Abstract
The Bcl-2 family of proteins serves as primary regulators of apoptosis. Myeloid cell leukemia 1 (Mcl-1), a pro-survival member of the Bcl-2 family of proteins, is overexpressed and the Mcl-1 gene is amplified in many tumor types. Moreover, the overexpression of Mcl-1 is the cause of resistance to several chemotherapeutic agents. Thus, Mcl-1 is a promising cancer target. This review highlights the current progress on the discovery of small molecule Mcl-1 inhibitors.
Keywords: Mcl-1, myeloid cell leukemia-1, inhibitors, small molecule, BH3-mimetic
1. Introduction
Apoptosis is a natural process for eliminating unwanted or damaged cells that represent a threat to the health of an organism. This process is highly regulated, and the B-cell lymphoma-2 (Bcl-2) family of proteins serve as the main regulators. Indeed, dysregulation and evasion of apoptosis is one of the hallmarks of cancer.(Hanahan & Weinberg, 2000, 2011)
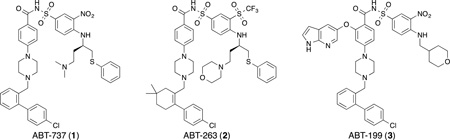
Members of the Bcl-2 family proteins share conserved sequences in regions known as Bcl-2 homology (BH) domains (BH1–BH4).(Korsmeyer, 1999; Pang, et al., 2012) Members within the same family can have opposite effects. The anti-apoptotic or pro-survival proteins, including Bcl-2, Bcl-XL, Bcl-w, Bfl-1/A1 and Mcl-1,(Adams & Cory, 2007) keep cells alive; whereas, the pro-apoptotic proteins (e.g., Bim, tBid, Bad, Puma, Noxa, Bak, and Bax)(Youle & Strasser, 2008) promote cell death. The relative levels of the anti- and pro-apoptotic proteins govern whether a cell will live or die. Recently, much has been learned about how the Bcl-2 proteins regulate apoptosis.(Burlacu, 2003; M. F. van Delft & Huang, 2006; Volkmann, et al., 2014) Upon triggering a death signal, a subset of the pro-apoptotic proteins with homology only in the BH3 region cause Bak and Bax to homo-oligomerize and form pores in the mitochondrial membrane leading to cytochrome c release into the cytosol. This activates the caspase cascade and causes cell death. The anti-apoptotic proteins block cell death by binding and sequestering, with varying specificity the BH3-only proteins.(L. Chen, et al., 2005) The binding specificity and affinity exhibited by the anti-apoptotic proteins for the pro-apoptotic proteins is defined by hydrophobic and electrostatic interactions between the BH3 region of the pro-apoptotic proteins and the binding groove formed by the BH1, BH2 and BH3 regions of the anti-apoptotic proteins.(Dutta, et al., 2010; Moldoveanu, et al., 2014; Sattler, et al., 1997) Of the BH3-only proteins, Bim and Puma are the least selective, binding to all five anti-apoptotic proteins. Bad binds strongly to Bcl-2, Bcl-XL and Bcl-w; whereas, Noxa binds exclusively to Mcl-1 and Bfl-1/A1. These observations suggest that apoptosis is regulated by the interactions between particular subsets of these proteins and that apoptosis can be initiated by the inhibition of the pro-survival members of the Bcl-2 family proteins. Indeed, this has been demonstrated by the BH3-mimetics ABT-737 (1)(Oltersdorf, et al., 2005) and its orally available derivative ABT-263 (2; navtioclax)(Tse, et al., 2008) which bind to Bcl-2, Bcl-XL and Bcl-w. As expected, ABT-737 and ABT-263 induce apoptosis in tumor cells that are dependent on Bcl-2 and Bcl-XL. More recently, a selective Bcl-2 inhibitor was discovered (ABT-199) that also demonstrates the utility of inhibitors of the Bcl-2 family.(Souers, et al., 2013) Indeed, Navitoclax and ABT-199 have shown efficacy in several clinical trials in patients with lymphoid malignancies that are believed to be Bcl-2 dependent.(Choo, et al., 2014; Roberts, et al., 2012; Tse, et al., 2008) However, there are some cancers that cannot be treated by these compounds alone. Several studies have shown that upregulation of Mcl-1 is a key factor in the development of resistance to ABT-737 and ABT-263 resistance in several tumor types.(Konopleva, et al., 2006; Tahir, et al., 2007; Mark F van Delft, et al., 2006)
Mcl-1 has a number of functions and features that make it unique among the anti-apoptotic Bcl-2 family members. Mcl-1 is essential for early embryogenesis(Rinkenberger, et al., 2000) as well as the development and maintenance of lymphocytes(Dzhagalov, et al., 2008; Opferman, et al., 2003), neurons(Arbour, et al., 2008), synovial fibroblasts(Liu, et al., 2005) and hematopoietic stem cells(Opferman, et al., 2005). Mcl-1 is also unique in that it has a very short half-life of <1–4 h, depending on cellular conditions(Yang-Yen, 2006), and multiple pathways tightly regulate Mcl-1 transcription, translation, and degradation.(Thomas, et al., 2010) Structurally, Mcl-1’s N-terminus also differs from that of the other anti-apoptotic Bcl-2 proteins in that it contains two PEST (proline/glutamic acid/serine/threonine–containing) regions.(Germain & Duronio, 2007) Indeed, the N-terminal region may serve as a regulatory domain for Mcl-1’s rate of turnover, localization, and phosphorylation, and may thus provide a mechanism to rapidly fine-tune the expression of Mcl-1 in response to environmental and cellular input.(Thomas, et al., 2010)
There is a lot of evidence to suggest that Mcl-1 is an important cancer target. For example, Mcl-1 overexpression is one of the most common genetic aberrations observed in human cancer,(Beroukhim, et al., 2010; G. Wei, et al., 2012) including lung(L. X. Song, et al., 2005), breast(Ding, et al., 2007), prostate(Krajewska, et al., 1996), pancreatic(Miyamoto, et al., 1999), ovarian and cervical cancers(Brotin, et al., 2010), as well as melanoma(Boisvert-Adamo, et al., 2009) and leukemia(Andersen, et al., 2005; Derenne, et al., 2002; Kang, et al., 2008). Furthermore, Mcl-1 overexpression induces resistance against the aforementioned Bcl-2-inhibitors, as well as a number of widely used anticancer therapies including paclitaxel,(Wertz, et al., 2011) vincristine(Wertz, et al., 2011) and gemcitabine(S.-H. Wei, et al., 2008). Moreover, RNA-mediated knockdown of Mcl-1 has shown tumor growth inhibition and cell death in Mcl-1 overexpressing lung, colon, ovarian and lymphoma cells.(Akgul, 2008; Boisvert-Adamo, et al., 2009; W. Chen, et al., 2010; Chetoui, et al., 2008; Hauck, et al., 2009; Keuling, et al., 2009; Konopleva, et al., 2006; Lucas, et al., 2012; Moulding, et al., 2000; Qin, et al., 2006; Thallinger, et al., 2003) Silencing of Mcl-1 also restores sensitivity in chemoresistant cells.(Lin, et al., 2007; Meng, et al., 2007; Taniai, et al., 2004) Given these data, Mcl-1 represents a very promising cancer target. An Mcl-1 inhibitor would be expected to be useful as a single agent against cancers that depend on Mcl-1 for survival and in combination with other drugs where Mcl-1 overexpression is the major resistance factor.
This Review focuses on the current state of Mcl-1 inhibitors. Although peptide-based inhibitors have been described, including stapled alpha-helix of Bcl-2 domains (SAHB)(Muppidi, et al., 2012; Stewart, et al., 2010), alpha-/beta-peptide foldamers(Smith, et al., 2013) and reverse BH3 (rBH3) peptides(Placzek, et al., 2011), we focus this review on small molecule inhibitors that have been reported to function as BH3-mimetics. As proposed by Lessene and coworkers true BH3-mimetics should exhibit Bak/Bax-dependant biological activity and high-affinity binding to at least one Bcl-2 family pro-survival protein, specifically Mcl-1 in the case of this review.(Lessene, et al., 2008) Therefore, Obatoclax (GX15-070)(Nguyen, et al., 2007), a putative pan-inhibitor that binds to all Bcl-2 family pro-survival proteins with low affinity(Nguyen, et al., 2007; Tse, et al., 2008) and kills wild-type and Bak/Bax-deficient cells with equal potency(Vogler, et al., 2009), as well as other chemical entities that exert their biological activities through possible off-target or unknown mechanisms of action are not included in this review.(Billard, 2013) In addition, this review does not cover molecular entities disclosed exclusively within patents. This information has been reviewed in detail elsewhere(Bajwa, et al., 2012).
2. S1
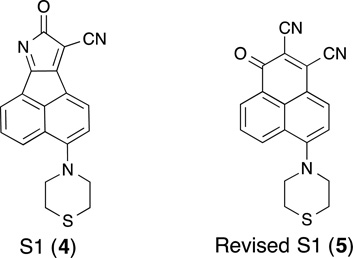
Efforts aimed at designing novel DNA intercalating agents led to the discovery of S1 (4), a rigid, planar chromophore, which exhibited anti-tumor activity yet surprisingly lacked the ability to intercalate into DNA.(Zhang, et al., 2007) The structure of S1 was originally reported as possessing an 8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (4) backbone. However, the structure was later revised by Song et al. to a 1-oxo-1H-phenalene-2,3-dicarbonitrile (5).(T. Song, Chen, et al., 2013) S1 has been touted as a pan-Bcl-2 family inhibitor as it has been reported to bind to Mcl-1 (Kd = 58 nM, Bid-BH3, FPA) and Bcl-2 (Kd = 310 nM, Bid-BH3, FPA), disrupt Bax/Bcl-2 and Bak/Mcl-1 complexes in a dose- and time-dependent manner, and induce Bax/Bak-dependent apoptosis.(Zhang, et al., 2010) However, Eastman and co-workers suggest that S1 does not function as a pan-Bcl-2 inhibitor in cells but rather it upregulates the BH3-only protein Noxa, which inhibits Mcl-1 and leads to its degradation and an increase in cellular sensitivity to apoptosis.(Albershardt, et al.) Furthermore, S1 has been shown to rapidly increase reactive oxygen species (ROS) which leads to the induction of endoplasmic reticulum (ER)-mediated stress.(Soderquist, et al., 2013) Finally, Zhong et al. have reported that S1-mediated cell death may be in part due to the induction of autophagy through (ER) stress and disruption of the interaction of Beclin 1 with Bcl-2.(Zhong, et al., 2012) Thus, S1-mediated cell death could be caused by several different mechanisms.
3. S1 Derivatives
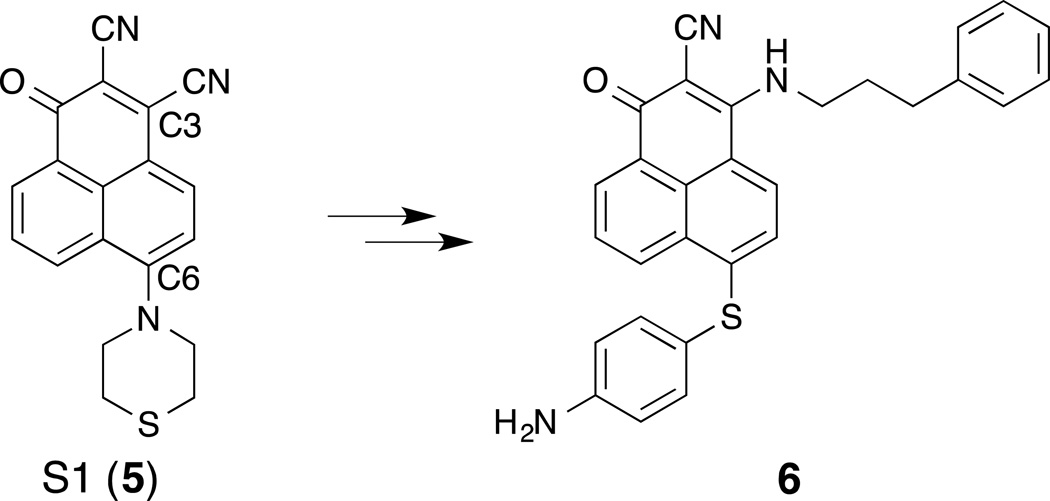
Zhang and co-workers have disclosed a number of S1 derivatives using scaffold hopping approaches. Based on their NMR-derived model for the binding of S1 to Mcl-1, Song et al. selected the C3 and C6 positions of S1 (5, Fig. 1) for synthetic elaboration in order to extend further along the hydrophobic BH3-binding groove.(T. Song, Li, et al., 2013; T. Song, et al., 2014) Structure–activity studies at the C3 and C6 positions led to the identification of 6, which binds Mcl-1 (IC50 = 10 nM, Bim-BH3, ELISA) and Bcl-2 (IC50 = 20 nM, Bim-BH3, ELISA) with increased affinity relative to S1 (Mcl-1, IC50 = 95 nM; Bcl-2, IC50 = 715 nM, Bim-BH3, ELISA). Preliminary cell studies indicate that compound 6 exhibits increased apoptotic activity compared to S1.
Figure 1.
SAR studies at C3 and C6 of S1 led to compound 6.
Additionally, Zhang and co-workers employed a fragment-based strategy to identify two novel Mcl-1 inhibitors. As shown in Figure 2, screening of several fragments derived from the dissection of the unrevised S1 structure using a fluorescence polarization assay led to the identification of two novel hits: a 2-cyanoacetamide (7)(Zhang, Song, et al., 2013), and a 2-hydroxynicotinonitrile (9)(Zhang, Liu, et al., 2013). Synthetic elaboration of these molecules yielded compounds 8, which is 6-fold more potent than S1, and 10, which is equipotent to S1.
Figure 2.
Fragments derived from the 4 led to two novel classes of Mcl-1 inhibitors.
4. A*STAR Compounds
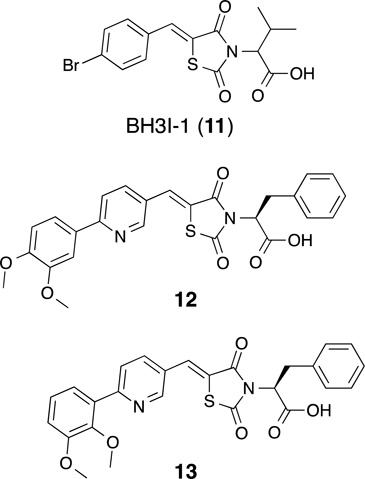
By synthesizing and screening a small, focused library of pyridine-based rhodanine derivatives of BH3I-1 (11)(Lugovskoy, et al., 2002), that binds to Bcl-2 at the BH3 site, Bernardo et al. identified two constitutional isomers, structurally-differentiated by the relative position of two methoxy groups. Compound 12 binds exclusively to Mcl-1 (Kd = 10 µM, ITC), and compound 13 binds Mcl-1 (Kd = 0.25 µM, ITC) with greater affinity and also binds to Bcl-XL (Kd = 3.4 µM, ITC). (Bernardo, et al., 2010) While NMR-guided docking studies suggest that compounds 12 and 13 bind to the BH3-binding groove, the interaction(s) responsible for the difference in selectivity observed as a result of such a subtle structural change have not yet been identified. Although Bernardo and coworkers did not provide data validating the biological activity of the these compounds, compounds 12 and 13 were evaluated alongside other putative Mcl-1-inhibitors by Varadarjan et al.(Varadarajan, et al., 2013) This follow-up study determined that neither compound killed cells, even at high concentrations (<30 nM), as a single agent or in combination with ABT-737.
5. Compounds from Takeda Pharmaceutical Company
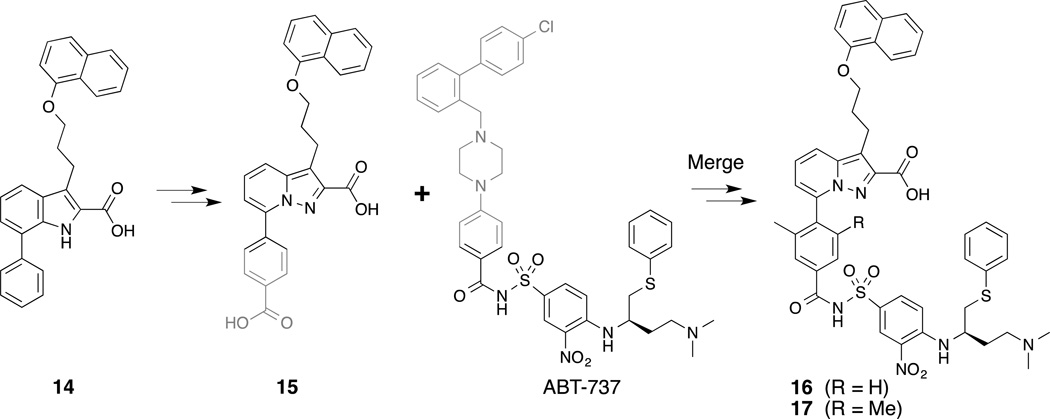
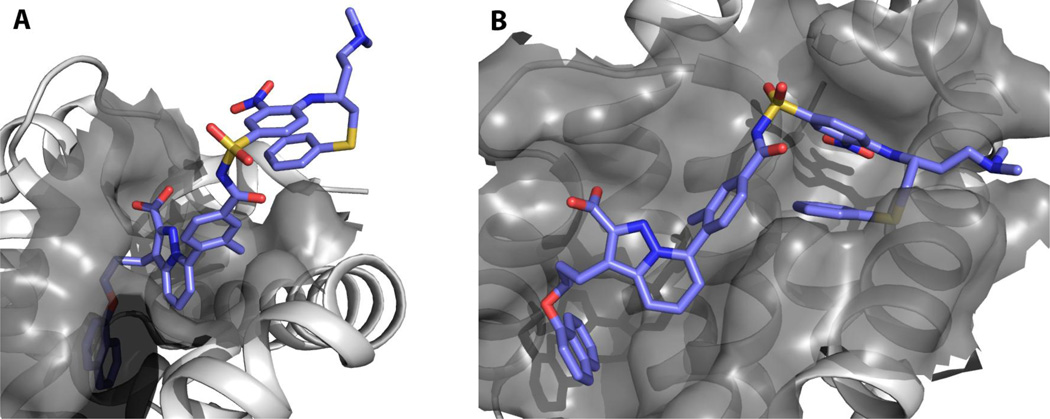
The desire to overcome Mcl-1 over-expression in cancer cells treated with Bcl-2 family inhibitors has prompted interest in the discovery of dual inhibitors, such as an Mcl-1/Bcl-XL dual inhibitor. To this end, Tanaka et al. analyzed known inhibitors of Mcl-1 such as compound 14(Elmore, et al., 2008) (Fig. 3) and the reported Bcl-XL inhibitor, ABT-737, for two complimentary binding motifs. Merging of the two scaffold fragments, 15 and the arylsulfonamide portion of ABT-737, resulted in compound 17, which binds to both Mcl-1 (IC50 = 88 nM, Bid-BH3, TR-FRET) and Bcl- XL (IC50 = 3.7 nM, Bid-BH3, TR-FRET).(Tanaka, et al., 2013) Compound 16, the des-methyl analog of 17, was cocrystallized with Mcl-1 (PDB ID 3WIY) and Bcl- XL (PDB ID 3WIZ) and, for the most part, confirmed the contribution of each half of the molecule for binding to the respective anti-apoptotic proteins (Fig. 4). The portion of the molecule derived from ABT-737 does not appear to contribute much towards the binding of Mcl-1.
Figure 3.
Merging of fragments derived from two known inhibitors of Mcl-1 and Bcl-XL resulted in novel dual-inhibitors.
Figure 4.
Crystal structures of 15 bound to Mcl-1 (A) and to Bcl-XL (B).
6. University of Michigan Compounds
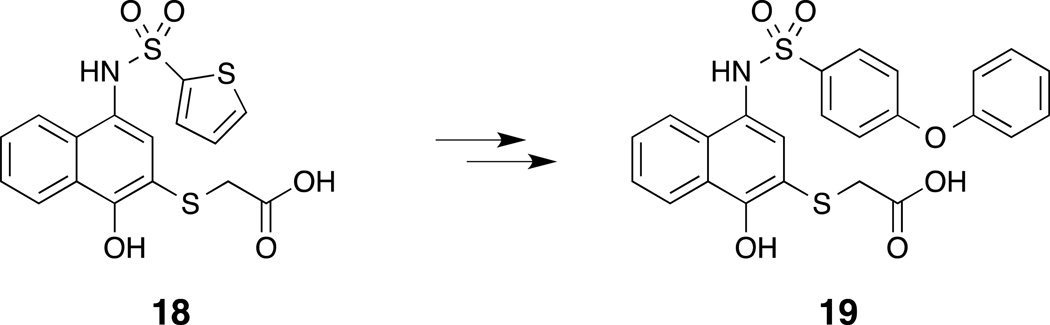
Abulwerdi et al. identified N-(4-hydroxynaphthalen-1-yl)arylsulfonamide 18 (Fig.5) as part of a high-throughput screen (HTS) of 53,000 synthetic small molecules using a fluorescence polarization assay.(Abulwerdi, et al., 2014) Aided by NMR-based docking models and SAR studies, elaboration of the initial hit led to compound 19, which exhibits selective affinity for Mcl-1 (Kd = 180 nM, Bid-BH3, FPA) over other pro-survival Bcl-2 family members (9- to 59-fold). Experiments in leukemia cell lines show that compound 19 inhibited cell growth and activated caspase-3 in a dose-dependent manner. Further, compound 19 shows moderate cytotoxicity against Bak/Bax-deficient cells, suggesting that apoptosis is Bak/Bax-dependent.
Figure 5.
Discovery of an N-(4-hydroxynaphthalen-1-yl)sulfonamide Mcl-1 inhibitor.
7. MIM1
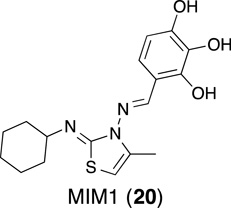
Cohen and coworkers screened a library of over 70,000 small molecules in a high-throughput competition fluorescence polarization-based assay for their ability to displace a fluorescently labeled Mcl-1 SAHBA(Stewart, et al., 2010) from Mcl-1.(Cohen, et al., 2012) In addition, the library was counter-screened for activity against Bcl- XL, and the resulting hits were excluded from the hit pool to select compounds that selectively bind to Mcl-1. Following a series of increasingly stringent confirmatory assays, 28 compounds were progressed into liposome- and cell-based assays. Mcl-1 Inhibitor Molecule 1 (20; MIM1) was ultimately selected based on a combination of biological and physicochemical properties. MIM1 was shown to bind selectively to Mcl-1 with modest affinity (IC50 = 4.78 µM, Bid-BH3, FPA), trigger Bax/Bak-dependent apoptosis in leukemia cells, and to act in concert with ABT-737 by down-regulation of Mcl-1. However, a recent evaluation of putative Mcl-1 inhibitors disclosed that MIM1 induced Bak-dependent apoptosis only at high concentrations (>10 µM) and that it failed to induce apoptosis in Mcl-1-, Bcl-2- and Bcl-XL-dependent cell lines.(Varadarajan, et al., 2013) Taken together, these data suggest that MIM1’s inhibitory effects may be cell-line dependent.
8. Marinopyrrole A (Maritoclax)
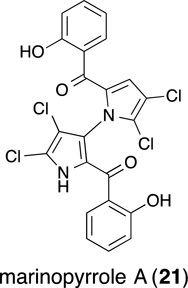
Marinopyrrole A (21), a natural product isolated from an obligate marine Streptomyces, has received considerable attention due to its promising antibiotic activity against methicillin-resistant Staphylococcus aureus (MRSA).(Haste, et al., 2011; Hughes, et al., 2009; Nicolaou, et al., 2011) Marinopyrrole A, which was named Maritoclax, was found to also selectively bind to Mcl-1 (IC50 = 10.1 µM, Bim-BH3, ELISA), decrease Mcl-1 protein levels via proteasomal degradation and induce apoptosis in Mcl-1-dependent, but not Bcl-2- and Bcl-XL-dependent, leukemia(Doi, et al., 2012) and melanoma cells(Pandey, et al., 2013). However, Eichhorn and coworkers disclosed that marinopyrrole A was equally effective against Bcl-2-dependent leukemia cells compared to Mcl-1-dependent cells, and that treatment with marinopyrrole A had no effect upon Mcl-1 expression levels.(Eichhorn, et al., 2013) Furthermore, the follow-up report indicates that marinopyrrole A does not lead to the degradation of Mcl-1 as no affect on Mcl-1 expression levels was observed upon treatment with this compound.
9. Compounds from Eutropics Pharmaceuticals
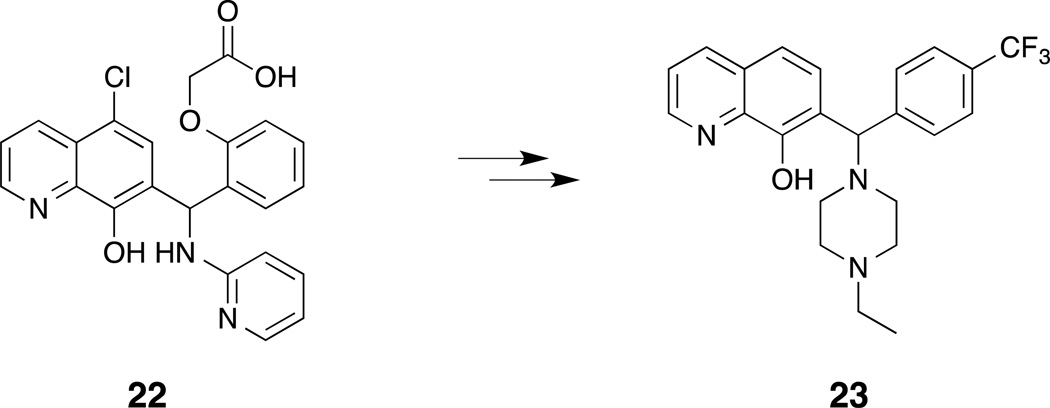
Richard et al. screened a library of 315,000 compounds in a high-throughput fluorescence polarization-based assay for the ability of compounds to inhibit Mcl-1.(Richard, et al., 2013) A subsequent FP assay was used as a counter-screen to the primary assay to identify compounds that displayed selectivity for Mcl-1 over Bcl-XL. Evaluation of the hits identified in the HTS campaign for their synthetic tractability and quality gave the team their lead compound, the 7-hydroxyquinoline 22 (Fig. 6). Analysis of compound 22 identified a number of perceived liabilities, namely, the carboxylic acid and the 4-chloro groups, which were subsequently modified or eliminated. Synthetic modification and further SAR studies resulted in compound 23, which yielded IC50s of 310 nM for Mcl-1 and 40 µM for Bcl-XL (Bim-BH3, FPA). Compound 23 was found to induce dose-dependent cytochrome c release and antiproliferative activity against several Mcl-1 dependent cell lines. Furthermore, the authors demonstrate that the cellular activity and selectivity of cell lines correlates with the degree of mitochondrial priming as determined by BH3 profiling(Certo, et al., 2006).
Figure 6.
Synthetic modification of 7-hydroxyquinoline 22 led to compound 23.
10. AbbVie Compounds
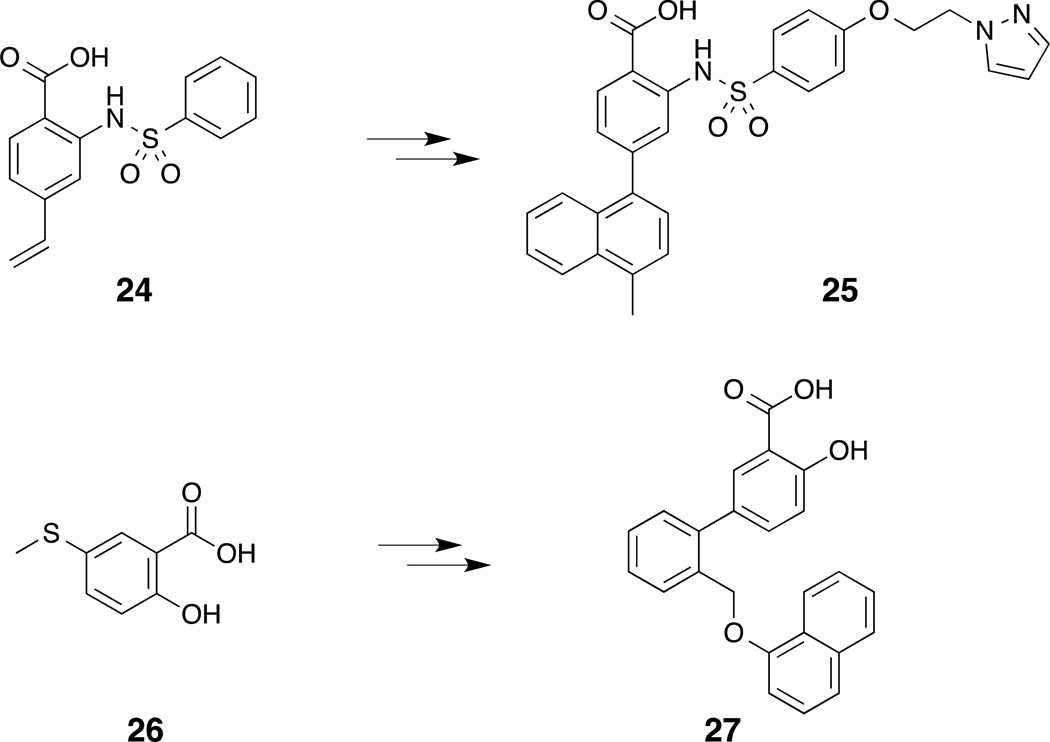
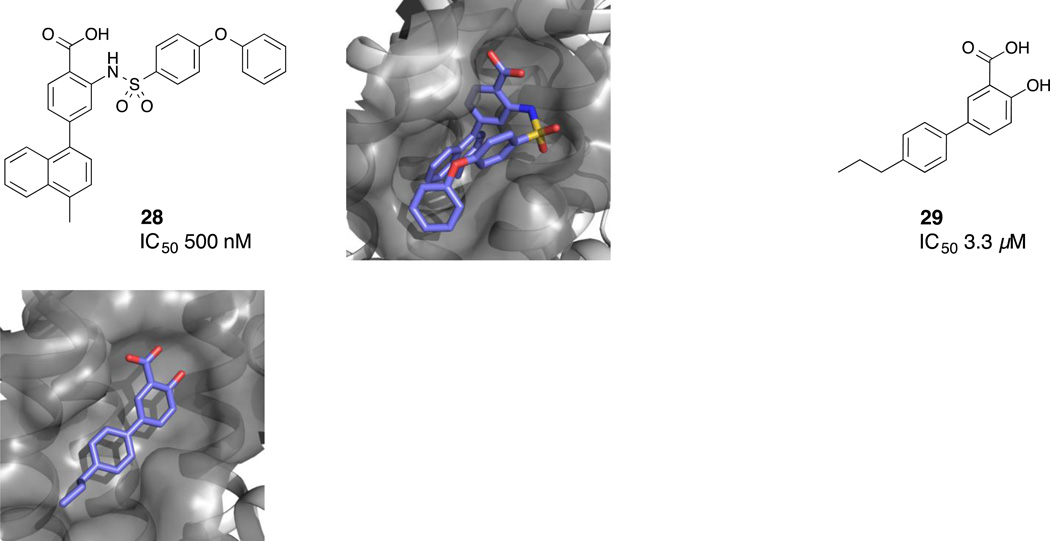
An NMR-based fragment screen against Mcl-1 of a 17,000 fragment library conducted by a team at AbbVie revealed a number of hits. Two of these hits were selected for additional studies based on the criteria of binding efficiency and synthetic tractability: (1) aryl sulfonamide 24 and (2) salicylic acid 26 (Fig. 7).(Petros, et al., 2014) In the absence of high resolution crystal structures, the binding modes for the respective fragments were determined by alternate means. The binding mode for the aryl sulfonamide fragment 24 was determined with the aid of nuclear Overhauser effect (NOE) restraint-driven docking and, in the case of the salicylic acid fragment 26, the binding mode was elucidated by simply docking the fragment into the BH3-binding groove guided by a single electrostatic-contact restraint. The aryl sulfonamide fragment was elaborated into compound 25, which exhibited an IC50 of 30 nM (Noxa-BH3, FPA) against Mcl-1, and the salicylic acid fragment was elaborated into compound 27, which yielded an IC50 of 570 nM (Noxa-BH3, FPA). Cocrystal structures of aryl sulfonamide 28 (PDB ID 4OQ5) and salicylate 29 (PDB ID 4OQ6) were subsequently obtained (Fig. 8). Notably, the acid moieties of both 28 and 29 are fixed in the same region, and the hydrophobic naphthyl moiety of the more potent aryl sulfonamide 28 is located deep within the hydrophobic pocket of Mcl-1.
Figure 7.
Fragments 24 and 26 were elaborated to give compounds 25 and 27.
Figure 8.
Cocrystal structures of aryl sulfonamide 28 and salicylate 29 with Mcl-1.
11. Vanderbilt University Compounds
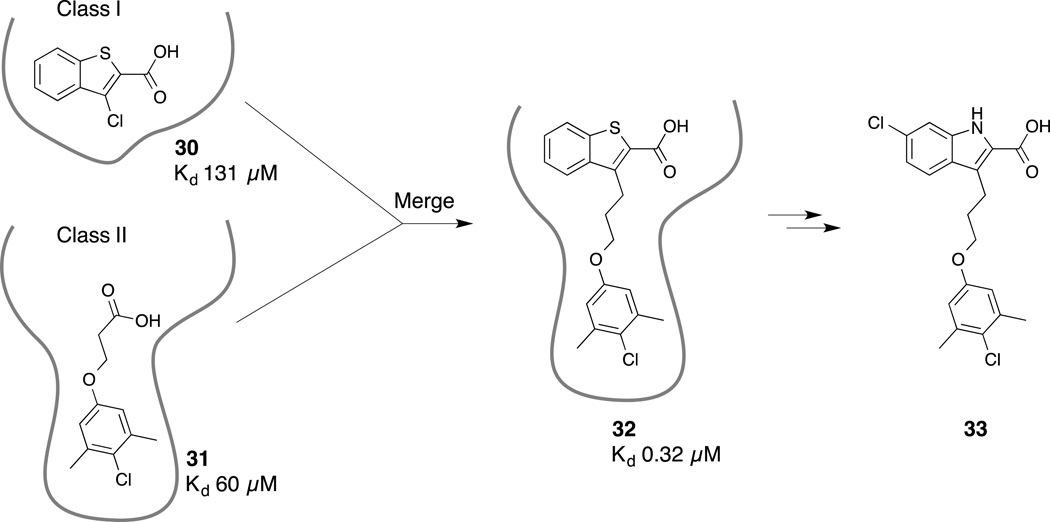
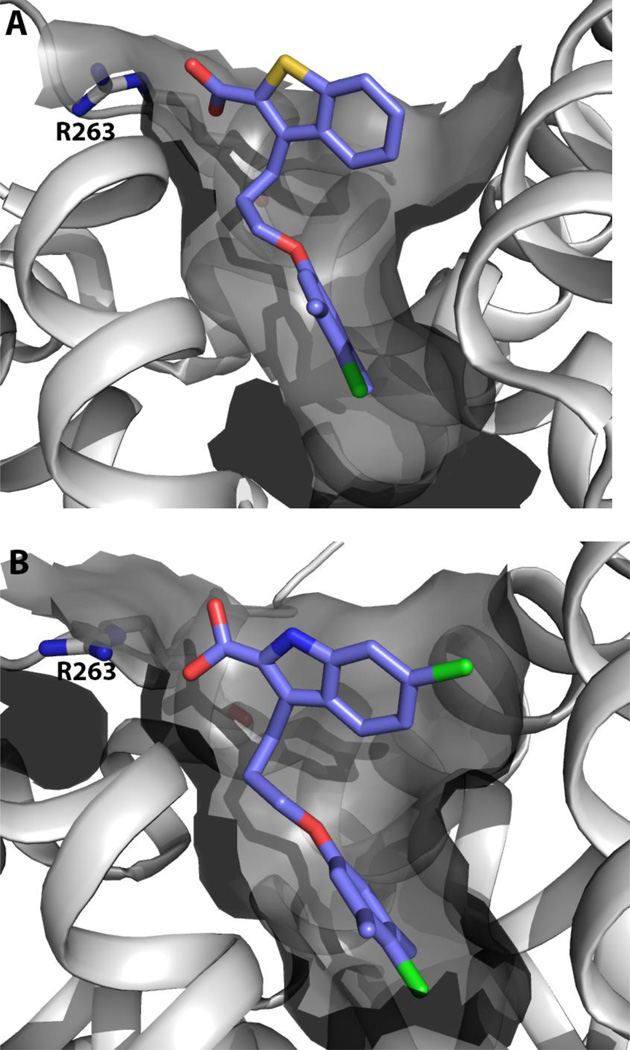
An NMR-based screen of a large fragment library (>13,800 compounds) by Friberg and coworkers led to the identification of several chemically distinct classes of fragment hits. Two of these hits, 5,6-ring-fused heterocyclic carboxylic acids, and a group of hydrophobic aromatics linked to a polar headpiece.(Friberg, et al., 2013) NMR-guided docking of the fragments revealed that the fragments bound in a mutually exclusive fashion in two closely situated binding sites within a large hydrophobic pocket. Based on this structural information, two fragments were merged together to produce compounds with markedly improved binding affinities (e.g., 30 and 31, Fig. 9). Further analoging led to the discovery of the indole-2-carboxylic acid 33, which was a potent inhibitor of Mcl-1 (Kd = 55 nM, Bak-BH3, FPA) that displayed a 16-fold selectivity over Bcl-2 and 270-fold over Bcl-XL (870 nM and >15 µM, respectively, Bak-BH3, FPA). Cocrystal structures of compounds 32 (PDB ID 4HW3) and 33 (PDB ID 4HW2) bound to Mcl-1 confirmed that the merged compounds occupy both pockets identified by the initial fragments and that the carboxylic acid moiety interacts with R263 (Fig. 10). Further analysis of the X-ray structure aided in rationalizing the SAR observed in the merged series and illuminated opportunities to improve the potency by accessing additional binding sites.
Figure 9.
Merging fragments from two distinct classes produced a compound with significantly improved binding affinity.
Figure 10.
Cocrystal structures of benzothiophene-2-carboxylic acid 32 (A) and indole-2-carboxylic acid 33 (B).
12. Conclusion
Significant effort has been directed towards the discovery of Bcl-2 and Bcl-XL inhibitors which has culminated in a number of very potent Bcl-2 family inhibitors such as ABT-737(Oltersdorf, et al., 2005), navitoclax (ABT-263)(Tse, et al., 2008), and ABT-199(Souers, et al., 2013). The remarkable in vitro and in vivo biological activities observed preclinically and in the clinic clearly demonstrates the feasibility of targeting the Bcl-2 family proteins with small molecule inhibitors. In contrast to the advances made in drugging Bcl-2 and Bcl-XL, the discovery of Mcl-1 inhibitors has lagged behind. This is unfortunate, since Mcl-1 appears to also be a promising cancer target. Encouragingly, the last half-decade has witnessed a rapid surge of interest towards the discovery of Mcl-1 inhibitors. This has resulted in a significant number of small molecule BH3-mimetics comprising a range of structurally diverse chemotypes. There remain, however, questions regarding the chemical liabilities, i.e., the inclusion of possible “bad actors”(Baell, 2010), and the non-drug-like physicochemical properties of some of the reported inhibitors. Also, the lack of in vivo data for the majority of proposed Mcl-1 inhibitors is striking.
The moderate potency of Mcl-1 inhibitors reported to date (Table 1) is likely responsible for the lack of convincing in vivo activity. It is tempting to speculate that the experiences observed in the discovery of Bcl-2 and Bcl-XL inhibitors(Oltersdorf, et al., 2005; Souers, et al., 2013; Tse, et al., 2008) will be reflected in the Mcl-1 inhibitors. By comparison, a clinically useful Mcl-1 inhibitor may need to exhibit in vitro affinities approaching the low picomolar range. At present, there are no reported Mcl-1 inhibitors, peptide or small molecule-based, exhibiting this level of affinity for Mcl-1.
Table 1.
Small molecule Mcl-1 inhibitors.
| Inhibition/Selectivity Data* |
||||
|---|---|---|---|---|
| Compound | Mcl-1 | Bcl-2 | Bcl-XL | Reference |
| S1 (5) | Kd 58 nM | Kd 310 nM | NA | T. Song, Chen, et al. (2013) |
| 6 | IC50 10 nM | IC50 20 nM | NA | T. Song, et al. (2014) |
| 8 | Kd 160 nM | NA | NA | Zhang, Song, et al. (2013) |
| 10 | IC50 54 nM | NA | NA | Zhang, Liu, et al. (2013) |
| 12 | Kd 10 µM | NA | Kd >750 µM | Bernardo, et al. (2010) |
| 13 | Kd 250 nM | NA | Kd 3.4 µM | Bernardo, et al. (2010) |
| 16 | IC50 610 nM | NA | IC50 4.4 nM | Tanaka, et al. (2013) |
| 17 | IC50 88 nM | NA | IC50 3.7 nM | Tanaka, et al. (2013) |
| 19 | Kd 180 nM | Kd 7.6 µM | Kd 10.6 µM | Abulwerdi, et al. (2014) |
| MIM1 (20) | IC50 4.8 µM | NA | IC50 >50 µM | Cohen, et al. (2012) |
| marinopyrrole A (21) | IC50 10 µM | NA | IC50 >80 µM | Doi, et al. (2012) |
| 23 | IC50 310 nM | NA | IC50 40 µM | Richard, et al. (2013) |
| 25 | IC50 30 nM | NA | NA | Petros, et al. (2014) |
| 27 | IC50 570 nM | NA | NA | Petros, et al. (2014) |
| 33 | Kd 55 nM | Kd 870 nM | Kd >15 µM | Friberg, et al. (2013) |
Abbreviations: NA, not available at time of writing.
Determined using varying competitive binding assays and ITC.
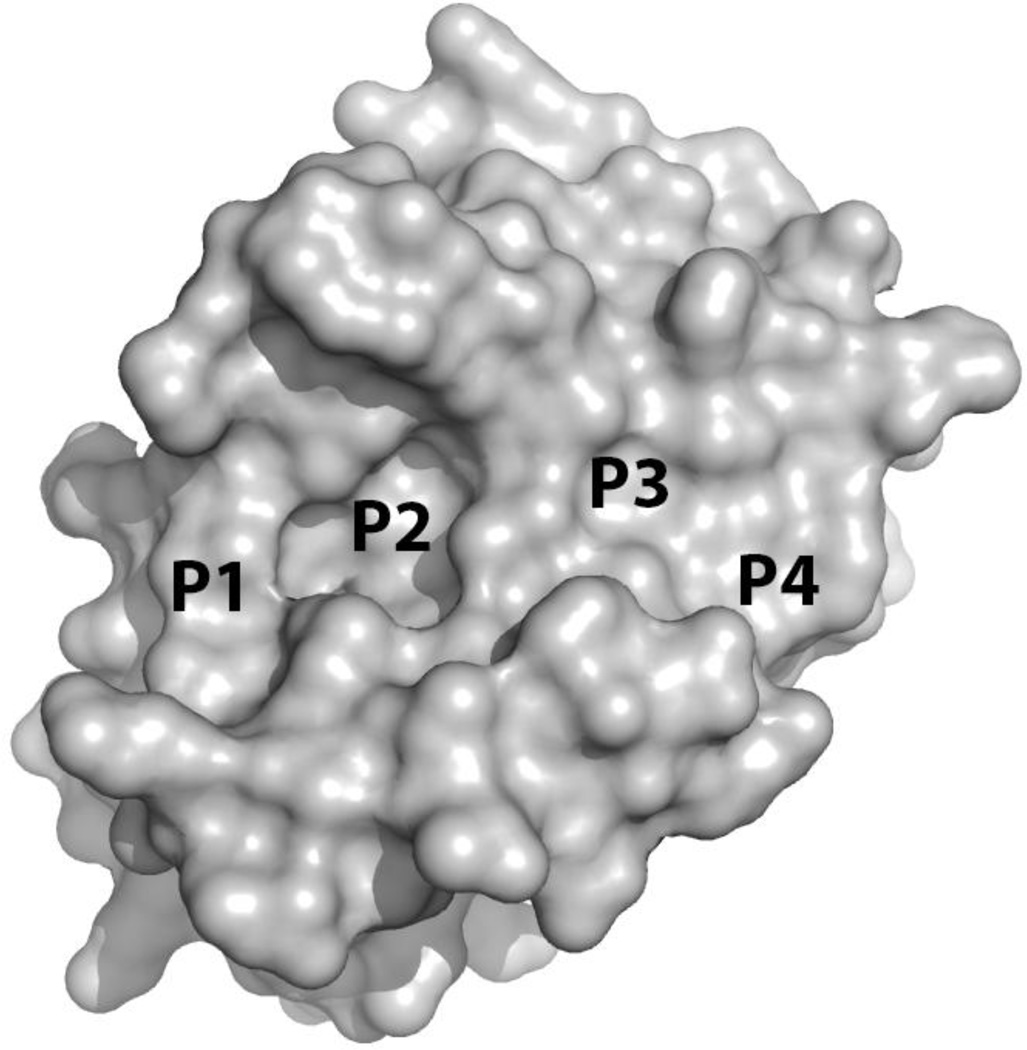
The discovery of potent Mcl-1 inhibitors is a unique challenge due to key structural differences between the BH3-binding grooves of Mcl-1 and Bcl-2 proteins. Unlike Bcl-2, the P2 pocket of Mcl-1 (Fig. 11), as confirmed by cocrystal structures of small molecule inhibitors bound to Mcl-1(Friberg, et al., 2013; Petros, et al., 2014; Tanaka, et al., 2013), has a high degree of plasticity. Indeed, the P2 pocket of Mcl-1 expands to form a large, hydrophobic cavity in the presence of ligands. The P4 pocket of Mcl-1, however, is less well defined and more solvent exposed.(Czabotar, et al., 2007) As such, this pocket is shallower and less hydrophobic than that found in Bcl-XL. The importance of the P2 pocket in Mcl-1 has been confirmed experimentally by the results of two NMR-based fragment screening campaigns against Mcl-1 conducted by Friberg et al. and Petros et al.(Friberg, et al., 2013; Petros, et al., 2014). These two studies disclosed that fragments, bind exclusively in P2. In contrast, the NMR-based screen leading up to the discovery of ABT-737 resulted in the discovery of two fragments bound to two distinct areas of Bcl-XL, P2 and P4.(Oltersdorf, et al., 2005) Future efforts to develop potent and specific ligands for Mcl-1 will need to fully exploit the binding opportunities available within the proximity of the P2 pocket as opportunities to gain significant contributions towards affinity from interactions outside of this region appear, at present, to be limited.
Figure 11.
X-ray structure of Mcl-1 (PDB ID 3MK8).
Significant advancements have been made over the past few years towards the discovery of Mcl-1 inhibitors. However, the Mcl-1 inhibitors described to date are still at a very early stage. Given the concerns described above, significant challenges still remain. With the recent increase in interest it is likely that these challenges will be overcome, and these efforts will lead to novel Mcl-1 inhibitors for the treatment of cancer.
Abbreviations
- Bad
Bcl-2-associated death promoter
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2 associated X
- Bcl-2
B-cell lymphoma-2
- BH
Bcl-2 homology
- BH3
Bcl-2 homology domain 3
- Bcl-XL
B-cell lymphoma-extra large
- Bim
Bcl-2 interacting mediator
- Bfl-1/A1
Bcl-2-related protein A1
- Mcl-1
myeloid cell leukemia-1
- Noxa
phorbol-12-myristate-13-acetate-induced protein 1
- Puma
p53 upregulated modulator of apoptosis
- SAHB
stapled alpha-helix of Bcl-2 domains
- SAR
structure activity relationships
- SPR
surface plasmon resonance
- tBid
truncated BH3-interacting domain death agonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Abulwerdi FA, Liao C, Mady A, Gavin J, Shen C, Cierpicki T, Stuckey JA, Showalter HDH, Nikolovska-Coleska Z. 3-Substituted-N-(4-Hydroxynaphthalen-1-yl)arylsulfonamides as a Novel Class of Selective Mcl-1 Inhibitors: Structure-Based Design, Synthesis, SAR and Biological Evaluation. J Med Chem. 2014;57:4111–4133. doi: 10.1021/jm500010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2008;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, Eastman A. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–24895. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MH, Becker JC, Straten PT. The antiapoptotic member of the Bcl-2 family Mcl-1 is a CTL target in cancer patients. Leukemia. 2005;19:484–485. doi: 10.1038/sj.leu.2403621. [DOI] [PubMed] [Google Scholar]
- Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, Slack RS. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell JB. Observations on screening-based research and some concerning trends in the literature. Future Medicinal Chemistry. 2010;2:1529–1546. doi: 10.4155/fmc.10.237. [DOI] [PubMed] [Google Scholar]
- Bajwa N, Liao C, Nikolovska-Coleska Z. Inhibitors of the anti-apoptotic Bcl-2 proteins: a patent review. Expert Opin Ther Patents. 2012;22:37–55. doi: 10.1517/13543776.2012.644274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo PH, Sivaraman T, Wan KF, Xu J, Krishnamoorthy J, Song CM, Tian LM, Chin JSF, Lim DSW, Mok HYK, Yu VC, Tong JC, Chai CLL. Structural Insights into the Design of Small Molecule Inhibitors That Selectively Antagonize Mcl-1. J Med Chem. 2010;53:2314–2318. doi: 10.1021/jm901469p. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard C. BH3 Mimetics: Status of the Field and New Developments. Mol Cancer Ther. 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 Is Required for Melanoma Cell Resistance to Anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotin E, Meryet-Figuiere M, Simonin K, Duval RE, Villedieu M, Leroy-Dudal J, Saison-Behmoaras E, Gauduchon P, Denoyelle C, Poulain L. Bcl-x(L) and MCL-1 constitute pertinent targets in ovarian carcinoma and their concomitant inhibition is sufficient to induce apoptosis. Int J Cancer. 2010;126:885–895. doi: 10.1002/ijc.24787. [DOI] [PubMed] [Google Scholar]
- Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen W, Bai L, Wang X, Xu S, Belinsky SA, Lin Y. Acquired activation of the Akt/cyclooxygenase-2/Mcl-1 pathway renders lung cancer cells resistant to apoptosis. Mol Pharmacol. 2010;77:416–423. doi: 10.1124/mol.109.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, Aoudjit F. Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res. 2008;6:42–52. doi: 10.1158/1541-7786.MCR-07-0080. [DOI] [PubMed] [Google Scholar]
- Choo EF, Boggs J, Zhu CQ, Lubach JW, Catron ND, Jenkins G, Souers AJ, Voorman R. The Role of Lymphatic Transport on the Systemic Bioavailability of the Bcl-2 Protein Family Inhibitors Navitoclax (ABT-263) and ABT-199. Drug Metab Dispos. 2014;42:207–212. doi: 10.1124/dmd.113.055053. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Stewart ML, Gavathiotis E, Tepper JL, Bruekner SR, Koss B, Opferman JT, Walensky LD. A Competitive Stapled Peptide Screen Identifies a Selective Small Molecule that Overcomes MCL-1-Dependent Leukemia Cell Survival. Chemistry & Biology. 2012;19:1175–1186. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DCS, Fairlie WD, Hinds MG, Colman PM. Structural insights into the degradation of Mcl-1 induced by BH3 domains. PNAS. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, Bataille R, Amiot M. Antisense strategy shows that Mcl-1 rather than Bcl-2 or BCI-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, Lee DF, Yang JY, Xie X, Liu JC, Hung MC. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, Krishnegowda G, Awwad A, Dewey A, Liu X, Amin S, Cheng C, Qin Y, Schonbrunn E, Daughdrill G, Loughran TP, Sebti S, Wang HG. Discovery of Marinopyrrole A (Maritoclax) as a Selective Mcl-1 Antagonist that Overcomes ABT-737 Resistance by Binding to and Targeting Mcl-1 for Proteasomal Degradation. J Biol Chem. 2012;287:10224–10235. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Gullá S, Chen TS, Fire E, Grant RA, Keating AE. Determinants of BH3 Binding Specificity for Mcl-1 versus Bcl-xL. J Mol Biol. 2010;398:747–762. doi: 10.1016/j.jmb.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn JM, Alford SE, Hughes CC, Fenical W, Chambers TC. Purported Mcl-1 inhibitor marinopyrrole A fails to show selective cytotoxicity for Mcl-1-dependent cell lines. Cell Death & Disease. 2013;4:e880. doi: 10.1038/cddis.2013.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SW, Souers AJ, Bruncko M, Song X, Wang X, Hasvold LA, Wang L, Kunzer AR, Park CM, Wendt MD. 7-substituted indole mcl-1 inhibitors. WO 2008/131000 A2. 2008 [Google Scholar]
- Friberg A, Vigil D, Zhao B, Daniels RN, Burke JP, Garcia-Barrantes PM, Camper D, Chauder BA, Lee T, Olejniczak ET, Fesik SW. Discovery of Potent Myeloid Cell Leukemia 1 (Mcl-1) Inhibitors Using Fragment-Based Methods and Structure-Based Design. J Med Chem. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Duronio V. The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J Biol Chem. 2007;282:32233–32242. doi: 10.1074/jbc.M706408200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haste NM, Hughes CC, Tran DN, Fenical W, Jensen PR, Nizet V, Hensler ME. Pharmacological Properties of the Marine Natural Product Marinopyrrole A against Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:3305–3312. doi: 10.1128/AAC.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck P, Chao BH, Litz J, Krystal GW. Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Mol Cancer Ther. 2009;8:883–892. doi: 10.1158/1535-7163.MCT-08-1118. [DOI] [PubMed] [Google Scholar]
- Hughes CC, Yang Y-L, Liu W-T, Dorrestein PC, La Clair JJ, Fenical W. Marinopyrrole A target elucidation by acyl dye transfer. J Am Chem Soc. 2009;131:12094–12096. doi: 10.1021/ja903149u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–595. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS ONE. 2009;4:e6651. doi: 10.1371/journal.pone.0006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvalo PP, Kitada S, Deng XM, Zhai DY, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling XY, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693–1700. [PubMed] [Google Scholar]
- Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, Fesik SW, Shen Y. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- Liu H, Eksarko P, Temkin V, Haines GK, 3rd, Perlman H, Koch AE, Thimmapaya B, Pope RM. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337–8345. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, Weninger W, Haass NK, Allen JD. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- Lugovskoy AA, Degterev AI, Fahmy AF, Zhou P, Gross JD, Yuan J, Wagner G. A novel approach for characterizing protein ligand complexes: molecular basis for specificity of small-molecule Bcl-2 inhibitors. J Am Chem Soc. 2002;124:1234–1240. doi: 10.1021/ja011239y. [DOI] [PubMed] [Google Scholar]
- Meng XW, Lee SH, Dai HM, Loegering D, Yu C, Flatten K, Schneider P, Dai NT, Kumar SK, Smith BD, Karp JE, Adjei AA, Kaufmann SH. MCL-1 as a buffer for proapoptotic BCL-2 family members during TRAIL-induced apoptosis - A mechanistic basis for sorafenib (bay 43-9006)-induced trail sensitization. J Biol Chem. 2007;282:29831–29846. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, Tsuji S, Nakajima S, Doi R, Kato M, Shimada Y, Imamura M. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci. 2014;39:101–111. doi: 10.1016/j.tibs.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood. 2000;96:1756–1763. [PubMed] [Google Scholar]
- Muppidi A, Doi K, Edwardraja S, Drake EJ, Gulick AM, Wang H-G, Lin Q. Rational Design of Proteolytically Stable, Cell-Permeable Peptide-Based Selective Mcl-1 Inhibitors. J Am Chem Soc. 2012;134:14734–14737. doi: 10.1021/ja306864v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Belec L, Billot X, Acoca S, Purisima E, Wiegmans A, Cluse L, Johnstone RW, Beauparlant P, Shore GC. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. PNAS. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou KC, Simmons NL, Chen JS, Haste NM, Nizet V. Total synthesis and biological evaluation of marinopyrrole A and analogs. Tetrahedron Lett. 2011;52:2041–2043. doi: 10.1016/j.tetlet.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Pandey MK, Gowda K, Doi K, Sharma AK, Wang H-G, Amin S. Proteasomal Degradation of Mcl-1 by Maritoclax Induces Apoptosis and Enhances the Efficacy of ABT-737 in Melanoma Cells. PLoS ONE. 2013;8:e78570. doi: 10.1371/journal.pone.0078570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang YP, Dai HM, Smith A, Meng XW, Schneider PA, Kaufmann SH. Bak Conformational Changes Induced by Ligand Binding: Insight into BH3 Domain Binding and Bak Homo-Oligomerization. Sci Rep-UK, 2. 2012 doi: 10.1038/srep00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Swann SL, Song D, Swinger K, Park C, Zhang H, Wendt MD, Kunzer AR, Souers AJ, Sun C. Fragment-based discovery of potent inhibitors of the anti-apoptotic MCL-1 protein. Bioorg Med Chem Lett. 2014;24:1484–1488. doi: 10.1016/j.bmcl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Placzek WJ, Sturlese M, Wu B, Cellitti JF, Wei J, Pellecchia M. Identification of a novel Mcl-1 protein binding motif. J Biol Chem. 2011;286:39829–39835. doi: 10.1074/jbc.M111.305326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced killing of melanoma cells by simultaneously targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–9645. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- Richard DJ, Lena R, Bannister T, Blake N, Pierceall WE, Carlson NE, Keller CE, Koenig M, He Y, Minond D, Mishra J, Cameron M, Spicer T, Hodder P, Cardone MH. Hydroxyquinoline-derived compounds and analoguing of selective Mcl-1 inhibitors using a functional biomarker. Bioorg Med Chem. 2013;21:6642–6649. doi: 10.1016/j.bmc.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DCS, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial Susceptibility of Chronic Lymphocytic Leukemia to BCL2 Inhibition: Results of a Phase I Study of Navitoclax in Patients With Relapsed or Refractory Disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-x(L)-Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Lee EF, Checco JW, Evangelista M, Gellman SH, Fairlie WD. Structure -Guided Rational Design of α/β - Peptide Foldamers with High Affinity for BCL - 2 Family Prosurvival Proteins. ChemBioChem. 2013;14:1564–1572. doi: 10.1002/cbic.201300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist R, Pletnev AA, Danilov AV, Eastman A. The putative BH3 mimetic S1 sensitizes leukemia to ABT-737 by increasing reactive oxygen species, inducing endoplasmic reticulum stress, and upregulating the BH3-only protein NOXA. Apoptosis. 2013;19:201–209. doi: 10.1007/s10495-013-0910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LX, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biology & Therapy. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- Song T, Chen Q, Li X, Chai G, Zhang ZC. Correction to 3-Thiomorpholin-8-oxo-8 H-acenaphtho[1,2- b]pyrrole-9-carbonitrile (S1) Based Molecules as Potent, Dual Inhibitors of B-Cell Lymphoma 2 (Bcl-2) and Myeloid Cell Leukemia Sequence 1 (Mcl-1): Structure-Based Design and Structure-Activity Relationship Studies. J Med Chem. 2013;56:9366–9367. doi: 10.1021/jm101181u. [DOI] [PubMed] [Google Scholar]
- Song T, Li X, Chang X, Liang X, Zhao Y, Wu GY, Xie SH, Su PC, Wu Z, Feng YG, Zhang ZC. 3-Thiomorpholin-8-oxo-8H-acenaphtho [1,2-b] pyrrole-9-carbonitrile (S1) derivatives as pan-Bcl-2-inhibitors of Bcl-2, Bcl-xL and Mcl-1. Bioorg Med Chem. 2013;21:11–20. doi: 10.1016/j.bmc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Song T, Li XQ, Yang Y, Wu GY, Xie SH, Su PC, Feng YG, Zhang ZC. 3-Thiomorpholin-8-oxo-8H–acenaphtho [1,2-b] pyrrole-9-carbonitrile (S1) derivatives as pan-Bcl-2-inhibitors of Bcl-2, Bcl-x(L) and Mcl-1 (vol 21, pg 11, 2013) Bioorg Med Chem. 2014;22:663–664. doi: 10.1016/j.bmc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park C-M, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir SK, Yang XF, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, Rosenberg SH, Tse C. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aikawa K, Nishida G, Homma M, Sogabe S, Igaki S, Hayano Y, Sameshima T, Miyahisa I, Kawamoto T, Tawada M, Imai Y, Inazuka M, Cho N, Imaeda Y, Ishikawa T. Discovery of Potent Mcl-1/Bcl-xL Dual Inhibitors by Using a Hybridization Strategy Based on Structural Analysis of Target Proteins. J Med Chem. 2013;56:9635–9645. doi: 10.1021/jm401170c. [DOI] [PubMed] [Google Scholar]
- Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- Thallinger C, Wolschek MF, Wacheck V, Maierhofer H, Gunsberg P, Polterauer P, Pehamberger H, Monia BP, Selzer E, Wolff K, Jansen B. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. 2003;120:1081–1086. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DCS. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20:1475–1484. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJS, Cohen GM. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, Shehata S, Kung AL, Beroukhim R, Golub TR. Chemical Genomics Identifies Small-Molecule MCL1 Repressors and BCL-xL as a Predictor of MCL1 Dependency. Cancer Cell. 2012;21:547–562. doi: 10.1016/j.ccr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S-H, Dong K, Lin F, Wang X, Li B, Shen J-j, Zhang Q, Wang R, Zhang H-Z. Inducing apoptosis and enhancing chemosensitivity to Gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62:1055–1064. doi: 10.1007/s00280-008-0697-7. [DOI] [PubMed] [Google Scholar]
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O’Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJC, Leong KG, Dueber EC, Maecker H, Huang DCS, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- Yang-Yen HF. Mcl-1: a highly regulated cell death and survival controller. J Biomed Sci. 2006;13:201–204. doi: 10.1007/s11373-005-9064-4. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jin L, Qian X, Wei M, Wang Y, Wang J, Yang Y, Xu Q, Xu Y, Liu F. Novel Bcl-2 Inhibitors: Discovery and Mechanism Study of Small Organic Apoptosis-Inducing Agents. ChemBioChem. 2007;8:113–121. doi: 10.1002/cbic.200600305. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu C, Li X, Song T, Wu Z, Liang X, Zhao Y, Shen X, Chen H. Fragment-based design, synthesis, and biological evaluation of N-substituted-5-(4-isopropylthiophenol)-2-hydroxynicotinamide derivatives as novel Mcl-1 inhibitors. Eur J Med Chem. 2013;60:410–420. doi: 10.1016/j.ejmech.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song T, Li X, Wu Z, Feng Y, Xie F, Liu C, Qin J, Chen H. Novel soluble myeloid cell leukemia sequence 1 (Mcl-1) inhibitor (E,E)-2-(benzylaminocarbonyl)-3-styrylacrylonitrile (4g) developed using a fragment-based approach. Eur J Med Chem. 2013;59:141–149. doi: 10.1016/j.ejmech.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song T, Zhang T, Gao J, Wu G, An L, Du G. A novel BH3 mimetic S1 potently induces Bax/Bak-dependent apoptosis by targeting both Bcl-2 and Mcl-1. Int J Cancer. 2010;128:1724–1735. doi: 10.1002/ijc.25484. [DOI] [PubMed] [Google Scholar]
- Zhong J-T, Xu Y, Yi H-W, Su J, Yu H-M, Xiang X-Y, Li X-N, Zhang Z-C, Sun L-K. The BH3 mimetic S1 induces autophagy through ER stress and disruption of Bcl-2/Beclin 1 interaction in human glioma U251 cells. Cancer Lett. 2012;323:180–187. doi: 10.1016/j.canlet.2012.04.009. [DOI] [PubMed] [Google Scholar]