Abstract
The susceptibility of reef-building corals to climatic anomalies is well documented and a cause of great concern for the future of coral reefs. Reef corals are normally considered to tolerate only a narrow range of climatic conditions with only a small number of species considered heat-tolerant. Occasionally however, corals can be seen thriving in unusually harsh reef settings and these are cause for some optimism about the future of coral reefs. Here we document for the first time a diverse assemblage of 225 species of hard corals occurring in the intertidal zone of the Bonaparte Archipelago, north western Australia. We compare the environmental conditions at our study site (tidal regime, SST and level of turbidity) with those experienced at four other more typical tropical reef locations with similar levels of diversity. Physical extremes in the Bonaparte Archipelago include tidal oscillations of up to 8 m, long subaerial exposure times (>3.5 hrs), prolonged exposure to high SST and fluctuating turbidity levels. We conclude the timing of low tide in the coolest parts of the day ameliorates the severity of subaerial exposure, and the combination of strong currents and a naturally high sediment regime helps to offset light and heat stress. The low level of anthropogenic impact and proximity to the Indo-west Pacific centre of diversity are likely to further promote resistance and resilience in this community. This assemblage provides an indication of what corals may have existed in other nearshore locations in the past prior to widespread coastal development, eutrophication, coral predator and disease outbreaks and coral bleaching events. Our results call for a re-evaluation of what conditions are optimal for coral survival, and the Bonaparte intertidal community presents an ideal model system for exploring how species resilience is conferred in the absence of confounding factors such as pollution.
Introduction
Reef corals tolerate only a narrow range of environmental conditions; hence widespread coral bleaching events, coupled with land-use impacts, have resulted in rapid and progressive degradation of coral reef habitats [1], [2]. Today, one third of coral species face an elevated risk of extinction [3] and those corals living in intertidal nearshore habitats are particularly threatened [4]. In addition to direct anthropogenic impacts (i.e. habitat modification, pollution, dredging, over-harvesting), intertidal coral communities must withstand multiple abiotic stressors including emersion during low tide, fluctuating temperature, light and wind conditions, physical damage from waves, and sediment and freshwater inundation [5–7]. These impacts may be rapid and pronounced in shallow reef communities [8], [9], hence intertidal fringing reef coral communities are increasingly impoverished [10] and often only the hardiest corals survive in the intertidal zone [11–13].
Scleractinian corals are critical components of the coral reef ecosystem, providing the structural framework of reefs; they contribute to primary production, nutrient recycling, and provide microhabitat and a food source for a wide diversity of coral reef organisms [14]. Hence, resource managers urgently need effective strategies to mitigate the risks imposed on corals to safeguard coral reef ecosystems [15], [16]. One promising approach is to identify existing coral communities that are hardened to climatic extremes and to determine how these communities tolerate stress [17]. To date, only a small number of populations of a restricted subset of species have been shown to tolerate climatic stress (e.g. Acropora hyacinthus in Ofu Island Lagoon, American Samoa [18]; back reef communities in the Western Caribbean [19]; and coral communities in the Persian/Arabian Gulf [20]).
In this study we examine the species composition and diversity of reef-building corals growing on intertidal fringing reef flats across three island groups in the Bonaparte Archipelago, Kimberley, north western Australia. We compare the environmental conditions and species diversity of these intertidal communities with those of other shallow fringing reef communities around Australia, and discuss how such a high diversity of coral is sustained in this dynamic and severe environmental setting.
Methods
Ethics Statement
All necessary permits were obtained for the described field studies. A coral collection permit was obtained from the Western Australian Fisheries Department, Permit Number—SPA 01/07.
Study Sites
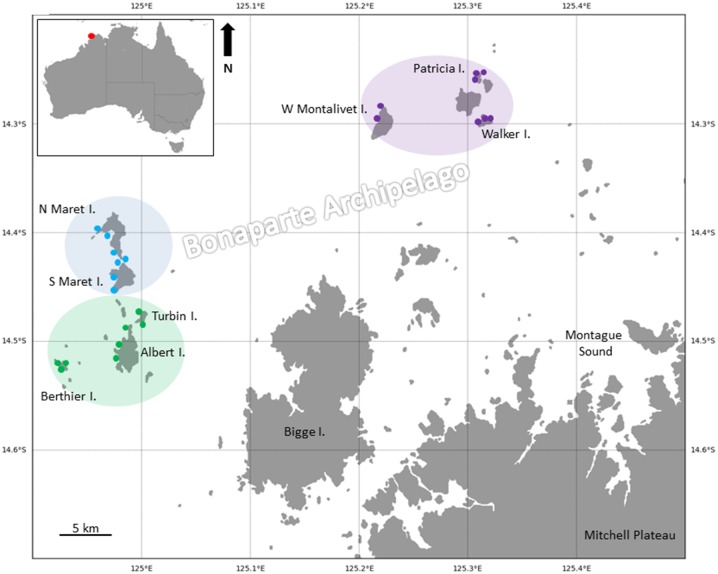
The Bonaparte Archipelago is located in north western Australia (Fig. 1) and is part of the Kimberley Bioregion [21]. The Kimberley consists of many island archipelagos with fringing reefs, platform reefs, submerged banks and offshore atolls [22]. Low energy, macro-tidal conditions characterize the region and the tidally-driven currents together with shelf position and the distance from rivers and estuaries influences the level of turbidity [23].
Fig 1. Map of study sites in the Bonaparte Archipelago, Kimberley, Australia.
Individual dots indicate survey sites within the three main island groups—Berthier (green), Maret (blue) and Montalivet (purple). Site names and details are listed in Table A of S1 File.
Field Surveys
In this study scleractinian coral biodiversity was recorded at 23 sites across ten islands (North and South Maret I., West Montalivet I., East Montalivet I.,Walker I., Patricia I., Berthier I., Albert I., Turbin I., Suffren I.) from three island groups (Maret, Berthier and Montelivet) in the Bonaparte Archipelago (Fig. 1, Table A in S1 File). Saltwater crocodiles (Crocodylus porosus) frequent these reefs and diving was prohibited under workplace safety regulations, hence only the intertidal habitat was examined, during low spring tides of 1st September–20th October 2007. Approximately 240 m2 of inner and outer reef flat and reef crest habitat was surveyed at each site and all coral species encountered were identified in situ or collected for later identification.
Coral diversity was surveyed using a rapid ecological assessment methodology. To determine the relative frequency of species occurrence, all species at each site were classified into one of the following five categories of abundance: Category 1–rare (1–2 colonies); Category 2–infrequent (3–5 colonies); Category 3–frequent (6–20 colonies); Category 4–common (21–50 colonies); and Category 5–dominant (51 or more colonies). Since accurate in-situ ID of many coral species is not possible we collected small (5–8cm) skeletal samples which were bleached in a 3% hypochlorite solution overnight and then air-dried and returned to the laboratory for ID. Identifications were carried out with comparison to known and type specimens in the Queensland Museum collection according to: [24] for Acropora and Isopora; [25] for Fungiidae; [26] for Psammocora; [27], [28] for Lobophylliidae, Merulinidae, Montastraeidae and Diploastraeidae; and [29] for all taxa that have not been revised recently. Moreover, the higher-level taxonomic classifications used in this study reflect the classifications listed in the World Register of Marine Species http://www.marinespecies.org/ as of September 2014. New distribution records were verified by discussion with JEN Veron and with comparison to the Corals of the World database: http://www.coralsoftheworld.com. Specimens have been deposited with the Queensland Museum.
Analyses
To examine the adequacy of local sampling at the Bonaparte Archipelago, a species accumulation curve was calculated using the “specaccum” function of the “vegan” library in R with jack-knifed standard errors http://www.r-project.org/.
To assess the proportion of coral species from our study not normally found in the intertidal zone, we looked for intertidal vs subtidal records for the same species in the Museum of Tropical Queensland database (>28,000 specimen-based records). To compare the diversity observed in the intertidal zone in the Kimberley with that seen in other more typical reef locations, we standardized the area surveyed to 100m2 and compared this with a semi-quantitative estimate of the level of diversity recorded elsewhere in the NE Indian Ocean to the NW Pacific Ocean (see Table B in S1 File).
A resemblance matrix based on Bray-Curtis similarities was constructed using square-root transformed abundance data from the 23 sites using PRIMER-E v6 [30]. Agglomerative CLUSTER analysis was used to group the sites according to the similarity in coral assemblage composition using group average linkage distances. We used Krustal’s non-metric multidimensional scaling (MDS) analysis to visualize the variation between sites as a 2-D plot.
Physical Variables
Hourly tidal predictions for North Maret I. (Bonaparte Archipelago, Kimberley, Australia); (Scott Reef, Offshore Atoll, Kimberley); Barrow I. (Pilbara, Western Australia); Lizard I. (Northern Great Barrier Reef, Australia) and Dent I. (Whitsundays, Central Queensland, Australia) (see Table C in S1 File for co-ordinates) were obtained from the National Tide centre of the Australian Bureau of Meteorology for the years 2002–2014 http://www.bom.gov.au/oceanography/projects/ntc/ntc.shtml. The average proportional occurrence of tidal amplitude per year (2002–2014) at 1 m intervals (± SD) was plotted to compare the distribution of tidal amplitudes between regions. The proportional occurrence of spring low tides (≤2 m) at hourly intervals for North Maret I. was also plotted to illustrate time of day and length of time intertidal corals are exposed to air during different lunar phases (2002–2014).
To provide environmental data we used satellite imagery captured by the MODerate resolution Imaging Spectroradiometer (MODIS) onboard NASA’s Aqua satellite. This sensor captures imagery at many spectral bands from the visible to the far infrared (http://modis.gsfc.nasa.gov/about/) on a near daily repeat cycle. Empirical algorithms are then applied to the spectral information to derive geophysical parameters such as sea surface temperature (SST) [24] and the diffuse down-welling attenuation coefficient {Kd(490 nm), [31]. We obtained eight day averaged global data at 4 km spatial resolution of SST and Kd(490 nm) from http://oceandata.sci.gsfc.nasa.gov/MODISA/Mapped/8Day/4km/ from July 2002 to June 2014. The SST data over a 12km × 12km region about a coordinate of 125.0°E/ 14.3750°S were averaged at each time stamp to obtain a SST time series for North Maret I. The same approach was used to obtain average SST estimates for the other four locations and co-ordinates listed in Table C in S1 File.
Kd(490) is a measure of the light penetrability in the water column and as such is a proxy for turbidity, where higher Kd(490) values pertains to lesser penetration of light into the water column and hence greater turbidity. The operational MODIS Kd(490) algorithm relies on a log-transformed ratio between the remote sensing reflectance at 488 and 547 nm and has been validated for oceanic waters with negligible bottom reflectance [32], [33]. Over optically shallow waters such as in coral reefs, the large contribution of bottom reflectance to the above-water radiances can over-estimate parameters that rely on this spectral ratio [34], [35]. To minimize this effect the Kd(490) values were taken over adjacent deep-water regions for each location and averaged to obtain a time series for each location. These values should therefore be interpreted as the average minimum turbidity at each location. Lastly, we conducted a series of one-way ANOVA’s to test the null hypothesis that there is a significant difference between the SST and Kd(490) time-series values between North Maret I., and Scott Reef, Barrow I., Lizard I. and Dent I.
Results and Discussion
Species Diversity
Based on a skeletal collection of 506 corals from 23 intertidal sites, we document 225 species of hard coral from 60 genera occurring in the northern sector of the Bonaparte Archipelago (Table D in S1 File). Seven of these species are newly recorded from Western Australia (Goniopora fruitcosa, Goniopora norfolkensis, Isopora crateriformis, Lobophyllia flabelliformis, Lobophyllia serratus, Platygyra acuta, Stylaraea punctata) and this study extends their distribution range from Indonesia and the NW Pacific to include the eastern Indian Ocean.
Our study provides a robust representation of the observed local species richness (see species accumulation curve—Figure A in S1 File); however this estimate is conservative because we only present data pertaining to species records that have been substantiated with a reference skeletal specimen. Furthermore, intertidal coral communities contain a subset of the local diversity (70–90%) [13], [36] hence we estimate a further 23–68 species could be expected if subtidal habitats were surveyed but further collection efforts are required to verify this.
By comparing the current intertidal records with over 28,000 specimen-based depth distribution records in the Museum of Tropical Queensland we document 34 species in the intertidal zone that have previously been recorded only from subtidal habitats (e. g. Echinopora gemmacea, Stylaraea punctata, Oulastrea crispata— Fig. 2f) (Table D in S1 File). Numerous other species we recorded in this inshore habitat were only previously recorded from offshore clear water habitats (e.g. Leptastrea pruinosa, Astreopora myriophthalma [29].
Fig 2. A high diversity of coral thrives in the Bonaparte intertidal zone.
(a) Thickets of branching Acropora aspera and A. muricata dominate the inner reef platform at north Patricia I., (b) On low tides corals are exposed to air for up to three and a half hours at a time, (c) A high diversity of Acropora species thrive on the outer fringing reef platform at north Patricia I., (d) An aggregation of juvenile and subadult corals inhabiting a steep granite cliff-face on the east side of Walker I., (e) Goniastrea coral heads dominate the inner platform at north-west Patricia I.,(f). Oulastrea crispata a rare and distinctive species that normally occurs subtidally was encrusting a granite boulder on the rocky shore of Walker I.
The diversity of hard corals in the three island groups of the Bonaparte Archipelago was similar to, or higher than a semi-quantitative estimate of the level of diversity in intertidal or shallow subtidal habitats (0–5 m depth) in other parts of the Indo-Pacific (Figure B in S1 File). The level of diversity was similar to that estimated for inshore fringing reefs in the central sector of the Great Barrier Reef (i.e. Dent I., Border I., Whitsundays) ~ 2 decades ago [37] prior to the well documented decline of coral cover and condition of the inshore mid to southern sections of the Great Barrier Reef [38–40].
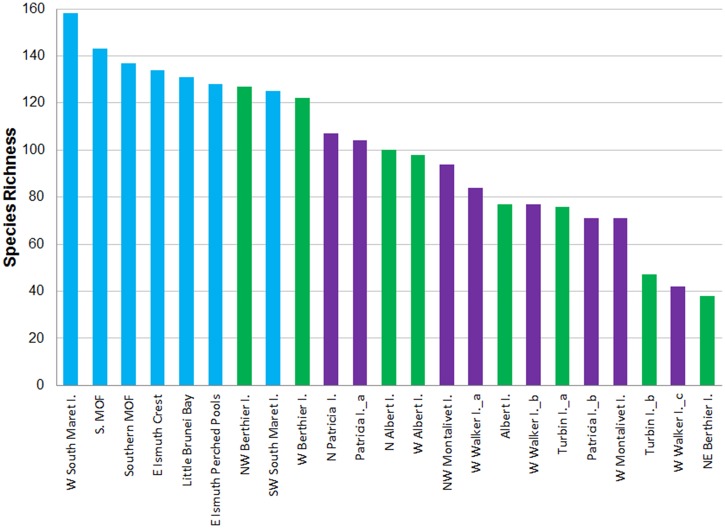
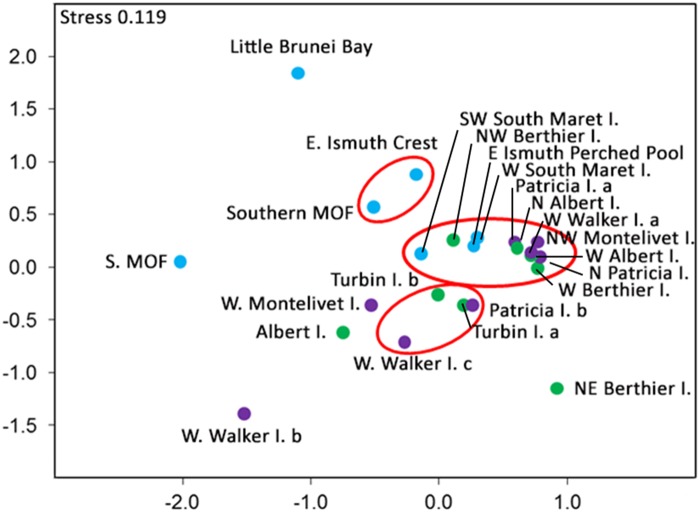
Species richness was highest within the Maret group and peaked on the western side of South Maret I. (n = 158) (Fig. 3). All Maret I. sites had over 120 species. The diversity of corals at sites in the Berthier group ranged from 38–127 species, while diversity of corals at sites within the Montalivet group ranged from 42–107 species. The spatial variation in the composition of coral assemblages is clear in the 2D nmMDS plot which shows Little Brunei Bay and S. MOF sites at Maret I. being distinctive from other sites in Maret I. (Fig. 4). There is a degree of overlap in the coral assemblages across the three island groups, with 11 of the sites grouping together with 60% similarity.
Fig 3. Spatial variation in the total species richness recorded at 23 sites spanning three island groups in the Northern sector of the Bonaparte Archipelago.
Maret Is. group (blue columns); Berthier Is. group (green columns) and Montalivet Is. group (purple columns).
Fig 4. Spatial variation in the composition of the coral assemblages at three island groups in the Bonaparte Archipelago.
Maret Group (blue dots); Berthier Group (green dots) and Montalivet Group (purple dots). Kruskal’s non-metric multidimensional scaling (nm-MDS), using Bray-Curtis similarity index of the coral assemblage at 23 sites based on relative abundance data. Linkages are based on weighted pair group averages and ellipses indicate those sites with 60% similarity at P < 0.001.
Seven species were locally widespread (Pocillopora damicornis; Symphyllia recta; Acropora hyacinthus, Galaxea astreata, Coelastrea aspera, Lobophyllia hemprichii and Stylophora pistillata) and 33 other species, including five Acropora species (A. aspera, A. millepora, A. intermedia, A. muricata and A. valida), were present at more than ¾ of sites surveyed. There were many rare species, seventeen of which were recorded at a single site only. One of these, Lobophyllia serratus, is listed as Endangered in the IUCN Red List of Threatened Species (www.iucn.redlist.org), and Stylaraea punctata is listed as Data Deficient.
The distinctiveness of the Bonaparte intertidal coral assemblage is exemplified by the high diversity of Acropora species living there (47 spp.) (Figure C in S1 File). Acropora are one of the most thermally sensitive and threatened coral genera [3], and it is increasingly rare to find diverse and abundant assemblage of Acropora on intertidal nearshore fringing reefs. On the Great Barrier Reef for example, nearshore reefs have been classified as “non-Acropora reefs” [41] due to the relative paucity of Acropora spp. Not only was Acropora the most diverse of the 60 genera of scleractinian coral recorded (Figure C in S1 File), but six Acropora species dominated the community (Fig. 2a-c). An abundance of Acropora spp. has been reported from other intertidal locations in the Kimberley (e.g. Turtle Reef at Talbot Bay and One-Arm Point, Cape Leveque, in the Western Kimberley [22]) indicating this region may provide a critical refuge for this increasingly threatened group of corals.
Physical Variables
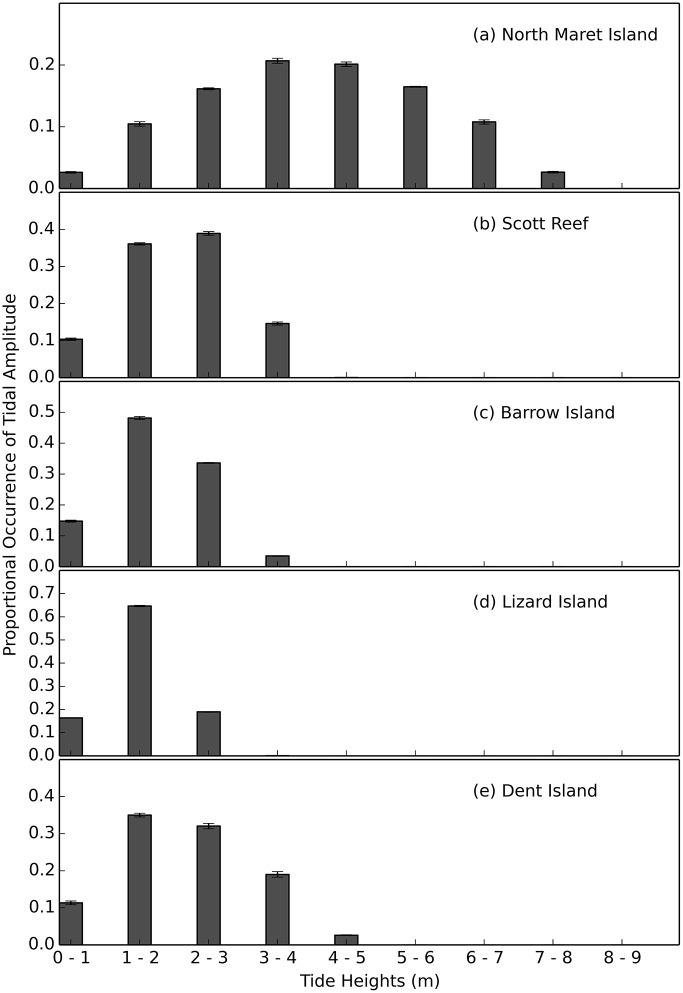
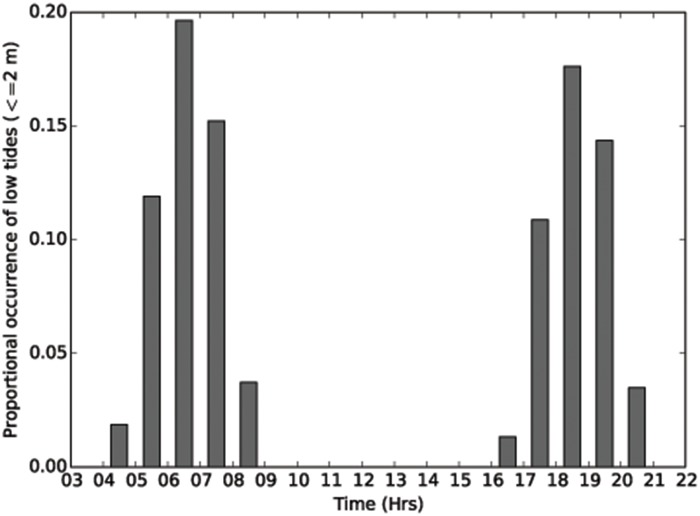
Hourly tide height data from North Maret I., shows the semidiurnal patterns of the tides which oscillate up to 8m over spring tides (Fig. 5a). When the mean proportional occurrence of tidal heights for North Maret I. is contrasted with four other more typical reef locations (Fig. 5b-e) it is evident tidal conditions in the vicinity of North Maret I. are more dynamic and reach amplitudes up to 3m greater than those in the other more typical reef locations with a similar level of diversity. During spring low tides (i.e. tides ≤ 2m), corals growing on the intertidal reef platform at North Maret I. are exposed to the air for up to 3.5 hours at a time (Figure D in S1 File). However, an important physical feature of the Bonaparte Archipelago is that the spring low tides occur in the early morning, 4am-9am, and late afternoon to early evening, 4pm–9pm (Fig. 6). Thus, corals remain submerged over the hottest parts of the day and are, buffered from the stresses arising from subaerial exposure. Nevertheless, even when corals are submerged, other environmental factors come into play such as sea-surface temperatures and turbidity.
Fig 5. Spatial comparison of tidal amplitude at 1 m intervals (± SD) from 2002–2014.
Presented is the proportional occurrence of tide heights for (a) North Maret I., (b) Scott Reef (c) Barrow I., (d) Lizard I, and (e) Dent Island.
Fig 6. Hourly tide height data of spring low tides for North Maret I. from 2002–2014.
The lengths of time corals are exposed for each day depends upon the lunar phase (see Figure D in S1 File) and maximum low tide exposure occurs in early morning and evening.
Eight-day average MODIS-derived SST from July 2002 to June 2014 shows that the average SST at North Maret I. ranged from 25.2 to 34.3°C (Table 1). The maximum mean summer SST over this period was 32.2 ± 1.0°C and the minimum mean winter SST was 26.5 ± 1.0°C encompassing a range of 5.7°C. The +1°C bleaching threshold (sensu NOAA Coral Reef Watch methods—see http://coralreefwatch.noaa.gov/satellite/index.php) at North Maret I. is 33.2°C and our data suggest SST remained above this threshold for two 8-day periods in January and February 2013 (Figure E in S1 File). When compared with more typical reef locations (Fig. 7) the mean SST in the vicinity of North Maret I. is significantly higher than Lizard I., Barrow I. and Dent I. but not significantly different from Scott Reef (Table E in S1 File). While Scott Reef has succumbed to bleaching events in the past [43], to date there is no evidence to suggest the intertidal coral communities in the Bonaparte Archipelago have experienced a bleaching event (despite NOAA issuing numerous bleaching alerts e.g Mar-Jun 2013). Even though this region is remote, the Bonaparte Archipelago is intermittently visited by scientists (WA Museum, Australian Institute of Marine Science, Department of Fisheries; Cygnet Bay Research Station); tourist vessels and the Australian customs service provide surveillance. While widespread bleaching was been reported across almost 2000 km of Western Australian coastline during the summer of 2010/11 [42] there is no suggestion that the inshore Kimberley reefs have experienced a widespread bleaching event to date.
Table 1. Summary statistics for SST parameters at North Maret I. in comparison to three other more typical coral reef locations (unit = °C).
| North Maret I. | Scott Reef | Barrow I. | Lizard I. | Dent I. | |
|---|---|---|---|---|---|
| Minimum SST | 25.193 | 25.120 | 21.714 | 22.147 | 20.040 |
| Maximum SST | 34.350 | 32.747 | 32.669 | 32.013 | 30.936 |
| Average SST | 29.216 | 29.170 | 26.439 | 26.743 | 25.414 |
| Standard Deviation SST | 1.790 | 1.623 | 2.733 | 2.036 | 2.619 |
| Max. Summer SST mean | 32.208 | 31.607 | 30.114 | 30.450 | 29.384 |
| Max. Summer SST Standard Deviation | 0.978 | 0.692 | 1.302 | 0.817 | 0.732 |
| Summer SST mean | 30.389 | 30.225 | 28.570 | 29.124 | 28.401 |
| Summer SST Standard Deviation | 1.328 | 1.113 | 1.458 | 0.925 | 0.941 |
| Min Winter SST mean | 26.504 | 26.635 | 22.407 | 23.808 | 21.077 |
| Min Winter SST Standard Deviation | 0.962 | 0.751 | 0.615 | 0.789 | 0.772 |
| Winter SST mean | 27.128 | 27.283 | 23.325 | 24.460 | 22.110 |
| Winter SST Standard Deviation | 0.989 | 0.782 | 0.896 | 0.792 | 1.005 |
Data relate to 8-day global averages derived from the MODIS aqua satellite at a 4-km spatial resolution from July 2002 to June 2014. See Table C in S1 File for site co-ordinates.
Fig 7. Spatial comparison of sea-surface temperatures from 2002–2014.
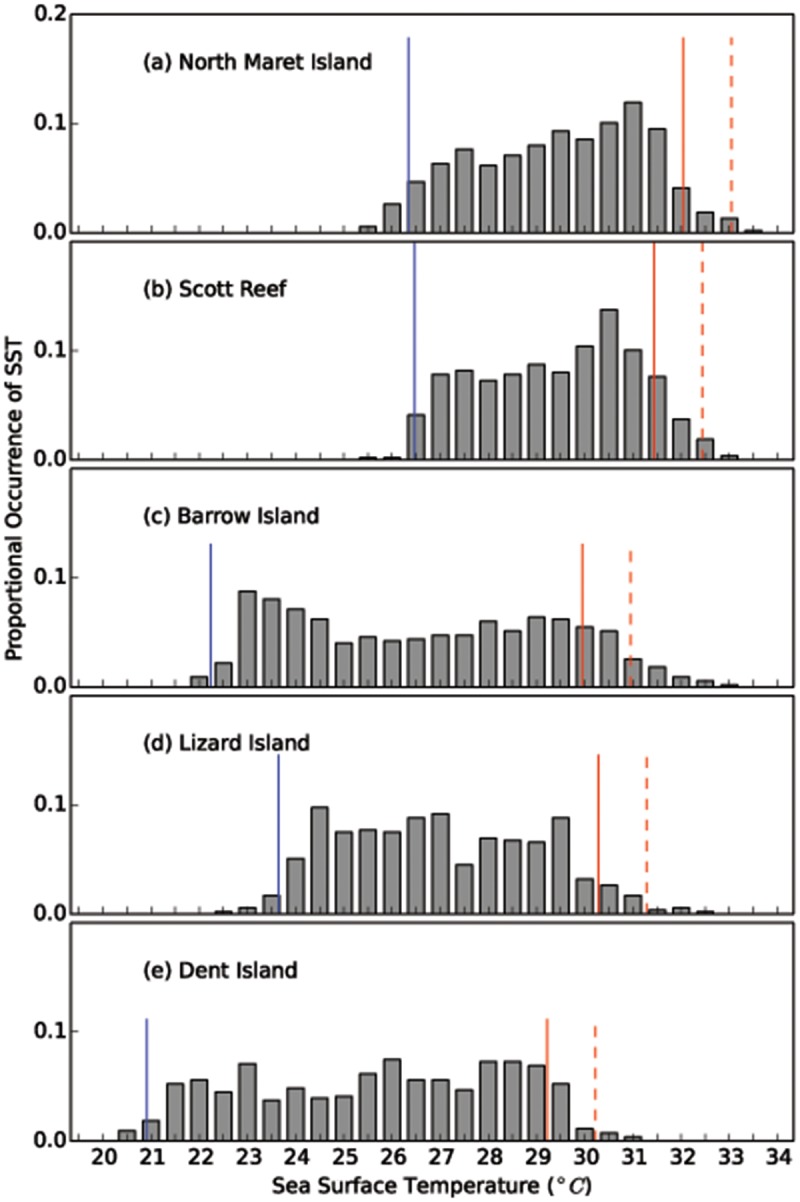
Presented is the proportional occurrence of SST (a) North Maret I., (b) Scott Reef (c) Barrow I., (d) Lizard I, and (e) Dent Island. The red line shows the mean max. summer SST; and the red dashed line (- - -) shows the + 1°C bleaching threshold (sensu NOAA); the blue line shows the mean min. winter SST.
The eight-day average of Kd(490) ranged from 0.03–0.18 m-1 at North Maret I. (Table 2). The Kd(490) values represent natural turbidity levels at North Maret Island. They are slightly lower than the values detected at Barrow I. (Pilbara), a location that has been exposed to a large multi-year dredging operation [44], [45] (Figure F in S1 File) and not significantly different from Dent Island (inshore Whitsundays, GBR) (Table E in S1 File). There are seasonal differences in the Kd(490) values obtained across all locations with the highest turbidity recorded in Winter at North Maret I. (Figure G in S1 File). It is likely the similarity of summer Kd(490) values across the locations (Table 2) reflects the effects of tropical cyclones and monsoonal storms on water quality.
Table 2. Summary statistics Kd(490) parameters at North Maret I. in comparison to three other more typical coral reef locations (unit = m-1).
| North Maret I. | Scott Reef | Barrow I. | Lizard I. | Dent I. | |
|---|---|---|---|---|---|
| Minimum K d (490) | 0.032 | 0.019 | 0.048 | 0.019 | 0.029 |
| Maximum K d (490) | 0.178 | 0.117 | 0.184 | 0.091 | 0.174 |
| Average K d (490) | 0.063 | 0.032 | 0.079 | 0.046 | 0.060 |
| Standard Deviation K d (490) | 0.017 | 0.009 | 0.023 | 0.011 | 0.015 |
| Winter-time K d (490) mean | 0.068 | 0.040 | 0.073 | 0.044 | 0.059 |
| Winter-time K d (490) Standard Deviation | 0.010 | 0.007 | 0.021 | 0.008 | 0.010 |
| Summer-time K d (490) mean | 0.061 | 0.029 | 0.086 | 0.048 | 0.060 |
| Summer-time K d (490) Standard Deviation | 0.021 | 0.006 | 0.023 | 0.012 | 0.017 |
| Autumn-time K d (490) mean | 0.068 | 0.030 | 0.094 | 0.050 | 0.072 |
| Autumn-time K d (490) Standard Deviation | 0.021 | 0.009 | 0.024 | 0.050 | 0.015 |
| Spring-time K d (490) mean | 0.055 | 0.027 | 0.065 | 0.043 | 0.052 |
| Spring-time K d (490) Standard Deviation | 0.012 | 0.004 | 0.009 | 0.007 | 0.009 |
Data relate to 8-day global averages derived from the MODIS aqua satellite at a 4-km spatial resolution from July 2002 to June 2014. See Table C in S1 File for site details.
Factors Driving Diversity
The findings of our preliminary comparison of regional diversity suggest that the level of coral diversity in the Bonaparte intertidal zone is roughly equivalent to, or greater than that documented from other more typical shallow-water reefs in the NE Indian Ocean and Western Pacific Ocean. It is important to note however that the environmental conditions in the Bonaparte Archipelago are far more extreme and dynamic than these other locations. Hence the question must be asked, how is the diversity of the Bonaparte community being sustained when intertidal coral communities all around the world are becoming increasingly impoverished due to coral bleaching and sediment impacts?
There are a number of possible explanations for the high diversity. Firstly, the Bonaparte Archipelago occurs at low latitude (~14° 24′ S) and in relatively close proximity to Indonesia (~500 km) where the greatest level of reef coral diversity is documented [46]. While it is well known that coral diversity increases towards the equator [47], [48] the diversity of corals occurring in the Kimberley region has been under-represented in studies of coral biogeography and biodiversity [49]. Hence the extent of faunal connectivity between Indonesia and NW Australia has not been quantified. The Timor and Banda Seas were continuously connected over the Quaternary via the Indonesian Throughflow Current [22] and this, coupled with our finding of a degree of affinity between the Kimberley and Indonesian coral faunas, supports the premise that the diversity of the Kimberley coral fauna is at least partly sustained through connections with Indonesia.
Secondly, there is a strong link between declining water quality, proximity to urban centres and the condition of coral reefs [50–52], hence we postulate that a low level of pollution and development helps to explain how this remarkable diversity of coral is sustained. The Kimberley region is sparsely populated with no major urban centres. In 2011 just over 34,000 people lived in the region (423,517 km²) (http://kdc.wa.gov.au/Statistics/Census-Profiles), compared to 1.1 million people living in the Great Barrier Reef catchment area (425 964 km2) in the same year [53]. The Kimberley has a large pastoral industry but only a relatively small area of land that is irrigated or used for horticulture (http://kdc.wa.gov.au/economic-activity/agriculture). Thus, in contrast to the east coast of Australia and in many other parts of the world [2], [54–56] there are no major urban centres in the Kimberley, and the input of agricultural-based nutrients and pesticides into the nearshore marine ecosystem is minimal.
Another factor likely to help explain how coral diversity is sustained is the distinctive tidal regime. The geographic position and shelf bathymetry of the Kimberley have resulted in the region being characterized by tides that reach their maximum spring and summer amplitudes in the early morning and late afternoon/evening (Fig. 6, Figure D in S1 File). This means that the intertidal corals are in effect, protected from subaerial emersion and desiccation-based stresses over the hottest parts of the day (10am-3pm). Furthermore, the apparent ability of corals to occupy a broad physiological niche may also relate to other environmental variables such as cloud cover, turbidity and the strong currents [57], [58].
The macro-tidal conditions dictate that the inshore Kimberley region is dynamic and even in the absence of flood or storm events, the large tides and subsequent currents create “water boils” during spring tides, when fine sediments on the seafloor are resuspended [22]. Thus, unlike the clear waters that characterize most coral reef ecosystems, this inshore Kimberley is characterized by turbid water. Ordinarily, high levels of suspended sediment are thought to restrict light availability and prevent the settlement and colonization of coral larvae [63]. However in the Kimberley, we postulate that the naturally high levels of suspended sediments may actually protect the shallow water corals from solar radiation [59] by back-scattering light and lowering the intensity of down-welling irradiance reaching the benthos [60], [61].
While tidal-driven cyclical variation in benthic irradiance could result in corals fluctuating between states of potential light limitation, to light stress [62]; overall, the Bonaparte corals maintain a positive energy balance. Thus in this system, the expected negative effects of heat, light and sediment may be transient and/or accommodated due to the large tides and strong currents which provide water movement and aeration. The Kimberley corals are also likely have adapted to the local conditions by the enhanced production of mucus and tissue inflation [64] and/or increased levels of heterotrophic feeding [65]; or via other physiological adaptations (see [66–69] for examples) however the traits that underpin survival remain to be explored.
Conclusion
Here we report the finding of an exceptionally diverse intertidal fringing reef community in the Kimberly region of north-western Australia, which thrives despite extreme environmental controls. The presence of this diverse community calls for a re-evaluation of what conditions are optimal for coral survival. Our results may elicit some optimism about the future of corals reefs because we demonstrate that in the absence of additional stressors, diverse assemblages of coral can thrive in atypical and dynamic environmental settings. The assemblage we report here provides an indication of which corals may have existed in other nearshore locations in the past and presents an ideal model system for exploring how resistance and resilience are conferred in the absence of confounding factors such as pollution. In the future, genetic material from the hardy Kimberley corals may help to boost the resilience of corals in other parts of the world (i.e. through natural gene flow or genetic translocation and preservation [17], [70]) and may circumvent the need to artificially design ‘smart reefs’ [71]. Nevertheless, the Kimberley coral communities are by no means immune from climatically or anthropogenically-imposed changes, hence ongoing local resource management and conservation action are vital.
Supporting Information
Table A. Details of the 23 study sites in the Bonaparte Archipelago. Table B. Location, co-ordinates, method and approximate area surveyed of our study sites and additional sites used for comparative purposes. Table C. Co-ordinates for physical variables. Table D. Annotated species list. Listed are the specimen accession numbers and site occupancy at local, group and regional scales including known depth zone i.e. <5m (intertidal) or >5m (subtidal) based upon the specimen-based records in the Queensland Museum coral database. Table E. Summary of significance results from one-way analysis of variance comparing the mean SST and Kd(490) time-series data between locations. Figure A. Permutated species accumulation curves. The local species diversity was adequately surveyed after approximately 20 sites were surveyed in the Bonaparte Archipelago. Figure B. Semi-quantitative spatial comparison of coral species diversity. This figure illustrates that the three Bonaparte Island groups (Maret I., Berthier I., and Montalivet I., in blue) have a similar level of diversity to that estimated for other more typical and less physically extreme reef locations such as Dent I. and Border I. on the Great Barrier Reef and Christmas I., an offshore oceanic location in the NE Indian Ocean. The level of diversity per 100m2 is higher than Ashmore Reef (Offshore Kimberley); Lizard I. (Northern GBR); Kosrae and Maju ro Atoll (Central Pacific) and the Red Sea. Data summarized from [37], [72–77]. Figure C. Species level diversity within genera at the 23 intertidal survey sites. Note: 27 genera were represented by a single species (Table D in S1 File). Figure D. Daily tidal cycle at North Maret Island on selected spring and neap tides over our survey period in October 2007. During spring low tides (i.e. tides ≤ 2m), corals growing on the intertidal reef platform at North Maret I. are exposed to the air for up to 3.5 hours at a time whereas at neap tide, corals remain submerged by at least 1m of water. See Fig. 5 for a time-series analysis showing the proportion of tides occurring at 1m intervals from 0–1m up to 7–8m. Figure E. Time series (2002–2014) showing SST data based on 8-day averages for 5 locations. This figure shows at North Maret I. SST surpassed the +1°C bleaching threshold in Feb-March 2013. Figure F. Spatial comparison of Kd(490), 2002–2014. Kd(490) represents the diffuse attenuation coefficient of down-welling irradiance at 490 nm and is used as a measure of turbidity. Figure G. Kd(490) time series for North Maret I. from 2002–2014. The blue line represents the average winter turbidity level and the red represents the average summer turbidity.
(DOC)
Acknowledgments
We thank M. Forde for fieldwork planning and J. E. N. Veron, A. Noreen and RPS staff, including F. Webster, for participation in the fieldwork. Thanks are due also to E. Turak for validating specimen identifications, J. E. N. Veron for cross-checking our distribution records against the Corals of the World database, B. Done for collection management. Thanks to Paul Davill from the National Tide Centre, Bureau of Meteorology for tidal data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was conducted under contract to INPEX as part of environmental monitoring for the Ichthys Gas Field Development Project. ZR was supported in the write-up phase of this project by Woodside Energy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Fr Res 50: 839–866. [Google Scholar]
- 2. Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, et al. (2008) A global map of human impact on marine ecosystems. Science 319: 948–952. 10.1126/science.1149345 [DOI] [PubMed] [Google Scholar]
- 3. Carpenter KE, Abrar M, Abey G, Aronson RB, Banks S, et al. (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321: 560–563. 10.1126/science.1159196 [DOI] [PubMed] [Google Scholar]
- 4. Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk—revisited. World Resources Institute: Washington DC. [Google Scholar]
- 5. Glynn PW (1976) Some physical and biological determinants of coral community structure in the eastern Pacific. Ecol Monogr 46: 431–456. [Google Scholar]
- 6. Dunne RP, Brown BE (2001) The influence of solar radiation on bleaching of shallow water reef corals in the Andaman Sea, 1993–1998. Coral Reefs 20: 201–210. [Google Scholar]
- 7. Le Tissier MDA, Brown BE (1996) Dynamics of solar bleaching in the intertidal reef coral Goniastrea aspera at Ko Phuket, Thailand. Mar Ecol Prog Ser 136: 235–244. [Google Scholar]
- 8. Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska, et al. (2011) The pace of shifting climate in marine and terrestrial ecosystems. Science 334: 652–655. 10.1126/science.1210288 [DOI] [PubMed] [Google Scholar]
- 9. Brown BE, Dunne RP, Scoffin TP, Le Tissier MD (1994) Solar damage in intertidal corals. Mar Ecol Prog Ser 105: 219–230. [Google Scholar]
- 10. Sweatman H, Delean S, Syms C (2011) Assessing loss of coral cover on Australia’s Great Barrier Reef over two decades, with implications for longer-term trends. Coral Reefs 30: 521–531. [Google Scholar]
- 11. Huston MA (1985) Patterns of species diversity on coral reefs. Ann Rev Ecol Syst 16: 149–177. [Google Scholar]
- 12. Brown B, Clarke K, Warwick R (2002) Serial patterns of biodiversity change in corals across shallow reef flats in Ko Phuket, Thailand, due to the effects of local (sedimentation) and regional (climatic) perturbations. Mar Biol 141: 21–29. [Google Scholar]
- 13. Karlson RH, Cornell HV, Hughes TP (2004) Coral communities are regionally enriched along an oceanic biodiversity gradient. Nature 429: 867–870. [DOI] [PubMed] [Google Scholar]
- 14. Knowlton N, Brainard RE, Fisher R, Moews M, Plaisance L, et al. (2010) Coral reef biodiversity. Life in the World’s Oceans: Diversity Distribution and Abundance, 65–74. [Google Scholar]
- 15. Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429: 827–833. [DOI] [PubMed] [Google Scholar]
- 16. Hughes TP, Graham NA, Jackson JB, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. TREE 25: 633–642. 10.1016/j.tree.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 17. Coles SL, Reigl BM (2013) Thermal tolerances of reef corals in the Gulf: A review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar Poll Bull 72:323–332. [DOI] [PubMed] [Google Scholar]
- 18. Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanism of Reef Coral Resistance to Future Climate Change. Science 344: 895–898. 10.1126/science.1251336 [DOI] [PubMed] [Google Scholar]
- 19. Castillo KD, Ries JB, Weiss JM, Lima FP (2012) Decline of forereef corals in response to recent warming linked to history of thermal exposure. Nat Cl Ch 2: 756–760. [Google Scholar]
- 20. Hume B, D’Angelo C, Burt J, Baker AC, Riegl B, et al. (2013) Corals from the Persian/Arabian Gulf as models for thermotolerant reef-builders: Prevalence of clade C3Symbiodinium, host fluorescence and ex situ temperature tolerance. Mar Poll Bull 72: 313–322. [DOI] [PubMed] [Google Scholar]
- 21. Thackway R, Cresswell ID (eds) (1998) Interim Marine and Coastal Regionalisation for Australia: and ecosystem-based classification for marine and coastal environments Version 3.3 Environment Australia. Commonwealth Department of Environment, Canberra. [Google Scholar]
- 22. Wilson B (2013) The biogeography of the Australian North West Shelf: Environmental Change and Life’s Response (Elsevier, Burlington, MA, USA: ). [Google Scholar]
- 23. McAlpine KW, Masini RJ, Sim SB, Daly T (2013) Background concentrations of selected metals and Total Suspended Solids in the coastal waters of the Kimberley Region In: Wilson B. The Biogeography of the Australian North West Shelf: Environmental Change and life’s Response (Elsevier, USA, 2013). [Google Scholar]
- 24.Wallace CC, Done, BJ, Muir PR (2012) Revision and catalogue of worldwide staghorn corals Acropora and Isopora (Scleractinia: Acroporidae) in the Museum of Tropical Queensland. Memoirs of the Queensland Museum, Nature. Queensland Museum
- 25. Gittenberger A, Reijnen BT, Hoeksema BW (2011) A molecularly based phylogeny reconstruction of mushroom corals (Scleractinia: Fungiidae) with taxonomic consequences and evolutionary implications for life history traits. Contrib Zool 80: 107–132. [Google Scholar]
- 26. Benzoni F, Stefani F, Pichon M, Galli P (2010) The name game: morpho‐molecular species boundaries in the genus Psammocora (Cnidaria, Scleractinia). Zool J Linn Soc 160: 421–456. [Google Scholar]
- 27. Budd AF, Fukami H, Smith ND, Knowlton N (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool J Lin Soc 166: 465–529. [Google Scholar]
- 28. Huang D, Benzoni F, Fukami H, Knowlton N, Smith ND, et al. (2014) Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zool J Lin Soc 171: 277–355. [Google Scholar]
- 29. Veron JEN (2000) Corals of the World. Vol. 1–3 Australian Institute of Marine Science, Townsville, Australia. [Google Scholar]
- 30.Clarke KR, Warwick, RM (2001). Change in marine communities: an approach to statistical analysis and interpretation. PRIMER v5: user manual/tutorial. PRIMER-E Limited, 2001.
- 31.Brown OB, Minnet PJ, Evans R, Kearns E, Kilpatrick K, et al. (1999) MODIS Infrared Sea Surface Temperature Algorithm. Algorithm Theoretical Basis Document http://modis.gsfc.nasa.gov/data/atbd/atbd_mod25.pdf
- 32. Mueller JL (2000) SeaWiFS algorithm for the diffuse attenuation coefficient, Kd (490), using water-leaving radiances at 490 and 550 nm, In: Hooker SB, Firestone ER (eds) SeaWiFS postlaunch calibration and validation analyses: Part 3, NASA Tech. Memo. 2000–206892, NASA Goddard Space Flight Center, Greenbelt, 11: 24–27 (2000). [Google Scholar]
- 33. Werdell PJ, Bailey SW (2005) An improved in-situ bio-optical data set for ocean color algorithm development and satellite data production validation. Rem Sens Env 98: 122–140. [Google Scholar]
- 34. Lee Z, Carder KL, Chen RF, Peacock TG (2001) Properties of the water column and bottom derived from Airborne Visible Infrared Imaging Spectrometer (AVIRIS) data. J Geoph Res 106: 11639–11651. [Google Scholar]
- 35. Cannizzaro JP, Carder KL (2006) Estimating chlorophyll a concentrations from remote-sensing reflectance in optically shallow waters. Rem Sens Env 101: 13–24. [Google Scholar]
- 36.Wolstenholme J, Dinesen ZD, Alderslade P (1997) Hard corals of the Darwin region, Northern Territory, Australia. In: Hanley JR, et al. (eds) Proceedings of the Sixth International Marine Biological Workshop. The marine flora and fauna of Darwin Harbour, Northern Territory, Australia. Museums and Art Galleries of the northern Territory and the Australian Marine Sciences Association: Darwin, 381–398, 1997.
- 37. DeVantier LM, De’Ath G, Done TJ, Turak E (1998). Ecological assessment of a complex natural system: a case study from the Great Barrier Reef. Ecological Applications 8: 480–496. [Google Scholar]
- 38. Thompson AA, Dolman AM (2010) Coral bleaching: one disturbance too many for near-shore reefs of the Great Barrier Reef. Coral Reefs 29: 637–648 [Google Scholar]
- 39. Sweatman H, Delean S, Syms C (2011) Assessing loss of coral cover on Australiaís Great Barrier Reef over two decades, with implications for longer-term trends. Coral reefs 30: 521–531. 10.1007/s00338-010-0715-1 [DOI] [Google Scholar]
- 40. De’ath G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences of the United States of America 109: 17734–17735. 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Done TJ (1982) Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1: 96–107. [Google Scholar]
- 42. Moore JA, Bellchambers LM, Depczynski MR, Evans RD, Evans SN, et al. (2012) Unprecedented mass bleaching and loss of coral across 12 of latitude in Western Australia in 2010–11. PloS One 7: e51807 10.1371/journal.pone.0051807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013). Recovery of an isolated coral reef system following severe disturbance. Science, 340: 69–71. 10.1126/science.1232310 [DOI] [PubMed] [Google Scholar]
- 44.Chevron-Australia (2009) Gorgon Gas Development and Jansz Feed Gas Pipeline Dredging and Spoil Disposal Management and Monitoring Plan. Chevron Australia, Perth, Western Australia. (G1-NT-PLNX0000373): 255.
- 45. Evans RD, Murray KL, Field SN, Moore JA, Shedrawi G, et al. (2012) Digitise this! A quick and easy remote sensing method to monitor the daily extent of dredge plumes. PloS one, 7(12), e51668 10.1371/journal.pone.0051668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veron JEN, Devantier LM, Turak E, Green AL, Kininmonth S, et al. (2009) Delineating the coral triangle. Galaxea 11: 91–100. [Google Scholar]
- 47. Stehli FG, Wells JW (1971) Diversity and age patterns in hermatypic corals. Syst Biol 20: 115–126. [Google Scholar]
- 48. Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Cornell University Press. [Google Scholar]
- 49. Wallace CC, Muir PR (2005) Biodiversity of the Indian Ocean from the perspective of staghorn corals (Acropora spp). Ind J Mar Sci 34: 42–49. [Google Scholar]
- 50. Wooldridge SA (2009) Water quality and coral bleaching thresholds: formalizing the linkage for the inshore reefs of the Great Barrier Reef Australia. Mar Poll Bull 58: 745–751 10.1016/j.marpolbul.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 51. De’ath G, Fabricius K (2010) Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol Appl 20: 840–850. [DOI] [PubMed] [Google Scholar]
- 52. D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. [in special issue: Environmental Change Issues] Cur Opin Environ Sust 7: 82–93. [Google Scholar]
- 53.Deloitte Access Economics (2013) Economic contribution of the Great Barrier Reef, Great Barrier Reef Marine Park Authority, Townsville.
- 54. van der Meij SE, Hoeksema BW (2010) Long-term changes in coral assemblages under natural and anthropogenic stress in Jakarta Bay (1920–2005). Mar Poll Bull 60: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 55. Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. Washington, DC: World Resources Institute; p. 114. [Google Scholar]
- 56. Waterhouse J, Brodie J, Lewis S, Mitchell A (2012) Quantifying the sources of pollutants in the Great Barrier Reef catchments and the relative risk to reef ecosystems. Mar Poll Bull 65: 394–406. [DOI] [PubMed] [Google Scholar]
- 57. Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob Ch Biol 10:1627–1641. [Google Scholar]
- 58. Mumby PJ, Chisholm JRM, Edwards AJ, Andrefouet S, Jaubert J (2001) Cloudy weather may have saved Society Islands reef corals during the 1998 ENSO event. Mar Ecol Prog Ser 222: 209–216. [Google Scholar]
- 59. Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62: 185–202. [Google Scholar]
- 60. Anthony K., Connolly SR, Hoegh-Guldberg O (2007) Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol Oceanog 52: 716–726. [Google Scholar]
- 61. Devlin MJ, Barry J, Mills DK, Gowen RJ, Foden J, et al. (2008) Relationships between suspended particulate material, light attenuation and Secchi depth in UK marine waters. Est. Coa Sh Sci 79: 429–439. [Google Scholar]
- 62. Mobley C (1994) Light and water: Radiative transfer in natural waters. Academic Press. [Google Scholar]
- 63. Anthony K., Ridd PV, Orpin AR, Larcombe P, Lough J (2004) Temporal variation of light availability in coastal benthic habitats: Effects of clouds, turbidity, and tides. Limnol Oceanog 49: 2201–2211. [Google Scholar]
- 64. Stafford-Smith MB, Ormond RFG (1992) Sediment-rejection mechanisms of 42 species of Australian scleractinian corals. Aust J Mar Fr Res 43: 683–705. [Google Scholar]
- 65. Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Expn Mar Biol Ecol 252: 221–253. [DOI] [PubMed] [Google Scholar]
- 66. Salih A, Larkum A, Cox G, Kuhl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408: 850–853. [DOI] [PubMed] [Google Scholar]
- 67. Howells EJ, Beltran VH, Larsen NW, Bay LK, Willis BL, et al. (2011) Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat Cl Ch 2: 116–120. [Google Scholar]
- 68. Brown BE, Downs CA, Dunne RP, Gibb SW (1992) Exploring the basis of thermotolerance in the reef coral Goniastrea aspera . Mar Ecol Prog Ser 242: 119–129. [Google Scholar]
- 69. Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, et al. (2013) Genomic basis for coral resilience to climate change. Proc Nat Acad Sci 110: 1387–1392. 10.1073/pnas.1210224110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, et al. (2008) Assisted colonization and rapid climate change. Science 321: 345–346. 10.1126/science.1157897 [DOI] [PubMed] [Google Scholar]
- 71. Mascarelli A (2014) Climate-change adaptation: Designer reefs. Nature 508: 444–446. 10.1038/508444a [DOI] [PubMed] [Google Scholar]
- 72. Richards ZT (2013) A comparison of proxy performance in coral biodiversity monitoring. Coral Reefs 32: 287–292. [Google Scholar]
- 73. Richards ZT, Beger M (2011) A quantification of the standing stock of marine debris in Majuro Lagoon, Republic of the Marshall Islands and its effect on coral communities. Mar Poll Bull 62: 1693–1701. 10.1016/j.marpolbul.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 74. Richards ZT (2014) The status of hard coral communities at Kosrae, Micronesia. Marine Biodiversity: 1–12. [Google Scholar]
- 75.Richards Z, Beger M, Hobbs J-P, Bowling T, Chong-seng K, et al. (2009) Ashmore Reef National Nature Reserve and Cartier Island Marine Reserve Marine Survey 2009. ARC Centre of Excellence for Coral Reef Studies. Produced for the Department of the Environment, Water, Heritage and the Arts.
- 76. Ryan NM, Richards ZT, Hobbs JPA (2014) Optimal monitoring of coral biodiversity at Christmas Island. Raffles Bulletin of Zoology, Supplement 30: 399–405. [Google Scholar]
- 77. Loya Y (1972) Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Mar Biol 13: 100–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A. Details of the 23 study sites in the Bonaparte Archipelago. Table B. Location, co-ordinates, method and approximate area surveyed of our study sites and additional sites used for comparative purposes. Table C. Co-ordinates for physical variables. Table D. Annotated species list. Listed are the specimen accession numbers and site occupancy at local, group and regional scales including known depth zone i.e. <5m (intertidal) or >5m (subtidal) based upon the specimen-based records in the Queensland Museum coral database. Table E. Summary of significance results from one-way analysis of variance comparing the mean SST and Kd(490) time-series data between locations. Figure A. Permutated species accumulation curves. The local species diversity was adequately surveyed after approximately 20 sites were surveyed in the Bonaparte Archipelago. Figure B. Semi-quantitative spatial comparison of coral species diversity. This figure illustrates that the three Bonaparte Island groups (Maret I., Berthier I., and Montalivet I., in blue) have a similar level of diversity to that estimated for other more typical and less physically extreme reef locations such as Dent I. and Border I. on the Great Barrier Reef and Christmas I., an offshore oceanic location in the NE Indian Ocean. The level of diversity per 100m2 is higher than Ashmore Reef (Offshore Kimberley); Lizard I. (Northern GBR); Kosrae and Maju ro Atoll (Central Pacific) and the Red Sea. Data summarized from [37], [72–77]. Figure C. Species level diversity within genera at the 23 intertidal survey sites. Note: 27 genera were represented by a single species (Table D in S1 File). Figure D. Daily tidal cycle at North Maret Island on selected spring and neap tides over our survey period in October 2007. During spring low tides (i.e. tides ≤ 2m), corals growing on the intertidal reef platform at North Maret I. are exposed to the air for up to 3.5 hours at a time whereas at neap tide, corals remain submerged by at least 1m of water. See Fig. 5 for a time-series analysis showing the proportion of tides occurring at 1m intervals from 0–1m up to 7–8m. Figure E. Time series (2002–2014) showing SST data based on 8-day averages for 5 locations. This figure shows at North Maret I. SST surpassed the +1°C bleaching threshold in Feb-March 2013. Figure F. Spatial comparison of Kd(490), 2002–2014. Kd(490) represents the diffuse attenuation coefficient of down-welling irradiance at 490 nm and is used as a measure of turbidity. Figure G. Kd(490) time series for North Maret I. from 2002–2014. The blue line represents the average winter turbidity level and the red represents the average summer turbidity.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.