Abstract
Objective
To evaluate the association of tumor-derived matrix metalloproteinase 2 (MMP-2) and stromal-derived MMP-2 expression with the prognosis of patients with ovarian cancer, a meta-analysis study was performed, which was aimed to comprehensively review the evidence of MMP-2 as prognostic biomarkers in ovarian cancers.
Methods
All relevant studies were searched in PubMed and Web of Science until May 30, 2014. Hazard ratios (HRs) with their 95% confidence intervals (CIs) were used to assess the association between MMP-2 expression (tumor-derived or stromal-derived) and prognosis of patients with ovarian cancer. Pooled odds ratios (ORs) and their 95% CIs were used to assess the correlation of MMP-2 expression with the clinicopathological features of patients with ovarian cancer.
Results
A total of 965 patients in 8 studies were included in this analysis. Among them, tumor-derived and stromal-derived MMP-2 expression was detected in 7 and 5 articles, respectively. The results revealed that ovarian cancer patients with positive tumor-derived MMP-2 expression showed a worse prognosis than did the ones with negative tumor-derived MMP-2 expression (HR, 1.52; 95% CI, 1.06–2.20). However, ovarian cancer patients with positive stromal-derived MMP-2 expression had not. In addition, we also found that tumor-derived MMP-2 expression was associated with distant metastasis (absent vs present; pooled OR, 4.52; 95% CI, 1.56–13.09; P = 0.001).
Conclusions
These results suggested that positive tumor-derived MMP-2 expression could predict a lower overall survival rate and could be an independent dangerous prognostic factor in patients with ovarian cancer.
Key Words: Tumor-derived, Stromal-derived, MMP-2, Ovarian cancer, Prognosis
Ovarian cancer is the leading cause of the death of all gynecologic tumors.1 Owing to lack of specific and early symptoms as well as effective diagnostic tests, most patients with ovarian cancer present with wide metastatic disease at diagnosis.2 Hence, the prognosis of ovarian cancer is very poor. The 5-year survival rate for patients with ovarian cancer is approximately 44%.3
Several clinicopathological features including International Federation of Gynecology and Obstetrics stage, grade of tumor, and distant metastasis have been recognized as effective factors for prognosis of patients with ovarian cancer.4,5These factors are useful at some time. However, in most situations, patients with ovarian cancer showed totally different outcomes even with the similar stage or treated with same therapies.6 They are insufficient to predict an individual patient’s prognosis. Thus, there is an urgent need for identification and validation of new biomarkers for predicting the prognosis of patients with ovarian cancer and helping physicians make the most appropriate medication choice.
Metastasis influenced significantly the survival rate of ovarian cancer. Invasion is a crucial step for tumor metastasis. In this process, there is tumor cell degradation of basement membranes and extracellular matrix (ECM),7 which depends on a series of complex interactions between tumor cells, host-derived stromal cells, and endothelial cells.8 Matrix metalloproteinases (MMPs) are a family of 24 secreted membrane-type proteases that mediate cell invasion, among which MMP-2 (gelatinase A) plays an important role in cancer cell invasion responsible for degradation of basement membranes and ECM.9
Expression of MMP-2 in cancer cells was reported to be significantly higher in advanced ovarian cancer than in their benign or premalignant counterparts.10 Up to now, large amount of studies confirmed that MMP-2 expression in cancer cells was correlated with advanced tumor grade and progression.11,12 However, not all the related studies showed consistent conclusions. Some showed no relation between tumor-derived MMP-2 and survival rate.13,14 In addition, some studies have revealed that MMPs are detected not only in tumor cells but also in stromal cells.15 A recent study showed that stromal MMP-2 expression was also an important predictor of recurrence-free survival in patients with ovarian adenocarcinomas.16 Nevertheless, the others showed that increased expression of MMP-2 is related with good ovarian cancer survival.17 Thus, whether tumor-derived or stromal-derived MMP-2 is a prognostic factor in ovarian cancer was still contradictory. In addition, these different conclusions were caused by their limited numbers of samples or other factors were not clear. Therefore, we conducted this meta-analysis to quantitatively inspect the significant of expression of MMP-2 in cancer cells or stromal cells for survival of patients with ovarian cancer.
MATERIALS AND METHODS
Search Strategy
All relevant studies were searched in PubMed and Web of Science. We combined the search terms “ovarian cancer,” “ovarian neoplasm,” “ovarian tumor,” “ovarian carcinoma,” “epithelial ovarian cancer,” “matrix metalloproteinase-2,” “MMP-2,” “prognosis,” “prognostic,” “survival,” and “outcome” for MMP-2 expression and ovarian cancer. The last search of these studies was updated on May 30, 2014.
Data Extraction
Data obtained from these studies included information as follows: name of the first author, year of publication, journal, study location, number of patients, method of detection, method of hazard ratio (HR) estimation, HR, as well as its 95% confidence interval (CI). If HR and its 95% CI were not given, we calculated the HRs from the Kaplan-Meier curve. These works were checked by 2 reviewers independently. In the case of conflicting evaluations, agreement was reached after discussion. All related statistical software and methods were performed as previously described.18
Statistical Analysis
The association of MMP-2 expression in cancer cells with grade, TNM stage and distant metastasis, ascites, and histopathological subtype was evaluated using the pooled odds ratios (ORs) and their 95% CIs. Hazard ratios and their 95% CIs were used to evaluate the correlation between tumor-derived or stromal-derived MMP-2 and the survival of patients with ovarian cancer. An HR greater than 1.0 indicated a worse prognosis. To assess the heterogeneity between the studies, a statistical test for heterogeneity was performed on the basis of Q test. When P value is greater than 0.10 and I2 is less than 50%, we deemed no heterogeneity, and fixed-effects model will be used.18 Otherwise, the random-effects model will be used. The potential publication bias was assessed through visual inspection of the funnel plots; an asymmetric plot suggested possible publication bias. The Begg and Egger test was also used to evaluate the publication bias, and publication bias exists when P < 0.05.
RESULTS
Characteristics of Studies Analyzed
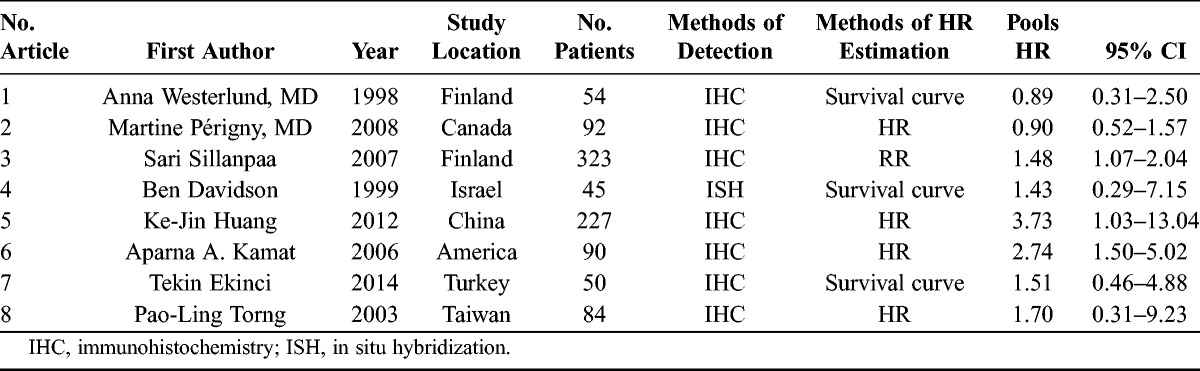
There were 52 articles relevant to searching strategy. Fourty-four of them were finally excluded for the following reasons: (a) no HR could be obtained according to the information supplied from these articles; (b) the articles were not written in English; (c) repetition of data; and (d) not ovarian cancer tissues. Finally, 8 studies were considered to be eligible, and the flow diagram of the selection process for relevant studies is shown in Figure 1. The 8 studies were used for the pooled analysis, and their detailed parameters are shown in Table 1. The 8 included studies were published from 1998 to 2014. A total of 965 patients from Finland, Canada, Israel, China, America, Chinese Taipei, and Turkey were included for the evaluation of HRs and 95% CIs. The follow-up times were from 80 to 120 months. Of these, 7 studies evaluated MMP-2 expression in cancer cells with 881 patients and 5 studies were included in the analysis of MMP-2 expression in stromal cells with 370 patients.
FIGURE 1.
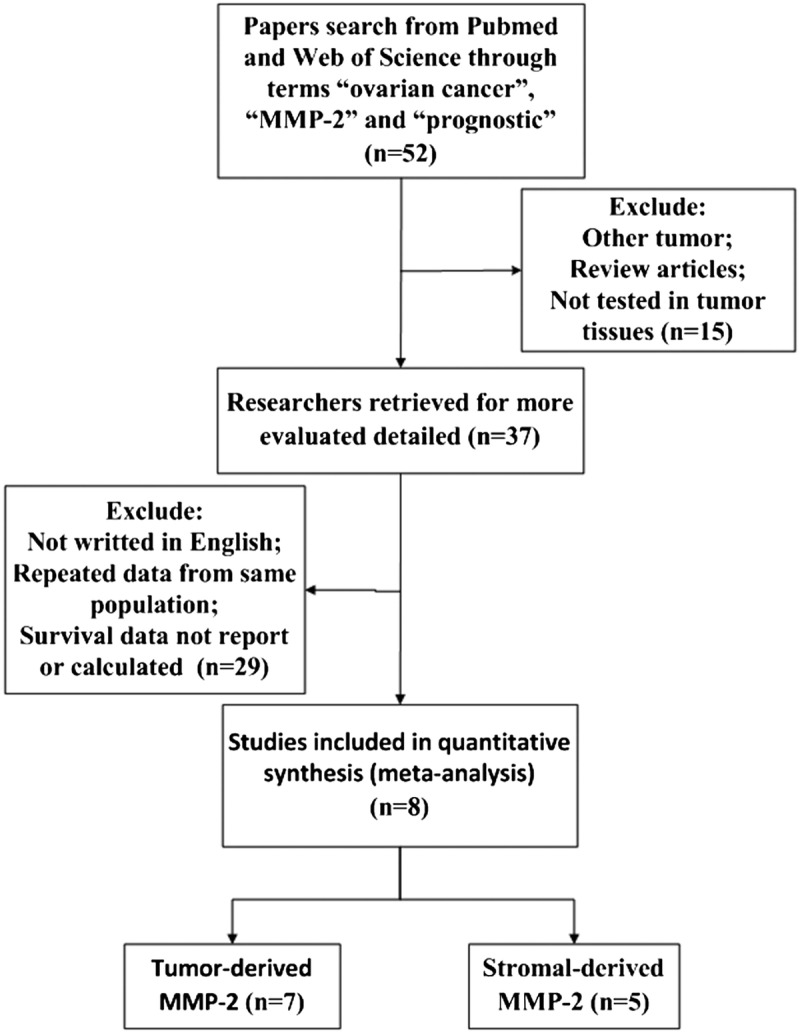
The flow diagram of studies selection.
TABLE 1.
Main characteristics and results of the eligible studies
Meta-Analysis of Expression of MMP-2 and Ovarian Cancer
We first analyzed the HR value of tumor-derived MMP-2 expression positive and negative group. Test of heterogeneity has shown the following results: χ2 = 10.05, I2 = 40.3%, I2 <50%, P = 0.122, and P > 0.1; hence, the fixed model was chosen. There was a difference between the 2 groups (HR, 1.52; 95% CI, 1.06–2.20), and MMP-2 positive expression was associated with poor overall survival, with its 95% CI overlapping with 1 (Fig. 2).
FIGURE 2.
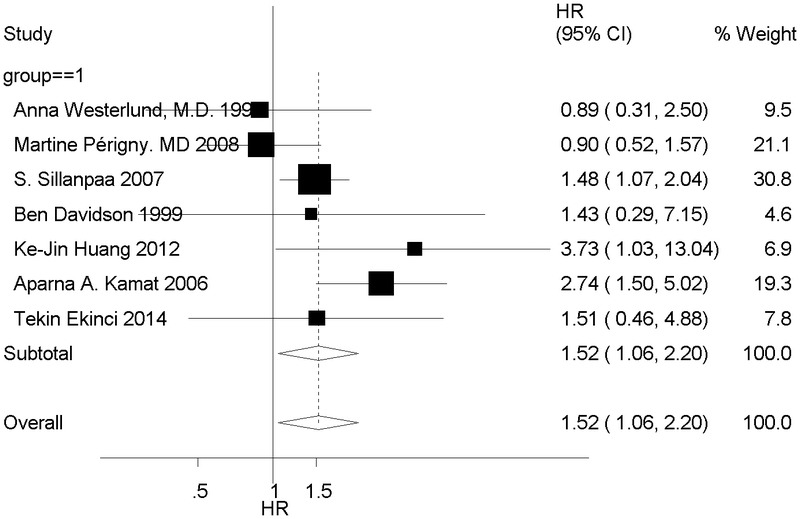
Forest plot shows that positive tumor-derived MMP-2 expression indicates a poor prognosis of patients with ovarian cancer.
We then evaluated the HR value of prognosis between MMP-2 positive and negative expression in stromal cells. The random model was chosen because of heterogeneity (I2 = 73.3%, P = 0.000, P < 0.1). No difference was observed between the 2 groups (HR, 1.28; 95% CI, 0.56–2.92), and stromal-derived MMP-2 positive expression was not associated with poor overall survival, with its 95% CI (Fig. 3).
FIGURE 3.
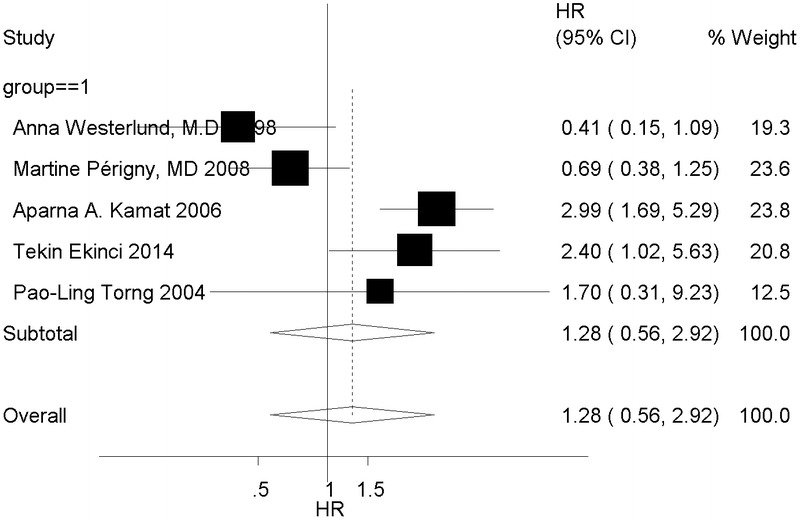
Forest plot shows the relation of positive stromal-derived MMP-2 expression with prognosis of patients with ovarian cancer.
Subgroup Analysis of Tumor-Derived MM-2 Increased Expression and Prognosis
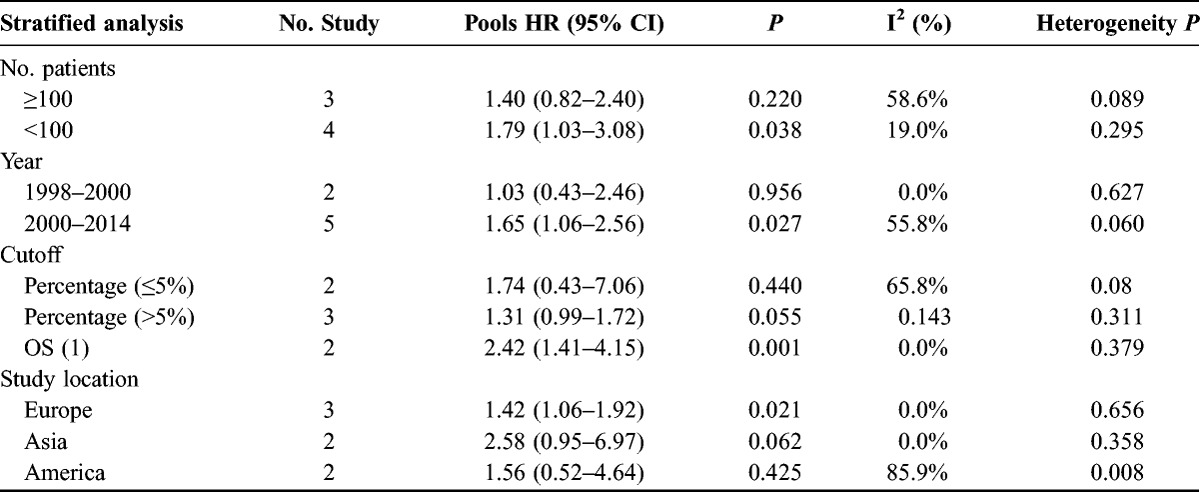
Subgroup analysis of number of patients, publication year, cutoff, and study location was then performed. The subgroup analysis indicated a significant relation between increased tumor-derived MM-2 expression and prognosis in studies with less than 100 patients (HR, 1.79; 95% CI, 1.03–3.08; P=0.038), but not in studies with more than 100 patients (HR, 1.40; 95% CI, 0.82–2.40) (Table 2). The subgroup analysis also suggested a significant relation between tumor-derived MMP-2 expression with prognosis in subgroup of Europe (HR, 1.42; 95% CI, 1.06–1.92; P = 0.021), publication year from 2000 to 2014 (HR, 1.65; 95% CI, 1.06–2.56; P = 0.027), and cutoff detection method with overall score (OS) (HR, 2.42; 95% CI, 1.41–4.15; P = 0.021).
TABLE 2.
Subgroup analysis of tumor-derived MM-2 increased expression and prognosis
The Correlation of Tumor-Derived MMP-2 Expression with Clinicopathological Features
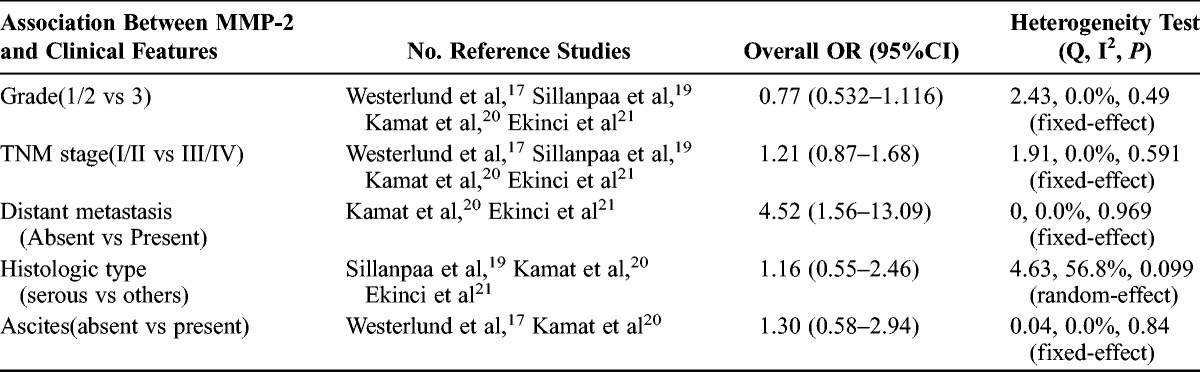
The analysis of association of MMP-2 expression in cancer cells with clinicopathological features was also performed. As shown in Table 2, no correlation was found between MMP-2 expression and clinicopathological features, such as grade (1/2 vs 3; pooled OR, 0.77; 95% CI, 0.53–1.16; P = 0.49), TNM stage (I/II vs III/IV; pooled OR, 1.21; 95% CI, 0.87–2.68; P = 0.59), ascites (absent vs present; pooled OR, 1.30; 95% CI, 0.58–2.94; P = 0.84), and histopathological subtype (serous vs others; pooled OR, 1.16; 95% CI, 0.55–2.46; P = 0.099). However, positive MMP-2 expression in cancer cells was correlated with distant metastasis (absent vs present; pooled OR, 4.52; 95% CI, 1.56–13.09; P = 0.001) (Table 3).
TABLE 3.
Main results for meta-analysis between tumor-derived MMP-2 and clinicopathological features
Publication Bias
Both Begg funnel plot and Egger tests were performed to assess the publication bias of the included literature (Fig. 4). Both Begg test and Egger test did not suggest any evidence of publication bias.
FIGURE 4.
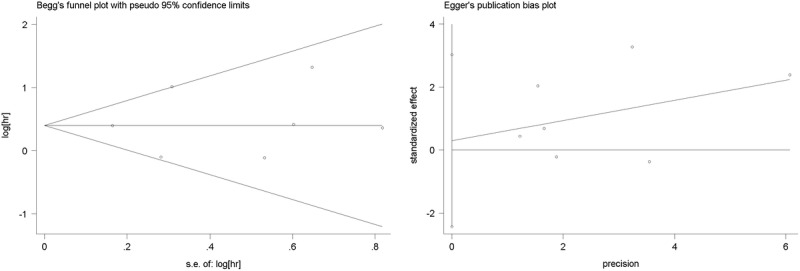
The Begg and Egger funnel plot evaluated the publication bias of the selected studies.
DISCUSSION
Current meta-analysis studies of MMP-2 expression mainly focus on tumor-derived MMP-2,22–24 and little analysis is available regarding the expression of MMP-2 in stromal cells of tumors. This meta-analysis, which combined results from 8 studies of 965 patients, explores the association of tumor-derived MMP-2 and stromal-derived MMP-2 with ovarian cancer prognosis.14,16,17,19–21,25,26 We demonstrated that the expression of MMP-2 in cancer cells was associated with a significant reduction of overall survival rate (HR, 1.52; 95% CI, 1.06–2.20), whereas the expression of MMP-2 in stromal cells did not appear to have an overall influence on the patients survival rate of ovarian cancer (HR, 1.28; 95% CI, 0.56–2.92).
Ovarian cancer is usually diagnosed in advanced stages. Metastasis is the most important characteristics of advanced cancer. It is known that tumor metastasis occurs by a series of steps including invasion, vessel formation, cell attachment, and cell proliferation.27 Invasion is one important step in the process of cancer metastasis. This process requires different cellular proteolytic enzymes, among which MMPs are one of important families of proteinases responsible for the ECM destruction. Among more than 20 identified MMPs, MMP 2 can efficiently degrade native collagen types IV and V, fibronectin, entactin, as well as elastin. Therefore, MMP 2 is considered one of crucial proteases for cell invasion. In our meta-analysis, tumor-derived MMP 2 have a relationship with distant metastasis (absent vs present; pooled OR, 4.52; 95% CI, 1.56–13.09; P = 0.001), indicating that MMP 2 has an important role in cancer metastasis.
According to our meta-analysis, there were several clinical significances. First, increased tumor-derived MMP-2 expression significantly predicted poor prognosis of patients with ovarian cancer, and tumor-derived MMP-2 could be a therapeutic target and a tool for assessing treatment. Second, the subgroup analysis indicated that increased tumor-derived MMP-2 expression significantly predicted poor prognosis of patients with ovarian cancer in Europe, but not in Asia and America. Third, the cutoff value with OS indicated that increased tumor-derived MMP-2 expression in ovarian cancer significantly predicted poor prognosis but not with percentage. It could be that the cutoff value with OS is more comprehensive than percentage. A conclusion remains to be seen in further investigations.
Matrix metalloproteinases can be produced by tumor cells and the surrounding stromal cells, such as fibroblasts and vascular endothelial cells.15 Although some of them showed that positive stromal MMP-2 expression was clearly associated with poor prognosis of patients with ovarian cancer, several reports also found otherwise. For example, Westerlund et al17 found a statistically significant correlation between the negative stromal fibroblast staining for MMP 2 and patient groups with poor prognosis of patients with ovarian cancer. Thus, we next investigated the association of MMP-2 expression in stromal cells with prognosis of ovarian cancer by a meta-analysis with 378 patients. Unexpectedly, no significant difference was observed in survival of ovarian cancer patients with stromal-derived MMP-2 positive and stromal-derived MMP-2 negative (HR, 1.23; 95% CI, 0.49–3.10). The role of stromal cells-derived MMP 2 in progression of ovarian cancer is poorly understood. This could be caused by their limited numbers of samples. Thus, more studies were needed to take into account to evaluate the real association between stromal cells-derived MMP 2 and survival of patients with ovarian cancer.
Although we did our best to conduct a comprehensive analysis, some limitations still exist. First, the sample size of some selected studies is limited, so it is difficult to make a definite conclusion on the prognostic value of MMP 2 among patients with ovarian cancer. Second, different cutoff value methods for positive MMP-2 expression were applied on these studies, resulting in inconsistent conclusions. In addition, some clinicopathological features of many studies were not given. Therefore, biases were unavoidable in this study, all of which seem to affect our analysis, so further studies are needed to take into account to evaluate the real association between MMP-2 expression and patients with ovarian cancer.
In conclusion, our meta-analysis revealed that tumor-derived MMP 2 was involved in the development of ovarian cancer and that positive tumor-derived MMP-2 expression was significantly associated with worse prognosis of patients with ovarian cancer. In terms of the present results, an evaluation of the tumor-derived MMP-2 expression of patients with ovarian cancer could provide relatively accurate prognostic information, and our results could be helpful to provide evidence for effective strategies of further treatment of ovarian cancer.
Footnotes
Supported by the National Natural Science Foundation of China (No. 81402139) and the Key Program of Science and Technology Development Fund of Nanjing Medical University (No. 2013NJMU145).
The authors declare no conflicts of interest.
Authors Ziyi Fu and Sujuan Xu contributed equally to this work.
REFERENCES
- 1. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000; 19: 3– 10. [DOI] [PubMed] [Google Scholar]
- 2. Nowak-Markwitz E, Pula B, Szajnik M, et al. Expression of survivin, SDF-1 and CXCR4 on tumor cells in ovarian cancer [in Polish]. Ginekol Pol. 2010; 81: 674– 677. [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012; 62: 283– 298. [DOI] [PubMed] [Google Scholar]
- 4. Clark TG, Stewart ME, Altman DG, et al. A prognostic model for ovarian cancer. Br J Cancer. 2001; 85: 944– 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman ML. Future directions in the surgical management of ovarian cancer. Gynecol Oncol. 2003; 90(2 pt 2) (2 pt 2): S33– S39. [DOI] [PubMed] [Google Scholar]
- 6. Oldenhuis CN, Oosting SF, Gietema JA, et al. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008; 44: 946– 953. [DOI] [PubMed] [Google Scholar]
- 7. Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008; 359: 2814– 2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997; 91: 439– 442. [DOI] [PubMed] [Google Scholar]
- 9. Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993; 4: 197– 250. [DOI] [PubMed] [Google Scholar]
- 10. Sakata K, Shigemasa K, Nagai N, et al. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol. 2000; 17: 673– 681. [PubMed] [Google Scholar]
- 11. Sheu BC, Lien HC, Ho HN, et al. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003; 63: 6537– 6542. [PubMed] [Google Scholar]
- 12. Guo CB, Wang S, Deng C, et al. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther. 2007; 11: 183– 192. [DOI] [PubMed] [Google Scholar]
- 13. Brun JL, Cortez A, Lesieur B, et al. Expression of MMP-2, -7, -9, MT1-MMP and TIMP-1 and -2 has no prognostic relevance in patients with advanced epithelial ovarian cancer. Oncol Rep. 2012; 27: 1049– 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perigny M, Bairati I, Harvey I, et al. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008; 129: 226– 231. [DOI] [PubMed] [Google Scholar]
- 15. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2: 161– 174. [DOI] [PubMed] [Google Scholar]
- 16. Torng PL, Mao TL, Chan WY, et al. Prognostic significance of stromal metalloproteinase-2 in ovarian adenocarcinoma and its relation to carcinoma progression. Gynecol Oncol. 2004; 92: 559– 567. [DOI] [PubMed] [Google Scholar]
- 17. Westerlund A, Apaja-Sarkkinen M, Hoyhtya M, et al. Gelatinase A-immunoreactive protein in ovarian lesions- prognostic value in epithelial ovarian cancer. Gynecol Oncol. 1999; 75: 91– 98. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Huang Y, Huang Y, et al. The prognostic value of SOX2 expression in non-small cell lung cancer: a meta-analysis. PLoS One. 2013; 8: e71140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sillanpaa S, Anttila M, Suhonen K, et al. Prognostic significance of extracellular matrix metalloproteinase inducer and matrix metalloproteinase 2 in epithelial ovarian cancer. Tumour Biol. 2007; 28: 280– 289. [DOI] [PubMed] [Google Scholar]
- 20. Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006; 12: 1707– 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ekinci T, Ozbay PO, Yigit S, et al. The correlation between immunohistochemical expression of MMP-2 and the prognosis of epithelial ovarian cancer. Ginekol Pol. 2014; 85: 121– 130. [DOI] [PubMed] [Google Scholar]
- 22. Shen W, Xi H, Wei B, et al. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysis. J Cancer Res Clin Oncol. 2014; 140: 1003– 1009. [DOI] [PubMed] [Google Scholar]
- 23. Yan Y, Liang H, Li T, et al. The MMP-1, MMP-2, and MMP-9 gene polymorphisms and susceptibility to bladder cancer: a meta-analysis. Tumour Biol. 2014; 35: 3047– 3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Li C, Zhu M, et al. Meta-analysis of MMP2, MMP3, and MMP9 promoter polymorphisms and head and neck cancer risk. PLoS One. 2013; 8: e62023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davidson B, Goldberg I, Gotlieb WH, et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999; 17: 799– 808. [DOI] [PubMed] [Google Scholar]
- 26. Huang KJ, Sui LH. The relevance and role of vascular endothelial growth factor C, matrix metalloproteinase-2 and E-cadherin in epithelial ovarian cancer. Med Oncol. 2012; 29: 318– 323. [DOI] [PubMed] [Google Scholar]
- 27. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011; 331: 1559– 1564. [DOI] [PubMed] [Google Scholar]


