Abstract
Immune cell entry into the virally infected central nervous system (CNS) is vital for promoting viral clearance yet may contribute to neuropathology if not rigorously regulated. We previously showed that signaling through the interleukin 1 receptor (IL-1R1) is critical for effector T cell reactivation and virologic control within the CNS during murine West Nile virus (WNV) encephalitis. WNV-infected IL-1R1−/− mice also display increased parenchymal penetration of CD8+ T cells despite lack of CD4-mediated full activation, suggesting dysregulation of molecular components of CNS immune privilege. Here, we show that IL-1 signaling regulates the CNS entry of virus-specific lymphocytes, promoting protective immune responses to CNS viral infections that limit immunopathology. Analysis of blood-brain barrier (BBB) function in the WNV-infected IL-1R1−/− mice revealed no alterations in permeability. However, parenchymal proinflammatory chemokine expression, including CCL2, CCL5 and CXCL10, was significantly upregulated, whereas microvasculature CXCL12 expression was significantly decreased in the absence of IL-1 signaling. We show that during WNV infection, CD11b+CD45hi infiltrating cells (macrophages) are the primary producers of IL-1β within the CNS and, through the use of an in vitro BBB model, that IL-1β promotes CXCR4-mediated T cell adhesion to brain microvasculature endothelial cells (BMECs). Of interest, IFNγ+ and CD69+ WNV-primed T cells were able to overcome CXCL12-mediated adhesion via down-regulation of CXCR4. These data indicate that infiltrating IL-1β-producing leukocytes contribute to cellular interactions at endothelial barriers that impart protective CNS inflammation by regulating the parenchymal entry of CXCR4+ virus-specific T cells during WNV infection.
Introduction
Leukocyte transmigration across the blood brain barrier (BBB) is a tightly regulated process and is central to inflammation and immune responses within the central nervous system (CNS). Under physiological conditions, a limited number of immune cells cross endothelial barriers as part of normal immune surveillance, yet remain localized to perivascular spaces within the leptomeninges or CNS parenchyma via expression of the chemokine CXCL12 (1-4). Thus, in neuroinflammation, a large number of immune cells accumulate within perivascular locations, distending the perivascular spaces (2-6). Within these perivascular cuffs, lymphocytes encounter antigen-presenting cells and other infiltrating leukocytes (7). These interactions ensure full activation of effector T cells and trigger their ability to migrate from the perivascular space into the CNS parenchyma (7, 8). These steps are essential to reduce or eliminate invading viruses from the CNS (4, 9, 10); however, the exact molecular mechanisms that regulate the entry of virus-specific lymphocytes, in particular, are not well defined.
West Nile Virus (WNV), a neurotropic flavivirus, has emerged globally as a significant cause of viral encephalitis (11). Both innate and adaptive immune defenses largely control WNV within the periphery; however, WNV can spread to the CNS, causing neuronal injury and inflammation that can lead to death in both humans and mice (12-16). In murine models of WNV infection, both T cells and macrophages traffic to the WNV-infected CNS soon after local viral replication begins, approximately 6 days post-infection, and are essential for virologic control at this site (17-20). In 5-week old mice, infiltrating leukocytes remain localized to perivascular spaces where CXCL12 levels remain elevated at this time-point. Later in the course of encephalitis, lymphocytes begin to enter the CNS after CXCL12 levels are down-regulated; however, this occurs too late to exert significant virologic control (4). Consistent with this, the early administration of a CXCR4 antagonist in 5-week old C57BL/6 mice promotes leukocyte entry into the CNS parenchyma and results in improved viral clearance, decreased immunopathology, and enhanced survival during WNV infection (4). In contrast, 8-week old mice have improved survival (~70%) with increased CNS entry of virus-specific T lymphocytes at early time-points (15, 21). While the mechanisms underlying age-related differences in T cell entry are unknown, the parenchymal presence of CD8+ T cells is crucial for preventing fatal encephalitis through clearance of WNV via mechanisms involving IFN-γ, TNF-α and perforin (22-25). The effectiveness of parenchymal CD8+ T cells in viral clearance also reflects the effects of CD4+ T cells, which promote the full activation, migration, and positioning of virus-specific CD8+ T cells within the CNS (26-28). Therefore, leukocyte interactions within the perivascular compartment are necessary for T-cell function and mobility within the CNS to ensure appropriate entry of T-cell subsets, which in turn would achieve the proper balance between protective versus pathogenic responses.
IL-1 signaling is critically involved in the regulation of inflammation and pathobiology of immune and inflammatory conditions (29, 30). A growing body of evidence suggests an important role for IL-1 signaling in the immunity against several viruses including influenza A, hepatitis B, Sendai and vesicular stomatitis virus (VSV) (31). Notably, IL-1 has been shown to drive host responses that regulate cellular infiltration to sites of viral infection (32, 33). In the context of CNS viral infections, IL-1 signaling has been associated with protection as well as enhancement of disease, depending on the virus. Thus, while IL-1β works synergistically with TNF-α to protect against HSV-1-induced encephalitis (33), IL-1β increases pathogenesis and lethality to Sindbis virus encephalitis (34, 35). During Theiler's murine encephalomyelitis virus (TMEV) infection, IL-1 signaling elevates pathogenic responses, whereas the lack of IL-1 signaling results in viral persistence due to insufficient T cell activation (36). During HIV encephalitis, IL-1β-producing macrophages promote CXCL12 expression by astrocytes resulting in increased neuropathogenesis (37). While studies in autoimmunity suggest that IL-1β-producing T cell infiltrates regulate the pattern of CXCL12 expression along the vasculature of the CNS (38), this has not been evaluated in viral models. Although little is known about IL-1 signaling in immunity to flavivirus infections, recent studies have clarified its protective role within the CNS. We and others, have shown that WNV infection is significantly exacerbated in mice deficient of the type 1 IL-1 receptor (IL-1R1) due to decreased neuronal antiviral activity as well as an inability to fully activate CD4+ T and dendritic cells specifically within the CNS, which is required for restimulation of infiltrating, virus-specific CD8+ T cells (26, 39). This latter study also found that ex vivo analysis of CNS virus–specific CD8+ T cells revealed no cell-intrinsic defects associated with loss of IL-1R1 activity. The study additionally demonstrated increased neuroinflammation in IL-1R1-deficient mice, suggesting that IL-1 signaling in WNV infection acts to regulate leukocyte interactions at the BBB that mediate protective immunity within the CNS and limit inappropriate T cell access to CNS parenchyma.
In this study, we reveal a novel role for IL-1 during WNV encephalitis. IL-1 signaling is critical for specifically regulating the perivascular localization of infiltrating T cells via CXCL12 expression, which promotes efficient T cell reactivation and entry, controlling viral replication and immunopathology within the WNV-infected CNS. Our observations link the production of IL-1β by infiltrating macrophages to the local control of lymphocyte function and fate at CNS endothelial barriers.
Materials and Methods
Virus
The West Nile Virus strain 3000.0259 was isolated in New York in 2000 (40) and passaged once in C6/36 Aedes albopitcus cells to generate an insect cell-derived stock. The stock titer was determined by using BHK21 cells for viral plaque assay as previously described (21).
Mouse experiments
C57BL/6 wild-type (WT) and IL-1R1−/− inbred mice were obtained commercially (Jackson Laboratories). All mice were housed and bred in the pathogen-free animal facilities of the Washington University School of Medicine. Experiments were performed in compliance with the guidelines and approval of the Washington University School of Medicine Animal Safety Committee. Matched 8 week-old mice were inoculated subcutaneously via footpad injection (50 μl) with 100 plaque-forming units (PFU) of WNV as previously described (41).
Viral load quantification
Quantification of WNV viral load was measured by analyzing positive-strand viral RNA levels using quantitative reverse transcription-PCR (qRT-PCR) as described previously (42).
Leukocyte isolation and stimulation
Cells were isolated from the CNS of WT and IL-1R1−/− mice at day 6 and 8 after infection and stained with fluorescently conjugated antibodies to CD4, CD8β, CD11b, CD11c and CD45 as previously described (3). Intracellular IFN-γ staining was performed on splenocytes from day 8 post-infection animals in an I-Ab–restricted NS32066 and NS31616 peptide and Db-restricted NS4B peptide restimulation assay as previously described (17, 43). Data collection and analysis were performed with a LSR flow cytometer (Becton Dickinson) using FlowJo software (Tree Star).
BBB permeability assay
At various day intervals after infection mice were injected IP with 100ul of 100mg/ml fluorescein sodium salt (Sigma-Aldrich) in sterile PBS. After 45 minutes, mice underwent extensive cardiac perfusion with PBS, followed by collection of blood and harvesting of CNS tissues. Tissue homogenates and serum were incubated overnight at 4°C at 1:1 dilution in 2% trichloroacetic acid (TCA, Sigma-Aldrich) to precipitate protein, which was pelleted by 10m centrifugation at 4000 rpm at 4C. Supernatants were then diluted in equal volumes of borate buffer, pH 11 (Sigma-Aldrich). Fluorescence emission at 538 nm was determined via a microplate reader Synergy™ H1 and Gen5™ software (BioTek Instruments, Inc.). Tissue fluorescence values were standardized against plasma values for individual mice.
Real-time quantitative RT-PCR for chemokines
Total RNA was prepared from the brains of WNV-infected WT and IL-1R1−/− mice using the RNeasy kit (Qiagen) according to the manufacturer's instructions. Reverse transcription and QPCR was performed as previously described (18). Calculated copies were normalized against copies of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh). All oligonucleotide primers used have been previously reported (18).
Immunohistochemistry and confocal microscopy
Mice were infected with 102 PFU of WNV and sacrificed at day 8 post-infection. CNS tissues were then isolated and frozen sections were permeabilized, blocked, and stained as previously described (3). IHC detection of CD3, CD31, GFAP, IBA-1, CXCL12 and pCXCR4 with nuclear ToPro3 counterstaining, were performed as previously described (4). In situ tetramer staining was performed on fresh brain tissues collected on day 8 after infection with WNV. 1 mm sections were incubated with 10 μl/section of the Db-NS4B tetramer at 37°C for 15 minutes. The tissue was then rinsed repeatedly at 37°C with PBS and then twice with ice-cold PBS prior to fixation with cold 2% paraformaldehyde for 20 minutes. After additional washes, anti-CD31 antibody (rat polyclonal, BD Biosciences) was applied for 1 hour. The tissues were then washed and incubated with anti-rat Alexa 488 (Molecular Probes). Tissues were then washed, mounted on a glass slide, and examined by confocal microscopy.
Western Blot
Brain microvascular endothelial cells (BMECs) were treated with IL-1β (10 ng/ml) overnight and protein lysates were isolated using RIPA buffer supplemented with protease and phosphatase-3 inhibitor cocktail (Sigma). Lysates were resolved on a 4-12% Bis-Tris gel and transferred on to an iBlot Nitrocellulose transfer membrane (Invitrogen) according to standard protocols. Blots were probed with polyclonal rabbit anti-CXCL12β (eBioscinec) and monoclonal mouse anti-β-tubulin (Sigma) antibodies flowed by incubation with IRDye®-conjugated secondary antibodies (LI-COR). Blots were imaged using the Odyssey fluorescent scanning system (LI-COR) and analyzed using ImageJ.
Migration Assay
In vitro BBB's were generated using a well-established transwell system as previously described (44). Following stimulation, both the CD4+ and CD8+ T lymphocytes were isolated via positive selection (MACS) and together yielded approximately 95% pure T lymphocytes: CD8, 40% and CD4, 55% (Fig. S1). Following selection, 5×105 lymphocytes in 200 μl of C5 media were added to the top chamber of each in vitro BBB (~5:1 ratio, lymphocyte to endothelial cell) and allowed to incubate for 6 h at 37°C. Medium was then harvested from bottom chambers and lymphocytes were collected by centrifugation (1500, 10 min, 4°C). Cell pellets were resuspended in 200 ul of FACS buffer (2% FBS, 0.5 mM EDTA, 0.02% sodium azide in sterile DPBS), and leukocyte subsets were identified and counted with LSRII flow cytometer (Becton Dickinson) using FlowJo software (Tree Star). For each leukocyte subset, migration was quantified as the number of cells present in the bottom chamber after 6 h divided by the total number of that cell type added to the top chamber (proportion migrating).
Statistical Analysis
Graphs were made and statistical analysis was performed via computerized software (GraphPad Prism). Depending on the data, an unpaired, two-tailed Student's t test or one-way ANOVA with Tukey-Kramer posttest was performed, with p<0.05 considered to be significant.
Results
Leukocyte infiltration is increased in the IL-1R1−/− WNV-infected CNS
In prior studies we showed that IL-1R1 signaling is required for APC-mediated T cell reactivation and subsequent virologic control specifically within the WNV-infected CNS (26). Accordingly, WNV RNA levels are significantly increased in the CNS of IL-1R1−/− mice compared with C57BL/6 wild-type (WT) mice on day 8 following footpad inoculation (100 PFU WNV) (Fig. 1A). Because prior studies in mice with neuroinflammation showed that IL-1 signaling is required for T cell migration from perivascular spaces (38), we evaluated the trafficking of immune cells within the brains of WNV-infected WT and IL-1R1−/− mice. As previously observed (26), flow cytometric analysis of infiltrating immune cells at this time-point revealed significantly increased numbers of CD45+ leukocytes within CNS tissues of WNV-infected IL-1R1−/− mice compared to those of similarly infected WT counterparts (Fig. 1B). In particular, total numbers of CD4+ (Fig. 1C) and CD8+ (Fig. 1D) T lymphocytes as well as the number of CD11b+ (Fig. 1E) leukocytes were increased in the IL-1R1-deficent mice on day 8 post-infection. There was no discernible difference between the genotypes on day 6 post-infection in the total number or in the numbers of infiltrating T lymphocytes or CD11b+ monocytes. Of note, there are limited numbers of inflammatory cells within the WNV-infected CNS prior to day 6 post-infection (19). Further evaluation of the locations of CD3+ T cells within the brains of WNV-infected IL-1R1−/− and WT mice with respect to the CD31+ microvasculature showed that parenchymal entry of T cells at day 8 post-infection was significantly increased in the WNV-infected IL-1R1−/− mice compared with similarly infected WT animals (p=0.0043) (Fig. 1F, G), despite their lack of reactivation and inability to clear virus. This surprising result suggested that IL-1 signaling is required to limit inappropriate T cell entry during viral infections of the CNS.
Figure 1. IL-1 signaling regulates virologic control and leukocyte trafficking within the CNS.
Examination of viral load and leukocyte entry in WT and IL-1R1−/− animals. 8-wk-old mice were infected with 100 PFU WNV via footpad injection. (A) Viral loads in the brain were assessed from WT (closed squares) or IL-1R1−/− mice (open squares) by Taqman based qRT-PCR on days 6 and 8 after infection using specific primers and probes to WNV envelope protein. (B-E) Leukocyte infiltration into the CNS was assessed by flow cytometry at days 6 and 8 post-infection (p.i.) with WNV. Total number of cells (B) recovered from perfused whole brain were stained with antibodies to CD4 (C), CD8 (D), and CD11b (E) and analyzed after gating on leukocyte population. (F, G) Histological analysis from day 8 p.i. brain tissue sections from WNV-infected mice. (F) Representative confocal microscopic images of CD3 (red) and CD31 (green) from the cerebral cortex of WT (left) and IL-1R1−/− (right) sections. Bars, 25 μm. (G) Quantitative analyses of parenchymal versus perivascular T cells within the brains of WNV-infected mice at day 8 p.i. Data are presented as a ratio of T cell location as determined by analyzing the associations of CD3+ cells with respect to CD31-stained vessels and counting the number of parenchymal versus perivascular cells in 10-15 low power confocal images for 4-6 mice per group. Data are shown as the mean ± S.E.M. for n = 4-6 mice per time point and is representative of 2-3 independent experiments. **p<0.001, ***p<0.0005.
Infiltrating leukocytes are the primary producers of IL-1β during WNV encephalitis
In the CNS, IL-1 can be produced by astrocytes, endothelial cells, infiltrating leukocytes, neurons and oligodendrocytes, but microglia are thought to produce the highest levels of IL-1 in response to infection or injury (45-48). We previously showed that IL-1β is detected within the CNS following WNV infection at approximately day 7 post-infection (26, 39). Infectious virus can be recovered from the WNV-infected CNS at day 4 to 5 post-infection before immune cell infiltration, which occurs approximately one week post-infection (49). To determine whether resident or infiltrating immune cells are the main producers of IL-1β, we examined IL-1β expression in cells isolated from 8 week-old WT mice that had been infected with 100 PFU of WNV on day 8 post-infection via footpad inoculation (Fig. 2). Flow cytometric analysis of cells isolated from the brains of WNV-infected mice showed that CD4+ T cells, CD11c+ antigen presenting cells (APCs), CD11b+CD45lo (microglia) and CD11b+CD45hi (macrophages) cells were all positive for IL-1β expression (Fig. 2A). The total number of CD45+ leukocytes expressing IL-1β was approximately 4.5×104 cells (Fig. 2B). However, analysis of total numbers of these cells revealed that macrophages had the highest number of IL-1β producers (~ 3×104 cells) (Fig. 2C). Thus, while CNS resident cells may produce IL-1β in response to inflammatory injury, infiltrating immune cells such as CD11b+ macrophages are the primary producers of the proinflammatory cytokine IL-1β during WNV encephalitis.
Figure 2. IL-1β is primarily produced by infiltrating macrophages during WNV encephalitis.
Examination of IL-1β production in the CNS of WT animals during WNV infection. Brains were harvested and analyzed by flow cytometry at day 8 p.i. with WNV and assessed for IL-1β expression by intracellular staining. (A) Representative histograms with percentages of CD4, CD8, CD11c, CD11b+CD45lo (microglia) and CD11b+CD45hi (macrophage) populations expressing IL-1β are shown. (B) Total number of IL-1β-expressing CD45+ leukocytes. (C) Total numbers of the indicated populations expressing IL-1β. Data are shown as the mean ± S.E.M. for n = 4-6 mice and is representative of 3 independent experiments.
BBB permeability is unaffected in the absence of IL-1R1 signaling
Since lymphocyte entry into the CNS was greatly increased in the IL-1R1−/− mice despite lack of full activation (26), we wondered whether BBB permeability might be altered in the absence of intact IL-1R1 signaling. To test this, we administered sodium fluorescein (NaFluor) intravenously on days 4, 6 and 8 post-infection to both WT and IL-1R1−/− mice and performed fluorometric analyses in various CNS regions including the olfactory bulb (Fig. S2A), cerebral cortex (Fig. S2B), cerebellum (Fig. S2C), and the spinal cord (Fig. S2D). The amount of NaFluor in each of the regions did not significantly differ between IL-1R1−/− mice compared to WT. These results suggest that the CNS entry of leukocytes across the BBB in WNV-infected IL-1R1−/− mice is not the result of barrier dysfunction.
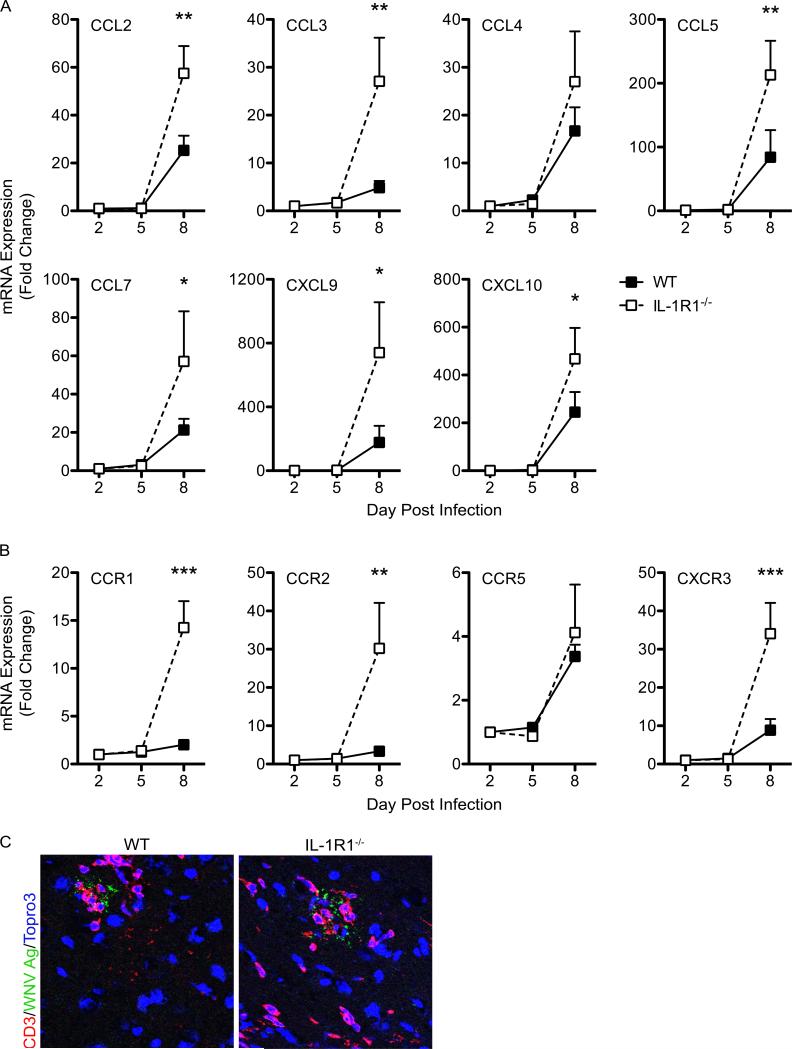
Inflammatory chemokine expression is increased in the absence of IL-1R1 signaling
Pro-inflammatory chemokines play a significant role in leukocyte trafficking into the CNS and facilitate the appropriate entry of virus-specific T cells (49, 50). Prior studies have demonstrated a positive correlation between viral loads within the CNS and pro-inflammatory chemokine mRNA expression, primarily by virally infected neurons (18, 51-53). We therefore assessed levels of pro-inflammatory chemokine mRNA expression within the WNV-infected CNS of IL-1R1-deficient mice compared with similarly infected WT controls on days 2, 5 and 8 following infection. By day 8 post-infection, we detected significant increases of CCL2, CCL3, CCL5, CCL7, CXCL9 and CXCL10 (Fig. 3A) within the CNS of the IL-1R1−/− mice. The mRNA levels of chemokine receptors that bind these ligands were also elevated in WNV-infected IL-1R1 deficient mice compared with similarly infected WT controls, specifically CCR1, CCR2 and CXCR3 (Fig. 3B). WNV-infected neurons directly induce the recruitment of virus-specific T cells for the purpose of viral clearance through pro-inflammatory chemokine expression (18, 51). Evaluation of CD3+ T cells within the CNS parenchyma revealed their juxtaposition with WNV-infected neurons in both IL-1R1-deficient and WT animals, however, a number of CD3+ T cells remained remote of WNV antigen in the absence of IL-1R1 signaling (Fig. 3C). Overall, these results are consistent with other studies that demonstrate that the extent of virally infected neurons within the CNS parenchyma determine levels of chemokine expression and provide a mechanism for the extensive lymphocyte migration observed within the CNS parenchyma of WNV-infected IL-1R1−/− mice (Fig. 1G).
Figure 3. Inflammatory chemokine signaling is increased in the absence of IL-1 signaling within the CNS during WNV infection.
Examination of inflammatory chemokine expression in the CNS. Brain tissue was harvested following cardiac perfusion from WNV-infected WT (closed squares) and IL-1R1−/− mice (open squares) at indicated time points and chemokine (A) and chemokine receptor (B) mRNA levels were analyzed via qRT-PCR, normalized to GAPDH and are presented as the mean fold change in mRNA levels over uninfected controls. Statistical significance of increased chemokine expression in WNV-infected IL-1R1−/− mice was determined in comparison with infected WT mice. (C) Representative confocal microscopic images from brain sections (brainstem region) from wild-type (left) and IL-1R1−/− (right) WNV-infected mice stained for WNV antigen (green), CD3 (red) and nuclei (blue). Images are representative of results from five independent mice. Data are averages of results for at least 4 mice per group and reflect at least two independent experiments and presented as mean values ± S.E.M. *p<0.05, **p<0.01, ***p<0.001
Loss of IL-1 signaling is associated with increased glial cell activation in the WNV-infected CNS
Stringent regulation of leukocyte entry into the brain is important for the success of antiviral immunity since lymphocytes can activate resident glial cells and promote immunopathology (3, 54, 55). To determine whether chemokine-mediated (see Fig. 3) increase in infiltrating leukocytes within WNV-infected IL-1R1−/− CNS (see Fig. 1) contributed to immunopathology, we assessed astrocyte and microglial activation at day 8 after infection; a time point at which IL-1R1−/− mice continue to develop immune cell infiltrates and WT mice begin to recover (26). Indeed, glial fibrillary acidic protein (GFAP), a marker for activated astrocytes, was widely expressed in IL-1R1−/− mice whereas GFAP expression detected in WT mice was limited (p=0.0232) (Fig. 4A, B). The significant increase astrocyte activation coincided with the presence of CD3+ T cells. We also observed increased expression of ionized calcium binding adaptor molecule 1 (Iba-1), a marker of activated microglia and macrophages, throughout the CNS tissue of WNV-infected IL-1R1−/− mice at day 8 after infection (p=0.0361) (Fig. 4C, D). This expression was most prominent in and around blood vessels and within the parenchyma of IL-1R1−/−, but not WT, brain tissue sections. These results suggest that increased infiltration is associated with neural glial cell activation observed in the CNS of the IL-1R1−/− animals (24). Thus, we conclude IL-1 dependent control of leukocyte infiltration in the CNS regulates the magnitude of inflammation during WNV infection to reduce immunopathology.
Figure 4. Neuroglial activation is increased in the absence of IL-1 signaling during WNV encephalitis.
Histological analysis of glial activation in the CNS of WT or IL-1R1−/− mice. Brain tissues from WNV-infected mice were collected on day 8 p.i. Confocal analysis of (A) GFAP (red) and CD3 (green) expression from cerbral cortex and of (C) IBA-1 (red) and CD3 (green) expression from brainstem of WT (left) and IL-1R1−/− mice (right). Quantitative analysis of GFAP (B) and IBA-1 (D) in both WT and IL-1R1−/− mice. Representative images shown are from 4-5 mice per group. Data are from at least 2 experiments in which 10 images were analyzed in each of the mice and presented as mean values ± S.E.M. *p<0.05 Bars, 25 μm.
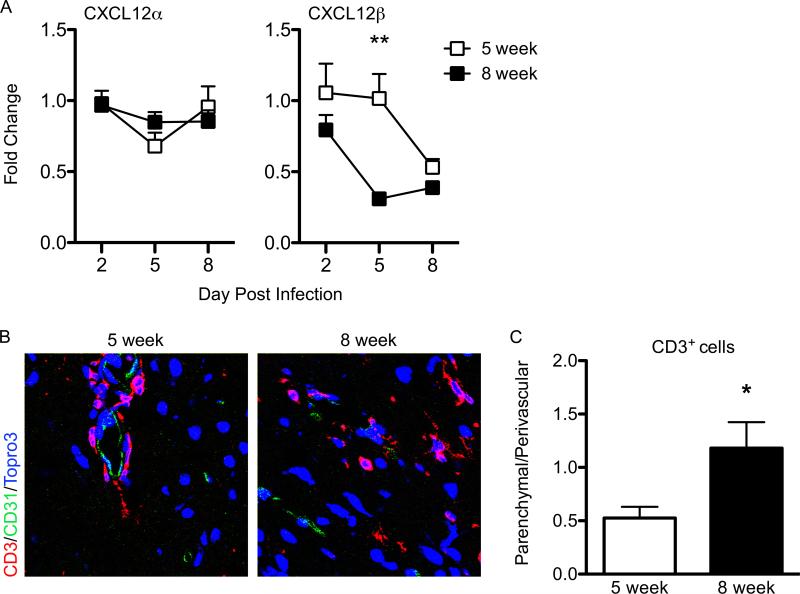
CXCL12 expression at the BBB is decreased by day 5 post-infection in 8-week-old mice during WNV encephalitis
In prior studies we showed CXCL12 localized lymphocytes to perivascular spaces, inhibiting their intraparenchymal migration, which limited their ability to control infection in 5-week-old animals, which universally succumb to WNV infection (4). In these studies, CXCL12 levels were observed to decrease at day 8 post-infection, a time-point when viral loads are already quite high within the CNS. Early administration of a specific antagonist of CXCR4, AMD3100 (56), led to improved CNS entry of WNV-specific CD8+ T cells with a 50% increase in survival. As 8-week-old animals have an ~70% survival rate with extensive CNS entry of T cells, we wondered whether CXCL12 levels are more efficiently decreased within the CNS of these older animals. Analysis of CXCL12α and β mRNA levels from WNV-infected 5-week-old and 8-week-old C57BL/6 WT mice at various days post infection revealed that CXCL12β, which is expressed by CNS endothelium (57), is decreased significantly as early as day 5 post-infection in 8-week-old animals, while decreased expression of CXCL12β in 5-week-old animals was delayed until day 8 (Fig. 5A). In contrast, no difference in expression of CXCL12α, which is expressed by neuronal subpopulations (57), was observed between the 5-week-old and 8-week-old animals (Fig. 5A). To determine whether decreased expression of CXCL12 at the BBB on day 5 post-infection affected the intraparenchymal trafficking of T cells, we examined the localization of CD3+ cells with respect to the CD31+ microvasculature in the CNS from both 5-week-old and 8-week-old mice at day 6 after WNV infection (Fig. 5B). The parenchymal migration of T cells at day 6 after infection significantly increased in 8-week-old WNV-infected mice compared with similarly infected 5-week-old animals (p=0.0102) (Fig. 5B, C). These results suggest that lymphocyte egress from the perivascular space is dependent on efficient alterations in the temporal expression of CXCL12 within the microvasculature of the CNS.
Figure 5. CXCL12 expression in the CNS during WNV encepahlits in 5-week old animals versus 8-week old animals.
(A) qRT-PCR analysis of CXCL12 expression in the CNS at indicated time points after infection with WNV in 5-week old WT (open squares) and 8-week old WT animals (closed squares). Data are mean values ± S.E.M. for n = 6 mice per group/per day across 3 independent experiments. (B) Confocal immunohistochemical analysis of brain tissue collected from WNV-infected 5-week old (left) and WNV-infected 8-week old (right) mice on day 6 p.i. Images were stained for CD3 (red), CD31 (green), and nuclei (blue). Representative images are shown for five sections from three mice in two separate experiments. (C) Quantitiave analyses of perivascular versus parenchymal T cells within brains of WNV-infected 5-week old (open bar) and 8-week old (closed bar) mice day 8 after infection. Data are presented as average percentages of T cells as determined by analyzing the associations of CD3+ cells with respect to CD31-stained vessels and counting the numbers of perivascular versus parenchymal cells in 10-12 low power confocal images for 4-5 mice per group. *p<0.05, **p<0.01
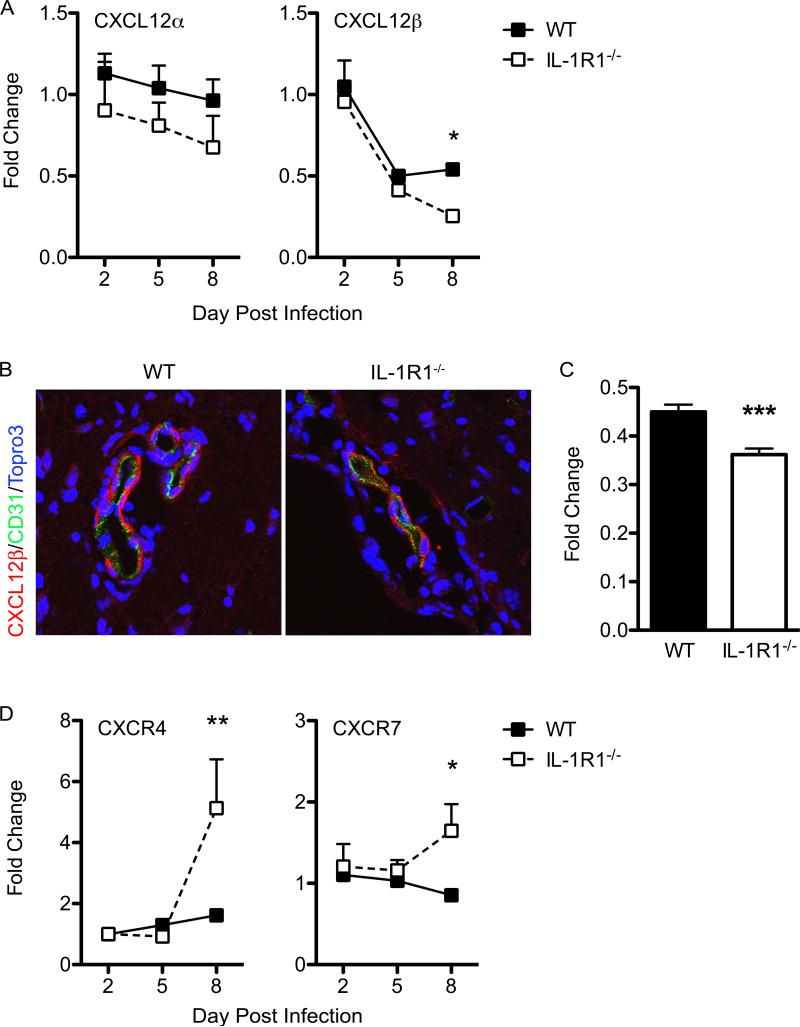
CXCL12 expression at the endothelium is decreased in the absence of IL-1R1 signaling
IL-1 has previously been shown to have a role in regulating expression of CXCL12 at CNS endothelial barriers (3, 6, 38, 58). Accordingly, levels of CXCL12β were significantly lower in IL-1R1−/− mice at day 8 post-infection compared with those of similarly infected WT animals (Fig. 6A). Although not significant, levels of CXCL12α were also lower in IL-1R1−/− mice compared with WT (Fig. 6A). Consistent with the RNA analyses, evaluation of CXCL12 protein expression within the brain microvasculature via quantitative confocal microscopy revealed a decrease in the intensity of staining in IL-1R1−/− mice compared with WT controls (p=0.0002) (Fig. 6B, C). CXCL12 binds two receptors, CXCR4 and CXCR7 (59, 60), which were both increased significantly in the IL-1R1−/− mice by day 8 post-infection (Fig. 6D). As endothelial cell expression of CXCR7 functions to regulate CXCL12-mediated retention of CXCR4+ infiltrates (6), these results suggest that IL-1 signaling regulates CXCR7-mediated internalization of CXCL12, which would impact the perivascular localization of CXCR4+ leukocytes (3, 4, 6). Taken together, these data show that loss of IL-1 signaling within the WNV-infected CNS leads to loss of lymphocyte localizing cues at the vasculature at day 8 post-infection, increased levels of chemoattractants within the parenchyma and significantly increased parenchymal entry of T lymphocytes.
Figure 6. IL-1 signaling is critical for regulating CXCL12 expression in the CNS during WNV infection.
Assessment of homeostatic chemokine expression in the CNS. Following cardiac perfusion, brain tissue was harvested and analyzed from WNV-infected WT (closed squares) and IL-1R1−/− mice (open squares) at indicated time points for CXCL12α/β (A), CXCR4, and CXCR7 (E) mRNA via qRT-PCR, normalized to GAPDH, and are presented as the mean fold change in mRNA levels over uninfected controls. Statistical significance of increased or decreased chemokine expression in WNV-infected IL-1R1−/− mice was determined in comparison with infected WT mice. Data are averages of results for at least 4 mice and reflect at least two independent experiments. (B) Confocal analysis of CXCL12β (red) and CD31 (green) expression from brainstem region of WNV-infected WT (left) and IL-1R1−/− mice (right) collected on day 8 p.i. (C) Quantitative analysis of CXCL12β expression in both WT and IL-1R1−/− mice brain tissue. Representative images are shown from 3 experiments in which 8-10 images were analyzed from 4-5 mice per group. *p<0.05, **p<0.01, ***p<0.001
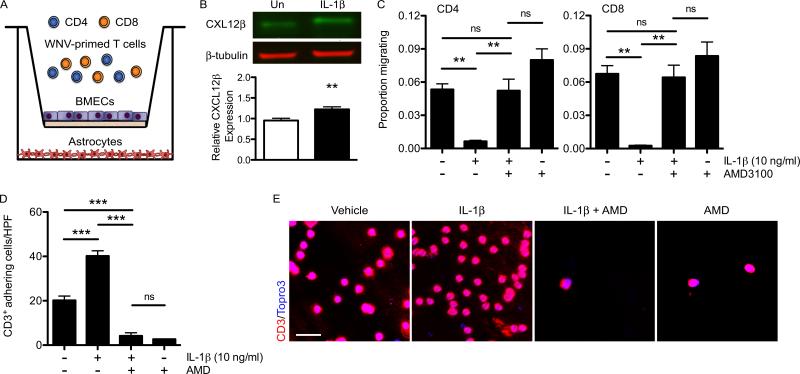
IL-1 signaling mediates leukocyte adhesion at BMECs
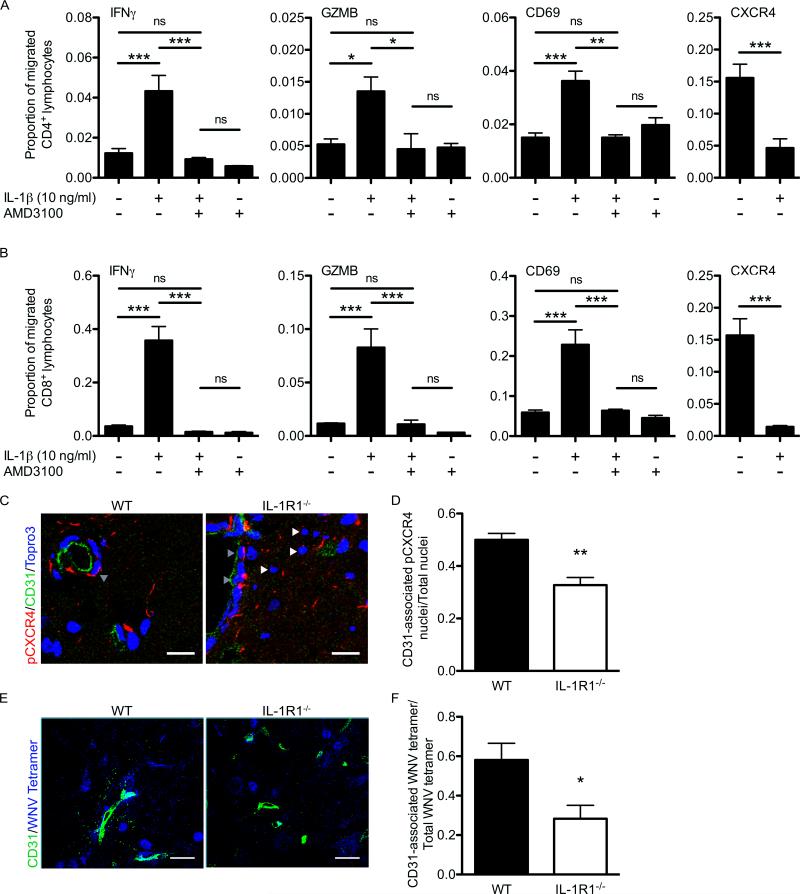
To determine whether IL-1β signaling mediates the localization of leukocytes to the perivascular space via CXCR4/CXCL12 interactions, we examined lymphocyte migration utilizing a well-established in vitro BBB model in which primary brain microvasculature endothelial cells (BMECs) and astrocytes derived from WT mice were seeded onto the top of a filter insert or on the bottom of a transwell plate, respectively, and co-cultured to generate high transendothelial resistances establishing similar barrier properties to those observed in vivo (61) (Fig. 7A). BMECs isolated from WT animals that had been treated with IL-1β (10 ng/ml) resulted in increased CXCL12β expression levels (p=0.0059) (Fig. 7B), and were subsequently used in the transwell co-culture (Fig. 7A). In order to simulate the lymphocyte reactivation that occurs at CNS endothelial barriers (26), WNV-primed CD4+ and CD8+ lymphocytes isolated from the spleens of WNV-infected mice were restimulated ex vivo with WNV-specific immunodominant peptides and then were added together to the top chamber of the in vitro BBB (Fig. 7A). Following IL-1β treatment (10 ng/ml) of the in vitro BBB, migration of both WNV-primed CD4+ and CD8+ lymphocytes into the bottom chamber significantly decreased compared to non-treated co-cultures (Fig. 7C). Lymphocyte migration in the IL-1β-treated co-cultures was restored to baseline levels when lymphocytes were pre-treated with the CXCR4 antagonist AMD3100 (5 μg/ml), before addition to the in vitro BBB, compared to non-treated lymphocytes, suggesting that the IL-1β-induced CXCL12 effects were abrogated. Pre-treatment of WNV-primed lymphocytes with AMD3100 in the absence of IL-1β treatment of the in vitro BBB also demonstrated similar migration of both CD4+ and CD8+ T cells compared to baseline migration levels. To determine whether IL-1β reduces lymphocyte migration via CXCL12-mediated retention at the brain microvasculature, we quantified the CD3+ cells adhering to the underside of the endothelial cell insert in untreated or IL-1β-treated co-cultures, with either untreated or AMD3100 pre-treated lymphocytes. In agreement with our previous results, IL-1β treatment of the co-culture led to a 2-fold increase in lymphocyte retention while AMD3100 pre-treated lymphocytes failed to adhere along the endothelial cell insert (Figure 7D, E). Lymphocyte adherence following pre-treatment with AMD3100 alone did not result in significant differences compared with AMD3100 pre-treated lymphocytes added to IL-1β-treated co-culture. These results confirm that IL-1β mediates the ability of CXCR4 expressing lymphocytes to adhere to BMECs via CXCL12 expression on their basolateral surface.
Figure 7. IL-1 signaling mediates lymphocyte adhesion at BMECs via CXCR4-CXCL12 interaction.
BMECs isolated from WT animals were used to generate an in vitro BBB model to assess WNV-primed T lymphocyte migration. (A) Schematic deptiction of in vitro BBB. Spleens were harvested from WNV-infected WT mice on day 6 p.i. Isolated leukocytes were restimulated ex vivo with I-Ab–restricted NS32066, NS31616 and Db-restricted NS4B peptides for 4 hrs. CD4+ and CD8+ T lymphocytes were positively selected and 5×105 cells (~60% CD4+ and ~40% CD8+ T cells) were added to coculture transwell system. The apical side of the transwell filter was seeded with 105 primary WT BMECs and 105 WT primary astrocytes were seeded in the bottom of 12-well plates. (B) CXCL12β protein expression analyzed via western blot in untreated (Un) or in IL-1β (10ng/ml) treated brain microvascular endothelial cell (BMEC) lysates. Data from 2 experiments with triplicates are presented as the relative expression after β-tubulin normalization. (C) Quantification of CD4+ or CD8+ lymphocytes collected in bottom chamber 6 hrs following addition to top chamber and analyzed by flow cytometry for respective surface markers. IL-1β (10 ng/ml) was added to both chambers of in vitro BBB culture 12 hrs before lymphocte addition and AMD3100 (5 μg/ml) was added to isolated CD4+ and CD8+ lymphocytes. The data are quantified as the number of CD4+ or CD8+ lymphocytes present in the bottom chamber after 6h divided by the total number of that cell type added to the top chamber (proportion migrating). (D) Quantitation of the number of CD3+ lymphocytes adhering to the basal side of the endothelial cell inserts. (E) CD3 adhesion was determined by visualing filter membranes labeled with CD3 (red) and nuclei (blue) and imaged by confocal microscopy. Data from 4 independent experiments in which 5 images were analyzed in each of three replicates per treatment group and are presented as mean values ± S.E.M. *p<0.05, **p<0.01, ***p<0.001, ns, not significant
IL-1 signaling promotes the entry of appropriately reactivated antiviral T lymphocytes in the CNS parenchyma of WNV infected mice
The parenchymal entry of WNV-specific CD8+ T cells is important for clearing WNV infection from the CNS (4, 19). Therefore, we hypothesized that fully activated WNV-primed T lymphocytes are able to overcome CXCL12-mediated endothelial cell retention via down-regulation of CXCR4 in the context of reactivation during perivascular localization. To test this, we measured intracellular IFN-γ and granzyme B (GZMB) expression, and surface CD69 and CXCR4 expression in lymphocytes that migrated to the bottom chamber of the in vitro BBB. We observed that IL-1β treatment of the co-culture resulted in a dramatic increase in the proportion of migrated CD4+ and CD8+ lymphocytes expressing IFN-γ, granzyme B, and CD69 compared to untreated or to AMD3100 pre-treated lymphocytes with or without IL-1β treatment (Fig. 8A, B). Importantly, IL-1β treatment also led to the decreased migration of CD4+ and CD8+ lymphocytes expressing CXCR4, supporting its role in retention of CXCR4+ lymphocytes (Fig. 8A, B). Detection of ligand-induced, phosphorylated CXCR4 (pCXCR4) within the WNV-infected brain demonstrated that pCXCR4 was present in cells within perivascular spaces (Fig. 8C, grey arrows) and that in the absence of IL-1 signaling there was an increase in cells negative for the phosphorylated form of CXCR4 (Fig. 8C, white arrows). In addition, the ratio of CD31-associated pCXCR4 cells to total cells evaluated was significantly lower in the IL-1R1-deficient mice (Fig. 8D). Since CXCR4 levels increased significantly in the absence of IL-1 signaling (see Fig. 7E), these data suggest that CXCR4-expressing lymphocytes are not limited to the perivascular space but instead are migrating into the parenchyma in the absence of IL-1 signaling. In situ tetramer staining with the WNV-specific Db-NS4B-tetramer (62) on fresh tissues demonstrated an increase in CD31-associated tetramer-binding CD8+ T lymphocytes within WNV-infected WT CNS compared with the WNV-infected IL-1R1−/− CNS (Fig. 8E, F). Taken together, these data support the notion that IL-1-mediated CXCL12 expression at the CNS microvasculature regulates the efficient parenchymal entry of anti-viral lymphocytes to control WNV infection.
Figure 8. IL-1 signaling is required for effectual T lymphocyte entry in the CNS of West Nile virus infected mice.
T lymphocytes that migrated through the coculutre transwell system or the in vitro BBB were analyzed for activation markers via flow cytometry. (A, B) Quantitation of the number of IFN-γ, GZMB, CD69, and CXCR4 expressing CD4+ (A) or CD8+ (B) lymphocytes collected from the bottom chamber of untreated and IL-1β, AMD3100, or IL-1β and AMD3100 treated transwell coculture systems and divided by the total number of CD4+ or CD8+ lymphocytes present in the bottom chamber and expressed as proportion migrated. Data are shown as mean ± S.E.M. for 2 independent experiments in each of three replicates per treatment group. (C) Confocal analysis of pCXCR4 (red) and CD31 (green) expression from brainstem region of WNV-infected WT (left) and IL-1R1−/− mice (right) collected on day 8 p.i. Representative images are shown from 2-3 experiments in which 8-10 images were analyzed from 4-5 mice per group. Bars, 25 μm. (D) Quantitative analyses of CD31-associated nuclei versus total nuclei within the brains of WNV-infected mice at day 8 p.i. (E) In situ tetramer staining for WNV-specific CD8+ T cells (blue) along with CD31 (green) was analyzed by confocal microscopy. Representative images are shown. Bars, 25 μm. (F) Quantitative analyses of CD31-associated WNV-tetramer labelled cells versus total WNV-tetramer within the brains of WNV-infected mice at day 8 p.i. Data are presented as a ratio in which 10-15 low-power confocal images were analyzed in each of the mice and presented as mean ± S.E.M. *p<0.05, **p<0.01, ***p<0.001
Discussion
Our observations support a model in which IL-1 signaling functions to control leukocyte interactions at the BBB to ensure their proper activation and entry into the virally infected CNS. In the absence of IL-1 signaling, leukocyte localization at endothelial barriers is impaired due to decreased microvasculature expression of CXCL12. Moreover, loss of CNS virologic control in IL-1R1−/− mice significantly upregulates parenchymal chemokine expression, enhancing the parenchymal entry of T lymphocytes with subsequent glial activation and immunopathology. In an in vitro BBB model, lymphocyte retention, specifically their ability to remain bound to BMECs, was augmented by treatment with IL-1β and reversed by CXCR4 antagonism. Although IL-1β treatment increased lymphocyte BBB retention, WNV-specific T lymphocytes expressing CD69, IFN-γ, and GZMB exhibited decreased levels of CXCR4, which limited their CXCL12-mediated localization and allowed them to migrate through the barrier. We conclude that IL-1 is fundamental for the control of WNV infection and immunity by promoting CXCL12-mediated leukocyte interactions at endothelial barriers that ensure the full activation and down-regulation of CXCR4 in effector T cells, which improves their ability to migrate from the perivascular spaces into the CNS parenchyma.
IL-1β mediates its effects through IL-1R1, which, in the un-inflamed brain, is predominantly found on vascular endothelial cells and in neurons in a few specific regions of the brain such as the amygdala, the hypothalamus, the trigeminal and hypoglossal motornuclei, and the area postrema (63, 64). Binding of IL-1β to endothelial IL-1R1 activates endothelial cells and triggers the up-regulation of cell adhesion molecules such as endothelial cell selectin (E-selectin) and intercellular adhesion molecule 1 (ICAM-1), pro-inflammatory cytokines such as IL-6 and TNF, and chemokines such as CXCL1, CXCL2, CXCL8, CX3CL1 and CXCL12 (6, 65-69). Both the presence of the IL-1R1 and IL-1 production have been shown to be critical for mediating CD4+ T cell recruitment into CNS tissues in EAE, the mouse model of multiple sclerosis (MS) (70) and in both acute and chronic-active MS lesions (71, 72). IL-1R1 signaling has also been shown to be instrumental in CD3+ lymphocyte recruitment into the CNS during infectious diseases such as HIV encephalitis (37), and cerebral listeriosis (73). Generally speaking, these studies present two distinct mechanisms in which IL-1R1 signaling mediates leukocyte migration into the CNS, either through increased ICAM-1 expression and/or increased CXCL12 expression along the microvasculature. In our studies, we found no significant changes in cell adhesion molecules within CNS tissues of WNV-infected IL-1R1-deficient mice compared with their WT counterparts (data not shown). However, CXCL12 expression along the CNS vasculature drops by day 5 post-infection during WNV encephalitis when lymphocytes begin to enter the CNS (see Fig. 5). This temporal decrease in CXCL12 expression permits leukocyte transmigration into the parenchyma and results in improved viral clearance, decreased immunopathology, and enhanced survival during WNV infection (4). By day 8 post-infection, CXCL12 levels plateau, preventing further migration of lymphocytes from the perivascular space and ensuring their full activation. In the absence of IL-1 signaling, however, CXCL12 levels continue to drop disrupting CNS immune privilege and increase by-stander lymphocyte entry resulting in increased immunopathology and decreased survival during WNV infection. Our results confirm that IL-1 signaling maintains CXCL12 expression at the microvasculature at critical time-points in 8-week old mice, fine-tuning the regulation of T cell infiltration during WNV infection in the CNS. They also suggest that lack of down-regulation of vascular CXCL12 may partly underlie limitations in T cell-mediated control of WNV infection within the CNS of 5-week-old animals. Further studies are needed to examine the developmental regulation of molecular mechanisms of immune privilege at the BBB.
It is well established that chemokines and their receptors modulate the recruitment of leukocytes into infected tissue. The contribution of IL-1 signaling to protective immunity against viral infections has largely been attributed to its ability to drive chemokine-signaling pathways that recruit fully activated antigen-specific immune cells to sites of viral replication (34, 35). For instance, the inflammatory chemokines CXCL10 and CCL2-5 are strongly induced within the parenchyma of the brain following WNV infection, due to virally infected neurons, which leads to enhanced trafficking of WNV-specific T cells from the perivascular space into the parenchyma that clear virus (4, 18, 51, 52, 74-76). We previously showed that WNV-specific T cells fail to be fully activated in the absence of IL-1R1 signaling (26) and therefore their ability to migrate from the perivascular space should be impaired despite the significant increase of inflammatory chemokines CXCL10, CCL5 and CCL2 over their WT counterparts. However, our results demonstrated that inflammatory chemokine expression positively correlated with increased leukocyte infiltration in the parenchyma and increased immunopathology suggesting that a crucial checkpoint is dysfunctional in the absence of IL-1 signaling. Our results suggest that IL-1 signaling establishes this checkpoint by localizing leukocytes at the BBB via CXCL12 during WNV encephalitis. In addition to CXCR4, CXCR7, which is expressed primarily on BMECS within the CNS, acts as a regulatory protein for CXCL12 (6). Endothelial CXCR7 serves as a scavenging receptor for CXCL12, sequestering the chemokine into lysosomal compartments, thereby negatively regulating its ability to localize CXCR4+ leukocytes within the perivascular space. Previous studies have demonstrated that IL-1β modulates CXCR7 expression and CXCL12 internalization during EAE (6). Our studies suggest that intact IL-1R1 signaling hinders CXCR7-mediated disruption of CXCL12-CXCR4 interactions at the microvasculature of the CNS promoting retention and subsequent full activation (3, 4, 6).
During WNV infection, we identified infiltrating myeloid cells, specifically CD11b+CD45hi monocytes, as the primary producers of IL-1β. Macrophages within the CNS increase over the course of WNV infection and are largely detected in perivascular regions (4). These IL-1β+ infiltrates may have an essential role in establishing selective barriers by orchestrating cell adhesion between infiltrating leukocytes and endothelial cells. Prior studies have shown that monocytes regulate the parenchymal penetration of effector T cells to the site of infection during viral encephalitis and rule out the mediation by matrix metalloproteinase (MMP) or the chemoattractants CXCL10 or CCL5, but do not address the role of IL-1 or CXCL12 (77). During EAE, myeloid cells within the perivascular space mediate cell-to-cell interactions via expression of ninjurin1, an adhesion molecule that, after cleavage, is similar in structure with chemokines such as CXCL12 (78, 79). Our data demonstrate that IL-1β+ infiltrating macrophages may be instrumental in facilitating leukocyte localization at endothelial barriers via CXCL12 expression. Indeed Yellow Fever virus (YFV), St. Louis encephalitis (SLEV) and WNV all induce IL-1β expression from myeloid cell populations in vitro (80, 81) suggesting IL-1β+ infiltrates may perform similar roles during encephalitis due to other flaviviruses. Taken together, infiltrating myeloid cells play a previously underappreciated role that is essential in establishing localizing cues, specifically CXCL12, within the perivascular space.
We found that IL-1β treatment of BMECs in our in vitro BBB model resulted in the reduced migration of lymphocytes beyond the barriers; nonetheless, increased migration of activated WNV-primed lymphocytes. The temporal and spatial arrest of leukocytes within the perivascular space may facilitate pivotal leukocyte encounters with antigen-presenting phagocytes or CD4+ T helper cells to guarantee full activation and effectual migration and positioning of CD8+ T cells within the brain (7). Indeed, CD4+ and CD8+ T cells activated by antigen-presenting cells (APCs) in the presence of IL-1β display enhanced cytokine production and migration into infected tissues (82). In support of this notion, we observed that IL-1β treatment enhanced the migration of CD8+IFN-γ+ and CD8+GZMB+cells, which are crucial for WNV control within the CNS (22-25). In the absence of IL-1R1 signaling, our results suggest that chemokine-mediated leukocyte entry, which may also include bystander non-specific or non-activated T cells (83), is destructive if not rigorously regulated through IL-1-mediated mechanisms. Moreover, increased CNS chemokine expression might also facilitate leukocyte retention within the CNS, which could lead to chronic inflammation. This is consistent with recent studies showing that IL-1R1 signaling is essential for recruitment of both proinflammatory and reparative monocytes in infarcted tissue (84) and that IL-1 signaling is associated with both neuroprotection and neuropathogenesis during TMEV-induced demyelinating disease (36). The effects of IL-1 within the CNS may therefore depend on the location and timing of its expression, especially during viral infections within the CNS. Indeed, our data demonstrate that IL-1, delivered to the CNS by infiltrating leukocytes, acts directly at the microvasculature to ultimately control lymphocyte migration and limit pathology.
In summary, our study provides evidence that IL-1 production at the CNS vasculature regulates leukocyte localization and interactions at this site during WNV encephalitis via CXCL12 expression. These vascular interactions ensure the full activation of lymphocytes, which trigger their migration beyond the vasculature into the parenchyma to effectively clear viral pathogen. Thus, IL-1-producing infiltrating cells may establish a protective barrier to enable selective transfer of trafficking leukocytes into the infected CNS parenchyma.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS052632 and P01NS059560 and National Institutes of Health/National Institute of Allergy and Infectious Diseases grant U19 AI083019 (all to R.S.K.)
References
- 1.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- 4.McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouffard JP, Riudavets MA, Holman R, Rushing EJ. Neuropathology of the brain and spinal cord in human West Nile virus infection. Clinical neuropathology. 2004;23:59–61. [PubMed] [Google Scholar]
- 6.Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208:327–339. doi: 10.1084/jem.20102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 8.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausmann J, Pagenstecher A, Baur K, Richter K, Rziha HJ, Staeheli P. CD8 T cells require gamma interferon to clear borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol. 2005;79:13509–13518. doi: 10.1128/JVI.79.21.13509-13518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez-Fernandez YV, Johnson AJ, Rodriguez M, Pease LR. Clearance of Theiler's virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur J Immunol. 2003;33:2501–2510. doi: 10.1002/eji.200324007. [DOI] [PubMed] [Google Scholar]
- 11.Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 13.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. Neurologic manifestations and outcome of West Nile virus infection. Jama. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 14.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Archives of neurology. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral immunology. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 17.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol. 2008;82:8956–8964. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrant DM, Robinette ML, Klein RS. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J Exp Med. 2013;210:503–516. doi: 10.1084/jem.20121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 28.Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J Virol. 2012;86:2416–2427. doi: 10.1128/JVI.06797-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis. 2007;196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 34.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim BS, Jin YH, Meng L, Hou W, Kang HS, Park HS, Koh CS. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J Neuroinflammation. 2012;9:217. doi: 10.1186/1742-2094-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCandless EE, Budde M, Lees JR, Dorsey D, Lyng E, Klein RS. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J Immunol. 2009;183:613–620. doi: 10.4049/jimmunol.0802258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M., Jr. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebel GD, Dupuis AP, 2nd, Ngo K, Nicholas D, Kauffman E, Jones SA, Young D, Maffei J, Shi PY, Bernard K, Kramer LD. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg Infect Dis. 2001;7:650–653. doi: 10.3201/eid0704.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engle MJ, Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77:12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BR, Silverman RH, Gale M, Jr., Diamond MS. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purtha WE, Myers N, Mitaksov V, Sitati E, Connolly J, Fremont DH, Hansen TH, Diamond MS. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007;37:1845–1854. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 44.Daniels BP, Cruz-Orengo L, Pasieka TJ, Couraud PO, Romero IA, Weksler B, Cooper JA, Doering TL, Klein RS. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J Neurosci Methods. 2013;212:173–179. doi: 10.1016/j.jneumeth.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eriksson C, Van Dam AM, Lucassen PJ, Bol JG, Winblad B, Schultzberg M. Immunohistochemical localization of interleukin-1beta, interleukin-1 receptor antagonist and interleukin-1beta converting enzyme/caspase-1 in the rat brain after peripheral administration of kainic acid. Neuroscience. 1999;93:915–930. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 46.Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20:489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- 47.Higgins GA, Olschowka JA. Induction of interleukin-1 beta mRNA in adult rat brain. Brain Res Mol Brain Res. 1991;9:143–148. doi: 10.1016/0169-328x(91)90139-o. [DOI] [PubMed] [Google Scholar]
- 48.Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein RS, Diamond MS. Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol Med. 2008;14:286–294. doi: 10.1016/j.molmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Hosking MP, Lane TE. The role of chemokines during viral infection of the CNS. PLoS Pathog. 2010;6:e1000937. doi: 10.1371/journal.ppat.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang B, Chan YK, Lu B, Diamond MS, Klein RS. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol. 2008;180:2641–2649. doi: 10.4049/jimmunol.180.4.2641. [DOI] [PubMed] [Google Scholar]
- 52.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr., Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J Virol. 2010;84:12125–12138. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 55.King NJ, Getts DR, Getts MT, Rana S, Shrestha B, Kesson AM. Immunopathology of flavivirus infections. Immunol Cell Biol. 2007;85:33–42. doi: 10.1038/sj.icb.7100012. [DOI] [PubMed] [Google Scholar]
- 56.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 57.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 61.Lundquist S, Renftel M. The use of in vitro cell culture models for mechanistic studies and as permeability screens for the blood-brain barrier in the pharmaceutical industry--background and current status in the drug discovery process. Vascul Pharmacol. 2002;38:355–364. doi: 10.1016/s1537-1891(02)00203-3. [DOI] [PubMed] [Google Scholar]
- 62.Pinto AK, Daffis S, Brien JD, Gainey MD, Yokoyama WM, Sheehan KC, Murphy KM, Schreiber RD, Diamond MS. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 65.Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Dev Comp Immunol. 2004;28:415–428. doi: 10.1016/j.dci.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 67.Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM, Herkenham M. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience. 2002;112:731–749. doi: 10.1016/s0306-4522(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 68.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 69.Ching S, He L, Lai W, Quan N. IL-1 type I receptor plays a key role in mediating the recruitment of leukocytes into the central nervous system. Brain Behav Immun. 2005;19:127–137. doi: 10.1016/j.bbi.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Li Q, Powell N, Zhang H, Belevych N, Ching S, Chen Q, Sheridan J, Whitacre C, Quan N. Endothelial IL-1R1 is a critical mediator of EAE pathogenesis. Brain Behav Immun. 2011;25:160–167. doi: 10.1016/j.bbi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brosnan CF, Cannella B, Battistini L, Raine CS. Cytokine localization in multiple sclerosis lesions: correlation with adhesion molecule expression and reactive nitrogen species. Neurology. 1995;45:S16–21. doi: 10.1212/wnl.45.6_suppl_6.s16. [DOI] [PubMed] [Google Scholar]
- 72.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 73.Deckert M, Virna S, Sakowicz-Burkiewicz M, Lutjen S, Soltek S, Bluethmann H, Schluter D. Interleukin-1 receptor type 1 is essential for control of cerebral but not systemic listeriosis. Am J Pathol. 2007;170:990–1002. doi: 10.2353/ajpath.2007.060507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 2010;201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim JK, Murphy PM. Chemokine control of West Nile virus infection. Experimental cell research. 2011;317:569–574. doi: 10.1016/j.yexcr.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savarin C, Stohlman SA, Atkinson R, Ransohoff RM, Bergmann CC. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. J Virol. 2010;84:4878–4888. doi: 10.1128/JVI.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahn BJ, Lee HJ, Shin MW, Choi JH, Jeong JW, Kim KW. Ninjurin1 is expressed in myeloid cells and mediates endothelium adhesion in the brains of EAE rats. Biochem Biophys Res Commun. 2009;387:321–325. doi: 10.1016/j.bbrc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 79.Ahn BJ, Le H, Shin MW, Bae SJ, Lee EJ, Wee HJ, Cha JH, Park JH, Lee HS, Lee HJ, Jung H, Park ZY, Park SH, Han BW, Seo JH, Lo EH, Kim KW. The N-terminal ectodomain of Ninjurin1 liberated by MMP9 has chemotactic activity. Biochem Biophys Res Commun. 2012;428:438–444. doi: 10.1016/j.bbrc.2012.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang DM, Shaio MF. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus. J Infect Dis. 1994;170:811–817. doi: 10.1093/infdis/170.4.811. [DOI] [PubMed] [Google Scholar]
- 81.Barros VE, Ferreira BR, Livonesi M, Figueiredo LT. Cytokine and nitric oxide production by mouse macrophages infected with Brazilian flaviviruses. Rev Inst Med Trop Sao Paulo. 2009;51:141–147. doi: 10.1590/s0036-46652009000300004. [DOI] [PubMed] [Google Scholar]
- 82.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, Paul WE. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phares TW, Stohlman SA, Hinton DR, Bergmann CC. Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J Neuroinflammation. 2012;9:269. doi: 10.1186/1742-2094-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.