Supplemental digital content is available in the text.
Key Words: Endometrial carcinoma, Tumor size, Magnetic resonance imaging, Prognosis
Abstract
Objective
The aim of this study was to explore the relation between preoperative tumor size based on magnetic resonance imaging (MRI) and the surgical pathologic staging parameters (deep myometrial invasion, cervical stroma invasion, and metastatic lymph nodes) and to assess the prognostic impact of tumor size in endometrial carcinomas. Interobserver variability for the different tumor size measurements was also assessed.
Methods/Materials
Preoperative pelvic MRI of 212 patients with histologically confirmed endometrial carcinomas was read independently by 3 radiologists. Maximum tumor diameters were measured in 3 orthogonal planes (anteroposterior, transverse, and craniocaudal planes [CC]), and tumor volumes were estimated. Tumor size was analyzed in relation to surgical staging results and patient survival. The multivariate analyses were adjusted for preoperative risk status based on endometrial biopsy. Intraclass correlation coefficients and receiver operating characteristics curves for the different tumor measurements were also calculated.
Results
Anteroposterior tumor diameter independently predicted deep myometrial invasion (P < 0.001), whereas CC tumor diameter tended to independently predict lymph node metastases (P = 0.06). Based on receiver operating characteristic curves, the following tumor size cutoff values were identified: anteroposterior diameter greater than 2 cm predicted deep myometrial invasion (unadjusted odds ratio [OR], 12.4; P < 0.001; adjusted OR, 6.7; P < 0.001) and CC diameter greater than 4 cm predicted lymph node metastases (unadjusted OR, 6.2; P < 0.001; adjusted OR, 4.9; P = 0.009). Large tumor size was associated with reduced progression/recurrence-free survival (P ≤ 0.005 for all size parameters), and CC diameter had an independent impact on survival (adjusted hazards ratio, 1.04; P = 0.009). The interobserver variability for the different size measurements was very low (intraclass correlation coefficient, 0.78–0.85).
Conclusions
Anteroposterior tumor diameter greater than 2 cm predicts deep myometrial invasion, and CC tumor diameter greater than 4 cm predicts lymph node metastases. Tumor size is a strong prognostic factor in endometrial carcinomas. Preoperative tumor measurements based on MRI may potentially improve preoperative risk stratification models and thus enable better tailored surgical treatment in endometrial cancer.
Endometrial cancer is the most common gynecologic malignancy in industrialized countries, and the incidence is increasing.1 Surgical treatment is planned based on preoperative assessment of histological subtype, grade, and depth of myometrial invasion. Surgical International Federation of Gynecology and Obstetrics (FIGO) stage is documented to be the strongest prognostic factor in endometrial carcinoma, thus guiding adjuvant therapy in addition to the assessment of histologic subtype and grade in the hysterectomy specimen.1–3
Magnetic resonance imaging (MRI) has long been considered the diagnostic imaging method of choice for preoperative staging of endometrial carcinomas.4–6 The presence of deep myometrial invasion and cervical stroma invasion could be visualized, and enlarged lymph nodes could be detected. However, conventional pelvic MRI has reportedly limitations in accuracy in the detection of the staging parameters, in particular for detecting lymph node metastases.6,7 Interobserver variation between radiologists for all staging parameters also represents a source of inaccuracy.8
As opposed to the cervical cancer FIGO staging system,6 FIGO staging for endometrial cancers does not include tumor size measurements. Nevertheless, large macroscopic tumor size, assessed in the hysterectomy specimen, has long been known to predict lymph node metastasis and poor survival in patients with endometrial carcinomas.9–13 Recent publications support that tumor volume based on preoperative MRI predicts lymph node metastases and has prognostic impact in endometrial cancer.14,15 However, the reproducibility of MRI-based tumor measurements has, to our knowledge, not yet been explored. Furthermore, the optimal cutoff value for risk assessment based on tumor size is not yet defined.
The primary objective of this study was to explore the relationship between different preoperative tumor size measurements using MRI and the surgical pathologic staging parameters deep myometrial invasion, cervical stroma invasion, and metastatic lymph nodes in endometrial carcinoma patients. The secondary objectives were to assess the interobserver variability for the different tumor measurements and to explore the value of these preoperative tumor size measurements to identify patients with poor outcome.
MATERIALS AND METHODS
Patient Series, Study Setting, and Clinical Outcome
This prospective study was conducted under institutional review board–approved protocols with informed consent from all patients. From April 2009 to November 2013, preoperative pelvic MRI was performed in 212 patients in whom the diagnosis of endometrial carcinoma was histologically verified at surgical staging. All patients were diagnosed and treated at the same university hospital serving a population of ~1 million inhabitants.
Follow-up data regarding recurrence, progression, and survival have been collected from patient records and from correspondence with the responsible primary physicians or gynecologists. The date of the last follow-up was July 2014, and the mean (range) follow-up for survivors was 25 (0–58) months.
Histological Diagnosis
All patients were surgically staged according to the 2009 FIGO staging criteria.2 The responsible surgeon decided the extent of sampling, balancing preoperatively known histologic risk factors and the patient’s comorbidity. The patient group without lymph node sampling is typically older with more myometrial invasion, otherwise not different from the sampled group.16 Surgical specimens were sectioned along the longitudinal plane of the uterus, and myometrial invasion and cervical stromal invasion were estimated grossly and confirmed microscopically according to standard procedures.17 Routine histopathology reports were generated without knowledge of preoperative MRI findings. The pathologists documented number and size of metastatic lymph nodes.
MRI Protocol
Contrast-enhanced (CE) MRI was performed on a 1.5-T Siemens Avanto Running Syngo MR B17 (Erlangen, Germany) using a 6-channel body coil8 in accordance with the guidelines of European Society of Urogenital Imaging.5 The mean (range) interval between MRI examination and surgical staging was 11.3 (0–98) days.
Data Analysis
All images were deidentified and read independently by 3 observers who were blinded for tumor stage, histological diagnosis, and patient outcome. Observer 1 and 2 are consultants with more than 10 years of experience with pelvic MRI. Observer 3 included 2 junior radiologists (both having more than 4 years of experience with pelvic MRI); one read the first 105 MRI examinations, and the other read the following 111 examinations.
All observers reported imaging findings on a standardized form. Presence of deep myometrial invasion (tumor invading half or more of the myometrial wall), cervical stroma invasion (disruption of the low-signal intensity cervical stroma on T2-weighted images), and enlarged pelvic or para-aortic lymph nodes (largest short-axis diameter >10 mm) were recorded. Maximum tumor diameters were measured in 3 orthogonal planes: anteroposterior (AP) and transverse (TV) diameters on axial CE T1-weighted oblique images (perpendicular to the long axis of the uterus) as well as craniocaudal (CC) diameters on sagittal T2-weighted images (Fig. 1). Tumor volume was then estimated based on these measurements of maximum tumor diameter in 3 orthogonal planes using the following equation: tumor volume = AP diameter × TV diameter × CC diameter/2.
FIGURE 1.
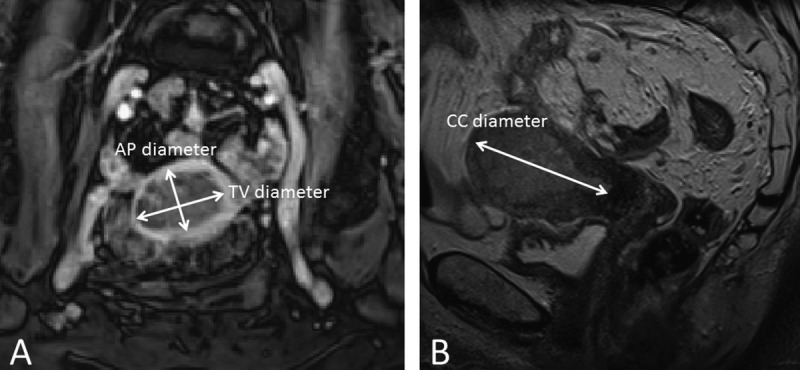
Axial oblique CE T1-weighted image (A) and sagittal T2-weighted image (B) for measurements of maximum tumor diameters in 3 orthogonal planes. AP and maximum TV diameters were measured on the axial oblique image (A), whereas CC diameters were measured on the sagittal image (B).
To establish the overall imaging findings based on the recordings by all 3 observers, we also computed a new data set (“consensus reading”) in which the value given by the majority of the observers was recorded for categorical variables, and the median value was recorded for continuous variables.
Statistical Analysis
Estimation of sample size was done by χ2 test using software East4 2005 (Cytel Software Corp). To achieve 90% power of detecting a 20% higher occurrence of positive markers in patients with metastatic lymph nodes (5% vs 25%) at a 5% significance level, 101 patients were needed for inclusion, defining the minimum number of patients to be included in the MRI study. To reach 90% power detecting a 30% difference in 5-year survival (90% for patients with markers within reference range vs 60% with pathologic markers) at a 5% level of significance, 65 patients were needed, assuming a positive to negative ratio of the markers of 1:3.
Clinical and histopathology staging parameters were analyzed in relation to tumor size measurements using Mann-Whitney U test, Kruskal-Wallis H test, Jonckheere-Terpsta trend test, χ2 test, and binary logistic regression analysis. Intraclass correlation coefficient was used to assess the consistency and reproducibility of tumor size measurements, and minimal detectable change (1.96 × standard error of the mean × square root of 2) for the measured diameters was also calculated.
Receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic value of the different tumor size measurements in identifying deep myometrial invasion, cervical stroma invasion, and lymph node metastases. The optimal cutoff values (rounded to centimeters) were determined for which the best separation in Youden index between groups was achieved.
Differences in time to recurrence (for patients considered cured by primary treatment) or progression (for patients known to have residual disease after primary treatment) were assessed by the Mantel-Cox (log-rank) linear trend test. The Cox proportional hazards model was used to study the effect on survival of continuous variables. The prognostic value of different tumor size categories was explored with univariate analyses using Kaplan-Meier, and groups with similar survival were merged. McNemar test was used for pairwise analysis for differences in sensitivity, specificity, and accuracy. The data were analyzed using SPSS 22.0 (Chicago, IL) and Stata 12.1 (College Station, TX). All reported P values were 2-sided and considered to indicate statistical significance when less than 0.05.
RESULTS
Patients
The median (mean) patient age in the study sample (n = 212) was 66 (66) years (range, 32-93), and 91% (193/212) of the patients were postmenopausal. Applying the FIGO 2009 staging criteria, 55% (116/212) were stage IA (<50% myometrial invasion), 23% (48/212) stage IB (≥50% myometrial invasion), 11% (23/212) stage II (cervical stroma invasion), 11% (24/212) stage III (local or regional tumor spread), and 0% (1/212) stage IV. The histological subtype was endometrioid in 81% (171/212) of which 50% (86/171) were grade 1, 29% (50/171) grade 2, and 17% (29/171) grade 3; whereas 4% (6/171) were ungraded. Clear cell histology was detected in 3% (6/212), serous in 10% (21/212), carcinosarcoma in 4% (9/212), and undifferentiated in 2% (5/212). Metastatic lymph nodes were more frequent in patients with deep myometrial invasion, cervical stroma invasion, high histologic grade, aneuploidy, and body mass index (BMI) greater than 25 (Table 1).
TABLE 1.
Clinical characteristics and MRI findings in relation to the presence of metastatic lymph nodes at surgical staging in 181 endometrial carcinoma patients
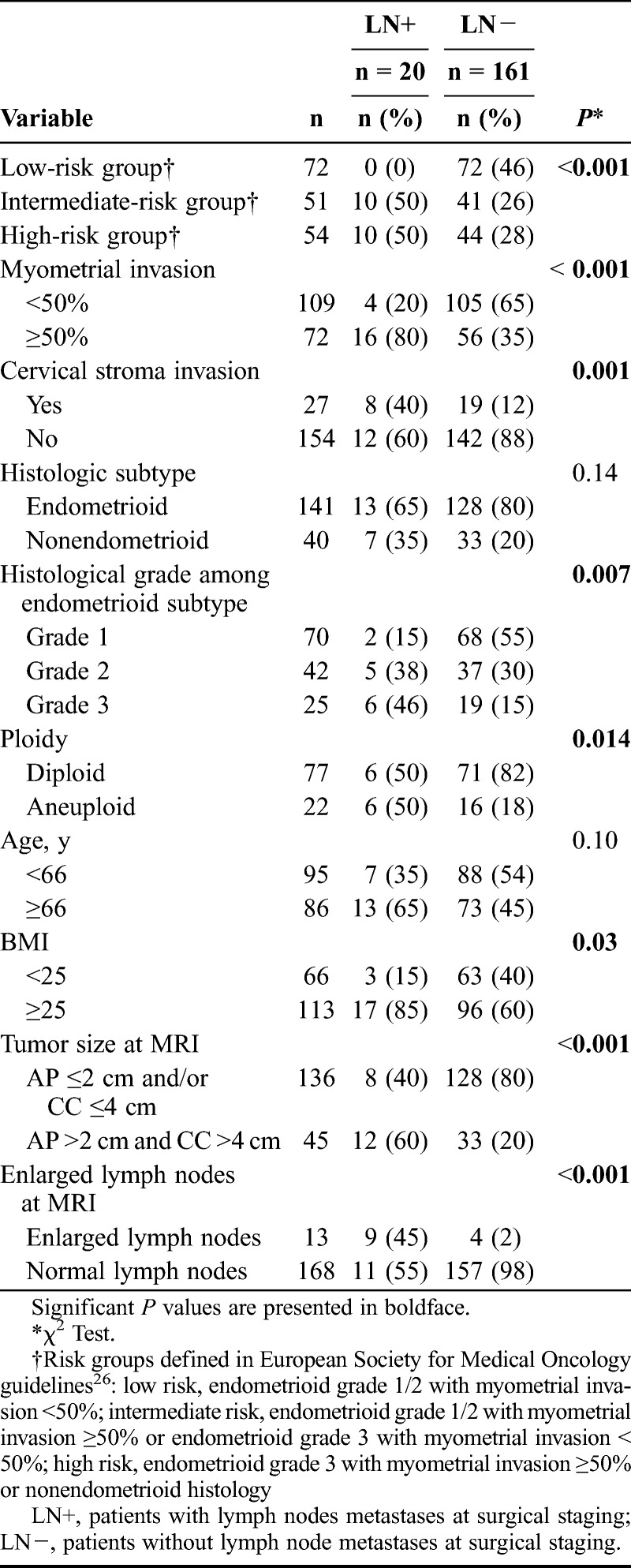
All patients were primarily treated with hysterectomy and bilateral salpingo-oophorectomy. Pelvic lymph node sampling was performed in 85% (181/212) as part of the routine surgical staging procedure. Adjuvant therapy was given to 33% (70/212), chemotherapy in 28% (59/212), pelvic radiation in 5% (10/212), and hormonal treatment in 0% (1/212).
Tumor Size Is Correlated to Surgicopathologic Findings
The mean (median, range) preoperative tumor diameters were 28 (26, 0–113) mm for axial TV diameter, 18 (16, 0–77) mm for axial AP diameter, and 35 (31, 0–102) mm for sagittal CC diameter. The mean (median, range) estimated tumor volume was 19 (6, 0–444) mL. Tumor volume was significantly higher in patients with deep myometrial invasion, cervical stroma invasion, and lymph node metastases at surgical staging and in patients with aneuploidy and high histologic grade (Table 2).
TABLE 2.
Tumor volume in relation to clinical and histologic characteristics in 212 endometrial carcinoma patients

Tumor measurements in the 3 orthogonal planes and tumor volume did all predict the presence of deep myometrial invasion at surgical staging (unadjusted odds ratios [ORs], 1.06–1.13; P < 0.001 for all); however, AP tumor diameter was the only size variable independently predicting deep myometrial invasion (adjusted OR, 1.14; P < 0.001). Craniocaudal tumor diameter was the only variable predicting cervical stroma invasion (adjusted OR, 1.04; P = 0.008). Although all size parameters predicted lymph node metastases in the univariate analyses (unadjusted ORs, 1.02–1.05; P ≤ 0.004 for all), only CC tumor diameter tended to independently predict lymph node metastases (adjusted OR, 1.04; P = 0.06).
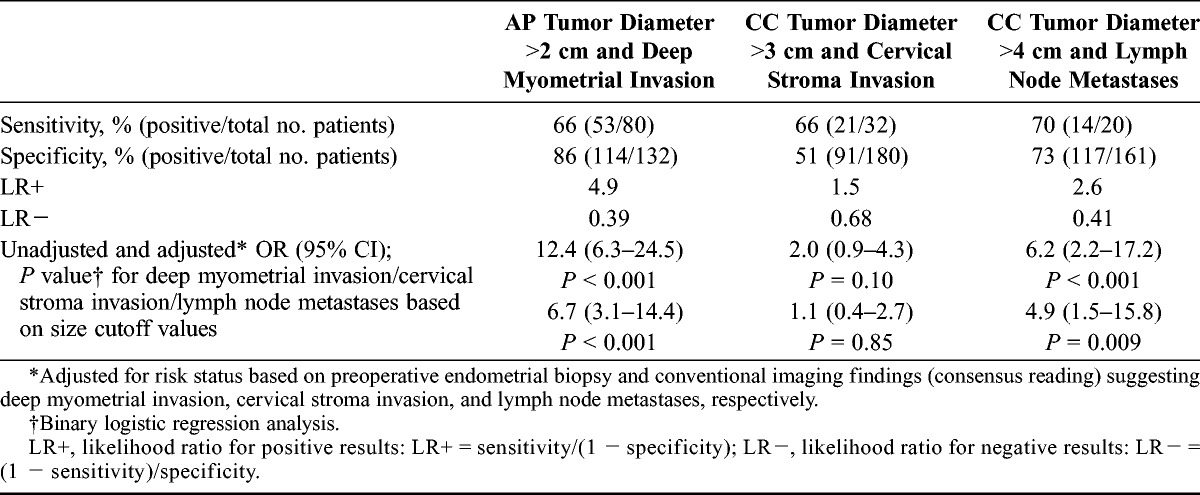
Receiver operator characteristic curves for the different size parameters in the prediction of deep myometrial invasion (Fig. 2A), cervical stroma invasion (Fig. 2B), and lymph node metastases (Fig. 2C) showed that AP diameter had the highest area under the curve (AUC, 0.82) for deep myometrial invasion, whereas CC diameter had the highest AUC for cervical stroma invasion (AUC, 0.66) and for lymph node metastases (AUC, 0.76). Based on these ROC curves, the following cutoff values were identified: AP tumor diameter greater than 2 cm predicts deep myometrial invasion yielding sensitivity/specificity of 66%/86% and an OR of 12.4, and CC tumor diameter greater than 4 cm predicts lymph node metastases yielding sensitivity/specificity of 70%/73% and an OR of 6.2, whereas CC tumor diameter greater than 3 cm tends to predict cervical stroma invasion yielding sensitivity/specificity of 66%/51% and an OR of 2.0 (Table 3). When adjusting for risk status based on preoperative endometrial biopsy and for conventional imaging findings (consensus reading) suggesting deep myometrial invasion, cervical stroma invasion, and lymph node metastases, respectively, AP diameter greater than 2 cm independently predicted deep myometrial invasion (adjusted OR, 6.7), and CC diameter greater than 4 cm independently predicted lymph node metastases (adjusted OR, 4.9), whereas CC diameter greater than 3 cm did not predict cervical stroma invasion (Table 3).
FIGURE 2.
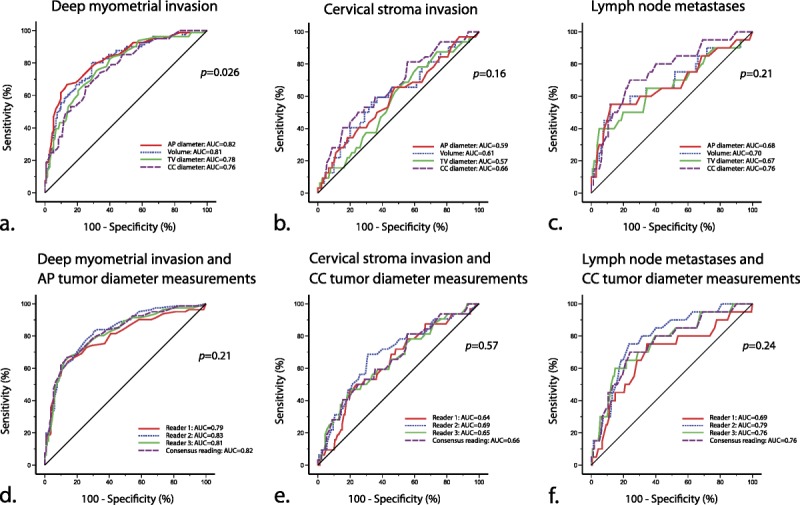
Receiver operator characteristic curves for the various tumor size measurements for identification of (A) deep myometrial invasion, (B) cervical stroma invasion, and (C) lymph node metastases and ROC curves for the different observers for (D) AP diameter to predict deep myometrial invasion, (E) CC diameter to predict cervical stroma invasion, and (F) CC diameter to predict lymph node metastases in patients with endometrial carcinoma. P values refer to the test of equal AUC values across tumor measurements.
TABLE 3.
Sensitivity, specificity, LR+, LR−, and OR for the prediction of deep myometrial invasion (by AP tumor diameter >2 cm), cervical stroma invasion (by CC tumor diameter >3 cm), and lymph node metastases (by CC diameter >4 cm) using surgical staging as the criterion standard
Tumor Size Predicts Progression/Recurrence-Free Survival
The 3 tumor diameter measurements and tumor volume did all predict progression/recurrence-free survival (P ≤ 0.01 for all size parameters) in endometrial carcinoma patients. In a multivariate analysis including all size parameters and preoperative risk status based on endometrial biopsy, only CC tumor diameter had an independent impact on survival (Table 4, Supplemental Digital Content, http://links.lww.com/IGC/A270). When stratifying patient groups according to the proposed cutoff values for size variables defined by the ROC analyses, patients with AP tumor diameter greater than 2 cm and patients with CC tumor diameter greater than 4 cm had significantly reduced progression/recurrence-free survival (P ≤ 0.03 for both; Figs. 3A, B). Combining these 2 size criteria yielded similar survival curves among patients with both or 1 size criterion below the cutoff values (the 2 survival curves are thus merged in Fig. 3C), whereas patients with both AP tumor diameter greater than 2 cm and CC tumor diameter greater than 4 cm had significantly reduced progression/recurrence-free survival (P = 0.004; Fig. 3C).
FIGURE 3.
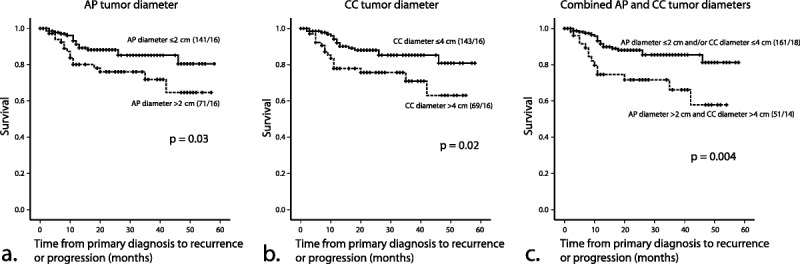
Kaplan-Meier survival curves depicting progression/recurrence-free survival according to (A) maximal AP tumor diameter (≤2 vs >2 cm), (B) maximal CC tumor diameter (≤4 vs >4 cm), and (C) a combination of AP and CC tumor diameters (AP ≤2 cm and/or CC ≤4 cm vs AP >2 cm and CC >4 cm). P values refer to the log-rank test for equality of survival distribution.
Interobserver Variability for Tumor Measurements
The interobserver variability for tumor diameter measurements by the 3 observers was low with intraclass correlation coefficients of 0.78 to 0.85 and minimum detectable change of 14 to 26 mm for the different tumor diameter measurements (Table 5, Supplemental Digital Content, http://links.lww.com/IGC/A270). Furthermore, the AUC values of the ROC curves for prediction of deep myometrial invasion, cervical stroma invasion, and lymph node metastases were not significantly different between observers (Figs. 2D-F).
DISCUSSION
In this large population-based study, we demonstrate a significant predictive value of preoperative tumor size measurements based on MRI to identify deep myometrial invasion and lymph node metastases. Furthermore, tumor size had a significant independent impact on survival also when adjusting for preoperative risk status based on endometrial biopsy. Based on the present study, we propose a risk model with cutoff values of AP tumor diameter greater than 2 cm indicating high risk for deep myometrial invasion and CC tumor diameter greater than 4 cm indicating high risk for lymph node metastases. Having established that the interobserver variability for these different tumor measurements at MRI was very low, we infer that these preoperative tumor measurements with corresponding cutoff values may represent robust biomarkers aiding in the preoperative risk stratification and in planning of tailored surgical treatment in endometrial cancer patients.
Presence of deep myometrial invasion in hysterectomy specimen at surgicopathological staging is associated with an increased risk of lymph node metastases, tumor recurrence, and distant relapse in endometrial carcinoma patients.3,18 We found that all size parameters predicted deep myometrial invasion, which is in accordance with the findings of Todo et al14 reporting high volume indexes (defined as the product of maximum AP, TV, and CC tumor diameters at MRI) to be associated with deep myometrial invasion. As opposed to Todo et al,14 we have also explored the independent impact of the different size variables in a multivariate model including preoperative risk status based on endometrial biopsy. Interestingly, AP diameter, which had the largest AUC (Fig. 2A), proved to be the only size variable independently predicting deep myometrial invasion.
Based on the ROC curve, the optimal cutoff value for prediction of deep myometrial invasion was AP diameter greater than 2 cm, and AP diameter greater than 2 cm independently predicted deep myometrial invasion even when adjusting for conventional imaging findings (consensus reading) suggesting the same. Interestingly, we found that AP diameter greater than 2 cm and MRI indicating deep myometrial invasion had comparable accuracy (sensitivity, specificity) for identification of deep myometrial invasion at surgical staging: 79% (66%, 86%) and 74% (70%, 77%), respectively, however, with significantly better specificity for AP diameter greater than 2 cm (86% vs 77%; P = 0.015, McNemar test). Thus, this relatively simple approach of measuring AP tumor diameter yields a diagnostic performance similar to or slightly better than conventional reading for prediction of deep myometrial invasion.
Several surgicopathological risk models for prediction of lymph node metastases have been proposed in endometrial cancer based on histologic grade, subtype, and tumor extent,10,19,20 among which 1 model includes tumor greater than 2 cm based on the gross inspection of hysterectomy specimen.10 These models are, however, limited by the fact that they rely on surgicopathological staging results, which are per definition not available preoperatively.
Tumor diameter greater than 2 cm in macroscopic fresh tissue has been reported to independently predict lymph node metastases and survival9; however, the independent impact on survival of tumor size greater than 2 cm has not been consistently reproduced in the literature.10,13 Based on macroscopic gross inspection of the cut-up of the uterus, maximum tumor dimension greater than 3.75 cm was recently reported an independent predictor of deep myometrial invasion, distant recurrence, and death.11 Because CC diameter was almost uniformly the largest tumor diameter in our study, our cutoff value for CC diameter greater than 4 cm seems to be in line with the proposed cutoff value of 3.75 cm. Direct comparison between tumor diameter measurements in macroscopic fresh tissue and preoperatively based on MRI is, however, difficult due to the differences in planes eligible for tumor measurements and the potential distortion and compression of tumor tissue in vivo compared with ex vivo. Thus, the optimal cutoff values for tumor size are not necessarily transferable from in vivo MRI-based assessment to the ex vivo gross section-based tumor measurements. Still, the metastatic potential and unfavorable prognostic impact of large tumor size in endometrial carcinomas is consistently supported by both in vivo and ex vivo studies.9–15
We found that the MRI-based parameters AP tumor diameter greater than 2 cm and CC tumor diameter greater than 4 cm both alone and combined are strongly associated with reduced progression/recurrence-free survival in endometrial carcinomas (Fig. 3). These tumor size parameters should in the future also be evaluated in relation to other preoperative biomarkers such as p53, hormone receptor, and DNA ploidy status in preoperative biopsies, prognostic markers assessed in blood samples,21 and on functional imaging by MRI or PET/CT, which have been shown to yield prognostic information.16,22–25
For all risk stratification models, high accuracy and reproducibility of the variables included in the model are essential. To our knowledge, this is the first study assessing the interobserver variability for the different tumor size measurements at MRI in endometrial cancer. Interestingly, we found that tumor size was measured with very low interobserver variability and with no striking difference related to the readers’ previous experience. Thus, tumor size measurements seem to represent robust biomarkers that are promising for potential inclusion in future risk stratification models in endometrial cancer.
This study has some limitations. First, the study was conducted in a single institution using a standardized imaging protocol. Thus, the potential impact of various imaging protocols on MRI-based tumor size measurements has not been assessed. However, our imaging protocol is based on the guidelines of the European Society of Urogenital Imaging and is thus expected to be quite similar to those applied at most centers treating endometrial cancer patients. Second, intraobserver variability was not assessed in this study. This is, however, expected to be lower than the observed interobserver variability, which was very low in this study, and the intraobserver variability is thus expected to be almost negligible.
In summary, tumor size assessed preoperatively by MRI predicts the presence of deep myometrial invasion and lymph node metastases and is a strong prognostic factor in endometrial carcinoma. Based on our findings, we propose cutoff values greater than 2 cm AP tumor diameter for predicting deep myometrial invasion and greater than 4 cm CC tumor diameter for predicting lymph node metastases, as well as poor survival for the combination of greater than 2 cm AP and greater than 4 cm CC tumor diameter. Preoperative tumor measurements at MRI may thus provide clinically relevant biomarkers for future risk stratification models guiding tailored surgical treatment in endometrial cancer.
Supplementary Material
Footnotes
Supported by the Western Norway Regional Health Authority; Research Funds at the Department of Radiology; Haukeland University Hospital; Norwegian Research Council; the University of Bergen; the Meltzer Foundation; the Norwegian Cancer Society (the Harald Andersen’s legacy); MedViz (www.medviz.uib.no); a medical imaging and visualization research and development cluster in Western Norway founded by Haukeland University Hospital, University of Bergen, and Christian Michelsen Research; and Bergen Research Foundation.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
The authors declare no conflicts of interest.
REFERENCES
- 1. Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005; 366: 491– 505. [DOI] [PubMed] [Google Scholar]
- 2. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105: 103– 104. [DOI] [PubMed] [Google Scholar]
- 3. Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012; 13: e353– e361. [DOI] [PubMed] [Google Scholar]
- 4. Frei KA, Kinkel K. Staging endometrial cancer: role of magnetic resonance imaging. J Magn Reson Imaging. 2001; 13: 850– 855. [DOI] [PubMed] [Google Scholar]
- 5. Kinkel K, Forstner R, Danza FM, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009; 19: 1565– 1574. [DOI] [PubMed] [Google Scholar]
- 6. Sala E, Rockall AG, Freeman SJ, et al. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013; 266: 717– 740. [DOI] [PubMed] [Google Scholar]
- 7. Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol. 2012; 67: 2– 12. [DOI] [PubMed] [Google Scholar]
- 8. Haldorsen IS, Husby JA, Werner HM, et al. Standard 1.5-T MRI of endometrial carcinomas: modest agreement between radiologists. Eur Radiol. 2012; 22: 1601– 1611. [DOI] [PubMed] [Google Scholar]
- 9. Schink JC, Rademaker AW, Miller DS, et al. Tumor size in endometrial cancer. Cancer. 1991; 67: 2791– 2794. [DOI] [PubMed] [Google Scholar]
- 10. Mariani A, Webb MJ, Keeney GL, et al. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002; 87: 274– 280. [DOI] [PubMed] [Google Scholar]
- 11. Chattopadhyay S, Cross P, Nayar A, et al. Tumor size: a better independent predictor of distant failure and death than depth of myometrial invasion in International Federation of Gynecology and Obstetrics stage I endometrioid endometrial cancer. Int J Gynecol Cancer. 2013; 23: 690– 697. [DOI] [PubMed] [Google Scholar]
- 12. Lee KB, Ki KD, Lee JM, et al. The risk of lymph node metastasis based on myometrial invasion and tumor grade in endometrioid uterine cancers: a multicenter, retrospective Korean study. Ann Surg Oncol. 2009; 16: 2882– 2887. [DOI] [PubMed] [Google Scholar]
- 13. Shah C, Johnson EB, Everett E, et al. Does size matter? Tumor size and morphology as predictors of nodal status and recurrence in endometrial cancer. Gynecol Oncol. 2005; 99: 564– 570. [DOI] [PubMed] [Google Scholar]
- 14. Todo Y, Watari H, Okamoto K, et al. Tumor volume successively reflects the state of disease progression in endometrial cancer. Gynecol Oncol. 2013; 129: 472– 477. [DOI] [PubMed] [Google Scholar]
- 15. Todo Y, Choi HJ, Kang S, et al. Clinical significance of tumor volume in endometrial cancer: a Japan-Korea cooperative study. Gynecol Oncol. 2013; 131: 294– 298. [DOI] [PubMed] [Google Scholar]
- 16. Trovik J, Wik E, Werner HM, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013; 49: 3431– 3441. [DOI] [PubMed] [Google Scholar]
- 17. Silverberg SG, Kurman RJ, Nogales F. Tumors of the uterine corpus. In: Tavassoli FA, Devilee P, eds. Tumours of the Breast and Female Genital Organs. World Health Organization Classification of Tumours. Pathology & Genetics. Lyon, France: IACR Press Inc; 2003: 217– 258. [Google Scholar]
- 18. Werner HM, Trovik J, Marcickiewicz J, et al. Revision of FIGO surgical staging in 2009 for endometrial cancer validates to improve risk stratification. Gynecol Oncol. 2012; 125: 103– 108. [DOI] [PubMed] [Google Scholar]
- 19. Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987; 60: 2035– 2041. [DOI] [PubMed] [Google Scholar]
- 20. Kang S, Lee JM, Lee JK, et al. How low is low enough? Evaluation of various risk-assessment models for lymph node metastasis in endometrial cancer: a Korean multicenter study. J Gynecol Oncol. 2012; 23: 251– 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staff AC, Trovik J, Eriksson AG, et al. Elevated plasma growth differentiation factor-15 correlates with lymph node metastases and poor survival in endometrial cancer. Clin Cancer Res. 2011; 17: 4825– 4833. [DOI] [PubMed] [Google Scholar]
- 22. Haldorsen IS, Gruner R, Husby JA, et al. Dynamic contrast-enhanced MRI in endometrial carcinoma identifies patients at increased risk of recurrence. Eur Radiol. 2013; 23: 2916– 2925. [DOI] [PubMed] [Google Scholar]
- 23. Haldorsen IS, Stefansson I, Gruner R, et al. Increased microvascular proliferation is negatively correlated to tumour blood flow and is associated with unfavourable outcome in endometrial carcinomas. Br J Cancer. 2014; 110: 107– 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Njolstad TS, Engerud H, Werner HM, et al. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013; 131: 410– 415. [DOI] [PubMed] [Google Scholar]
- 25. Kang S, Kang WD, Chung HH, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean gynecologic oncology group study. J Clin Oncol. 2012; 30: 1329– 1334. [DOI] [PubMed] [Google Scholar]
- 26. Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 (suppl 6): vi33– vi38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


