Abstract
In addition to the core circadian oscillator, located within the suprachiasmatic nucleus, numerous peripheral tissues possess self-sustaining circadian timers. In vivo these are entrained and temporally synchronized by signals conveyed from the core oscillator. In the present study, we examine circadian timing in the lung, determine the cellular localization of core clock proteins in both mouse and human lung tissue, and establish the effects of glucocorticoids (widely used in the treatment of asthma) on the pulmonary clock. Using organotypic lung slices prepared from transgenic mPER2::Luc mice, luciferase levels, which report PER2 expression, were measured over a number of days. We demonstrate a robust circadian rhythm in the mouse lung that is responsive to glucocorticoids. Immunohistochemical techniques were used to localize specific expression of core clock proteins, and the glucocorticoid receptor, to the epithelial cells lining the bronchioles in both mouse and human lung. In the mouse, these were established to be Clara cells. Murine Clara cells retained circadian rhythmicity when grown as a pure population in culture. Furthermore, selective ablation of Clara cells resulted in the loss of circadian rhythm in lung slices, demonstrating the importance of this cell type in maintaining overall pulmonary circadian rhythmicity. In summary, we demonstrate that Clara cells are critical for maintaining coherent circadian oscillations in lung tissue. Their coexpression of the glucocorticoid receptor and core clock components establishes them as a likely interface between humoral suprachiasmatic nucleus output and circadian lung physiology.
It is well established that the suprachiasmatic nucleus (SCN), located in the anterior hypothalamus, is the principle circadian pacemaker in mammals, driving circadian rhythms of behavior and activity. These rhythms are approximately 24 h in period, and are created and maintained by interactive positive and negative transcriptional and translational feedback loops (reviewed in Ref. 1). A number of core clock genes are involved, which in mammals include: Clock and Bmal, the protein products of which drive the rhythmic expression of 3 Period genes (Per1, Per2, and Per3) and 2 cryptochrome genes (Cry 1 and Cry2). In turn, their protein products, PER and CRY, form multimeric complexes that translocate to the nucleus, where CRY inhibits the transcription of CLOCK and/or BMAL, and PER regulates the transcription of BMAL. Additional components contribute to the clock, including two orphan nuclear receptors, REV-ERB α and ROR α, which repress and activate BMAL1 transcription, respectively, through a shared element (2).
Circadian timers are recognized as a pervasive feature of most organs. Tissues such as the liver, lung, pituitary, and kidney all exhibit robust circadian rhythms in culture (3). In SCN-lesioned animals, these peripheral oscillators rapidly lose synchrony and dampen. Thus, the SCN serves an important role in maintaining a temporally synchronized circadian repertoire throughout the body.
The understanding of how peripheral clocks drive physiology is relatively poor. Circadian timers are known to be important for lung function. For example, there is a well-documented link between diurnal variations in lung physiology (i.e. airway narrowing and inflammation) and nocturnal asthma, and from this authors have presumed an underlying circadian component to the timing and severity of asthma attacks (4-6). Glucocorticoid hormones, both endogenous and therapeutic, are known to modulate the phase of circadian gene expression in peripheral tissues (7). Inhaled glucocorticoids are also widely used as an integral part of the treatment of asthma. Therefore, it is important to understand the effects of these compounds on the pulmonary clock.
Although previous studies have clearly established the presence of a circadian oscillator in the lung, primarily through Northern blot for mRNA expression (8, 9) and by bioluminescence reporting (3), little is known of the cellular localization of circadian clocks. In particular, it is not known whether every cell in the lung contains a circadian oscillator or whether specific cell types are involved. In normal adult lung, the alveolar epithelium is populated by two different cell types: type I and type II pneumocytes. Type I pneumocytes are very thin, with a large apical surface, and function to provide a gas permeable barrier. These cells are abundant in the alveolar compartment, occupying about 97% of the surface area. Type II pneumocytes make up the other 3%, and are found interspersed among the type I cells, usually in groups of two or three. They have a rounded appearance, and contain a rounded nucleus with a prominent nucleolus. Type II pneumocytes self-renew, and are also progenitors for type I cells, which are prone to injury. Other cell types specific to the lung include Clara cells, which are tall columnar, nonciliated cells lining respiratory bronchioles. They secrete a variety of products, including Clara cell secretory protein (CCSP) and a component of the lung surfactant. Clara cells function to detoxify harmful substances inhaled into the lungs.
Here, we describe the regional specificity in expression of two of the core clock proteins (PER2 and CLOCK) within the lung. We demonstrate that Clara cells and type II pneumocytes coexpress these clock proteins with the glucocorticoid receptor (GR), and that they play a key role in the glucocorticoid-mediated resetting of the pulmonary clock.
Materials and Methods
Animals
All experimental procedures were within the guidelines of the Animals (Scientific Procedures) Act, 1986. Wild-type (C57/BL6J) and PER2::Luc transgenic mice (age 8–24 wk) were housed at an ambient temperature of 20–22 C, and maintained in a 12-h light, 12-h dark lighting schedule.
Preparation of ex vivo lung slices
Ex vivo lung slices were cultured from PER2::Luc mice. Mice were killed using cervical dislocation, and the trachea and diaphragm exposed. The lung perfusion technique was adapted from an established method (10). Briefly, a small incision was made in the upper trachea and fine tubing (OD: 0.96 mm; Harvard Apparatus, Ltd., Kent, UK) inserted. One milliliter of 2% agarose (ultra low gelling temperature agarose; Sigma-Aldrich, Dorset, UK; in Hanks’ buffered salt solution warmed to 37 C) was perfused through the tubing into the lungs, and the trachea tied off to prevent leakage. The mouse was cooled at 4 C for 15 min to allow the agarose to set fully before the lungs were removed on ice. Using a vibraslice (Integraslice 7550 MM; Campden, Loughborough, UK), 275-μm serial sections of lung lobes were cut in 4 C Hanks’ buffered salt solution. Sections were transferred to Glutamax DMEM (Invitrogen, Paisley, UK) (containing Pen Strep) and warmed to 37C in an incubator (5% CO2). After four changes of media (to remove any traces of agarose), the tissue was left overnight, and prepared for bioluminescence recording the next day.
Bioluminescence real-time recording
One hour before bioluminescence recordings, lung slices were synchronized by dexamethasone (DEX) (200 nm) or forskolin (10 μm; used in the DEX phase response studies). One hour later, the tissue was transferred onto a Millicell insert (Millipore Corp., Billerica, MA) within a 35-mm culture dish containing 1 ml DEX-free recording medium (containing luciferase) (11, 12), covered, and placed under a photomultiplier tube (PMT) (H6240 MOD1; Hamamatsu Photonics, Shizuoka, Japan) in a light-tight box for bioluminescence recording. Phase and period of the slices were analyzed by rhythm analyzing software using the cosinor method, based on Fourier analysis, specific for circadian rhythms (13). For phase response experiments, single slices (collected from the same experimental animal, 12 slices per animal, n = 3) were used for each time point, recording was stopped temporarily, and DEX (stock solution in dimethylsulfoxide, final concentration 200 nm) was added to the recording medium, the lid resealed, and recording continued. The first treatment of DEX was applied after one full circadian cycle of PER2 expression (peak to peak), and then at two hourly intervals after the second peak. After DEX treatment, slices remained in the PMT system for five further cycles. The phase shift was calculated as follows: the period of each slice was determined from bioluminescence data generated before the treatment. Using this period the predicted timing of the third peak was calculated and compared with the actual timing of the peak after DEX. The difference between the timing of the predicted and actual peak was measured in solar hours, and termed as a phase shift. Statistical analysis on control (untreated) lung slices confirmed that the period calculated using the first two peaks of the cycle does not differ significantly from the period calculated from subsequent adjacent peaks or from all cycles recorded (one-way ANOVA, P = 0.676). Data for phase shift studies are presented as time relative to PER2 peak (solar hours) rather than circadian time.
In some instances, once recording was stopped, the lung slice was preserved for histological analysis by fixing overnight in 4% paraformaldehyde (PFA) and processing as described for whole mouse lung (see below).
Lung tissue preparation for histology
Mouse tissue
Mice were killed by cervical dislocation, and the heart, lungs, and trachea removed en bloc. The trachea was cannulated with fine tubing (OD: 0.96 mm; Harvard Apparatus) attached to a reservoir containing 4% PFA, held 20 cm above the lung preparation. The lungs were pressure filled, and the trachea was tied off. Individual lung lobes were removed and processed before embedding in paraffin wax. Four-micrometer sections were cut and mounted onto slides.
Human tissue
Samples were taken from three nonsmoking patients undergoing surgical resection for suspected or confirmed lung cancer (two male, one female, mean age 51 yr). All subjects gave written informed consent. The study was approved by the local research ethics committee. Fresh lung tissues as far distal to tumor as possible were fixed in formalin and paraffin embedded. Four-micrometer sections were cut and mounted.
Immunohistochemistry
Sections were dewaxed and rehydrated. For antigen retrieval, slides were boiled in citrate buffer [10 mm trisodium citrate (pH 6.0)] for 12 min and cooled to room temperature before further processing. After washing in PBS and then 0.03% Triton X-100 in 0.1 m PBS, endogenous peroxidase was quenched (1% H2O2 in 0.03% Triton X-100 in 0.1 m PBS, 30 min). After further washes, sections were blocked using 2% normal goat serum (Sigma-Aldrich). Subsequently, the primary antibody was applied: CCSP (07-623; Chemicon, Hampshire, UK); Pro-Surfactant Protein C (Ab28744; Abcam plc, Cambridge, UK); PER2 (produced in rabbit, a gift from Hajime Tei; Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan); CLOCK (Ab3517; Abcam plc); and GR (recognizing α and β-isoforms; SC-1004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Slides were left overnight at 4 C. The next day, sections were washed before incubation with biotinylated goat antirabbit IgG (1:200, 30 min; Vector Laboratories, Burlingame, CA). After further washes, sections were incubated with peroxidase-conjugated avidin-biotin complex (Vectastain Elite ABC kit; Vector Laboratories) as per kit directions. The enzymatic reaction product was detected using 3′3-diaminobenzidine (DAB) or vasoactive intestinal polypeptide (VIP) as a substrate (Vector Laboratories). Sections were counterstained with hematoxylin QS (Vector Laboratories), dehydrated, and cleared before coverslipping. Omission of the primary antibody was used as a control to check for nonspecific binding of the secondary antibody. Where available, the antigenic peptide was used to show specificity of the antibody, and the relevant concentration of rabbit polyclonal IgG was used as a control.
For immunostaining on mouse brain, the tissue was fixed in PFA and cryoprotected in sucrose. Sagittal sections (30 μm) were cut on a freezing microtome. Staining was performed on free-floating sections using the methods and reagents described previously.
Immunofluorescence
For dual immunofluorescence in mouse tissue, a tyramide signal amplification system was used (PerkinElmer, Inc., Wellesley, MA) that permits visualization of two antibodies made in the same host species (14). The system was used as per kit instructions, using streptavidin-horse radish peroxidize complex to amplify the signal produced from the fluorophore-tyramide complex (Cy3, 1:50). Subsequently, sections were washed and blocked again before a second primary antibody (CCSP) was applied and left overnight at 4 C. The following day a fluorophore-labeled secondary antibody [fluorescein isothiocyanate (FITC) Vector Laboratories] was applied before further washes, brief dehydration, and clearing, before coverslipping with DePeX (VWR Internatinal, Leicestershire, UK) (15). For dual immunofluorescence in human tissue, sections were incubated with anti-CLOCK that was detected with an Alexa 488 conjugated antirabbit secondary antibody (Invitrogen) before incubation with a rat monoclonal antibody specific for human CCSP (MAB4218; R&D Systems, Abingdon, UK) and detection with an antirat secondary antibody (Texas Red; Vector Laboratories). Slides were stored at 4 C in darkness until they were viewed.
Western blot
Wild-type mice were transferred from the 12-h light, 12-h dark lighting schedule to continuous darkness for a single 24-h period, and lung tissue was collected thereafter at timed intervals. The tissue was minced and homogenized on ice in 1 ml 5× radioimmunoprecipitation assay buffer containing Complete Mini protease inhibitor cocktail tablet (Roche Molecular Biochemicals, Indianapolis, IN). Samples were centrifuged (30 min, 13,000 rpm, 4 C), and the supernatant was collected and respun (10 min 13,000 rpm, 4 C). The supernatant was collected and stored at −80 C. The protein content of each sample was determined using the bicinchoninic acid protein assay (Sigma-Aldrich). A total of 100 μg protein was mixed with 6× Laemmli buffer and denatured at 100 C for 5 min. Samples were loaded onto an 8% sodium dodecyl sulfate polyacrylamide gel. Protein was wet-transferred onto a nitrocellulose membrane overnight. The membrane was washed in Tris-buffered saline plus 0.1% Tween before blocking for 2 h with 5% milk and overnight incubation at 4 C with the primary antibody diluted in blocking solution. The following day, the primary antibody was washed off, and a horseradish peroxidase-linked secondary antibody applied for 1 h. Bands were visualized using the ECL Advance system (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). GR expression levels in lung tissue (measured as band intensity, corrected for α-tubulin levels) were compared between time points using one-way ANOVA.
Primary culture of mouse Clara cells
Clara cells were cultured using the method of Oreffo (16) and Bingle et al. (17). In brief, the lungs were perfused free of blood. After repeated bronchiolar lavage with saline, the lungs were filled, via the trachea, with warmed 0.25% trypsin and left for 15 min at 37 C. The lobes from several sets of lungs were removed and pooled, newborn calf serum (Invitrogen) was added, and the tissue minced finely. Bovine deoxyribonuclease I was added, and the mixture filtered through 100 and 40-μm cell strainers. The suspension was spun, and the pellet resuspended in DMEM-1 (Geneflow Ltd., Staffordshire, UK) and plated out. After 2 h, the suspension was removed from the flask (leaving macrophages and fibroblast-like cells attached) and spun. The pellet was resuspended in 3 ml DMEM-1 containing 10% newborn calf serum and plated out into 35-mm culture plates or coverslips coated with 3% PureCol (Nutacon, Leimuiden, The Netherlands). For bioluminescence recording, these cells were synchronized with a 1-h treatment of DEX (200 nm) before changing the media for recording medium. For immunocytochemistry, coverslips were rinsed in PBS before fixing in ice-cold methanol, blocking in goat serum, incubation overnight with anti-CCSP, and then labeling with a FITC-linked secondary antibody. After the secondary antibody, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) to identify cell nuclei and then the coverslips mounted onto slides.
Naphthalene treatment in vivo
To determine the contribution of Clara cells to the pulmonary clock, female wild-type mice were administered 300 mg/kg naphthalene (Sigma-Aldrich) dissolved in corn oil (20 mg/ml; Sigma-Aldrich) ip using a well-established method (18). Naphthalene is known to selectively kill Clara cells, allowing ablation of this cell type in vivo (19). Naphthalene was administered midmorning, and then 48 h later, the mice were killed and the lungs prepared for either immunohistochemistry or agarose inflation for culture.
Results
Evidence for a glucocorticoid-sensitive circadian clock in the lung
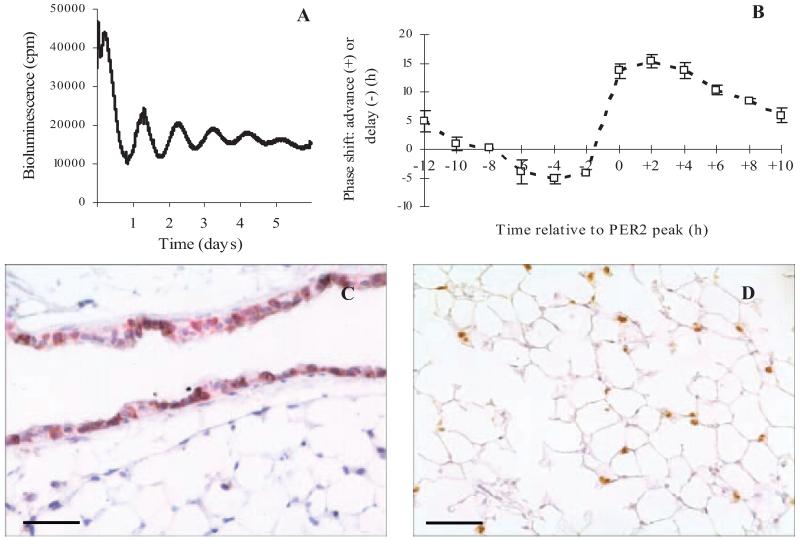
Ectopic lung slices from PER2::Luc mice exhibited robust circadian rhythms of PER2 expression, which dampened after several days (Fig. 1A). The mean period of the oscillations was 24.9 ± 0.19 h and ranged from 23.8–25.9 (n = 12, calculated using bioluminescence data collected over 5 d). Application of DEX reset the PER2 oscillations in a phasic manner, with maximal phase delays and advances of 5 and 15 h, respectively (Fig. 1B). Histological examination of lung slices after recording demonstrated that the characteristic structure of the tissue remained, and that specific cell types (Clara cells and type II pneumocytes) could still be clearly identified (Fig. 1, C and D, respectively).
FIG. 1.
Circadian oscillations in PER2 levels in precision cut lung slices from PER2::Luc mice. A, Sample bioluminescence recorded from a lung slice. B, Lung slices showed a typical phase response curve in response to timed application of DEX (200 nm); values are mean ± sem plotted according to the time of DEX application in relation to the peak of PER2 expression (n = 3 repeats). After at least 5 d in the PMT system, lung slices maintain their morphology and still stain positive for Clara cells (CCSP; VIP, purple staining) in the bronchioles (C) and type II pneumocytes (SP-C; DAB, brown staining) in the alveoli (D). Cell nuclei are stained blue with hematoxylin. Scale bars represent 50 μm. cpm, Counts per minute.
Cellular localization of clock-related proteins and GR in lung
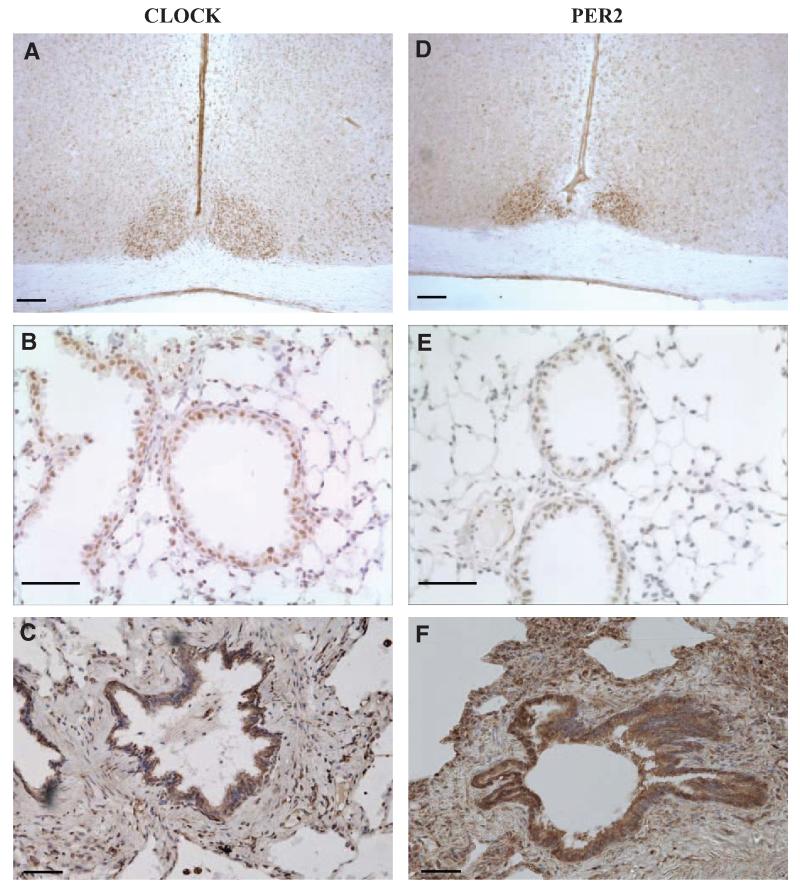
Immunohistochemistry for CLOCK and PER2 on mouse brain slices (positive control) resulted in intense staining in the SCN, confirming the specificity of these antibodies (Fig. 2, A and D). Staining on mouse lung tissue showed that CLOCK is also expressed abundantly in the lung, and is present within the bronchioles and to some extent in the alveoli (Fig. 2B). In human lung, a similar distribution of CLOCK was seen with tissue expression concentrated around the bronchiolar lining (Fig. 2C). In the mouse and human lung, distribution of PER2 was similar to that of CLOCK (Fig. 2, E and F). Control slides showed no positive staining (primary antibody negative and IgG only; data not shown).
FIG. 2.
Immunohistochemistry to localize CLOCK (A–C) and PER2 (D–F) in mouse and human tissue. In the mouse brain, both CLOCK and PER2 show intense staining in the SCN (DAB, brown staining; A and D). In the mouse lung, CLOCK and PER2 localize to the nuclei of cells surrounding the bronchioles with some alveolar staining (DAB, brown staining; B and E). Similarly, in human tissue most CLOCK and PER2 positive cells are found lining the bronchioles (DAB, brown staining; C and F). Cell nuclei are stained blue with hematoxylin (lung sections only). Scale bars, 50 μm.
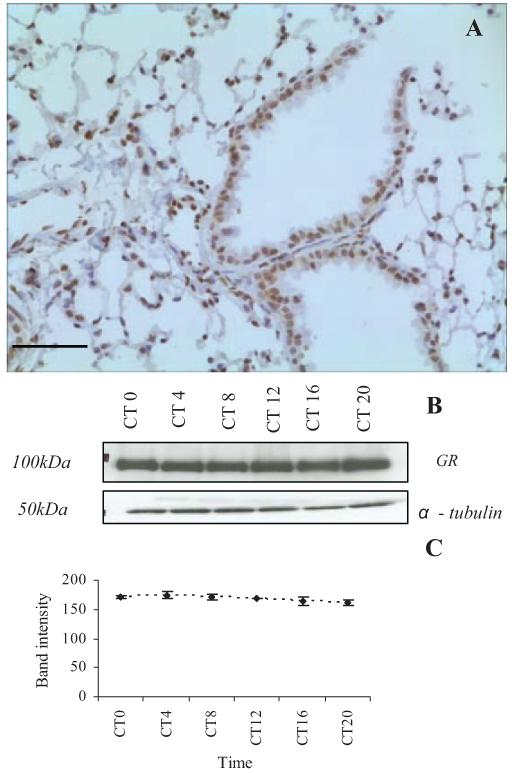
The GR was identified at high levels throughout the lung tissue in the bronchioles and alveoli (Fig. 3A) both in the cell nucleus and the cytoplasm. Control experiments with the blocking peptide showed no positive staining (data not shown). Results from Western blotting (Fig. 3, B and C) indicate that GR expression in the lung is consistent throughout the circadian day.
FIG. 3.
Expression of GR (isoforms α and β) in mouse lung tissue (A). The GR was found to be expressed throughout the mouse lung (DAB, brown staining). Cell nuclei are stained blue with hematoxylin (scale bar represents 50 μm). B, GR expression levels in lung tissue extracted from wild-type mice at timed intervals from circadian time zero (start of the subjective day) to CT20 (Western blot). C, Mean band intensity (corrected for α-tubulin; n = 4) did not vary significantly between time points (values are mean ± sem, one-way ANOVA; P > 0.05).
Bronchiolar Clara cells express CLOCK and PER2
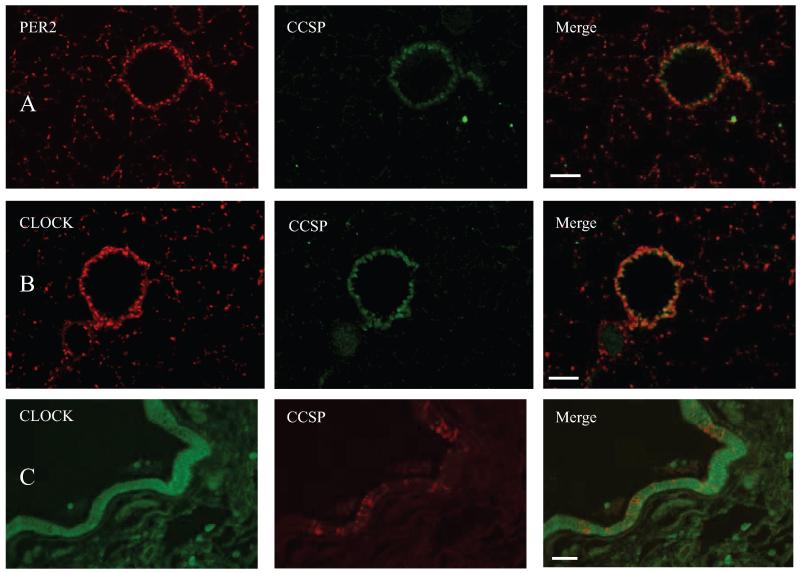
In the mouse lung, both CLOCK and PER2 are clearly expressed in the Clara cells lining the bronchioles (Fig. 4, A and B). Of note, CLOCK and PER2 positive cells are also evident in the alveoli. Analysis of the morphology and distribution of these cells indicate that they are likely to be type II pneumocytes (Figs. 1 and 4). In human lung tissue, expression of CLOCK is found within the cells lining the bronchioles, which consist of both Clara and non-Clara cells (Fig. 4C).
FIG. 4.
Dual immunofluorescence. PER2 (A) and CLOCK (B) in mouse lung; PER and CLOCK (Cy3, red) colocalize with CCSP expression (FITC, green) in the bronchioles. C, CLOCK in human lung; CLOCK (FITC, green) is expressed throughout the bronchiolar lining, but only a limited number of these cells express CCSP (Texas Red). Scale bars represent 50 μm.
Cultures of murine Clara cells show circadian rhythmicity
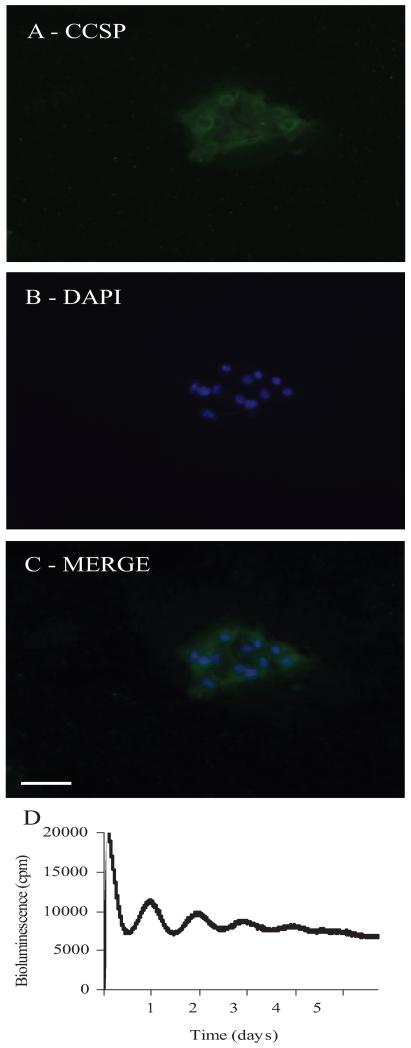
Because the epithelial cells that line the bronchioles express both CLOCK and PER2, experiments were performed on isolated murine bronchiolar epithelial cells. All the cultured cells stained positively for CCSP in the cytoplasm, confirming that they were Clara cells (Fig. 5, A–C). Further experiments using isolated Clara cells from PER2::Luc mice, recorded from on the PMT system, showed that the PER2 protein oscillates in a circadian manner in these cells (Fig. 5D). The period of the oscillations was measured at 23.8 ± 0.4 h (n = 3 separate experiments, cells pooled from four to five mice each time).
FIG. 5.
Isolated mouse nonciliated bronchiolar epithelial cells. A–C, Immunofluorescence shows CCSP expression (FITC, green) in the cytoplasm of the cells after 3 d in culture. The nuclei are stained with DAPI (blue). Scale bar represents 50 μm. D, In the PMT system, CCSP positive cells taken from PER2::Luc mice show clear circadian oscillations in PER2 expression. cpm, Counts per minute.
Influence of Clara cells on the lung clock
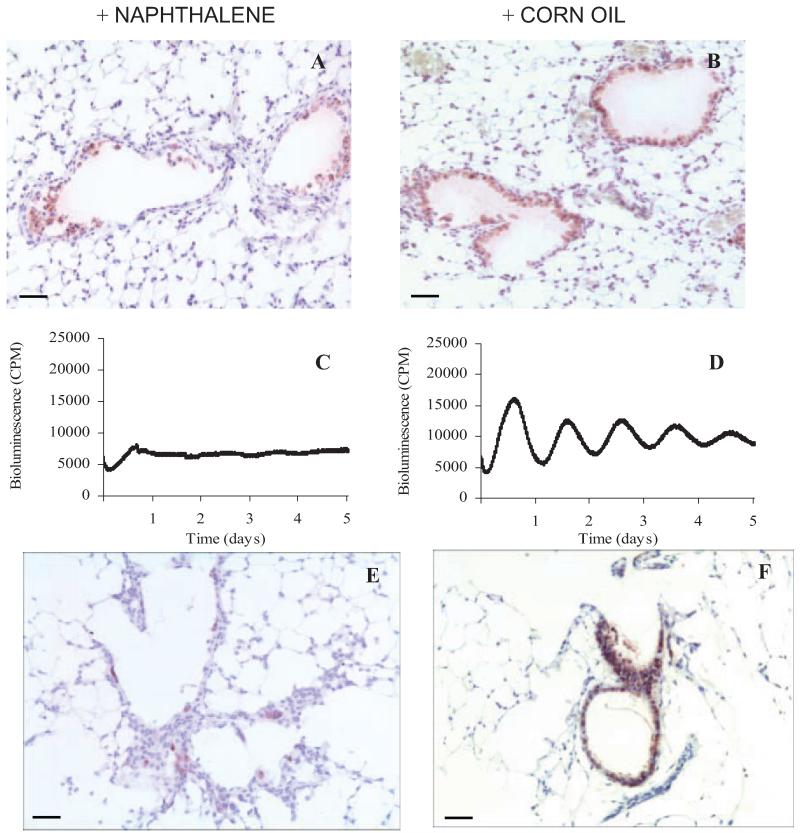
Naphthalene is known to selectively kill Clara cells, allowing ablation of this cell type in vivo (19). Preliminary experiments established that the naphthalene dose and length of exposure caused selective loss of Clara cells within the bronchioles compared with vehicle-treated controls (Fig. 6, A and B). As expected these CCSP positive cells (stained with VIP, purple) exfoliate away from the bronchiolar wall. Some CCSP positive cells can still be seen after naphthalene treatment, which are in the process of detaching or exfoliating away from the bronchiolar wall (Fig. 6A). Ectopic lung slices were prepared from naphthalene and vehicle-treated mice, and luciferase activity was measured. The loss of Clara cells severely dampened the circadian rhythm of PER2 expression in this tissue, whereas control tissue continued to oscillate with a period of 24 h (Fig. 6, C and D). These tissue slices were subsequently fixed in preparation for immunohistochemistry. Figure 6, E and F, is an image taken from these same slices, confirming the loss of CCSP staining after naphthalene treatment.
FIG. 6.
Effects of naphthalene treatment. In vivo application of naphthalene causes the loss of Clara cells from the bronchioles as identified by immunohistochemical staining for CCSP (A); vehicle treatment has no effect (B) (VIP, purple staining). Lung slices from naphthalene-treated mice (C) show dampened rhythms in the PMT system compared with controls (D). Fixation of this tissue after PMT analysis and staining for CCSP (VIP; purple staining) confirms that minimal Clara cells are present in the naphthalene-treated lung after 5 d in the PMT system (E) compared with controls (F). Nuclei are stained blue with hematoxylin. Scale bars, 50 μm. CPM, Counts per minute.
Discussion
This study has shown clear evidence for a glucocorticoid-sensitive circadian clock within the lung. We have specifically identified Clara cells as key components of the pulmonary circadian clock, and destruction of these cells results in the loss of circadian rhythmicity in ectopic lung tissue slices.
Previous work has shown circadian oscillations of PER2 in the lung (3). Our development of an ex vivo slice preparation, which preserves normal tissue architecture, has allowed us to investigate circadian oscillations at the cellular level in the lung. The rhythmic expression of PER2 is considered to be a defining feature of molecular oscillators generating circadian rhythms in mammalian cells, and analysis of the expression of mPer2 mRNA and PER2 have previously been used to identify clock cells in both neural and nonneural tissues (20).
The resetting effects of DEX on peripheral clocks are well established. In 2000, Balsalobre et al. (7) demonstrated that DEX can phase shift the liver clock, and suggested that glucocorticoid secretion in vivo may be an important humoral link between the SCN and peripheral timers. Our in vitro experiments here compliment a recent study on mice in which inhalation of DEX at Zeitgeber time zero phase advanced the expression of Per1, Per2, and Bmal genes in the lungs (but not the liver) and did not affect daily locomotor activity (21). In our studies, application of DEX to cultured lung slices induced phase shifts in the expression profile of PER2, demonstrating that like the liver, the pulmonary clock is responsive to glucocorticoids. The phase response curve we derived is comparable to an earlier study on Rat-1/Pmper2::dLuc cells (22), in which similar phase shifts were observed (maximal phase advances of ~12 h and maximal phase delays of ~8 h). In this, and our current study, the clock was responsive to DEX throughout the circadian day, with no unresponsive “dead zone.” One aspect of our study is that the mean phase shifts at times of maximal advance (0–2 h after the peak in PER2) include individual data points that exceed 12-h shifts; these might also be presented as delays. A likely explanation is that at this transition point, some slices are still in the delay portion, whereas others have moved to the advance portion of the phase response curve.
CLOCK and PER2 were expressed most strongly in the bronchioles in both murine and human tissue. Limited data are available describing which specific pulmonary cell types express clock gene products. A study examining expression of the mammalian homolog of the Drosophila melanogaster TIMELESS in murine lung found that expression was limited to the type II pneumocytes, endothelial and smooth muscle cells surrounding the respiratory blood vessels, and bronchiolar Clara cells (23). A more recent study (8) demonstrated for the first time that PER2 immunoreactivity shows circadian rhythmicity in mouse respiratory tissue, and noted expression of this protein in pseudocolumnar respiratory ciliary epithelial cells of the bronchioles, with some expression in the alveolar cells. Our work directly compliments this study, confirming expression of PER2 in the bronchioles of the mouse lung and demonstrating for the first time that CLOCK is also expressed in the same cells. It is also evident that both CLOCK and PER2 are present in the alveoli, and by cell morphology and distribution, it appears that expression is principally seen in type II pneumocytes. Results from dual-immunofluorescence experiments have enabled us to demonstrate that in the mouse, the principle PER2/CLOCK expressing cells in the bronchioles are CCSP positive, and, thus, can be defined as Clara cells.
Differences in the structure, morphology, and localization of Clara cells exist throughout both the tracheobronchial and bronchiolar epithelium. However, in human airways, Clara cells make up 15–20% of distal airway epithelial cells and are limited to the bronchioles (24). The predominant secretory cells in human tracheobronchial airways are goblet cells (18). This is reflected in our results, which demonstrate that in human lung tissue, only a minority of CLOCK positive cells lining the bronchioles are Clara cells. The similar pattern of clock protein expression within the bronchiolar lining in mice and humans suggests that this anatomical location is important and is retained despite the change in cell population through evolution.
Using an antibody that recognizes both α and β-isoforms of the GR, we have shown that Clara cells also express the GR. These findings are supported by histological studies performed in young mice (embryonic d 18.5), in which the GR is detected at high levels in both airway and alveoli cells (25), and in human lung tissue, in which the GR expressing cells are identified as mononuclear cells, fibroblasts, and epithelial cells (26). Therefore, Clara cells are likely targets for the phase resetting effects of the glucocorticoid analog DEX. There is evidence to suggest that expression levels of the mRNA encoding the GR oscillate in some tissues (white and brown adipose tissue), but not others (liver and muscle) (27). In our study, Western blotting established that levels of the GR protein in the lung remained unchanged throughout the day. Therefore, lung sensitivity to glucocorticoids is likely to be stable.
We have demonstrated, for the first time, that cultured murine Clara cells are able to sustain a circadian rhythm. Critically, we have established that ablation of the Clara cells results in the loss of circadian rhythmicity from lung slices, revealing that Clara cells are a crucial component of the pulmonary clock. The physiological functions of these cells include: secretion of the hypophase layer of bronchoalveolar fluid, local stem cell function within the bronchial epithelium (being progenitors of themselves and of ciliated cells within the bronchioles), modulation of the pulmonary immune response (28), and metabolism of xenobiotics through their abundant P450 cytochrome-dependent mixed function oxygenases (29). It is as a consequence of this latter function, geared toward metabolizing xenobiotics, that it is possible to selectively target Clara cells with naphthalene, which produces toxic metabolites, and results in exfoliation of Clara cells into the airway lumen (19, 28, 30). Results presented here suggest that type II pneumocytes also express CLOCK and PER2, however, whether these cells have the ability to oscillate has yet to be tested. That ablation of Clara cells dampened the pulmonary rhythm of PER2 to such an extent suggests that these cells may be a major oscillatory cell type in the lung, or alternatively, may play a dominant role in coordinating the circadian rhythm in this tissue.
Our findings suggest that a number of the physiological functions performed by Clara cells may be under circadian control. For example, there are daily fluctuations in circulating levels of some inflammatory cytokines, such as IL-6, released by bronchiolar epithelial cells (31, 32). Because there are strong diurnal variations in airway inflammation in asthma patients (6), it is likely that Clara cells may regulate any circadian-related gating of this pulmonary inflammatory response.
In summary, we show that pulmonary Clara cells are critical for maintaining coherent circadian oscillations in isolated lung slices. Their coexpression of the GR and core clock components reveals that they act as key elements of the phasic resetting response to glucocorticoids, and are, therefore, a likely interface between humoral SCN output and lung physiology. Because bronchiolar epithelial cells are the target for inhaled glucocorticoids, their use therapeutically is likely to result in unexpected changes in lung circadian physiology.
Acknowledgments
We thank Dr. Graham Sturton for his assistance with the design of the precision cut lung slices methodology in mouse, and Professor Terry D. Tetley for her assistance setting up the methodology for culturing cells. We also thank Hajime Tei for his kind gift of the PER2 antibody.
Abbreviations
- CCSP
Clara cell secretory protein
- DAB
3′3-diaminobenzidine
- DAPI
4′,6-diamidino-2-phenylindole
- DEX
dexamethasone
- FITC
fluorescein isothiocyanate
- GR
glucocorticoid receptor
- PFA
paraformaldehyde
- PMT
photomultiplier tube
- SCN
suprachiasmatic nucleus
Footnotes
Disclosure Statement: S.F. is employed by GlaxoSmithKline. The other authors have nothing to disclose.
References
- 1.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 2.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 3.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebowitz MD, Krzyzanowski M, Quackenboss JJ, O’Rourke MK. Diurnal variation of PEF and its use in epidemiological studies. Eur Respir J Suppl. 1997;24:49S–56S. [PubMed] [Google Scholar]
- 5.Jarjour NN. Circadian variation in allergen and nonspecific bronchial responsiveness in asthma. Chronobiol Int. 1999;16:631–639. doi: 10.3109/07420529908998732. [DOI] [PubMed] [Google Scholar]
- 6.Martin RJ. Location of airway inflammation in asthma and the relationship to circadian change in lung function. Chronobiol Int. 1999;16:623–630. doi: 10.3109/07420529908998731. [DOI] [PubMed] [Google Scholar]
- 7.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 8.Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci. 2007;27:4359–4365. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendova Z, Sumova A. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res. 2006;55:623–632. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- 10.Moreno L, Perez-Vizcaino F, Harrington L, Faro R, Sturton G, Barnes PJ, Mitchell JA. Pharmacology of airways and vessels in lung slices in situ: role of endogenous dilator hormones. Respir Res. 2006;7:111. doi: 10.1186/1465-9921-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci USA. 2003;100:16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto K, Onai K, Ishiura M. RAP, an integrated program for monitoring bioluminescence and analyzing circadian rhythms in real time. Anal Biochem. 2005;340:193–200. doi: 10.1016/j.ab.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Shindler KS, Roth KA. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem. 1996;44:1331–1335. doi: 10.1177/44.11.8918908. [DOI] [PubMed] [Google Scholar]
- 15.Espada J, Juarranz A, Galaz S, Canete M, Villanueva A, Pacheco M, Stockert JC. Non-aqueous permanent mounting for immunofluorescence microscopy. Histochem Cell Biol. 2005;123:329–334. doi: 10.1007/s00418-005-0769-2. [DOI] [PubMed] [Google Scholar]
- 16.Oreffo VI, Morgan A, Richards RJ. Isolation of Clara cells from the mouse lung. Environ Health Perspect. 1990;85:51–64. doi: 10.1289/ehp.85-1568317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingle L, Richards RJ, Fox B, Masek L, Guz A, Tetley TD. Susceptibility of lung epithelium to neutrophil elastase: protection by native inhibitors. Mediators Inflamm. 1997;6:345–354. doi: 10.1080/09629359791488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Driskell RR, Engelhardt JF. Stem cells in the lung. Methods Enzymol. 2006;419:285–321. doi: 10.1016/S0076-6879(06)19012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol. 1999;21:44–53. doi: 10.1165/ajrcmb.21.1.3630. [DOI] [PubMed] [Google Scholar]
- 20.Amir S, Lamont EW, Robinson B, Stewart J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayasaka N, Yaita T, Kuwaki T, Honma S, Honma K, Kudo T, Shibata S. Optimization of dosing schedule of daily inhalant dexamethasone to minimize phase shifting of clock gene expression rhythm in the lungs of the asthma mouse model. Endocrinology. 2007;148:3316–3326. doi: 10.1210/en.2007-0010. [DOI] [PubMed] [Google Scholar]
- 22.Izumo M, Sato TR, Straume M, Johnson CH. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput Biol. 2006;2:e136. doi: 10.1371/journal.pcbi.0020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao J, Li C, Zhu NL, Borok Z, Minoo P. Timeless in lung morphogenesis. Dev Dyn. 2003;228:82–94. doi: 10.1002/dvdy.10346. [DOI] [PubMed] [Google Scholar]
- 24.Plopper CG, Hill LH, Mariassy AT. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res. 1980;1:171–180. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- 25.Thompson A, Han VK, Yang K. Differential expression of 11β-hydroxysteroid dehydrogenase types 1 and 2 mRNA and glucocorticoid receptor protein during mouse embryonic development. J Steroid Biochem Mol Biol. 2004;88:367–375. doi: 10.1016/j.jsbmb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Oakley RH, Webster JC, Jewell CM, Sar M, Cidlowski JA. Immunocytochemical analysis of the glucocorticoid receptor α isoform (GRα) using GRα-specific antibody. Steroids. 1999;64:742–751. doi: 10.1016/s0039-128x(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Elizur A, Adair-Kirk TL, Kelley DG, Griffin GL, deMello DE, Senior RM. Clara cells impact the pulmonary innate immune response to LPS. Am J Physiol Lung Cell Mol Physiol. 2007;293:L383–L392. doi: 10.1152/ajplung.00024.2007. [DOI] [PubMed] [Google Scholar]
- 29.Singh G, Katyal SL. Clara cell proteins. Ann NY Acad Sci. 2000;923:43–58. doi: 10.1111/j.1749-6632.2000.tb05518.x. [DOI] [PubMed] [Google Scholar]
- 30.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol. 2002;160:315–327. doi: 10.1016/S0002-9440(10)64375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 32.Khair OA, Davies RJ, Devalia JL. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J. 1996;9:1913–1922. doi: 10.1183/09031936.96.09091913. [DOI] [PubMed] [Google Scholar]