Abstract
Purpose
Researchers studying the response of mice to stress generally use mice housed under standard, nationally-enforced conditions as controls. Few investigators are concerned whether basic, physical aspects of mouse housing could also be a source of stress, capable of influencing the subsequent impact of an experimentally applied stressor. We have recently become aware of the potential for standard housing conditions to influence important physiological and immunological properties in mice.
Materials and Methods
Here, we sought to determine whether housing mice at standard temperature (ST; 22°C) versus thermoneutral temperature (TT; 30°C) influences baseline expression of heat shock proteins (HSPs) and their typical induction following a whole body heating.
Results
There were not significant differences in baseline expression of HSPs at ST and TT. However, in several cases, we induction of HSP70, HSP110, and HSP90 in tissues of mice maintained at ST was greater than at ST, following 6 hours of heating which elevated core body temperature to 39.5°C. This loss of HSP induction was also obtained when mice housed at ST were given propranolol, a β-adrenergic receptor antagonist, used clinically to treat hypertension and stress.
Conclusions
Taken together, these data show that housing temperature has a significant influence on the expression of HSPs in mice after whole body heating and should be considered when stress responses are studied in mice.
Keywords: whole body hyperthermia, thermoneutrality, heat shock protein, cold stress
Introduction
Stress proteins are a large class of molecules involved in numerous cellular processes that occur in both normal and pathological settings. These proteins function to maintain homeostasis as well as to prevent protein aggregation and misfolding in the face of environmental stress. The two major categories of stress proteins include the HSPs, which were first characterized by their induction following exposure of cells and organisms to increased temperature, and the glucose regulated proteins (GRP) which, in addition to responding to environmental stress stimuli, also sense nutrient deprivation (1–3). Moreover, these molecules are also induced by other forms of stress including changes in pH and increased levels of oxidative free radicals (4).
In addition to their roles in regulating the stress response, the HSPs also mediate numerous other cellular activities (5). Specifically, these proteins have been widely studied in inflammation, cellular metabolism, and tumorigenesis (6–10). Many of the molecular interactions involving HSPs have been investigated using in vitro culture systems. However, elucidation of in vivo HSP function relies heavily on the use of mouse models. Due to a high surface-area-to-volume ratio, mice have a remarkable capacity for heat exchange with their surrounding environment, allowing for rapid increases in their core temperature when heated (11). In a previous study, our group examined the expression of three members of the HSP70 superfamily– HSP70 (HSP72), HSP110, and GRP170 - in normal tissues after exposing 8 week old female BALB/c mice to several hours of whole body hyperthermia (WBH). This work helped elucidate the post-heating expression of HSP70 and HSP110 and showed that they were detectable at baseline levels in various tissues and organs prior to heating (12).
However, the rate of murine heat exchange also creates largely unrecognized problems for mice housed under standard conditions. In nearly all research facilities, mice are housed at standard, ambient temperatures between 20–26°C as directed by guidelines set forth by the National Research Council (13). This occurs despite the fact that their preferred, thermoneutral temperature, or the temperature at which basal metabolic rate is sufficient to maintain core temperature at 37°C, is approximately 29–31°C (14). Under standard housing temperatures, laboratory mice continuously lose heat to their environment and, thus, must expend more metabolic energy to generate sufficient heat to maintain their body temperature. The response to mild cold stress is regulated specifically by norepinephrine (NE), a stress hormone which can drive heat production through adaptive thermogenesis and other metabolic changes (15,16). This metabolic stress creates several important physiological changes in mice (14,17). Our lab has recently described the significant impact mild cold stress induced by cool housing temperature has on tumor growth and anti-tumor immunity (18,19) as well as therapeutic responsiveness (Eng et al., manuscript submitted).
Environmental factors, such as temperature, have been known to enhance or blunt HSP induction in response to severe stress stimuli in various organisms including fish (20,21) and lizards (22). Since stress proteins, such as HSPs, play such prominent roles in maintaining normal cellular homeostasis, we wondered whether their expression in response to hyperthermia changed as a result of the mild, but chronic cold stress that mice experience from their standard housing conditions. Therefore, we tested whether housing mice at thermoneutrality (TT; 30°C) affects expression of HSPs following WBH compared with mice housed at standard temperature (ST; 22°C). The results obtained here suggest that unrecognized physiological stresses, like housing temperature, can influence the outcome of experimentally applied stressors. Thus, it is important that investigators who are studying stress responses in mice take these other factors into account when they assess stress protein induction.
Materials & Methods
Animals
6 week old female BALB/c mice were purchased from the National Cancer Institute. The mice were implanted with subcutaneous temperature probes (Bio Medic Data Systems) and maintained on ad libitum standard chow diets and water in temperature controlled vivarium set to either 22°C (ST) or 30°C (TT) for 2 weeks prior to whole body hyperthermia.
Hyperthermia
Fever range whole-body hyperthermia was induced for 6hrs in mice using a Wisconsin Oven incubator. Prior to hyperthermia, mice were injected with 1mL of saline. Mice were then heated at 38.5°C until core temperature reached 39.5°C. Afterwards, incubator temperatures were adjusted in order to maintain core temperatures between 39.5 to 40°C. Following 6hrs of heating, mice were either euthanized at 0hrs or were housed back in ST or TT-maintained vivarium for 24 or 48hrs.
Tissue Collection
Immediately following heating, both control groups and 0hr post-whole body hyperthermia groups were sacrificed, while the remaining groups were returned to the same housing temperatures for 24 and 48hr time points. Tissues were harvested and immediately snap frozen in liquid nitrogen. Frozen samples were stored at −80°C until they were processed.
ELISA
Whole blood was collected with 0.5M EDTA by cardiac puncture at the time of sacrifice. Samples were centrifuged at 16,100rcf in a table top centrifuge to separate the plasma fractions. Plasma samples were snap frozen and stored at −80°C until they were processed. ELISAs were performed to assess the levels of plasma norepinephrine (Rocky Moutain Diagnostics) and plasma HSP70 (R&D Systems). Experimental reactions were read at 450nM on a Biotek Synergy HT plate reader.
Western Blot
Protein lysates were prepared and quantitated as previously described (Ostberg et al, 2002). Samples were run on 10% acrylamide gels and transferred to PVDF membranes (EMD Millipore). Membrane blots were blocked with 1% nonfat milk in TBS for 1hr. Antibodies to HSP70 (HSP72/HSPA1A) (Stressgen), HSP90 (Cellsignaling), and HSP110 (Stressmarq) were diluted in TBS-T (1:1000) and blots were incubated overnight at 4°C. Membranes were incubated with anti-rabbit Dylight 790 (Jackson) and anti-mouse Dylight 680 (Jackson) secondary antibodies diluted in TBS-T (1:15000) and then developed by Odyssey Scanner (Li-Cor). Fluorescence intensity was assessed with Image Studio Lite Software (Li-Cor) was normalized to GAPDH (GeneTex).
In vivo Propranolol Studies
Four groups of 6 week old female BALB/c mice were acclimated to either ST or TT for 2 weeks as previously described prior to heating. Additionally, four groups of mice were simultaneously acclimated to ST or TT and treated once daily with 10mg/kg of propranolol (Sigma Aldrich) by intraperitoneal injections.
Results
Norepinephrine levels serum levels
Previous work has established that housing mice at prescribed sub-thermoneutral ambient temperatures does not affect their normal body temperature although it does increase the amount of metabolic effort needed to maintain a normal body temperature. To confirm that mice in our study housed at ST or TT did not affect body temperature, we first measured the core temperatures of healthy mice housed at these two temperatures. As predicted from previous work (18,19), the body temperature of the mice did not differ while being housed at ST versus TT (Figure 1A). However, previous studies have also demonstrated that even moderately cool temperatures are sufficient to induce stress in mice (23–25). Since the response to mild cold stress is regulated specifically by norepinephrine (NE), we next assessed the levels of NE in the serum of mice housed at ST and TT to determine if baseline expression differed in these two groups. Naïve BALB/c mice were first acclimated for two weeks at ST or TT. Analysis of the plasma revealed that norepinephrine levels were significantly higher in mice at ST compared with mice at TT, supporting that animals housed at the standard temperature conditions were, in fact, cold stressed (Figure 1B).
Figure 1.
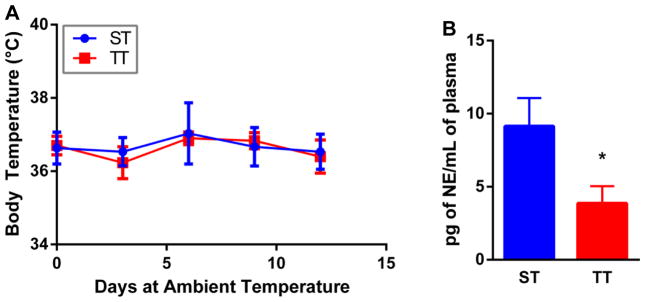
Body temperature does not change in mice housed at ST and TT, but plasma norepinephrine levels are increased in mice maintained at ST. (A) core body temperature of mice housed at ST and TT for 12 days. No significant differences by two-way Anova; n = 3. (B) Plasma levels of norepinephrine from mice acclimated to standard room temperature and thermoneutrality for 14 days. *p < 0.05 by Student’s t test; n = 8.
Heat shock protein expression in visceral organs and the brain
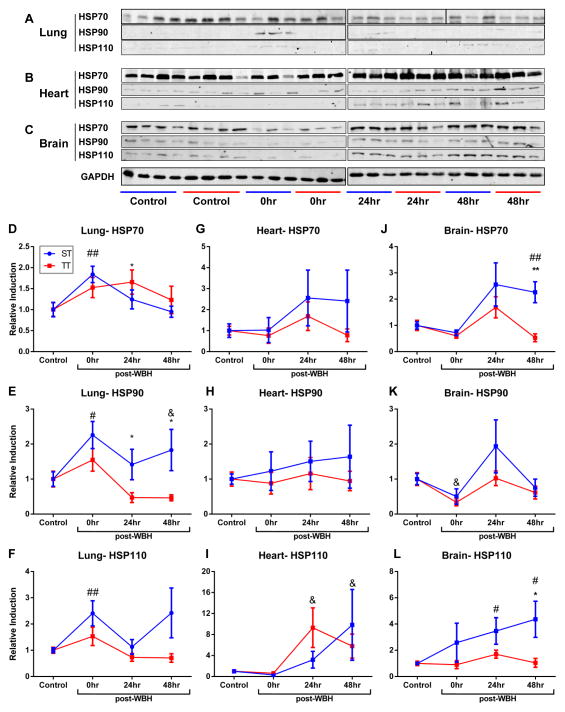
Next, we sought to investigate if chronic stress from sub-thermoneutral housing temperatures could affect the induction of HSPs when body temperature was raised to approximately 39–40°C. As demonstrated above, the body temperatures of mice is the same when they are maintained for extended time periods at ST or TT. After 2 weeks of being housed at either condition, we heated mice for 6hrs (well known to induce HSPs) and then harvested tissues 0, 24 or 48hrs following heat treatment. Control mice did not receive WBH and were held at either ST or TT until euthanized. Tissues were analyzed for the expression of total HSP70, HSP90, and HSP110. We found no significant differences in baseline HSP expression between mice housed at ST and TT in the brain, lung, and heart (Figure 2A–C). Confirming our previous findings (12), the expression of the HSPs significantly increased in certain tissues immediately and 24hrs after heat treatment when mice were maintained at ST (Figure 3). However, the induction of HSP70, HSP90 and HSP110 following WBH was lower in the lungs (Figure 3A, 3D–F), heart (Figure 3B, 3G–I) and brain (Figure 3C, J–L) of mice housed at TT compared to ST. Of note, we did not observe significant differences in the expression of HSP70, HSP90, or HSP110 in the spleen or kidney of mice at ST and TT following WBH (Supplemental Figure 1). Additionally, we examined the concentration of plasma HSP70 in mice housed at ST and TT following WBH. Interestingly, plasma HSP70 levels in mice housed at TT increased more rapidly following heating compared with mice housed at ST; however, while the differences were statistically significant, the change in heat shock protein levels were relatively minimal (Supplemental Figure 2).
Figure 2.
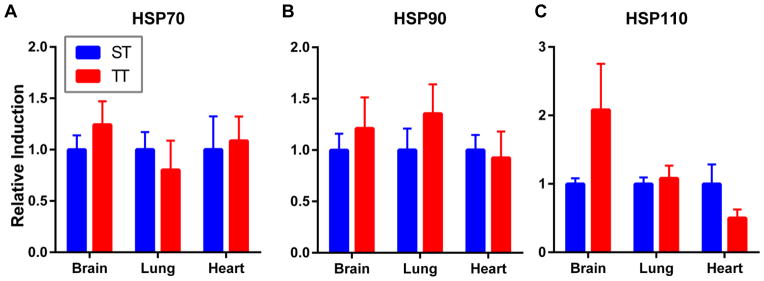
Housing mice at thermoneutrality does not significantly alter baseline expression of HSPs. Brain, lung, and heart expression of (A) total HSP70 (B) HSP90 (C) HSP110 of mice housed for 14 days at standard temperatures and thermoneutrality. No statistically significant differences by Student’s t test. n = 3–4/experiment, experiments were performed twice and data were combined.
Figure 3.
The expression of heat shock proteins is decreased in visceral organs of mice housed at TT compared to mice housed at ST after WBH. (A) Lung, (B) heart, and (C) brain expression of HSP70, HSP90 and HSP110 following 6hrs of whole body hyperthermia. (D–L) Fluorescence intensity of heat shock protein expression relative to unheated ST or TT controls. *p <0.05, **p <0.01 (ST versus TT), #p < 0.05, ##p < 0.01 (heated ST versus unheated ST control), &p < 0.05 (heated TT versus unheated TT control) by Student’s t test; n = 3–4/experiment, each experiment was performed twice and combined.
Effect of β-blockers on heat shock protein expression
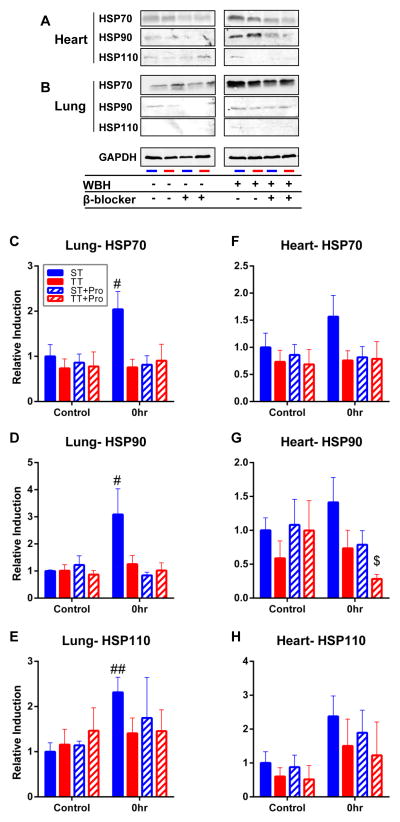
As previously reported, maintenance of body temperature depends heavily on the biochemical process of thermogenesis. Under conditions of cold stress, rodents increase systemic production of the catecholamine, norepinephrine, to drive UCP1-mediated heat production in brown adipose tissue (24,26,27). To determine if the differences observed in HSP expression were driven by catecholamine levels, we pre-treated mice housed at ST and TT with the β-blocker, propranolol, for 2 weeks during the standard acclimation period prior to WBH. We hypothesized that if HSP expression was being altered by mild cold stress, then addition of a β-blocker to mice house at ST should make their HSP response more similar to that seen at TT. Notably, pre-treatment with β-blockers reduced the overall induction of HSPs in the lungs and hearts, similar to that seen in untreated mice housed at TT given WBH (Figure 4). However, β-blocker use did not influence HSP expression in mice housed at TT, strongly suggesting that β-adrenergic signaling in tissues due to sub-thermoneutral housing temperatures is regulating the heat shock response (Figure 4C–H).
Figure 4.
The induction of heat shock proteins is abrogated by propranolol in mice housed at ST after WBH. A) Lung and B) heart expression of HSP70, HSP90 and HSP110 following 6hrs of whole body hyperthermia with and without the β-blocker, propranolol. C–H) Densitometry quantifications of heat shock protein expression relative to unheated ST only, TT only, ST + propranolol, and TT + propranolol. ##p<0.01 (heated ST versus unheated ST control), $p<0.05 (heated TT+ propranolol compared to unheated TT+ propranolol) by Student’s t-test; n = 3–4/experiment, each experiment was performed twice and combined.
Discussion
The use of mouse models in biomedical research has led to a massive breadth of knowledge about physiological interactions in complex living organisms. Many of these studies have further fueled significant clinical advances, particularly in the development and testing of novel therapeutics. However, the growing reality that the housing conditions of mice in research facilities could impact experimental results has become a major concern (17,28). Since these suboptimal conditions can induce baseline physiological stress in mice, the impact on outcome of both systemic and cellular stress responses could be influenced by housing conditions and is generally underappreciated by investigators using these models.
In our study, we explored whether the stress imparted on mice by suboptimal housing temperature in research facilities impacts expression of HSPs in response to mild (fever-range) whole body hyperthermia. Our data demonstrate that mice housed at standard temperatures have significantly elevated levels of norepinephrine, the key catecholamine involved in regulating adaptive thermogenesis. Evidence from previous studies has shown that norepinephrine can directly modulate the production and even secretion of HSPs in various tissue types (29–31).
In a previous study examining the effects of methamphetamines on the induction of HSP expression in the brain, the authors reported greater increases in HSP70 expression in mice housed at thermoneutrality compared to those housed at the standard room temperature (32). However, they reported greater expression of HSP70 was seen in the brains of mice housed at thermoneutrality. These discrepancies in the expression of HSPs with our findings may come about due to the significantly higher body temperature induced by methamphetamines (33). Following injection of methamphetamine, the authors of this study observed rectal temperatures climb to 43°C within 90 minutes. This suggests that at these much higher temperatures, where protein aggregation and greater cellular damage can occur, that HSP induction in thermoneutral adapted mice would be potentially greater than animals housed at standard temperatures and function as a protective mechanism. Perhaps more similar to our studies, mild heating (resulting in a 2°C increase in core temperature) five days prior to 42°C heat shock blunted HSP72, an important regulator of stress induced apoptosis, expression in mice (34). These findings correlate with our own data, suggesting that the diminished HSP induction in mice housed at TT following WBH may also be a result of heat acclimation. A second possibility for the lack of induction of HSPs in mice, even though their core body temperature is elevated by WBH, may be due to the effects of adrenergic receptor signaling on HSP induction. Several studies in various mammals and vertebrates have shown that circulating catecholamines can enhance the expression of HSPs in various tissues through currently unknown mechanisms (35–37).
In particular, work in trout and porcine models have shown that HSP induction by stressors such as high temperatures or hypoxia, respectively, can be mitigated or even abrogated by use of β1 and β2-adrenergic receptor antagonists (35,37). Overall, both our work, and the previous reports show the importance of fully understanding the role of ambient temperature in data interpretation of HSP expression studies.
In addition to changes in HSP expression in the brain, we observed the greatest shifts in HSP expression in the hearts and lungs of mice housed at ST versus TT. Since heart and lung tissues are highly innervated by sympathetic fibers, it is not surprising that baseline systemic stress could affect responses to heat stress in these cells. These findings are in line with previous studies showing that expression of HSP70 in brown adipose tissues could be induced by β-adrenergic receptor activation (38). In our studies, the differences were readily abrogated by prior administration of the β-blocker, propranolol, suggesting that β-adrenergic receptors play an important role in regulating HSP expression under mild stress conditions, as well. Furthermore, these findings also highlight the fact that even moderate cold stress could significantly alter the induction of HSPs, which could have important scientific implications for modeling pathologies including myocardial infarctions, infections, and even malignancies.
Much more investigation will be required to fully tease out the implications of housing temperature on HSP biology, including study of additional and a wider range of temperatures used to induce thermal stress. Furthermore, the work presented here does not extend to different murine strains. Other immunecompetent stains including C3H and C57BL/6 will need to be examined to determine how HSP expression is impacted by housing temperature. Other important work should be directed at immunocompromised mice such as SCID and NUDE which are often used for tumor xenograft experiments. We expect particularly insightful findings from NUDE and other hairless mice (i.e. SKH1) as they may be even more sensitive to cool housing temperatures. Moreover, very little is still known about whether the α-adrenergic receptors also play a role in regulating the induction of HSPs in response to stress. These receptors have a greater binding affinity for norepinephrine, but are primarily located at peripheral vascular sites and the genitourinary tract (39,40). Thus, these receptors may also contribute to the differences in HSP expression in cold stressed mice at these different tissue sites. A recent study showed that HSP70 inhibited the inflammatory response through IL-10 driven downregulation of CCAAT/enhancer-binding proteins (41). As these transcription factors are involved in the regulation of various cellular functions including proliferation, differentiation and cytokine production, activation of this pathway may have wide-ranging off target effects. Because HSPs may serve as targets for therapeutic intervention, it will ultimately be important to examine the impact of ambient temperatures on HSP expression in mouse models for diseases, including cancer (42–44), autoimmunity (45) and infection (46).
Conclusion
In summary, the findings presented here highlight the surprising influence that mild chronic stress can have on basic physiological responses. In particular, this work demonstrates that stress induced by housing temperature has a significant, yet underappreciated, effect on HSP induction in mice following WBH. Our data indicate that the metabolic impact of housing temperature should be considered when any type of stress response is being studied in mice. Overall, it would be prudent for investigators working on HSPs or any stress proteins in animal models to perform these studies at more than one ambient temperature to fully gauge the complete range of responses.
Supplementary Material
The rate of plasma HSP70 induction after heating is slower in mice housed at ST compared to TT. A) Total HSP70 levels in the serum of mice housed at ST and TT following 6hrs of WBH. *p<0.05 (ST versus TT), #p < 0.05 (ST versus unheated ST control), &p < 0.05, &&p<0.01 (TT versus unheated TT control) by Student’s t test n = 3–4/experiment, experiments were performed twice and data were combined.
The induction of heat shock proteins in the spleen and kidney does not significantly differ in mice housed at ST compared to TT. A) Spleen and B) kidney expression of HSP70, HSP90 and HSP110 following 6 hrs of WBH. No significant differences by two way ANOVA; n = 3–4/experiment, experiments were performed twice and data were combined.
Acknowledgments
We thank Drs. Bonnie Hylander and John Subjeck for their discussion of the data, and Jeanne Prendergast for laboratory assistance.
Footnotes
Declarations of Interest
This work was supported by National Institute of Health Grants R01 CA135368 and T32 CA 085183. Work in this manuscript used shared resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA 016056. The authors have no conflicts of interest to report.
References
- 1.Subjeck JR, Shyy TT. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- 2.Gabai VL, Kabakov AE. Rise in heat-shock protein level confers tolerance to energy deprivation. FEBS Lett. 1993;327(3):247–250. doi: 10.1016/0014-5793(93)80997-9. [DOI] [PubMed] [Google Scholar]
- 3.Whelan SA, Hightower LE. Differential induction of glucose-regulated and heat shock proteins: Effects of pH and sulfhydryl-reducing agents on chicken embryo cells. J Cell Physiol. 1985;125(2):251–258. doi: 10.1002/jcp.1041250212. [DOI] [PubMed] [Google Scholar]
- 4.Georgopoulos C, Welch W. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9(1):601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 5.Singh IS, Hasday JD. Fever, hyperthermia and the heat shock response. Int J Hyperthermia. 2013 Aug;29(5):423–435. doi: 10.3109/02656736.2013.808766. [DOI] [PubMed] [Google Scholar]
- 6.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000 Apr;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Metzler B, Jahangiri M, Mandal K. Molecular chaperones and heat shock proteins in atherosclerosis. Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H506–14. doi: 10.1152/ajpheart.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng L, Hunt C, Yaglom JA, Gabai VL, Sherman MY. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011;30(25):2836–2845. doi: 10.1038/onc.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. 2011 doi: 10.1586/erv.11.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, et al. Up-regulation of PPAR g, heat shock protein-27 and-72 by naringin attenuates insulin resistance, b-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr. 2011;106(11):1713–1723. doi: 10.1017/S000711451100225X. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol. 2002;43(1):33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 12.Ostberg JR, Kaplan KC, Repasky EA. Induction of stress proteins in a panel of mouse tissues by fever-range whole body hyperthermia. Int J Hyperthermia. 2002 Nov-Dec;18(6):552–562. doi: 10.1080/02656730210166168. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011 [Google Scholar]
- 14.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol. 2012 Dec;37(8):654–685. [Google Scholar]
- 15.Myers R. Catecholamines and the regulation of body temperature. In: Szekeres L, editor. Adrenergic Activators and Inhibitors. Berlin: Springer; 1980. pp. 549–567. [Google Scholar]
- 16.Gordon CJ. Temperature Regulation in Laboratory Rodents. 1. Cambridge University Press; 1993. [Google Scholar]
- 17.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. The Journal of Experimental Medicine. 2012 Jun 04;209(6):1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA. Stressful Presentations: Mild Chronic Cold Stress in Mice Influences Baseline Properties of Dendritic Cells. Front Immunol. 2014;5:23. doi: 10.3389/fimmu.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20176–81. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightower LE, Norris CE, Diiorio PJ, Fielding E. Heat shock responses of closely related species of tropical and desert fish. Am Zool. 1999;39(6):877–888. [Google Scholar]
- 21.White CN, Hightower LE, Schultz RJ. Variation in heat-shock proteins among species of desert fishes (Poeciliidae, Poeciliopsis) Mol Biol Evol. 1994 Jan;11(1):106–119. doi: 10.1093/oxfordjournals.molbev.a040085. [DOI] [PubMed] [Google Scholar]
- 22.Zatsepina OG, Ulmasov KA, Beresten SF, Molodtsov VB, Rybtsov SA, Evgen’ev MB. Thermotolerant desert lizards characteristically differ in terms of heat-shock system regulation. J Exp Biol. 2000 Mar;203(Pt 6):1017–1025. doi: 10.1242/jeb.203.6.1017. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011 Nov 20;480(7375):104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011 Jan 15;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Shiuchi T, Inada H, Minokoshi Y, Tominaga M. Metabolic adaptation of mice in a cool environment. Pflügers Archiv-European Journal of Physiology. 2010;459(5):765–774. doi: 10.1007/s00424-010-0795-3. [DOI] [PubMed] [Google Scholar]
- 26.Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed) 2011 Jan 1;3:352–371. doi: 10.2741/s156. [DOI] [PubMed] [Google Scholar]
- 27.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006 Apr;86(2):435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 28.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega E, Giraldo E, Hinchado MD, Martín-Cordero L, García JJ. Heat Shock Proteins and Whole Body Physiology. Springer; 2010. 72 kDa Extracellular Heat Shock Protein (eHsp72), Norepinephrine (NE), and the Innate Immune Response Following Moderate Exercise; pp. 327–350. [Google Scholar]
- 30.Giraldo E, Multhoff G, Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav Immun. 2010;24(4):672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Lacoste A, Malham SK, Cueff A, Poulet SA. Stress-Induced Catecholamine Changes in the Hemolymph of the Oyster< i> Crassostrea gigas. Gen Comp Endocrinol. 2001;122(2):181–188. doi: 10.1006/gcen.2001.7629. [DOI] [PubMed] [Google Scholar]
- 32.Kiyatkin EA, Sharma HS. Expression of heat shock protein (HSP 72 kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J Neural Transm. 2011;118(1):47–60. doi: 10.1007/s00702-010-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Kuperman DI, Freyaldenhoven TE, Schmued LC, Ali SF. Methamphetamine-induced hyperthermia in mice: examination of dopamine depletion and heat-shock protein induction. Brain Res. 1997;771(2):221–227. doi: 10.1016/s0006-8993(97)00710-5. [DOI] [PubMed] [Google Scholar]
- 34.Sareh H, Tulapurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress and Chaperones. 2011;16(3):297–307. doi: 10.1007/s12192-010-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy SJ, Song D, Welsh FA, Wilson DF, Pastuszko A. The effect of hypoxia and catecholamines on regional expression of heat-shock protein-72 mRNA in neonatal piglet brain. Brain Res. 1996 Jul 15;727(1–2):145–152. doi: 10.1016/0006-8993(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 36.Paroo Z, Noble EG. Isoproterenol potentiates exercise-induction of Hsp70 in cardiac and skeletal muscle. Cell Stress Chaperones. 1999 Sep;4(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 37.Templeman N, LeBlanc S, Perry S, Currie S. Linking physiological and cellular responses to thermal stress:β2-adrenergic blockade reduces the heat shock response in fish. Journal of Comparative Physiology B. 2014 Aug 1;184(6):719–728. doi: 10.1007/s00360-014-0831-2. [DOI] [PubMed] [Google Scholar]
- 38.Matz JM, Blake MJ, Tatelman HM, Lavoi KP, Holbrook NJ. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am J Physiol. 1995 Jul;269(1 Pt 2):R38–47. doi: 10.1152/ajpregu.1995.269.1.R38. [DOI] [PubMed] [Google Scholar]
- 39.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999 Dec 7;100(23):2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- 40.Schwinn D. The role of α1-adrenergic receptor subtypesin lower urinary tract symptoms. BJU Int. 2001;88(s2):27–34. doi: 10.1046/j.1464-410x.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- 41.Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPbeta and C/EBPdelta. Int J Hyperthermia. 2013 Aug;29(5):455–463. doi: 10.3109/02656736.2013.798037. [DOI] [PubMed] [Google Scholar]
- 42.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005 Summer;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderwood SK, Gong J, Stevenson MA, Murshid A. Cellular and molecular chaperone fusion vaccines: targeting resistant cancer cell populations. Int J Hyperthermia. 2013 Aug;29(5):376–379. doi: 10.3109/02656736.2013.792126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia. 2013 Aug;29(5):399–408. doi: 10.3109/02656736.2013.807439. [DOI] [PubMed] [Google Scholar]
- 45.Van Herwijnen MJ, Van Der Zee R, Van Eden W, Broere F. Heat shock proteins can be targets of regulatory T cells for therapeutic intervention in rheumatoid arthritis. Int J Hyperthermia. 2013 Aug;29(5):448–454. doi: 10.3109/02656736.2013.811546. [DOI] [PubMed] [Google Scholar]
- 46.Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999 Jan;12(1):19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The rate of plasma HSP70 induction after heating is slower in mice housed at ST compared to TT. A) Total HSP70 levels in the serum of mice housed at ST and TT following 6hrs of WBH. *p<0.05 (ST versus TT), #p < 0.05 (ST versus unheated ST control), &p < 0.05, &&p<0.01 (TT versus unheated TT control) by Student’s t test n = 3–4/experiment, experiments were performed twice and data were combined.
The induction of heat shock proteins in the spleen and kidney does not significantly differ in mice housed at ST compared to TT. A) Spleen and B) kidney expression of HSP70, HSP90 and HSP110 following 6 hrs of WBH. No significant differences by two way ANOVA; n = 3–4/experiment, experiments were performed twice and data were combined.