ABSTRACT
Objective
The aim of this study was to elucidate clinical trial efficacy, safety, and dosing practices of abobotulinumtoxinA (ABO) treatment in adult patients with upper limb spasticity (ULS).
Methods
A systematic literature review was performed to identify randomized controlled trials and other comparative clinical studies of ABO in the treatment of adult ULS published in English between January 1991 and January 2013. Medical literature databases (PubMed, Cochrane Library, and EMBASE) were searched, and a total of 295 records were identified. Of these, 12 primary publications that evaluated ABO for the management of ULS were included in the final data report.
Synthesis
Total ABO doses ranged between 500 and 1500 U for ULS. Most of the studies in ULS showed statistically significant benefits (reduction in muscle tone based on Ashworth score) of ABO vs. placebo. Statistical significance was reached for most evaluations of spasticity using the Modified Ashworth Scale. Statistically significant effects on active movement and pain were demonstrated, albeit less consistently. ABO was generally well tolerated across the individual studies; most adverse events reported were considered unrelated to treatment. Adverse events considered associated with ABO treatment included fatigue, tiredness, arm pain, skin rashes, flu-like symptoms, worsening of spasm, and weakness.
Conclusions
On the basis of data extracted from 12 randomized clinical studies, a strong evidence base (9/12 studies) exists for the use of ABO to reduce ULS caused by stroke.
Key Words: AbobotulinumtoxinA, Botulinum Toxin, Upper Limb, Spasticity
The use of botulinum toxin type A (BoNT-A) as a safe and effective focal intervention for reduction of spasticity is supported by a robust body of evidence. Guidelines recommend that BoNT-A should be offered as a treatment option in adult upper and lower limb spasticity as standard clinical practice1–4 and that the exact pattern of spasticity should be considered when selecting specific muscles for injection.1 In upper limb spasticity (ULS), BoNT injections can be made into a variety of muscles to reduce adductor tone in the shoulder and/or reduce flexor tone at the elbow, the wrist, and the fingers. Injections can be made with the aim of increasing range of motion (passive and/or active), reducing pain, and/or achieving other functional goals (hygiene/ease of dressing).
Although there have been a number of reviews of the efficacy of BoNT-A in the management of ULS,4,5 none of these have provided detailed information about the specific products available. This is important because the dosing schemes of each product are not interchangeable. Education on the specifics of each product is a key unmet need in the medical community because the lack of direct product comparability leads to confusion and therefore, potentially, suboptimal treatment. AbobotulinumtoxinA (ABO) has been used to treat ULS in many countries outside the United States for many years.6 The necessary clinical trials required for registration by the Food and Drug Administration for this common indication are now underway.
The aims of this systematic review were therefore to elucidate clinical trial efficacy, safety, and dosing practices of ABO treatment in adult patients with ULS. Such information informs the design of new clinical trial programs and provides an evidence-based resource for clinical practice.
METHODS
The systematic literature review presented here is one part of a larger systematic review of all potential indications for ABO, the results of which will be presented separately per each relevant indication. The literature search strategy and methods for this systematic review were specified in advance and documented in a protocol. Components of the protocol include the literature search strategy, screening criteria, data extraction methods, and risk for bias appraisal used to assess studies selected for inclusion.
Screening Criteria
Specific study characteristics of interest were defined in the protocol. They include study type—randomized controlled trials (RCTs) and other comparative clinical studies; patient population—adult patients with ULS; treatment—ABO; and outcomes—primary and secondary efficacy, safety, and dosing.
Literature Search Strategy and Data Sources
The literature search strategy was developed using a combination of Medical Subject Headings terms and key words. Key words of relevance to the review of ULS were AbobotulinumtoxinA (alternative spellings included Abobotulinumtoxin A and Abobotulinum Toxin A), Dysport, spasticity, and clinical trial. Language (English only) and date limits (January 1991 to January 2013) were also applied. The search was performed in three foundational and comprehensive electronic medical literature databases (PubMed, Cochrane Library, and EMBASE). Bibliographic reference lists of systematic reviews identified during screening were searched to identify any relevant studies that were not identified through the electronic database searches.
Study Selection
At level 1 screening, all publications reporting preclinical, phase 1, prognostic/biomarker, genetic retrospective, registry, case report, and/or noncomparative studies were excluded, as were letters, consensus reports, editorials, and nonsystematic reviews. Although systematic reviews and meta-analyses were not included in their own right, they were used for identification of additional primary studies. At level 2 screening, all publications that reported only biochemical or immunologic endpoints were excluded. Also at this stage, nonrandomized controlled phase 2 or 3 clinical trials, comparative long-term follow-up studies (e.g., open-label follow-up of randomized controlled clinical trials), and comparative prospective phase 4 postmarketing trials were excluded, provided that adequate information from randomized phase 2 and phase 3 trials had been identified. Publications reporting secondary and post hoc analyses from a previously published article were not included. The systematic literature review process of study selection was depicted in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.4
Data Extraction
Study methodology, patient, and treatment-level data were extracted from the full text publications under predefined headings. Each included study underwent quality assessment of risk for bias based on Cochrane metrics. The quality assessment for RCTs systematically addresses six types of bias: selection, performance, detection, attrition, reporting, and other sources of bias not covered by other domains. If non-RCTs or other study types were deemed relevant for data extraction, quality assessment was performed using Transparent Reporting of Evaluations with Nonrandomized Designs appraisal criteria for non-RCTs.7
Role of the Funding Source
The study was partially funded by Ipsen for data collection and editorial support. Dr Khashayar Dashtipour developed the protocol, and data collection was coordinated and designated by RTI Health Solutions. Aside from procuring the data collection and editorial support, Ipsen did not contribute to the study conduct or reporting of results. All authors had full access to all data, contributed to manuscript revisions, and had final approval for submission. Dr Khashayar Dashtipour wrote the initial draft and had final responsibility for the decision to submit the paper for publication.
RESULTS
Publications Identified
A total of 295 records were identified from the medical literature databases. Of these, 12 primary publications that evaluated ABO for the management of ULS in adult patients were included in the final data report.8–19 Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram for the full systematic review of all RCTs of ABO. Most of the studies fulfilled criteria for low-risk reporting bias. The studies used a wide range of outcome measures including measures of spasticity (usually assessed with the Modified Ashworth Scale [MAS]), range of movement (passive and active), global clinical impression, activities of daily living (ADLs), goal attainment, and caregiver burden.
FIGURE 1.
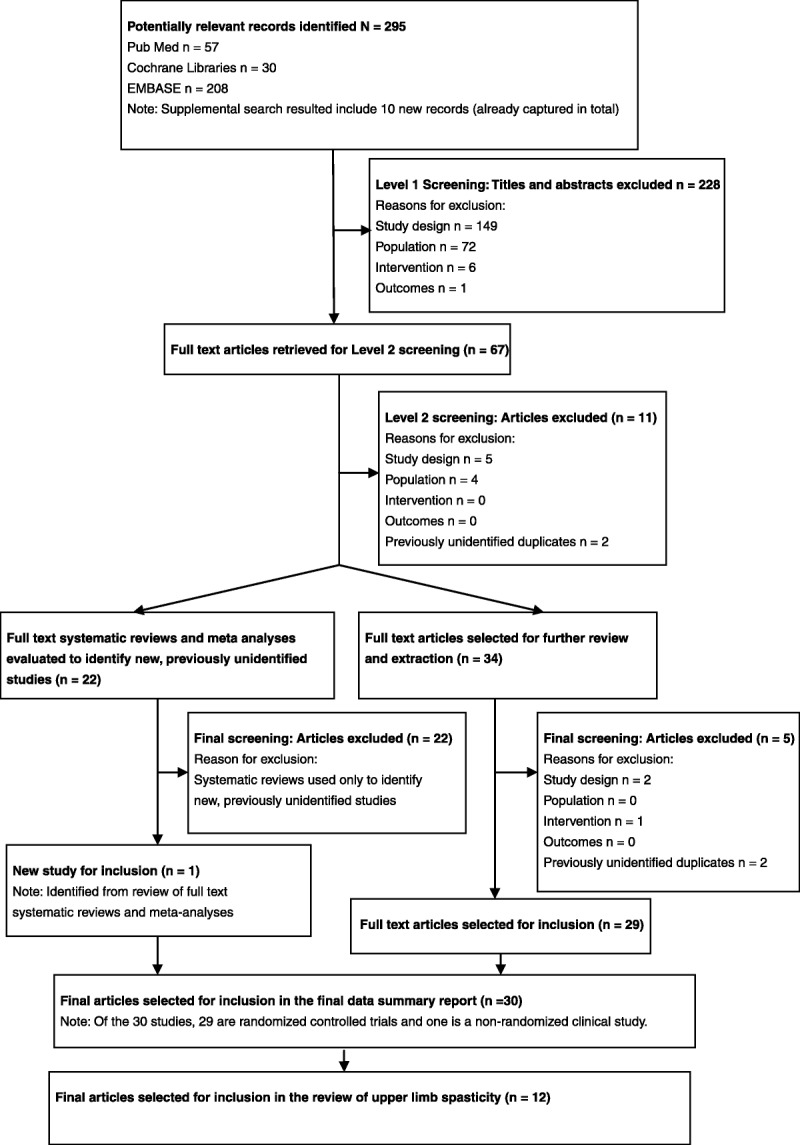
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram reporting the results of the systematic literature search.
Efficacy in ULS
Most of the studies (9/12) in ULS showed statistically significant benefits of ABO in decreasing muscle tone, measured by the MAS, vs. placebo (PBO). Statistical significance was reached for most of the evaluations of spasticity using the MAS. Statistically significant effects on active movement and pain were harder to achieve (studies showed positive trends). Studies that evaluated the effect on caregiver burden showed significant benefits in favor of treatment. Table 1 provides an overview of efficacy and safety outcomes from each of the studies (http://links.lww.com/PHM/A96).
Bakheit and colleagues9 (2000) investigated effective and safe dose ranges in the management of spasticity in individuals with ULS secondary to stroke. Eighty-two subjects were recruited and randomized to receive injections with PBO, or three doses of ABO: 500, 1000, or 1500 U. The subjects received the following distribution of injections: biceps brachii (BB), 200, 400, or 600 U (500, 1000, and 1500 U groups, respectively), and the flexor digitorum profundus (FDP), the flexor digitorum superficialis (FDS), the flexor carpi ulnaris (FCU), and the flexor carpi radialis (FCR) each received 75, 150, or 225 U (500, 1000, and 1500 U groups, respectively). All injections were performed using anatomic landmarks for guidance. The investigators noted a reduction in spasticity as measured on the MAS for all three doses of Dysport at week 4. In addition, they noted an increase in range of motion at the elbow, the wrist, and the fingers for all study groups, but no significant difference was noted between treatment groups. Secondary outcomes included pain measured on a visual analog scale (VAS) as well as functional status as measured by the Barthel Index (BI) and the Rivermead Motor Assessment (arm section) scale; these outcomes did not show significant differences. It was noted in the group receiving 1500 U of ABO that 15.8% of the patients reported loss of the ability to voluntarily extend their fingers. The investigators concluded that treatment with ABO at doses of 500, 1000, and 1500 U is effective and safe; however, for those individuals with residual voluntary movement in the affected limb, the optimal dose of ABO is 1000 U to achieve adequate spasticity control without negatively impacting voluntary movement.
Bakheit and colleagues8 (2001) further evaluated the efficacy and safety of ABO in 59 individuals with ULS secondary to stroke. Thirty-two patients were randomized to the PBO group and 27 patients were randomized to the ABO group. The ABO group received 1000 U of ABO in 2 ml of normal saline injected into the BB (300–400 U) and the FDS (150–250 U) as well as 150 U each into the FDP, the FCU, and the FCR using anatomic landmarks for guidance. The PBO group received injections of PBO reconstituted in normal saline into the same muscle groups. These investigators found that injection of ABO resulted in a statistically significant reduction in spasticity based upon the MAS score at week 4 when compared with PBO. Other outcome measures, including active range of motion at the elbow, the wrist, and the fingers; pain; BI; as well as goal attainment, did not show significance between group differences; however, both the patients’ and the investigators’ global assessment of benefit demonstrated “some or much improved” for the patients receiving ABO. The authors concluded that, although secondary outcomes including pain and functional status as measured by the BI did not show improvement, the subjects receiving ABO likely demonstrated a clinically significant benefit from the reduction in spasticity given the significant improvement in the global assessment of benefit.
Bhakta and colleagues10 evaluated the effect of ABO on ULS as well as the impact on disability and caregiver burden in individuals who had experienced a stroke. Twenty subjects received 1000 U of ABO injected into upper limb muscles, and 20 subjects received PBO. The injections were performed using anatomic landmarks, and the muscle selection and doses injected into individual muscles were at the discretion of the single injector for the study. Spasticity was measured using the MAS, and muscle power, joint movement, and pain were also assessed. Level of disability (eight items) and caregiver burden (four items) were measured using predefined activities, such as cleaning the palm, cutting fingernails, and dressing. All outcome measures were collected at two baseline time points (1 wk before injection and on the day of injection) as well as at weeks 2, 6, and 12. The individuals who received ABO injections demonstrated improvement in disability, with less disability reported at week 6 compared with the PBO group, and a 22% improvement in disability score at week 6, compared with a 4.7% improvement in the PBO group. In addition, the ABO group was noted to have a reduction in caregiver burden at week 2 and continued through week 12. Evaluation of spasticity noted a significant improvement in finger flexor spasticity from week 2 through week 12. Reduction in the MAS score was noted in the elbow flexors in the ABO group at week 2, but this was not maintained at weeks 6 and 12. No difference was noted between the ABO and the PBO group in regard to shoulder adductor spasticity. There were no significant differences noted between the groups in terms of active or passive range of motion at the shoulder or the elbow, pain, or muscle strength. These authors concluded that the use of ABO might be beneficial in stroke patients who have difficulty with self-care as a result of ULS and may also reduce caregiver burden.
Bhakta and colleagues11 later reported the effect of ABO injections on associated reactions (ARs) in the study population above. ARs were defined as involuntary flexion movements in the paretic arm caused by various activities, including coughing, sneezing, and yawning. ARs were measured in the paretic forearm musculature using surface electromyography (EMG), whereas ARs performed on the unaffected arm were elicited by maximum voluntary grip. Patient-reported outcomes of effect of ABO on ARs were also recorded. These investigators reported a significantly greater reduction in ARs at week 2 in the ABO group compared with PBO. The effect of ARs on ADLs was also evaluated. At baseline, 33 subjects reported daily ARs, with 24 noting that ARs interfered with ADLs. Ten of 12 subjects in the ABO group reported a reduction in the interference of ARs with ADLs, compared with 2 of 12 in the PBO group. These investigators concluded that ARs can interfere with ADLs and that ABO may reduce these involuntary movements.
Hesse and colleagues12 investigated the effect of ABO with or without electrical stimulation (ES) treatment after injection on ULS in 24 subjects with a history of stroke. Six subjects were randomized into each treatment group: 1000 U of ABO followed by ES treatment (30 mins, 3 days per week 3 days after injection), 1000 U of ABO without ES, PBO injections followed by ES, and PBO injections without ES. Injections were performed into the following muscles using EMG guidance: BB, 250 U; brachialis, 250 U; FCR, 125 U; FCU, 125 U; FDP, 125 U; and FDS, 125 U. The study results indicated that there were no significant differences in MAS scores across the groups; however, the group receiving ABO + ES treatment demonstrated the greatest reduction in MAS scores (P = 0.011). The results of other measures, including limb position at rest and ability to perform three identified ADLs, were variable. There was a significant improvement in the ability to clean the palm of the affected hand as rated by the subject or the caregiver in the ABO + ES treatment group compared with the ABO without ES and PBO groups. The ABO + ES group also trended toward better limb position at rest, specifically at the wrist/fingers (P = 0.068/P = 0.059). Contrary to other studies of ABO in ULS, this study did not demonstrate a significant reduction in spasticity, which the authors attributed to selection of subjects with severe spasticity (mean MAS scores, 3), as well as the fact that the subjects did not participate in postinjection rehabilitation. The authors did suggest that ES of injected muscles might enhance the effectiveness of ABO in the treatment of ULS.
Kong and colleagues13 investigated the effects of ABO on shoulder pain in 17 subjects with spastic hemiplegia caused by stroke. Eight subjects were randomized into the treatment group and received 250 U of ABO into the pectoralis major and 250 U into the BB using anatomic landmarks to guide the injections, and nine subjects received PBO injections into the same sites. The investigators noted that both the ABO and PBO groups experienced a 2- to 3-point reduction in shoulder pain on the VAS, with no statistically significant difference between the groups. In terms of spasticity, the ABO group was noted to have a statistically significant reduction in tone of 1 grade on the Ashworth Scale at the shoulder adductors and the elbow flexors at week 4. Passive shoulder abduction was also measured at all time points and did not demonstrate any significant differences between the ABO and PBO groups. These investigators concluded that, although ABO effectively reduced spasticity in the shoulder adductors and the elbow flexors, shoulder pain might not have been improved because of low study enrollments and other potential etiologies for shoulder pain other than spasticity.
The efficacy of ABO on management of shoulder pain in spastic hemiplegia after stroke was also investigated by Marco and colleagues.14 Fourteen subjects were randomized to receive 400 U of ABO into four sites in the pectoralis major muscle using EMG guidance, and 15 subjects received PBO into the same locations. After the injections, both groups received 6 wks of conventional transcutaneous electrical nerve stimulation therapy. Outcome measures were obtained at baseline, 7 days, 1 mo, 3 mos, and 6 mos. Pain scores were measured using the 100-mm VAS, and on completion of the study, the subjects in the ABO group demonstrated a statistically significant reduction in pain, with a mean reduction of 46.2 mm, whereas the PBO group noted a 21.9-mm reduction on the VAS. Secondary outcome measures including range of motion with shoulder flexion and abduction, spasticity according to the MAS, as well as motor potential latency of the pectoral nerve did not demonstrate statistically significant differences between the groups; however, range of motion with external rotation was significantly improved, and pectoral nerve motor amplitudes were significantly reduced at the 1-mo point in the ABO group. The authors concluded that ABO is effective in treating shoulder pain and improving external rotation range of motion in patients with spastic hemiplegia secondary to stroke.
McCrory and colleagues15 evaluated the effect of ABO injections on quality-of-life in individuals with ULS secondary to cerebrovascular accident in a multicenter RCT. Fifty-four subjects were randomized into the ABO group and were given doses of 750–1000 U of ABO at the initial treatment and then 500–1000 U 12 wks later; 42 subjects were randomized to the PBO group. The subjects received injections of ABO or PBO into the “principal spastic muscles of the distal upper limb”; muscle selection, number of injection sites per muscle, and use of injection guidance with EMG or ES were at the individual clinician’s discretion. At the 12-wk point, the subjects received reinjection with the same agent (ABO or PBO), with ABO doses ranging from 500 to 1000 U. The primary outcome for this study was the quality-of-life and well-being of the subjects as measured on the Assessment of Quality of Life Scale; the change in Assessment of Quality of Life Scale scores from baseline to end of study follow-up showed no significant difference between the ABO and PBO groups. Other patient-centered outcomes including pain and mood also did not show a difference between the groups. Muscle spasticity as measured on the MAS was noted to be significantly reduced in the ABO group at all time points. The achievement of individual goals selected from the Patient Disability Scale reflected a functional benefit in the ABO group at week 20; in addition, modest improvements were noted in the Caregiver Burden Scales and the Patient Disability Scale; however, these measures did not reach statistical significance. Global Assessment of Benefit as reported by both investigators and subjects or their guardians suggested that the ABO group had a higher proportion of subjects with overall benefit. Of note, in this study, the investigators reported a wide range in the number of postinjection therapy sessions attended by the subjects (0–91), with a mean of 10.8 sessions; however, 33% of the patients did not receive any therapy. These authors concluded that, although ABO injections did not result in change in quality-of-life, ABO is safe and effective at decreasing ULS.
Smith and colleagues16 conducted a double-blind, PBO-controlled, dose-ranging study to evaluate dose-response relationships in 21 subjects with stroke (n = 19) or head injury (n = 2). The subjects were randomized to receive injections with PBO and 500, 1000, or 1500 U of ABO, and four of the six PBO group subjects were rerandomized, allowing 25 randomizations from 21 subjects. Two-thirds of the total ABO or PBO dose was allocated to muscles above the elbow, with the remaining one-third distributed into the wrist flexors, the finger flexors, and the thumb adductors/flexors. Outcomes measured at baseline and 2, 6, and 12 wks included passive and active range of motion; MAS scores at the elbow, wrist, and metacarpophalangeal joints; patient disability as determined by the upper body dressing time and the Frenchay Arm Test; as well as the patient-reported Global Assessment Scale. Using combined dose data, these investigators reported a significant overall improvement in MAS scores at the wrist and the fingers in the subjects treated with ABO, as well as improved passive range of motion increased at the wrist at week 6 follow-up, and an increase in finger curl distance. No significant differences were noted in the dressing time, the Frenchay Arm Test, or postural alignment. Fifteen subjects reported improvement in global rating scale scores in the ABO group, compared with only two subjects in the PBO group. Data from individual doses revealed a significant increase in passive range of motion at the elbow in the 1500-U ABO group. Spasticity as measured on the MAS was significantly reduced at the wrist in the 500-U ABO group and trended toward a reduction in the 1500-U ABO group (P = 0.06). These investigators concluded that ABO injections increased passive range of motion and decreased ULS in individuals with spasticity caused by stroke or head injury, and increasing the dose tended to increase the magnitude of response at the wrist and the elbow.
Shaw and colleagues17 conducted a multicenter trial evaluating the clinical effectiveness and cost-effectiveness of ABO injections in treating ULS in 333 individuals with spasticity secondary to stroke. The subjects were randomized to receive PBO injections or ABO injections into upper limb muscles18 by trained clinicians at each site, with total ABO dosing of 1000 U or less at each injection visit. Both groups completed a 4-wk upper limb therapy program after injection. Outcome assessments were completed at baseline as well as at 1, 3, and 12 mos, and clinical reassessment was completed at 3, 6, and 9 mos to determine whether the subjects required repeated injections. The effect of ABO injections on upper limb function as determined by the Action Research Arm Test was the primary outcome measure for the study. Additional measures, including upper limb impairment and activity limitations, upper limb pain, as well as overall quality-of-life, were also obtained using various measures. These investigators reported that there was no significant difference between the ABO and PBO groups for improved arm function at 1 mo as determined by the Action Research Arm Test (ARAT); however, several other measures did show significance between group differences. The ABO group demonstrated a significant improvement in MAS score at the elbow at 1 mo when compared with the PBO group; however, no between-group differences were noted at 3 and 12 mos. The ABO group was also found to have an improvement in upper limb strength as determined by the Motricity Index at 3 mos; however, no differences were noted at 1 or 12 mos. Functional measures (including dressing a sleeve, cleaning the palm, and opening the hand to cut fingernails) were significantly improved in the ABO group (see Table 1, http://links.lww.com/PHM/A96, for details). In terms of pain, there were no significant between-group differences at 1 or 3 mos; however, the ABO group had a 2-point reduction in pain on the VAS at 12 mos compared with no change in the PBO group. Cost analysis indicated that the addition of ABO injections to upper limb therapy was not a cost-effective strategy. These authors concluded that the addition of ABO injections to a therapy program did not result in upper extremity functional improvements; however, muscle tone, limb strength, as well as specific functional activities and pain may improve.17
Suputtitada and Suwanwela19 conducted a study in patients with ULS caused by various neurologic etiologies (ischemic/hemorrhagic stroke, n = 48, and cerebral embolism, n = 2), with the goal of determining the lowest effective dose of Dysport in the treatment of ULS. Fifty subjects were enrolled and randomized to receive PBO or ABO in total doses of 350, 500, or 1000 U. Subjects were excluded if they had complete plegia of the upper extremity, which was defined as muscle strength of less than 2 of 5 in a target segment. Injections were performed using EMG guidance into the biceps (150, 200, or 400 U) as well as the FCU, the FCR, the FDS, and the FDP (50, 75, or 150 U). After injections, the subjects received therapy 3 days per week for the 6-mo study period. Spasticity was assessed using the MAS, and pain was assessed using the VAS at baseline as well as 2, 4, 8, 16, and 24 wks. Dexterity was evaluated using the Action Research Arm Test, and ADLs were assessed using the BI at baseline as well as 8 and 24 wks. These investigators found that all three doses of ABO significantly decreased the MAS score at week 8, with the largest change in MAS scores in the 500- and 1000-U groups. Each of the ABO groups demonstrated a statistically significant decrease in VAS scores at weeks 8 and 24 when compared with PBO, with the 500- and 1000-U groups showing a greater reduction than the 350-U group. Statistically significant improvement in performance of ADLs as measured by the BI was noted in the 350- and 500-U groups, with the 500-U group demonstrating the higher change compared with the 350-U group at weeks 8 and 24. Dexterity as measured by the Action Research Arm Test was statistically significantly increased at weeks 8 and 24 in the 500-U group, whereas the 1000-U group demonstrated a significant decrease at weeks 8 and 24. These authors concluded that 500 U is the optimal dose for treatment of ULS in individuals with residual voluntary movement.
Yelnik and colleagues20 investigated the efficacy of ABO in treating shoulder pain in subjects with spastic hemiplegia secondary to stroke. Ten subjects were randomized into the ABO group, with ten patients in the PBO group. Injections of 500 U of ABO or PBO were targeted into the subscapularis muscle on the affected side using ES guidance, followed by nonstandardized therapy on weekdays. These investigators noted that pain improved as early as 1 wk after injection, with a statistically significant reduction in the ABO group at week 4. Lateral (external) rotation and abduction of the arm were assessed in both groups. External rotation was noted to improve at weeks 2 and 4 in the ABO group; however, no statistically significant difference was noted between groups in terms of abduction. Spasticity was measured using MAS scores at all time points in the subscapularis muscle by passively moving the arm into lateral rotation and abduction. In addition, MAS scores were recorded for the arm muscles including the elbow flexors, the wrist flexors, and the finger flexors. Only the finger flexors showed a statistically significant reduction in spasticity at the 4-wk point in the ABO group; these researchers suggest that the finger flexor spasticity reduction was related to the reduction in shoulder pain and that “spasticity in [upper limb] muscles remote from the injection point is related to shoulder pain.” These researchers concluded that injection of ABO into the subscapularis muscle is helpful in managing shoulder pain; however, management of ULS likely requires ABO injections directly into all muscles affected.
Safety in ULS
ABO was generally well tolerated across the individual studies. Most adverse events reported were considered unrelated to treatment. Adverse events considered associated with ABO treatment included fatigue, tiredness, arm pain, skin rashes, flu-like symptoms, worsening of spasm, and weakness. Treatment-related serious adverse events were infrequent. One study reported a case of dysphagia, which was considered potentially related to treatment.
Dosing Across Indications
Total ABO doses ranged between 500 and 1500 U for ULS. The most commonly injected muscles were the BB. Other injected muscles included the FDS, the FDP, the FCU, the FCR, the flexor pollicis longus, the pronator teres, and the pectoralis major. Dose ranges for individual muscles are summarized overall in Table 2 (http://links.lww.com/PHM/A96) and by each individual study in Table 3 (http://links.lww.com/PHM/A96).
DISCUSSION
Efficacy of ABO
This is the first systematic review of well conducted trials of ABO in the management of ULS in adult patients. Other reviews2,3 have included all BoNT formulations (which have different dosing schemes) and have therefore not been able to provide detailed information on the dosing schemas used per muscle. This is essential information because dosing units of one BoNT-A product are not interchangeable and cannot be converted or compared with dosing units of another product. In this review, all studies showed reductions in spasticity. The studies generally showed that clinically significant (≥1 point on the MAS)2 reductions in muscle tone were achieved within 2 wks after injection and that the therapeutic effect lasted approximately 3–4 mos.
By contrast, none of the individual trials were able to demonstrate meaningful effects of ABO on functional improvement. Interestingly, a recent meta-analysis of post-stroke RCTs that included some sort of an activity outcome (e.g., Disability Assessment Scale, Action Research Arm Test, and BI) found that treatment with BoNT was associated with a moderate treatment effect (standard mean difference = 0.536 ± 0.094, 95% confidence interval = 0.352–0.721; P < 0.0001).5 This positive finding was based on a pooled analysis of studies (7/16 studies reviewed were with ABO), and the authors noted that there was substantial variation of effect sizes in the individual studies because of the use of differing outcome measures.5 Indeed, a recent review of upper limb function measurement methods found that none of the methods currently used to assess function after BoNT treatment for ULS satisfactorily fulfill all the criteria for a relevant outcome measure when used on their own.21 Further work on developing relevant functional outcome measures is a key unmet need in spasticity research because the MAS measures muscle tone, not spasticity. This is the fundamental problem of most of the spasticity studies. In addition, although MAS scores are ordinal, many of the studies used descriptive statistics as a primary analysis to determine statistical significance. Although there are limitations to this approach, the results do provide an indication of central tendency (e.g., improvement from baseline or compared with PBO), which the authors believe to be clinically meaningful.
ABO Safety and Dosing
Current studies provide strong evidence that ABO is a safe therapeutic modality for ULS. As with all botulinum toxins, an area of concern is the diffusion of toxin into unwanted muscles. Obviously, overdosing can lead to more diffusion and unwanted effects such as weakness.22 This systematic review provides the dose ranges of ABO that have been safely used in the various trials. It is worth mentioning that many of the studies used predefined doses and standardized injection sites but that the pattern of ULS varies between patients. At least five characteristic arm post-stroke spasticity patterns have been defined,23 and the number of muscles and the amount of injection per patient will frequently differ depending on the pattern of the spasticity, size of the patient, and the residual functionality of the affected limb.
Limitations
In this systematic review, a quality assessment that included the risk for bias criteria presented in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.024 was applied. This resulted in the exclusion of large uncontrolled studies and other studies that did not meet the predefined assessment criteria, which eliminated some relevant information. It is notable that most of the trials of ULS did not meet the criteria for inclusion in this review because they were mostly exploratory in nature. Excluding these large uncontrolled studies affected this study’s sample size as well. However, a fundamental purpose of systematic review methodology is to avoid bias, and this is best accomplished by including only controlled trials.
The aim of this project was to produce a comprehensive, evidenced-based data report that provides information on the injection schema used and associated outcomes for ABO. This systematic literature review focused on identifying comparative clinical trials across countries. The heterogeneity of outcome measures (and lack of a good functional outcome measure) makes it difficult to directly compare studies. Similarly, limitations result from the heterogeneity of the timing of both the development of post-stroke spasticity and the administration of ABO. None of the studies posed a specific definition in their inclusion criteria with respect to the elapsed time between treatment and the onset of the spasticity. Differences can be expected between the results of injection in patients with spasticity that occurs in the early phase after stroke, during the first weeks, vs. late-onset spasticity at 3–6 mos after stroke.25 Another limitation is the fact that most of the studies applied only a single injection with no long-term follow-up.
Finally, it should be noted that most of the studies on the treatment of spasticity caused by etiologies other than stroke, such as multiple sclerosis, traumatic brain injury, and cerebral palsy, were excluded because they did not meet the randomized controlled study inclusion criteria.
CONCLUSIONS
On the basis of data extracted from 12 randomized clinical studies, a strong evidence base already exists for the safe and effective use of ABO in post-stroke–related ULS. Future clinical trial programs required for ABO registration in the United States will be able to use the information on injection schema to optimize trial design.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lee-Ann Braun (RTI Health Solutions, funded by Ipsen) for assistance with conducting the literature search and Anita Chadha-Patel, PhD (ACP Clinical Communications, funded by Ipsen), for editorial support, including referencing.
Footnotes
Funded by Ipsen. Jack Chen has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc., Khashayar Dashtipour has received compensation/honoraria for services as a consultant or an advisory committee member or speaker from Allergan, Inc., Ipsen Biopharmaceuticals, Inc., Lundbeck Inc., Merz Pharmaceuticals, Teva Pharmaceutical Industries Ltd., UCB Inc., and US World Meds., Michael Lee has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc., and Merz Pharmaceuticals, Heather Walker has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc., and Merz Pharmaceuticals.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
REFERENCES
- 1.Royal College of Physicians, British Society of Rehabilitation Medicine, Chartered Society of Physiotherapy, Association of Chartered Physiotherapists Interested in Neurology. Spasticity in adults: management using botulinum toxin. National guidelines. London: RCP, 2009. [Google Scholar]
- 2. Esquenazi A, Mayer NH, Elia AE, et al. : Botulinum toxin for the management of adult patients with upper motor neuron syndrome. Toxicon 2009; 54: 634– 8 [DOI] [PubMed] [Google Scholar]
- 3. Simpson DM, Gracies JM, Graham K, et al. : Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review). Neurology 2009; 73: 736– 7; author reply 737–8 [PubMed] [Google Scholar]
- 4. Esquenazi A, Albanese A, Chancellor MB, et al. : Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013; 67: 115– 28 [DOI] [PubMed] [Google Scholar]
- 5. Foley N, Pereira S, Salter K, et al. : Treatment with botulinum toxin improves upper-extremity function post stroke: A systematic review and meta-analysis. Arch Phys Med Rehabil 2013; 94: 977– 89 [DOI] [PubMed] [Google Scholar]
- 6. Hubble J, Schwab J, Hubert C, et al. : Dysport (botulinum toxin type A) in routine therapeutic usage: A telephone needs assessment survey of European physicians to evaluate current awareness and adherence to product labeling changes. Clin Neuropharmacol 2013; 36: 122– 7 [DOI] [PubMed] [Google Scholar]
- 7. Des Jarlais DC, Lyles C, Crepaz N, et al. : Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am J Public Health 2004; 94: 361– 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bakheit AM, Pittock S, Moore AP, et al. : A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol 2001; 8: 559– 65 [DOI] [PubMed] [Google Scholar]
- 9. Bakheit AM, Thilmann AF, Ward AB, et al. : A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 2000; 31: 2402– 6 [DOI] [PubMed] [Google Scholar]
- 10. Bhakta BB, Cozens JA, Chamberlain MA, et al. : Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: A randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry 2000; 69: 217– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhakta BB, O’Connor RJ, Cozens JA: Associated reactions after stroke: A randomized controlled trial of the effect of botulinum toxin type A. J Rehabil Med 2008; 40: 36– 41 [DOI] [PubMed] [Google Scholar]
- 12. Hesse S, Reiter F, Konrad M, et al. : Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: A randomized, double-blind, placebo-controlled trial. Clin Rehabil 1998; 12: 381– 8 [DOI] [PubMed] [Google Scholar]
- 13. Kong KH, Neo JJ, Chua KS: A randomized controlled study of botulinum toxin A in the treatment of hemiplegic shoulder pain associated with spasticity. Clin Rehabil 2007; 21: 28– 35 [DOI] [PubMed] [Google Scholar]
- 14. Marco E, Duarte E, Vila J, et al. : Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J Rehabil Med 2007; 39: 440– 7 [DOI] [PubMed] [Google Scholar]
- 15. McCrory P, Turner-Stokes L, Baguley IJ, et al. : Botulinum toxin A for treatment of upper limb spasticity following stroke: A multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J Rehabil Med 2009; 41: 536– 44 [DOI] [PubMed] [Google Scholar]
- 16. Smith SJ, Ellis E, White S, Moore AP: A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil 2000; 14: 5– 13 [DOI] [PubMed] [Google Scholar]
- 17. Shaw L, Rodgers H, Price C, et al. : BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess 2010; 14: 1– 113, iii–iv [DOI] [PubMed] [Google Scholar]
- 18. Shaw LC, Price CI, van Wijck FM, et al. : BoTULS Investigators. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: Effect on impairment, activity limitation, and pain. Stroke 2011; 42: 1371– 9 [DOI] [PubMed] [Google Scholar]
- 19. Suputtitada A, Suwanwela NC: The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil 2005; 27: 176– 84 [DOI] [PubMed] [Google Scholar]
- 20. Yelnik AP, Colle FM, Bonan IV, et al. : Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J Neurol Neurosurg Psychiatry 2007; 78: 845– 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashford S, Turner-Stokes L: Systematic review of upper-limb function measurement methods in botulinum toxin intervention for focal spasticity. Physiother Res Int 2013; 18: 178– 89 [DOI] [PubMed] [Google Scholar]
- 22. Ramirez-Castaneda J, Jankovic J, Comella C, et al. : Diffusion, spread and migration of botulinum toxin. Mov Disord 2013; 28: 1775– 83 [DOI] [PubMed] [Google Scholar]
- 23. Hefter H, Jost WH, Reissig A, et al. : Classification of posture in poststroke upper limb spasticity: A potential decision tool for botulinum toxin A treatment? Int J Rehabil Res 2012; 35: 227– 33 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT: Green S (eds): Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 [Google Scholar]
- 25. Cousins E, Ward A, Roffe C, Rimington L, et al. : Does low-dose botulinum toxin help the recovery of arm function when given early after stroke? A phase II randomized controlled pilot study to estimate effect size. Clin Rehabil 2010; 24: 501– 13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


