.e. after treatment of cells with pronase to remove extracellular epitopes). Comparison of total cell lysates and cell surface immunoprecipitates show that at the pro-erythroblast stage (T0) band 3 is undetectable at the surface, consistent with confocal imaging (Figure 1) and our previously published flow cytometry data 6,9. As differentiation progresses, the surface expression of band 3 increases but the internal band 3 pool, which initially increases, decreases at the latest stages of differentiation (T120-T168, particularly post enucleation). Immunoblotting of the BRIC6 immunoprecipitates from total cell lysates demonstrates that protein 4.2 was co-immunoprecipitated with the intracellular pool of band 3 before the appearance of band 3 at the cell surface (Figure 4D). Protein 4.2 was found associated with intracellular band 3 throughout erythropoiesis. This demonstrates for the first time that the association between band 3 and protein 4.2 occurs in an intracellular compartment, prior to delivery to the membrane. Levels of co-immunoprecipitated protein 4.2 mimic band 3 levels throughout erythropoiesis at both the surface and internal pools further confirming the dependence of protein 4.2 expression on band 3.
Band 3 and protein 4.2 Interact in the early Golgi or ER
To identify the intracellular compartment where band 3 and protein 4.2 interact, immunoprecipitations were performed on pronase treated erythroblasts differentiated for 48h that had been pre-treated for 3 hours with brefeldin A (BFA; a secretory pathway inhibitor), or the protein synthesis inhibitor cycloheximide. BFA treatment results in Golgi collapse, preventing trafficking from the ER to the Golgi and from the Golgi to the plasma membrane and is thus predicted to result in increased ER localized band 3. As expected, a slight increase in band 3 (6.62% +/− 1.1%, n=3) is observed due to the BFA block in anterograde trafficking (compare lane 2 and 3 Figure 5A) which was more pronounced in the immunoprecipitations (compare lane 6 and lane 7 Figure 5A; 16.1% +/− 4.7%, n=3). In these experiments there was no discernable alteration in the amount of protein 4.2 co-immunoprecipitated with band 3, indicating that the initial association does not occur post exit from the Golgi (i.e. between Golgi and plasma membrane) since trafficking out of the Golgi is blocked by BFA (Figure 5A and B). Importantly, flow cytometry experiments showed the concentration of BFA used in Figure 5A is sufficient to block trafficking of band 3 to the plasma membrane (Figure 5B) and also caused Golgi collapse in erythroblasts (Supplemental Figure 3).
Figure 5. The pronase resistant internal pool of band 3 in basophilic erythroblasts is EndoH sensitive.
A) 1.5×107 cells differentiated for 48 hours were either left untreated (lane 1, 5), treated with pronase only (lane 2, 6), brefeldin A + pronase (lane 3, 7) or cycloheximide + pronase (lane 4, 8) as described in Materials and Methods. Cells were then lysed and used to immunoprecipitate band 3 using BRIC6 (lanes 5-8). Immunoprecipitated proteins were detected by immunoblotting with polyclonal antibodies raised against protein 4.2 and the C-terminus of band 3 sequentially without stripping. Lanes 1-4 represents the total cell lysate of 1/15th of the immunoprecipitation input. B) Cells differentiated for 48 hours were left untreated or pre-treated with 100ng/ml cycloheximide or indicated concentrations of brefeldin A (BFA) for 3 hours followed by 500μg/ml pronase treatment. Reappearance of the BRIC6 (band 3) epitope at the cell surface was monitored at the indicated timepoints by flow cytometry. Data expressed as mean fluorescence intensity (arbitrary units) n=2 for each treatment. C and D) Cells removed from culture after 48 hours of differentiation were treated or not with 500μg/ml pronase and lysed. C) BRIC6 was used to immunoprecipitate the internal pool of band 3. Immunoprecipitates were treated with EndoH, PNGase or water as a control as detailed in Materials and Methods and proteins separated on 6% gels. Blots were stained with anti-band 3 C-terminal antibody. Note that the pronase insensitive BRIC6 immunoprecipitated band 3 is sensitive to both EndoH and PNGase. D) Total cell lysates from the pronase untreated sample were treated with EndoH, PNGase or water as a control and proteins separated SDS PAGE. Blots were stained with anti-band 3 C-terminal antibody. Note that the majority of band 3 is sensitive PNGase but not Endo H.
As previously shown, incubation with cycloheximide prevents de novo protein synthesis of band 3 (see Figure 3C) but not trafficking of band 3 through the secretory pathway to the plasma membrane and thus is predicted to result in a time dependent decrease in the intracellular band 3 pool. Cells incubated with cycloheximide for 3 hours and then pronase treated showed a reduced level of protein 4.2 co-immunoprecipitating with band 3 (Figure 5A), accompanied by a concomitant drop in intracellular band 3 expression. Taken together, the experiments in Figure 4D and 5A suggest that the intracellular compartment in which newly synthesized band 3 and protein 4.2 interact is most likely to be the ER or an early Golgi compartment. This conclusion is further supported by our observation that the pronase inaccessible intracellular band 3 pool is sensitive to Endo H and so is the high mannose (or core glycosylated form). This indicates that this pool of band 3 has not reached the medial Golgi compartment where complex glycosylation occurs (Figure 5C) unlike the majority of band 3 visible in the pronase untreated cells which is PNGase sensitive but largely EndoH insensitive (Figure 5D).
Rh and RhAG interact at the plasma membrane
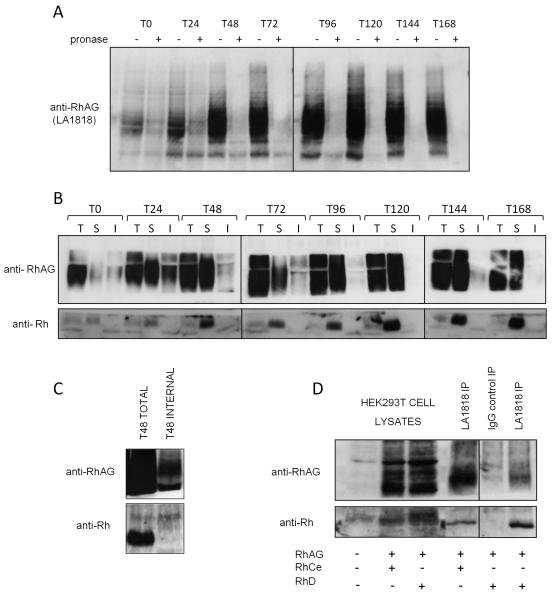
Similar experiments to those conducted on band 3, were performed using the RhAG extracellular antibody LA1818 to determine when and where RhAG associates with Rh polypeptides. The significant intracellular staining of RhAG at the pro and basophilic erythroblast stage observed by confocal immunofluorescence was confirmed by the presence of uncleaved RhAG detectable with LA1818 in pronase treated erythroblasts (Figure 6A and 6B). As differentiation progresses the internal pool of RhAG is depleted almost entirely, with very little intact protein detectable between 120 and 168 hours. Importantly, an increasing amount of Rh polypeptide is co-immunoprecipitated with RhAG from the plasma membrane surface as differentiation progresses (Figure 6B). There was consistently more Rh polypeptide present when RhAG was immunoprecipitated from the cell surface than from total cell lysates. This suggests that the association between Rh and RhAG at the cell surface is more stable than in cell lysates. This stabilization may be due to the presence of antibodies used for immunoprecipitation. This is the first demonstration that RhAG and Rh establish their association at the plasma membrane early during erythroid terminal differentiation and that this interaction is maintained throughout the whole differentiation process as expression levels increase.
Figure 6. RhAG binds Rh at the plasma membrane in basophilic erythroblasts.
A) 1×106 cells from indicated timepoints in differentiation were either treated (internal) or not (total) with 500μg/ml pronase before lysis. Proteins were immunoblotted with LA1818. Uncleaved pronase resistant intracellular RhAG is detectable at the early stages of erythropoiesis B) 1.5×107 cells at indicated timepoints in differentiation were used for total, internal or surface immunoprecipitations respectively using the RhAG antibody LA1818 as described in Materials and Methods. Proteins were detected by immunoblotting with polyclonal antibodies raised against the C-termini of Rh and RhAG C) Lysate from 3×107 erythroblasts differentiated for 48 hours and treated or not with 500μg/ml pronase were used for RhAG (LA1818) immunoprecipitations. Rh is co-immunoprecipitated with RhAG from total cell lysates but not from pronase treated cell lysates (internal pool IP). D) Co-immunoprecipitation of RhD (lane 4) and RhCe (lane 6) with RhAG using LA1818 (RhAG, surface IP, see Materials and Methods) but not with IgG1 control IP (lane 5) from the plasma membrane of HEK293T cells transfected with the indicated combinations of expression constructs encoding human RhD, RhCe and RhAG. Lanes 1-3 represent 1/30th of the total cell lysates used in the immunoprecipitations.
It is notable that despite our ability to efficiently immunoprecipitate intracellular RhAG in the first 0-72 hours of differentiation, no detectable Rh protein was co-immunoprecipitated with the intracellular pool (Figure 6B). This could indicate that unlike the observed association of protein 4.2 and band 3 in an intracellular compartment, Rh and RhAG may first traffic to the plasma membrane independently, before association is initiated (surface immunoprecipitation, Figure 6B). However, since only low levels of Rh polypeptides were co-immunoprecipitated with RhAG from total cell lysates using LA1818 during erythropoiesis, the amount of Rh polypeptide immunoprecipitated from internal pools may be too low to detect. Doubling the number of cells used for immunoprecipitation, which significantly increased the amount of Rh co-immunoprecipitated from total cell lysates using LA1818, did not result in co-immunoprecipitation of Rh with RhAG from intracellular pools (Figure 6C). Interestingly, RhAG plasma membrane surface immunoprecipitation from HEK293T cells transiently transfected with plasmids encoding RhD or RhCe together with RhAG, showed that both RhD and RhCe were co-immunoprecipitated with RhAG from the plasma membrane (Figure 6D). This demonstrates that the interaction between Rh and RhAG at the plasma membrane is not critically dependent on other erythroid proteins (Figure 6D).
Dependency for Rh surface expression on RhAG is established early in erythropoiesis
The dependency of Rh expression on RhAG expression is well documented in erythrocytes [20-23] but the point at which this dependency is established during erythropoiesis has never been addressed. We demonstrate here that RhAG is delivered to the cell surface predominantly during the first 72h of differentiation and becomes associated with Rh polypeptides as judged by co-immunoprecipitation at this early stage. To investigate whether Rh expression is dependent on the presence of RhAG from the onset of erythropoiesis, RhAG was depleted in erythroblasts using siRNA. Transient transfection with a pool of three siRNAs against RhAG resulted in a confirmed depletion of RhAG by western blot (Supplemental Figure 4) and a ~55% decrease in RhAG at the cell surface after 24h, as assessed by flow cytometry which was ~30% after 72h (Figure 7A-B). Surface staining with anti-Rh antibody BRIC69 showed a similar reduction in Rh surface levels, which was evident at the earliest basophilic stage. Band 3 surface levels at 72h were unaffected by knockdown of RhAG (Figure 7A-B) demonstrating both that band 3 surface expression is unaffected by a 50% reduction in RhAG during erythropoiesis and that this level of knockdown of RhAG causes no delay in erythroid differentiation. These data show for the first time that the dependency of Rh on RhAG is established early during erythropoiesis at a point where these two proteins associate in the plasma membrane.
Figure 7. Rh dependency on RhAG is established early in erythropoiesis.
A) Flow cytometry histograms showing knockdown of RhAG and effect on band 3 and Rh. 1.5×106 expanding erythroblasts were transfected with control siRNA or pooled siRNAs directed against RhAG as described in Materials and Methods. Cells were reseeded in differentiation medium RhAG (LA1818) and Rh (BRIC69) surface expression levels monitored after 24 and 72 hours of differentiation and band 3 after 72 hours B) Quantification of RhAG and Rh knockdown at 24 and 72 hours in differentiation as shown in A, normalized against cells transfected with non targeting siRNA. Stars indicate statistical significance (Student T-Test).
Discussion
In this study we have used confocal imaging, flow cytometry and biochemical techniques to define the dynamics of localization and associations of several key erythroid proteins throughout terminal erythroid differentiation. We have previously shown using our in vitro culture system that expression of erythroid specific proteins increases dramatically during the initial stages of differentiation 9. We now show that this flood of newly synthesized protein results in a considerable intracellular pool of band 3 and RhAG at the early basophilic and polychromatic stages of erythropoiesis which is depleted by the orthochromatic and reticulocyte stage. Our results suggest that an expression window exists within the first 0-72 hours of differentiation during which the bulk of band 3 and RhAG are synthesized and rapidly delivered to the plasma membrane. Delivery to the plasma membrane was attenuated at the orthochromatic stage and was negligible immediately prior to and post enucleation within the recovery period studied. This suggests that trafficking of band 3 and RhAG to the surface at later stages of differentiation occurs relatively slowly, hence the weak delivery within the 3 hour time frame. This fits with published 32 and our unpublished data suggesting that the secretory pathway is lost during these later stages of erythropoiesis, leaving only the endocytic pathways to explain any alterations in protein delivery. It seems likely that since the most active delivery of RhAG occurs during the first 72 hours, the increased Rh and RhAG surface expression we previously observed by flow cytometry during the later stages of differentiation 9 reflects an increased epitope availability of Rh and RhAG possibly due to increased stability of these proteins upon incorporation into existing membrane protein complexes, further consolidation of membrane complexes or perhaps reflecting a progressive increase in cytoskeletal attachment 33.
Importantly, whilst proteins are being delivered to the plasma membrane, the assembly of specific plasma-membrane protein complexes composed of peripheral (cytosolic) proteins and membrane proteins is also occurring. Alongside our previous observation that band 3 and protein 4.2 associate early in erythroblast differentiation in parallel with their expression 9 our BFA and cycloheximide experiments now show for the first time that protein 4.2 associates with band 3 initially in the ER or early Golgi compartment prior to delivery to the plasma membrane. This work now raises the possibility that in human erythroblasts a core complex of ‘band 3-protein 4.2-ankyrin’ may be assembled in the ER before moving to the plasma membrane as a complex, since Gomez et al. 34 used metabolic labeling to report that band 3 interacts with ankyrin in the ER or the first Golgi compartment in the mouse MEL cell line. However, metabolic labeling studies used in chick erythroblast suggests that ankyrin binding to band 3 occurs after recycling to the Golgi apparatus from the plasma membrane 35. Unfortunately, we were unable to specifically co-immunoprecipitate ankyrin with band 3-protein 4.2 from human progenitors during terminal differentiation using the immunoprecipitation conditions and antibodies available to us (unpublished observation).
We showed for the first time that an association between Rh and RhAG is detectable at the plasma membrane during erythropoiesis coincident with the appearance of Rh and RhAG on the cell surface during erythroblast differentiation. The ability to co-immunoprecipitate Rh with RhAG at the plasma membrane increased proportionally with the expression levels of both proteins and the amount of these proteins present at the cell surface. Interestingly, unlike band 3 and protein 4.2, we were unable to detect an intracellular association of Rh with RhAG. We observed that a plasma membrane immunoprecipitation (where an extracellular epitope-specific antibody is incubated first with intact cells prior to lysis) was more efficient than an immunoprecipitation conducted after cells had been lysed. Although our data suggest that the interaction is established at the plasma membrane, we cannot exclude the possibility of a weak intracellular interaction existing below the level of detection or that is disrupted upon lysis. Interestingly, the HEK293T cell experiments demonstrate that at least within the plasma membrane, Rh and RhAG can interact in a non-erythroid environment. This is the first report of an observable interaction between these proteins outside erythrocytes and indicates that the two proteins probably interact directly.
The total absence of Rh polypeptides in erythrocytes from patients with Rh null syndrome of the regulator type (RhAG deficiency [20-23]) and in RhAG knockout mice 36 shows the dependency of expression of Rh on RhAG. It has also been suggested that the stability of RhD is post-translationally regulated by RhAG 27. In mice Rh appears to exist as part of the junctional complex, based on the observation that in protein 4.1 knockout mice, Rh but not RhAG is reduced, suggesting that Rh expression is not solely dependent on RhAG 37. The RhAG knockdown experiments we conducted show that the dependency of Rh on RhAG becomes established early in erythropoiesis at the basophilic stage, consistent with the observed interaction at the plasma membrane between these two proteins at this stage of differentiation. Therefore we speculate that the deficiency in Rh induced by RhAG mutations in Rh null patients probably arises during the early stages of erythropoiesis. This hypothesis needs to be addressed through expansion and differentiation of erythroblasts from an Rh null patient in the future.
The observations made here are important for our general understanding of both erythrocyte membrane biogenesis and the pathophysiology behind hemolytic anemias. The numerous transitions that a proerythroblast must undergo during terminal differentiation to form a nascent reticulocyte and then the final biconcave erythrocyte, means that there are multiple stages at which the absence of a protein due to a specific genetic mutation can result in the secondary loss of other proteins within a particular protein complex, leading to specific membrane protein deficiencies observed in hereditary elliptocytosis and hereditary spherocytosis. Since key membrane protein associations and dependencies are beginning to establish from the onset of erythropoiesis, it is likely that characteristic secondary changes observed in patients (e.g. band 3 deficiency, protein 4.2 null, Rh null) will also begin to manifest at these early stages. The fact that we recently observed characteristic changes evident in protein 4.2 null erythrocytes occurring early during erythropoiesis further supports this idea 9. Since further remodeling and connection to the cytoskeleton occurs at later stages, both during and post enucleation, missorting of proteins during enucleation or remodeling processes remains a further point at which these and other proteins can potentially be lost in such diseases 38.
In summary, we have generated sufficient numbers of erythroblasts to begin to study the cellular localization of erythroid proteins during terminal differentiation, and also determine at what stage specific protein-protein interactions and dependencies become established in erythropoiesis. Our data supports a model of protein complex assembly during erythropoiesis in which the critical interactions that form the core of multiprotein complexes and the associated protein dependencies they impart begin at an early stage where the rate of synthesis and delivery to the plasma membrane is greatest. The presence of residual intracellular pools of proteins in late stage enucleated reticulocytes but not erythrocytes suggests that further membrane remodeling and cytoskeletal binding may still be occuring at this penultimate stage.
Supplementary Material
Acknowledgments
This work was funded by a NHSBT Wellcome Trust Fellowship to AMT, a BBSRC DTA NHSBT Case Studentship for TJS (AMT), Wellcome Trust PhD studentship for AB and NHSBT project grants for EvdA and SP (GD).
The authors thank Dr. Rosey Mushens for monoclonal BRIC/BRAC antibodies and Dr. Marieke von Lindern for cell culture reagents.
Footnotes
The authors declare no competing financial interests.
References
- 1.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang H, Langer PJ, Lodish HF. Asynchronous synthesis of erythrocyte membrane proteins. Proc Natl Acad Sci U S A. 1976;73(9):3206–3210. doi: 10.1073/pnas.73.9.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods CM, Boyer B, Vogt PK, Lazarides E. Control of erythroid differentiation: asynchronous expression of the anion transporter and the peripheral components of the membrane skeleton in AEV- and S13-transformed cells. J Cell Biol. 1986;103(5):1789–1798. doi: 10.1083/jcb.103.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods CM, Lazarides E. The erythroid membrane skeleton: expression and assembly during erythropoiesis. Annu Rev Med. 1988;39:107–122. doi: 10.1146/annurev.me.39.020188.000543. [DOI] [PubMed] [Google Scholar]
- 5.Wickrema A, Koury ST, Dai CH, Krantz SB. Changes in cytoskeletal proteins and their mRNAs during maturation of human erythroid progenitor cells. J Cell Physiol. 1994;160(3):417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]
- 6.Southcott MJ, Tanner MJ, Anstee DJ. The expression of human blood group antigens during erythropoiesis in a cell culture system. Blood. 1999;93(12):4425–4435. [PubMed] [Google Scholar]
- 7.Bony V, Gane P, Bailly P, Cartron JP. Time-course expression of polypeptides carrying blood group antigens during human erythroid differentiation. Br J Haematol. 1999;107(2):263–274. doi: 10.1046/j.1365-2141.1999.01721.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A. 2009;106(41):17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Akker E, Satchwell TJ, Pellegrin S, et al. Investigating the key membrane protein changes during in vitro erythropoiesis of protein 4.2 (−) cells (mutations Chartres 1 and 2) Haematologica. 95(8):1278–1286. doi: 10.3324/haematol.2009.021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce LJ, Beckmann R, Ribeiro ML, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101(10):4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 11.Toye AM, Williamson RC, Khanfar M, et al. Band 3 Courcouronnes (Ser667Phe): a trafficking mutant differentially rescued by wild-type band 3 and glycophorin A. Blood. 2008;111(11):5380–5389. doi: 10.1182/blood-2007-07-099473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Akker E, Satchwell TJ, Williamson RC, Toye AM. Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mol Dis. 45(1):1–8. doi: 10.1016/j.bcmd.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Peters LL, Shivdasani RA, Liu SC, et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86(6):917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 14.Inaba M, Yawata A, Koshino I, et al. Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest. 1996;97(8):1804–1817. doi: 10.1172/JCI118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartron JP. RH blood group system and molecular basis of Rh-deficiency. Baillieres Best Pract Res Clin Haematol. 1999;12(4):655–689. doi: 10.1053/beha.1999.0047. [DOI] [PubMed] [Google Scholar]
- 16.Eyers SA, Ridgwell K, Mawby WJ, Tanner MJ. Topology and organization of human Rh (rhesus) blood group-related polypeptides. J Biol Chem. 1994;269(9):6417–6423. [PubMed] [Google Scholar]
- 17.Ridgwell K, Eyers SA, Mawby WJ, Anstee DJ, Tanner MJ. Studies on the glycoprotein associated with Rh (rhesus) blood group antigen expression in the human red blood cell membrane. J Biol Chem. 1994;269(9):6410–6416. [PubMed] [Google Scholar]
- 18.Gruswitz F, Chaudhary S, Ho JD, et al. Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci U S A. 107(21):9638–9643. doi: 10.1073/pnas.1003587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH(3) into red blood cells. Proc Natl Acad Sci U S A. 2004;101(49):17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. Faseb J. 2008;22(1):64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- 21.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci U S A. 2009;106(13):5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton NM, Anstee DJ. Structure, function and significance of Rh proteins in red cells. Curr Opin Hematol. 2008;15(6):625–630. doi: 10.1097/MOH.0b013e328311f422. [DOI] [PubMed] [Google Scholar]
- 23.Cherif-Zahar B, Raynal V, Gane P, et al. Candidate gene acting as a suppressor of the RH locus in most cases of Rh-deficiency. Nat Genet. 1996;12(2):168–173. doi: 10.1038/ng0296-168. [DOI] [PubMed] [Google Scholar]
- 24.Cherif-Zahar B, Raynal V, Le Van Kim C, et al. Structure and expression of the RH locus in the Rh-deficiency syndrome. Blood. 1993;82(2):656–662. [PubMed] [Google Scholar]
- 25.Hyland CA, Cherif-Zahar B, Cowley N, et al. A novel single missense mutation identified along the RH50 gene in a composite heterozygous Rhnull blood donor of the regulator type. Blood. 1998;91(4):1458–1463. [PubMed] [Google Scholar]
- 26.Huang CH, Liu Z, Cheng G, Chen Y. Rh50 glycoprotein gene and rhnull disease: a silent splice donor is trans to a Gly279-->Glu missense mutation in the conserved transmembrane segment. Blood. 1998;92(5):1776–1784. [PubMed] [Google Scholar]
- 27.Mouro-Chanteloup I, D’Ambrosio AM, Gane P, et al. Cell-surface expression of RhD blood group polypeptide is posttranscriptionally regulated by the RhAG glycoprotein. Blood. 2002;100(3):1038–1047. [PubMed] [Google Scholar]
- 28.van den Akker E, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34(−) cells, outweighing the contribution of CD34(+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 95(9):1594–1598. doi: 10.3324/haematol.2009.019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douay L, Giarratana MC. Ex vivo generation of human red blood cells: a new advance in stem cell engineering. Methods Mol Biol. 2009;482:127–140. doi: 10.1007/978-1-59745-060-7_8. Prepublished on 2008/12/18 as DOI 10.1007/978-1-59745-060-7_8. [DOI] [PubMed] [Google Scholar]
- 30.Wu F, Saleem MA, Kampik NB, et al. Anion Exchanger 1 Interacts with Nephrin in Podocytes. J Am Soc Nephrol. doi: 10.1681/ASN.2009090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Guo X, Mohandas N, Chasis JA, An X. Membrane remodeling during reticulocyte maturation. Blood. 115(10):2021–2027. doi: 10.1182/blood-2009-08-241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson ST, Li J, Kang JA, Wickrema A, Williams DB, Reithmeier RA. Loss of specific chaperones involved in membrane glycoprotein biosynthesis during the maturation of human erythroid progenitor cells. J Biol Chem. 2009;284(21):14547–14557. doi: 10.1074/jbc.M809076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodippili GC, Spector J, Kang GE, et al. Analysis of the kinetics of band 3 diffusion in human erythroblasts during assembly of the erythrocyte membrane skeleton. Br J Haematol. 150(5):592–600. doi: 10.1111/j.1365-2141.2010.08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez S, Morgans C. Interaction between band 3 and ankyrin begins in early compartments of the secretory pathway and is essential for band 3 processing. J Biol Chem. 1993;268(26):19593–19597. [PubMed] [Google Scholar]
- 35.Ghosh S, Cox KH, Cox JV. Chicken erythroid AE1 anion exchangers associate with the cytoskeleton during recycling to the Golgi. Mol Biol Cell. 1999;10(2):455–469. doi: 10.1091/mbc.10.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goossens D, Bony V, Gane P, Colin Y, Cartron JP. Generation of mice with inactivated Rh or Rhag genes. Transfus Clin Biol. 2006;13(1-2):164–166. doi: 10.1016/j.tracli.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Salomao M, Zhang X, Yang Y, et al. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci U S A. 2008;105(23):8026–8031. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomao M, Chen K, Villalobos J, Mohandas N, An X, Chasis JA. Hereditary spherocytosis and hereditary elliptocytosis: aberrant protein sorting during erythroblast enucleation. Blood. 116(2):267–269. doi: 10.1182/blood-2010-02-264127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.