Abstract
Objective
Association between endometriosis and ovarian cancer has been well established. Nonetheless, endometriosis may also be associated with endometrial cancer because of shared etiological mechanisms of both estrogen stimulation and chronic inflammation; however, the association between these 2 disorders has rarely been investigated.
Methods
The National Health Insurance Research Databases in Taiwan were retrieved and analyzed. The case cohort consisted of patients with a diagnosis of endometriosis between January 1997 and December 2000 (N = 15,488). For the construction of control cohort, 8 age- and sex-matched control patients for every patient in the case cohort were selected using a random sampling method (n = 123,904). All subjects were tracked for 10 years from the date of entry to identify whether they had developed endometrial cancer. The Cox proportional hazards regression model was used to evaluate 10-year event occurrence of endometrial cancer.
Results
During the 10-year follow-up period, 392 participants developed endometrial cancer, with 104 (0.7%) distributed in the case cohort and 288 (0.2%) in the control cohort. Multivariable Cox regression modeling demonstrates a higher risk for developing endometrial cancer in the case cohort than in the control cohort (adjusted hazard ratio [aHR], 2.83; 95% confidence interval [CI], 1.495.35; P < 0.01). Age at diagnosis of endometriosis shows a moderator effect: when 40 years or younger, the risk for developing endometrial cancer was comparable between the case cohort and the control cohort (aHR, 1.42; 95% CI, 0.55–3.70; P = 0.226), whereas when older than 40 years, the risk for developing endometrial cancer was higher in the former group than in the latter group (aHR, 7.08; 95% CI, 2.33–21.55; P = 0.007).
Conclusions
Patients diagnosed with endometriosis may harbor an increased risk for developing endometrial cancer in their later life. Closer monitoring is advised for this patient population.
Key Words: Endometrial cancer, Endometriosis, Population-based cohort study
In the United States, endometrial cancer is the most common gynecological malignancy, with 46,470 new cases and 8120 deaths from the disease in 2011.1 Various conditions, including anovulation, polycystic ovarian syndrome, obesity, estrogen-only hormone replacement therapy, and tamoxifen use, lead to high levels of unopposed estrogen exposure, which have been linked to the pathogenesis of endometrial cancer.2,3 Although the exact mechanisms involved in endometrial carcinogenesis due to chronic estrogen exposure are unclear, it is thought that prolonged estrogen stimulation might enhance the pro-proliferative and inflammatory gene performance, which further induce DNA-damaging effects.4
Endometriosis is a common disease that affects 5% to 10% of women of reproductive age.5 Chronic inflammation has been linked to the establishment and progression of endometriosis, largely through the secretion of proinflammatory cytokines, inducing proliferation of peritoneal macrophages and mesothelial cells.6 Furthermore, several lines of evidence have linked endometriosis to excessive 17-estradiol signaling in the ectopic tissue. It was reported that elevated expression of P450 aromatase (CYP19A1) in endometriotic tissue leads to increased local production of 17-estradiol, which in turn promotes growth of the ectopic lesions.7–9
Endometriosis has been found to be associated with some histological subtypes (ie, clear cell and endometrioid carcinoma) of epithelial ovarian carcinoma, known as endometriosis-associated ovarian cancer (EAOC), which is etiologically distinct from other subtypes of ovarian cancer in several aspects.10 Studies have documented that endometriosis is associated with an approximately 3-fold risk for developing endometrioid and clear cell subtype. Patients with EAOC had a lower stage of cancer, a distribution of histological subtypes that differs from the general population, predominantly lower-grade endome triosis lesions, and significantly better overall survival as compared with other ovarian carcinomas.11 Although the association between endometriosis and some subtypes of ovarian cancer has been well established, the association between endometriosis and endometrial cancer is not as well defined as that in EAOC. However, clinical observation has shown that simultaneously detected endometrial and ovarian carcinomas are most often associated with endometrioid subtype, and ovarian endometriosis was identified in approximately 30% of these cases.12,13 Hence, there exists a potential association between endometriosis and endometrial cancer.
From the above descriptions, it seems that endometriosis and endometrial cancer share common etiological mechanisms, including estrogen stimulation and chronic inflammation. As such, there is a putative association between these 2 disorders. In this work, we tested the hypothesis that endometriosis may increase the risk for developing endometrial cancer. We used a population-based national health registry database in Taiwan to explore the relationship between endometriosis and risk for subsequent development of endometrial cancer.
MATERIALS AND METHODS
Data Sources
The Taiwan National Health Insurance program was established on March 1, 1995, by the Bureau of National Health Insurance. More than 99% of the Taiwanese population is enrolled into this program.14 The National Health Research Institutes was commissioned to National Health Insurance Research Databases (NHIRDs) for research proposals. The identification codes of beneficiaries were scrambled by a computer.
The NHIRDs consist of comprehensive health care data provided to researchers, including ambulatory care records, inpatient care records, registration files, catastrophic illness files, and various data regarding drug prescriptions. In this study, we used the Longitudinal Health Insurance Database, which is a sub–data set of NHIRDs and contains 1 million beneficiaries randomly selected from those enrolled in the insurance program. The Longitudinal Health Insurance Database contained insurant information, outpatient and inpatient visits, and medical treatment records between January 1, 1996, and December 31, 2010.
The NHRI reported that there were no statistically significant differences in age or sex between the randomly sampled group and all beneficiaries of the NHI program. To improve claims data accuracy, the NHRI invited expert reviews on a random sample of every 50 to 100 ambulatory and inpatient claims in each hospital, and clinic routine practice of performing cross-checks and validations of medical claims ensures the accuracy of the NHIRDs diagnostic coding.
Patient Selection and Ascertainment of Outcome
The design of the current work was a population-based retrospective cohort study. We selected patients with the diagnosis of endometriosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 617.X) from January 1, 1997, to December 31, 2000, as the case cohort. Accordingly, each patient in the case cohort was matched on the basis of age, sex, and index year to 8 randomly identified beneficiaries without endometriosis to build the control cohort. To minimize the influence of possible “reverse causation,”15 we excluded those subjects with a diagnosis of endometrial cancer before the diagnosis of endometriosis. The date of the initial diagnosis of endometriosis was assigned as the baseline date for each patient. To improve data accuracy, the endometriosis selection criteria required that all case ICD-9 codes are assigned by a gynecologist and the patients must have the diagnosis of endometriosis for at least 2 times in the same year in outpatient clinic record. Selection criteria for endometrial cancer patients (ICD-9-CM code 182) were assigned by a gynecologic oncologist. We selected endometrial cancer cases in this study only if they received 2 or more endometrial cancer diagnoses for ambulatory care visit or 2 or more diagnoses for inpatient care. All study subjects were followed from the baseline date to the first event, which was defined as occurrence of endometrial cancer up to the end of 2010.
Patients diagnosed with endometrial cancer before or after the study period were excluded from both cohorts. We also identified relevant comorbidities, including hypertension (ICD-9-CM 401.X-405.X), diabetes mellitus (ICD-9-CM 250.X), and hyperlipidemia (ICD-9-CM 272.X) for both the case cohort and the control cohort.
Identification of Level of Urbanization
For the investigation of urbanization, all 365 townships in Taiwan were stratified into 7 levels according to the standards established by the Taiwanese NHRI based on a cluster analysis of the 2000 Taiwan census data, with 1 referring to the most urbanized area and 7 referring to the least urbanized. The criteria on which these strata were determined included the population density (persons per square kilometer), the number of physicians per 100,000 people, the percentage of people with a college education, the percentage of people older than 65 years, and the percentage of agricultural workers. Because levels 4, 5, 6, and 7 contained few endometriosis cases, they were combined into a single group and were recoded as level 4.
Statistical Analysis
All data processing and statistical analyses were performed with SPSS 20 (SPSS, Chicago, IL) and SAS 8.2 (SAS System for Windows; SAS Institute, Cary, NC). The Pearson χ2 test was used to compare differences in geographic location, monthly income, and urbanization level of patients’ residences between the case and control cohorts. Event occurrence (defined as occurrence of endometrial cancer) was analyzed using the Kaplan-Meier method. The elapsed period was calculated for the patients who had endometriosis until the occurrence of endometrial cancer or the end of the study period (December 31, 2010), whichever came first. After adjusting for urbanization level, monthly income, resident region, and comorbidities as potential confounders, we performed a Cox proportional hazards analysis stratified by age at first diagnosis of endometriosis to investigate the risk for developing endometrial cancer during the 10-year follow-up period in both cohorts. We further classified the age factors in both groups. Hazard ratio and 95% confidence interval (CI) were calculated to quantify the risk for developing endometrial cancer. The results of comparisons with a 2-sided P value of less than 0.05 were considered to represent statistically significant differences.
Ethical Approval
Insurance reimbursement claims adopted in this study were from Taiwan’s NHIRDs, which are available for research purposes. This study was conducted in accordance with the Helsinki Declaration. This study was also evaluated and approved by the institutional review board of Taipei Veterans General Hospital.
RESULTS
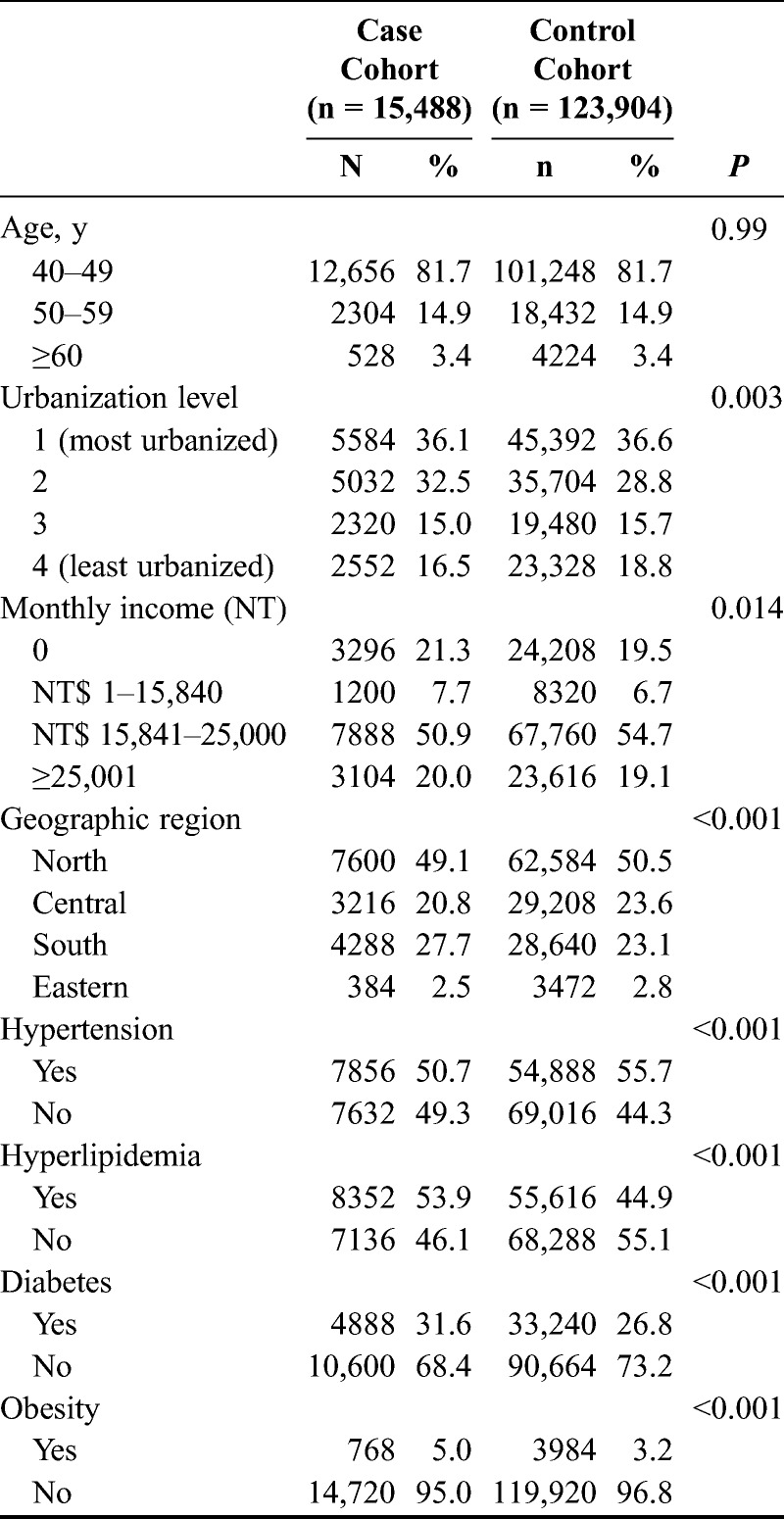
The case cohort contained 15,488 patients diagnosed with endometriosis, whereas 123,904 patients were included in the control cohort. Distributions of demographic characteristics and comorbidities for both the case and the control cohort are shown in Table 1. Hyperlipidemia (P < 0.001), diabetes mellitus (P < 0.001), and obesity (P < 0.001) were more prevalent in the case cohort than in the control cohort. The case cohort also harbored a greater tendency to earn a lower monthly income (P = 0.014), reside in the southern area of Taiwan, and reside in the middle levels of urbanization communities (P = 0.003) compared with the control cohort. In contrast, the control cohort has a higher rate of hypertension (P < 0.001).
TABLE 1.
Demographic characteristics for the recruited participants, stratified by presence/absence of endometriosis from 1997 to 2000
In total, there were 392 participants who were newly diagnosed with endometrial cancer during the 10-year follow-up, with 104 in the case cohort (0.7%) and 288 in the control cohort (0.2%). The Kaplan-Meier survival curves demonstrate significantly lower event-free rates in the case cohort than in the control cohort (P = 0.001, log-rank test) (Fig. 1). Moreover, the incidence density was also higher in the case cohort (0.68 per 1000 patient-years) than in the control cohort (0.23 per 1000 patient-years).
FIGURE 1.
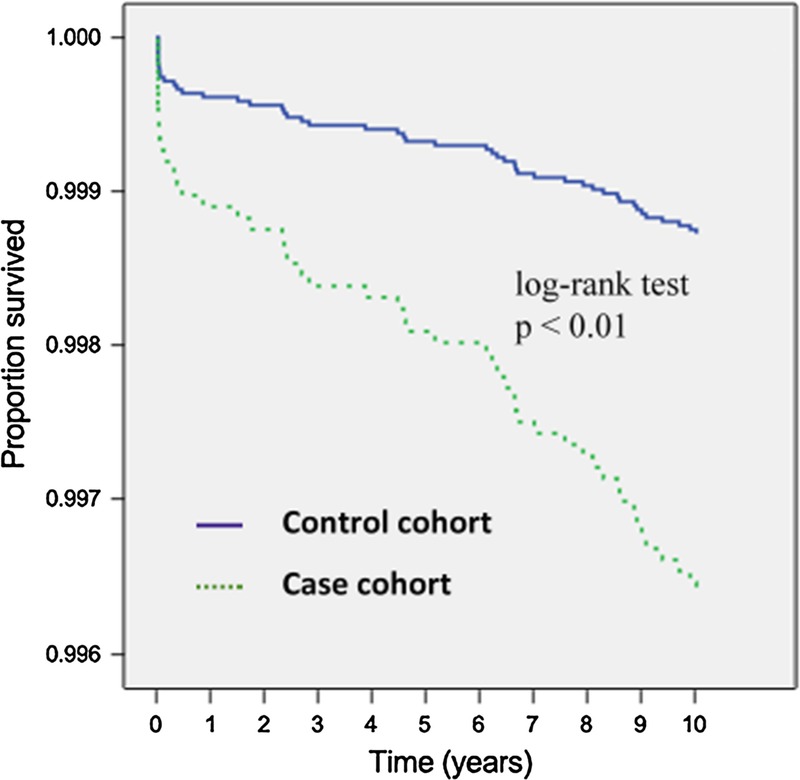
Distribution of endometrial cancer–free rates between the case and the control cohort from 1997 to 2000.
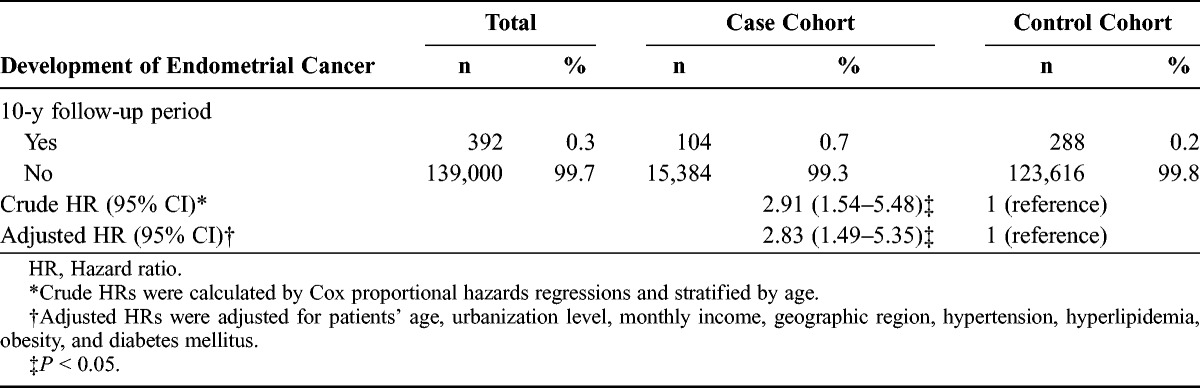
The crude hazard ratio for event occurrence of endometrial cancer was 2.91-fold higher for the case cohort than for the control cohort (95% CI, 1.54–5.48; univariable Cox regression analysis). Furthermore, after adjusting for potential confounders, the hazard ratio did not change significantly, with a 2.83-fold greater risk in the case cohort than that in the control cohort (95% CI, 1.49–5.35; multivariable Cox regression analysis) (Table 2).
TABLE 2.
Hazard ratios of endometrial cancer among endometriosis patients during the 10-year follow-up period from the index ambulatory visits or inpatient care from 1997 to 2000
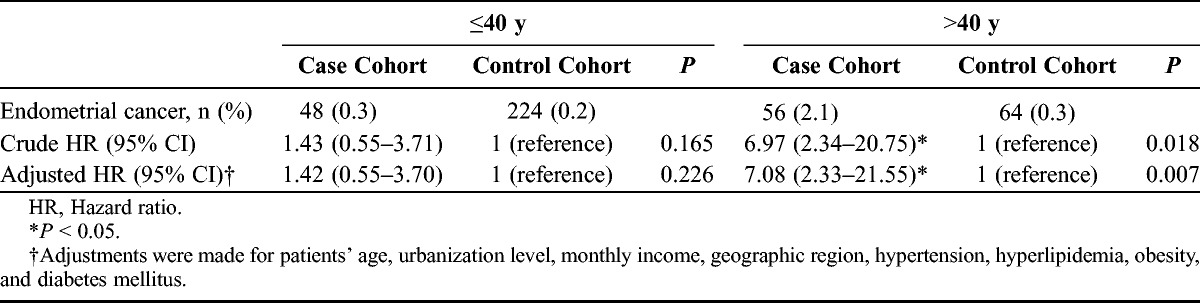
Next, we asked whether age at diagnosis of endometriosis posed any moderator effect on the occurrence of endometrial cancer. Age at diagnosis of endometriosis was divided into 2 groups (≤40 years vs >40 years). When the age at diagnosis of endometriosis is 40 years or younger, there is a non–statistically different 1.42-fold higher adjusted hazard ratio in the case cohort than in the control cohort (95% CI, 0.55–3.70). Whereas when the age at diagnosis of endometriosis is older than 40 years, there is a statistically different 7.08-fold higher adjusted hazard ratio in the case cohort than in the control cohort (95% CI, 2.33–21.55) (Table 3).
TABLE 3.
Hazard ratios for endometrial cancer among the case cohort and the control cohort by age group
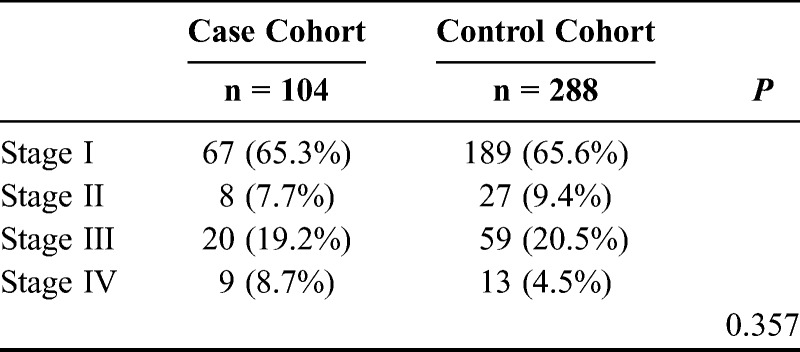
Lastly, we evaluated whether there is a difference for stage distribution between the case cohort and the control cohort. For the occurrence of endometrial cancer, there is no difference with respect to stage distribution between the case cohort and the control cohort (Table 4).
TABLE 4.
Distribution of endometrial cancer among the case cohort and the control cohort by stage
DISCUSSION
The result of our work indicates that patients with endometriosis harbor a higher risk for developing endometrial cancer. Although endometriosis is a benign disease, still, multiple lines of evidence suggest that endometriosis could be viewed as a neoplastic process, including the increased susceptibility to develop some subtypes of epithelial ovarian cancer as well as molecular similarities between endometriosis and cancer.16 It is estimated that ovarian endometriosis has a 0.7% malignant transformation risk17 and 4.2 times greater risk for developing ovarian cancer.18 Furthermore, a study by Zaino et al13 showed that endometriosis was identified in approximately 30% of the cases with synchronous endometrioid type endometrial and ovarian cancers.
Although the association between endometriosis and ovarian cancer has been extensively investigated, in contrast, the association between endometriosis and endometrial cancer has rarely been reported.18–21 The results of our work show discrepancy with the published articles. The potential reasons may include selection bias (because most endometriosis cases are underdiagnosed), different study design (cohort study design of our work vs case-control design of the published articles), different ethnic group, and different baseline demographics.
The underlying molecular mechanisms by which these 2 disorders are connected remain uncertain. However, there are 2 putative shared mechanisms: estrogen stimulation and chronic inflammation. For the first mechanism, like uterine or breast cancer, endometriosis behaves as an estrogen-dependent disorder, specifically adapting to estrogen-induced signaling, by increased local production of estrogen through enhanced expression of aromatase cytochrome P450 expression but deficient 17β-hydroxysteroid dehydrogenase type 2 expression (which impairs inactivation of potent estradiol to less-potent estrone).22 Evidence has further shown that there is pathological overexpression of estrogen receptor β (ERβ) in endometriotic stromal cells, resulting from deficient methylation of the ERβ promoter, which also suppress estrogen α (ERα) expression.23 Studies in endometrial carcinoma has demonstrated paralleled high ERβ-to-ERα ratio, with the amount of ERα messenger RNA significantly lower in poorly differentiated endometrial cancer.24–26 Furthermore, progesterone receptor has been viewed as a classic ERα target gene. High ERβ-to-ERα ratio in endometriotic stromal cells might contribute to the suppressed progesterone receptor and thus causes progesterone resistance, which might contribute to progesterone treatment failure in patients with endometriosis and/or endometrial cancer.23
For the second mechanism, the impact of chronic inflammation may play another critical role. Endometriosis tissue is associated with overproduction of prostaglandins, cytokines, and chemokines.27 Among these, cyclooxygenase 2 (COX-2), a rate-limiting enzyme in the biosynthesis of prostaglandin E2, increased in both endometriosis and endometrial cancer patients.28 Prostaglandin E2 promotes the initial carcinogenesis process and further consolidates tumor progression by increasing cell proliferation and neoangiogenesis while decreasing in situ immune performance.29
The impact of estrogen stimulation and chronic inflammation may not be mutually exclusive. Association between COX-2 and aromatase expression with increased estrogen production has been investigated. The process regulates COX-2 in a positive feedback loop.30,31 Fowler et al28 found aromatase expression in 65% of endometrial cancer patients compared with nonexpression in normal endometrium; the results showed no difference in different histology groups. A study by Collins et al25 found a high ratio of COX-2 detected in patients with ER-positive endometrial cancer. On the basis of the above descriptions, the interconnection between COX-2, ER, and aromatase is close and might exert a synergetic effect, and, as such, there is association between endometriosis and endometrial cancer via the link by chronic inflammation.
The strengths of our study include its use of a population-based database that is highly representative of the general population. Still, certain limitations to our findings should be considered. First, we selected patients only by ICD code; potential bias including patient selection and diagnosis criteria might be present. Second, the NHIRDs data set does not contain detailed information regarding parity, menstrual status, and hormonal use, all of which may be potential risk factors of endometriosis and/or endometrial cancer. These unmeasured variables may produce a confounding bias if it is associated with the studied exposure and disease simultaneously.32 Third, the evidence derived from a retrospective cohort study is generally lower in research quality than that from randomized trials. To this end, further prospective cohort studies with adequate sample size are needed to verify the temporal association between endometriosis and endometrial cancer.
To summarize, the findings in this study included increased association of endometriosis with endometrial cancer. The pathogenesis in endometriosis and endometrial cancer is complicated and the etiopathogenesis of both disorders is multifactorial, but there may exist a yet unidentified common link. The putative linking mechanisms may contain both estrogen stimulation and chronic inflammation. However, much work is still needed to fully explain the exact mechanisms between these 2 disorders.
ACKNOWLEDGMENTS
The authors thank the Task Force on Endometriosis Research group, Lilly Wen, MD (Catholic Cardinal Tien Hospital, Taipei); Kuo-Chang Wen, MD; Wei-Min Hu, MD; Hsiao-Wen Tsai, MD; Pi-Lin Sung, MD; Wei-Lun Hsu, MD; Chih-Yu Chen, MD; Jen-Yu Huang, MD; Chia-Ming Chang, MD; Peng-Hui Wang, MD; Nae-Fong Twu, MD; and Hsiang-Tai Chao, MD (Veterans General Hospital, Taipei).
Footnotes
Supported by the National Science Council, Taiwan (Grants No. NSC 101-2623-E-010-001-NU).
The authors declare no conflicts of interest.
This study is based in part on data from the NHIRDs provided by the Bureau of NHI, Department of Health, Taiwan, and managed by the NHRI. The interpretations and conclusions reported herein do not represent those of the Bureau of NHI, Department of Health, or the NHRI.
REFERENCES
- 1. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61: 212– 236. [DOI] [PubMed] [Google Scholar]
- 2. Munstedt K, Grant P, Woenckhaus J, et al. Cancer of the endometrium: current aspects of diagnostics and treatment. World J Surg Oncol. 2004; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000; 13: 295– 308. [DOI] [PubMed] [Google Scholar]
- 4. Groothuis PG, Dassen HH, Romano A, et al. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007; 13: 405– 417. [DOI] [PubMed] [Google Scholar]
- 5. Song M, Karabina SA, Kavtaradze N, et al. Presence of endometrial epithelial cells in the peritoneal cavity and the mesothelial inflammatory response. Fertil Steril. 2003; 79(suppl 1): 789– 794. [DOI] [PubMed] [Google Scholar]
- 6. Kyama CM, Overbergh L, Debrock S, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006; 85: 1667– 1675. [DOI] [PubMed] [Google Scholar]
- 7. Bulun SE. Endometriosis. N Engl J Med. 2009; 360: 268– 279. [DOI] [PubMed] [Google Scholar]
- 8. Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003; 30: 1– 19, vii. [DOI] [PubMed] [Google Scholar]
- 9. Giudice LC, Kao LC. Endometriosis. Lancet. 2004; 364: 1789– 1799. [DOI] [PubMed] [Google Scholar]
- 10. Missmer SA, Hankinson SE, Spiegelman D, et al. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004; 82: 1501– 1508. [DOI] [PubMed] [Google Scholar]
- 11. Worley MJ, Welch WR, Berkowitz RS, et al. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013; 14: 5367– 5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stern RC, Dash R, Bentley RC, et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001; 20: 133– 139. [DOI] [PubMed] [Google Scholar]
- 13. Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001; 83: 355– 362. [DOI] [PubMed] [Google Scholar]
- 14.Bureau of National Health Insurance, Department of Health, Executive Yuan. March 1, 1995. Available at: http://nhird.nhri.org.tw/ Accessed February 28, 2014.
- 15. Kramer MS, Moodie EE, Dahhou M, et al. Breastfeeding and infant size: evidence of reverse causality. Am J Epidemiol. 2011; 173: 978– 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varma R, Rollason T, Gupta JK, et al. Endometriosis and the neoplastic process. Reproduction. 2004; 127: 293– 304. [DOI] [PubMed] [Google Scholar]
- 17. Nishida M, Watanabe K, Sato N, et al. Malignant transformation of ovarian endometriosis. Gynecol Obstet Invest. 2000; 50(suppl 1): 18– 25. [DOI] [PubMed] [Google Scholar]
- 18. Brinton LA, Gridley G, Persson I, et al. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997; 176: 572– 579. [DOI] [PubMed] [Google Scholar]
- 19. Rowlands IJ, Nagle CM, Spurdle AB, et al. Gynecological conditions and the risk of endometrial cancer. Gynecol Oncol. 2011; 123: 537– 541. [DOI] [PubMed] [Google Scholar]
- 20. Brinton LA, Sakoda LC, Sherman ME, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev. 2005; 14: 2929– 2935. [DOI] [PubMed] [Google Scholar]
- 21. Zucchetto A, Serraino D, Polesel J, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev. 2009; 18: 316– 321. [DOI] [PubMed] [Google Scholar]
- 22. Bulun SE, Zeitoun KM, Takayama K, et al. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J Mol Endocrinol. 2000; 25: 35– 42. [DOI] [PubMed] [Google Scholar]
- 23. Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012; 30: 39– 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skrzypczak M, Bieche I, Szymczak S, et al. Evaluation of mRNA expression of estrogen receptor beta and its isoforms in human normal and neoplastic endometrium. Int J Cancer. 2004; 110: 783– 787. [DOI] [PubMed] [Google Scholar]
- 25. Collins F, MacPherson S, Brown P, et al. Expression of oestrogen receptors, ERalpha, ERbeta, and ERbeta variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERalpha. BMC Cancer. 2009; 9: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boney-Montoya J, Ziegler YS, Curtis CD, et al. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol. 2010; 24: 346– 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasir A, Boulware D, Kaiser HE, et al. Cyclooxygenase-2 (COX-2) expression in human endometrial carcinoma and precursor lesions and its possible use in cancer chemoprevention and therapy. In Vivo. 2007; 21: 35– 43. [PubMed] [Google Scholar]
- 28. Fowler JM, Ramirez N, Cohn DE, et al. Correlation of cyclooxygenase-2 (COX-2) and aromatase expression in human endometrial cancer: tissue microarray analysis. Am J Obstet Gynecol. 2005; 192: 1262– 1271; discussion 1271–1273. [DOI] [PubMed] [Google Scholar]
- 29. Tuynman JB, Hulscher JB, Steller EP, et al. Cyclooxygenase(COX)-2-inhibition in the prevention and treatment of colorectal carcinoma. Ned Tijdschr Geneeskd. 2003; 147: 2207– 2212. [PubMed] [Google Scholar]
- 30. Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002; 80: 203– 212. [DOI] [PubMed] [Google Scholar]
- 31. Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003; 86: 501– 507. [DOI] [PubMed] [Google Scholar]
- 32. Lee WC. Bounding the bias of unmeasured factors with confounding and effect-modifying potentials. Stat Med. 2011; 30: 1007– 1017. [DOI] [PubMed] [Google Scholar]


