Abstract
Background and objective: Antineuronal antibodies have been implicated in tic and obsessive compulsive disorders (OCD) associated with group A streptococcal infections. We investigated antineuronal autoantibody levels as well as antibody-mediated neuronal cell signaling activity, as previously reported for Sydenham chorea and pediatric autoimmune neuropsychiatric disorder associated with streptococci (PANDAS), to determine immunological profiles for a large cohort of children with tics and/or OCD.
Methods: Study participants (n=311; ages 4–27 years, 66% male) were selected from a larger group of individuals with self-reported neuropsychiatric symptoms (n=742) and included only those with accurate knowledge of group A streptococcal infection status, except for four individuals in whom streptococcal infection status was unknown. Healthy control samples (n=16; ages 5–14 years, 81% male), came from the National Institute of Mental Health and Yale University. In addition to serum donations, participants and/or legal guardians provided neuropsychiatric and related medical histories of symptoms that had lasted >1 year. Antineuronal immunoglobulin G (IgG) titers were measured by standard enzyme-linked immunosorbent assay (ELISA) and compared with mean titers of normal age-matched sera against lysoganglioside, tubulin, and dopamine receptors (D1R and D2R). Antibody-mediated signaling of calcium calmodulin dependent protein kinase II (CaMKII) activity in a human neuronal cell line (SK-N-SH) was tested in serum.
Results: Of 311 individuals, 222 (71%) had evidence of group A streptococcal infection, which was associated with tics and/or OCD status (p=0.0087). Sera from individuals with tics and/or OCD (n=261) had evidence of elevated serum IgG antibodies against human D1R (p<0.0001) and lysoganglioside (p=0.0001), and higher serum activation of CaMKII activity (p<0.0001) in a human neuronal cell line compared with healthy controls (n=16). Furthermore, patients with tics and OCD had significantly increased activation of CaMKII activity compared with patients with only tics or only OCD (p<0.033 for each).
Conclusion: Our study suggested a significant correlation of streptococcal-associated tics and OCD with elevated anti-D1R and antilysoganglioside antineuronal antibodies in serum concomitant with higher activation of CaMKII in human neuronal cells. Youth and young adults with chronic tics and OCD may have underlying infectious/immunologic etiology.
Introduction
In the last few decades, there has been a growing interest in the association of infections, autoimmunity, and behavioral changes, and their impact on the genesis of neuropsychiatric disorders (Murphy et al. 2012). In 1998, a link between obsessive-compulsive disorder (OCD) and group A streptococcal infections was identified by Swedo at the National Institute for Mental Health (NIMH) (Swedo et al. 1998). These disorders may be identified as pediatric autoimmune neuropsychiatric disorder associated with streptococci (PANDAS) (Swedo et al. 1998) or pediatric acute-onset neuropsychiatric syndrome (PANS) in the presence of other causes, including infections (Swedo et al. 2012). This discovery was a result of two parallel studies conducted at the NIMH, including investigation of children with OCD and tics and investigation of children with Sydenham chorea (SC), the major neurological manifestation of acute rheumatic fever (ARF), which presents with involuntary movements and neuropsychiatric disturbances, including obsessive-compulsive symptoms, hyperactivity, and emotional lability (Marques-Dias et al. 1997). Swedo and coworkers identified a cohort of patients who experienced a sudden acute onset of obsessions and compulsions that followed a relapsing–remitting symptom course. Five diagnostic criteria emerged from careful observation of these patients: 1) Presence of OCD (by Diagnostic and Statistical Manual of Mental Disorders IV criteria) or a tic disorder, 2) symptom onset between 3 years of age and puberty, 3) episodic course of illness, with abrupt and substantial symptom exacerbations, 4) symptom onset and exacerbations associated temporally with group A streptococcal infections, and 5) presence of neurological abnormalities, including choreiform movements during symptom exacerbations (Swedo et al. 1998).
Murphy also describes neurological symptoms in acute onset OCD/tic patients, including severe hyperactivity, loss of fine motor skills (handwriting deterioration), or adventitious movements, such as choreiform movements (Murphy et al. 2012). Psychiatric symptoms as described by Murphy et al. included irritability, frequent mood changes, separation anxiety, hyperactivity, late-onset attention problems, personality change, oppositional behaviors, sleep disturbances, and deterioration in mathematical skills. Although streptococcal infections have been closely associated with a PANDAS onset, there is debate in the literature whether group A streptococcal infections are coincidental or causal. Historical accounts from the first 50 cases of PANDAS indicate that at least some cases of PANDAS occurred immediately following or during a group A streptococcal infection (Swedo et al. 1998). Whether PANDAS is a variant of ARF is still debated (Kurlan et al. 2008). Although there is still discussion as to the exact mechanism of PANDAS, current PANDAS criteria cite that a history of RF is exclusionary for a PANDAS diagnosis. However, controversy does exist among some neurologists regarding the validity of PANDAS as a subset of OCD/tics versus its being a forme fruste of RF (SC). In SC, neuropsychiatric symptoms predate choreoathetoid movements. PANDAS is described as including choreiform piano-playing movements of the fingers and toes (Swedo et al. 1998).
Since the initial identification of the PANDAS subgroup, it has been proposed that the disorder may develop as a result of postinfectious autoimmune processes (Swedo et al. 1998; Kirvan et al. 2006b). We hypothesize that antistreptococcal antibodies produced in response to group A streptococcal infection cross-react with neuronal targets of susceptible hosts through the process of molecular mimicry (Kirvan et al. 2003). We suggest that the pathogenesis of PANDAS could be similar to that of SC, which was delineated by studying human monoclonal antibodies (mAbs) derived from an SC patient. Three human mAbs reacted with the surface of neuronal cells and demonstrated antibody cross-reactivity with the group A carbohydrate epitope N-acetyl-β-D-glucosamine (GlcNAc) and lysoganglioside. mAb 24, one of these mAbs, possessed the highest avidity for lysoganglioside, and also induced elevated calcium calmodulin dependent protein kinase II (CaMKII) levels in SK-N-SH, a human neuronal cell line. Subsequent studies of acute and convalescent sera from SC patients demonstrated that antibodies in the acute sera had immunoglobulin G (IgG) reactivity profiles similar to that of mAb 24 (Kirvan et al. 2003). Autoantibodies against tubulin and lysoganglioside were also present in PANDAS (Kirvan et al. 2006b). Additionally, both SC and PANDAS sera stimulated neuronal cells in culture in an IgG- dependent reaction, leading to activation of CaMKII.
Mouse and rat animal models lend support to the streptococcal autoimmune hypothesis. Group A streptococcal-immunized mice were found to have serum immunoreactivity to deep cerebellar nuclei (DCN) and increased IgG deposits in DCN (Hoffman et al. 2004). Streptococcal immunized mice with anti-DCN antibodies developed distinct motoric and behavioral disturbances that correlated with level of immunoreactivity to the DCN (Hoffman et al. 2004). The validity of the autoimmune hypothesis in the murine model was further demonstrated through passive transfer of antistreptococcal sera from immunized mice to naïve mice (concomitant with lipopolysaccharide [LPS] to break the blood–brain barrier) (Yaddanapudi et al. 2010). It was also found that passive serum antibody transfer recipients developed motor and behavioral disturbances. Repetitive behaviors in both Group A β-hemolytic streptococci (GABHS) donor and passive transfer mice are reminiscent of tics, obsessions, and compulsions (Yaddanapudi et al. 2010).
In an investigation of the specific neural and immune characteristics in a Lewis rat model, Brimberg et al. reported that exposure of male Lewis rats to GABHS antigens led to OCD/tic-like behavior, as well as immunological and neural characteristics similar to those of SC and PANDAS (Brimberg et al. 2012). Behaviorally, streptococcal-exposed rats were impaired in manipulating food and in traversing a narrow, but not a wide, beam. Serum studies of the immunized rats to GABHS led to antibodies that activated CaMKII signaling, and sera from GABHS rats reacted more significantly with human D1 and D2 dopamine receptor membrane antigens than that from control rats. The reactivity of the GABHS rat sera with the D1 and D2 receptor antigen was confirmed by Western immunoblot. Impaired food manipulation and increased induced grooming in GABHS-exposed rats were alleviated by the administration of the D2 blocker haloperidol, which is used to treat motor and compulsive symptoms in SC and PANDAS (Brimberg et al. 2012).
Evidence for autoantibodies in PANDAS and SC includes several studies that have demonstrated significantly higher concentrations of antineuronal antibodies in patients with SC, OCD, and Tourette's syndrome compared with healthy controls, (Husby et al. 1976; Kiessling et al. 1993, 1994; Singer et al. 1998); however, further studies did not replicate these results (Black et al. 1998). In further support of autoantibodies and the autoimmune hypothesis, there are several reports of immunosuppressive treatments (e.g. plasmapheresis, intravenous,immunoglobulin [IVIG], and prednisone) resulting in immediate and strong suppression of acute childhood OCD and Tourette's syndrome (Kondo and Kabasawa, 1978; Matarazzo 1992; Swedo 1994; Allen et al. 1995; Perlmutter et al. 1999).
Autoantibody detection and the identification of their neuronal targets in SC and PANDAS has been the object of scrutiny for some time (Husby et al. 1976; Church et al. 2002; Kirvan et al. 2003; Singer et al. 2005; Dale et al. 2006; Brilot et al. 2011; Dale et al. 2012; Mohammad et al. 2013; Pathmanandavel et al. 2013; Ramanathan et al. 2013). The autoantibody hypothesis has been debated in the literature with inconsistent results, that is, with both positive (Church et al. 2003; Kirvan et al. 2006b; Pavone et al. 2006) and negative findings (Singer et al. 2005; Morris et al. 2009). There are several challenges associated with the investigation into these autoantibodies, including clinical distinction of PANDAS from other presentations of OCD or tics (Murphy et al. 2012), and there is a lack of prospective studies examining any temporal relation between antecedent bona fide GABHS infections and the onset or exacerbation of tics and obsessive-compulsive symptoms (Leckman et al. 2011). Most recently, autoantibodies against the D1 and D2 receptors were confirmed in SC by an independent study in which the ratio of anti-D1/anti-D2 receptor antibodies correlated with symptoms (Ben-Pazi et al. 2013) and another study of SC mAb and serum from youth with SC and PANDAS that demonstrated reaction with the D2R and the chorea-derived human mAb targeted dopaminergic neurons in transgenic (Tg) mice (Cox et al. 2013).
The purpose of this study was to determine if our previous findings applied to a large cohort of patients from the general community with self-reported neuropsychiatric symptoms. We hypothesized that youth with OCD and/or tics would have higher rates of GABHS infection history than those without, and higher levels of lysoganglioside, tubulin, dopamine D1 receptor, and dopamine D2 receptor antineuronal autoantibodies than healthy controls. Additionally, we hypothesized that serum activation of CaMKII levels in youth with OCD and/or tics would be elevated and be correlated with a positive GABHS infection history.
Methods
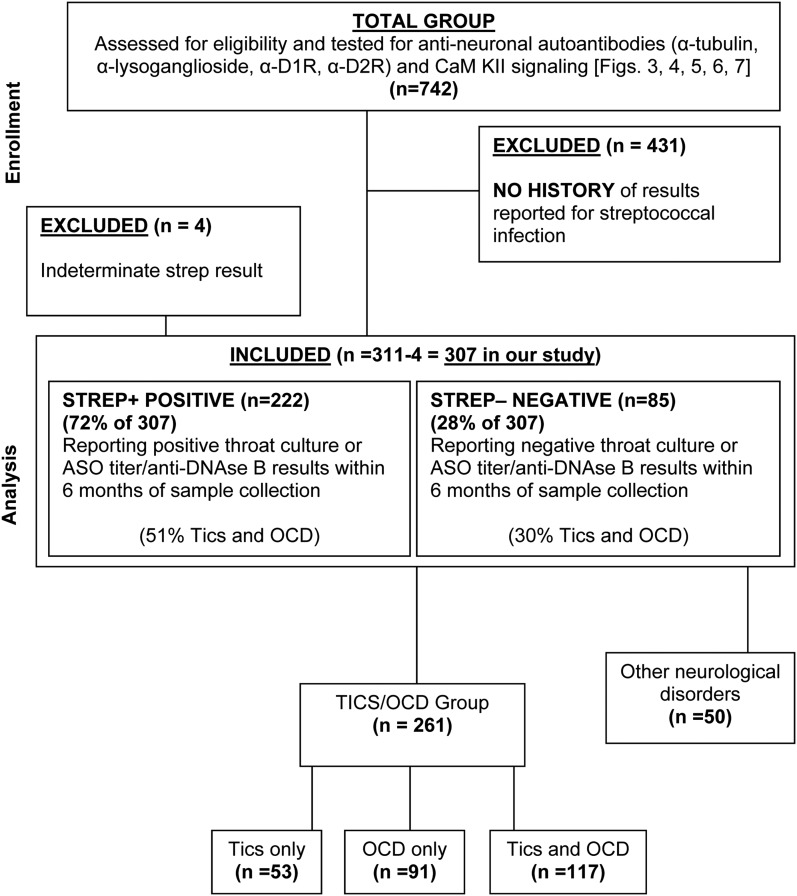
Our investigation (n=742) was conducted to evaluate the presence of elevated antineuronal autoantibodies in patients with tics and/or OCD. This study included data from the subset of the 311 in the current report (see consort diagram, Fig. 1). The study inclusion criteria were a report of a tic disorder, OCD, or both, by parent and /or physician. Recruitment was weighted toward subjects with a detailed history of streptococcal infection, either positive or negative for cultures or antistreptococcal antibody titers (antistreptolysin O [ASO] or anti-deoxyribonuclease B [anti-DNAse B]). Patients on psychotropic medication, antibiotics, or steroids for their condition were not excluded. Additionally, patients who had previously received intravenous immunoglobulin or plasma exchange in the past but were experiencing symptoms were not excluded. Only patients with accurate group A streptococcal infection information (n=311) were included in this study. Of the 311, all but 4 were categorized with or without history of streptococcal infection. There were 261 patients who had tics/OCD, and the remaining had other neuropsychiatric symptoms and were not included in the analysis (see Fig. 1). Sixteen healthy controls selected at the National Institute of Mental Health and Yale University had normal physical examination findings; no lifetime personal history for the participant or any first degree relative with a Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM IV) diagnosis of a tic disorder, Tourette syndrome, OCD, or attention-deficit/hyperactivity disorder (ADHD); and had ASO titers ranging between 70 and 513 (Todd units) (American Psychiatric Association 1994). Figure 1 is a consort diagram depicting recruitment of study subjects.
FIG. 1.
Consort diagram depicting recruitment of study subjects.
Study procedures
Our study was conducted at the University of Oklahoma Health Sciences Center. This study was approved by the University of Oklahoma Health Sciences Center human subjects institutional review board. Although the study was not formally advertised, parents were informed about the study from initial participants who had contacted our research laboratory about research studies for PANDAS. Before participation, parents or legal guardians gave written consent for subjects years of age. Oral assent was given by youth ≥7 years, and when age appropriate (≥13 years), subjects gave written assent. Subjects >18 years of age gave informed consent. Blood samples were collected and the samples and consent forms were returned overnight to the research laboratory from areas throughout the United States.
Streptococcal infection status
The status of streptococcal infection was determined for 311 patients: 222 (72%) were streptococcus positive, 85 (28%) were streptococcus negative, and 4 (1%) had undetermined status. The streptococcal infection status was determined using either throat culture, ASO antibody assay, or anti-DNASeB antibody assay, or a combination of the three tests. When the age of the participant was known and either ASO and anti-DNASe B antibody assays were available, their thresholds were age adjusted as previously described. (Kaplan et al. 1998). Where ages were unavailable, thresholds used were ≥200 for ASO and ≥240 for anti-DNASe B (Murphy et al. 2012).
Antineuronal antibody titers in the enzyme-linked immunosorbent assay (ELISA)
Ninety-six well Immunolon-4 microtiter plates (Dynatech Laboratories) were coated with antigen: lysoganglioside (Sigma, 20 μg/mL), tubulin (MP Biomedical, 10 μg/mL), human dopamine receptor D1antigen (Perkin Elmer, 10 μg/mL), and human dopamine receptor D2L antigen (Perkin Elmer, 10 μg/mL), and incubated overnight at 4°C. ELISA was performed on patient and control sera as previously described (Cox et al. 2013).
Calcium calmodulin (CaM) kinase assay
Cell culture
SK-N-SH human neuroblastoma cells obtained from American Type Culture Collection (ATCC) were treated with serum after growth in F12-Dulbecco's Modified Eagle Medium (DMEM) (complete with 10% fetal bovine sera and Pen/Strep) as previously described (Kirvan et al. 2003). Cell extracts were centrifuged at 15,000 rpm for 20 minutes at 4°C. Protein concentrations of the cell lysates were determined by Bradford assay (Bio-Rad Protein Assay Kit II #500-0002).
CaM KII activity assay
Protein kinase activity was measured using CaM kinase assay system (signa-TECT CaM Kinase assay kit, Promega) according to the manufacturer's instructions. In brief, 5 μL of cell lysate was incubated with 50 μM peptide substrate and [γ-32P] adesnosine triphosphate (ATP) for 2 minutes at 30°C as previously described (Kirvan et al. 2003). Sera were tested at 1:100 (Brimberg et al. 2012; Cox et al. 2013).
Statistical analysis
Means of symmetric distributions were compared between groups using an independent t test. The distributions of continuous measures that were positively skewed were compared between two independent groups using a nonparametric Wilcoxon rank sum test, and were compared among more than two groups using a Kruskal–Wallis test. Proportions were compared between and among groups using a χ2 test or Fisher's exact test (when >20% of expected frequency counts were <5). A two sided 0.05 α level was used to define statistical significance, with adjustment for multiple pairwise comparisons using a Bonferroni correction of the calculated p value.
Results
A total of 311 out of 742 patient volunteers were selected for our current report, on the basis of having data regarding streptococcal infection history. Of these 311 volunteers, age was only available for 85% and race/ethnicity was available for 62%. Age of the patients ranged from 4 to 27 years with an average age of 9.91 years (standard deviation,±3.95 years), with 8 patients who ≥19 years of age. In our study group, 66.6% of the patients were male, and 90% were Caucasian. Healthy control subjects (n=16) were 5–14 years of age, with an average age of 10±3 years, and 13 (81%) were male. Demographic data, diagnoses (OCD and/or tics), and streptococcal infection data for the 311 participants are presented in Table 1.
Table 1.
Demographic and Symptom Characteristics for 311 Participants: Comparison of Males versus Females (Distribution Was Not Significant)
| Characteristics | All patients (n=311) | Male (n=206) | Female (n=105) | p value* |
|---|---|---|---|---|
| Age (years, mean [±standard deviation]) | 9.91 (±3.95) | 9.70 (±3.95) | 10.29 (±3.95) | 0.24 |
| Sex (male, count [%]) | 206 (66%) | — | — | 0.16 |
| Race/Ethnicity (count [%]) | ||||
| African American | 5 (3%) | 3 (2%) | 2 (3%) | |
| Asian/Pacific Islander | 9 (5%) | 9 (7%) | 0 | |
| Caucasian | 169 (90%) | 111 (87%) | 58 (95%) | |
| Hispanic | 4 (2%) | 3 (2%) | 1 (2%) | |
| Native American | 1 (<1%) | 1 (<1%) | 0 | |
| Race/Ethnicity unknown (count [%]) | 123 (40%) | 79 (38%) | 44 (42%) | |
| Symptoms (count [%]) | 0.84 | |||
| Tics only | 53 (20%) | 35 (20%) | 18 (20%) | |
| OCD only | 91 (35%) | 57 (33%) | 33 (37%) | |
| Tics and OCD | 117 (44%) | 79 (46%) | 38 (43%) | |
| 261 | ||||
| No tics and no OCD | 2 (<1%) | 2 (1%) | 0 | |
| Other neurological symptoms (count [%]) | 48 (15%) | 33 (16%) | 16 (15%) | |
| 50 | ||||
| 311 Total | ||||
| Group A streptococcal infection (307/311 participants) (count [%]) | 0.89 | |||
| Positive | 222 (72%) | 148 (73%) | 74 (72%) | |
| Negative | 85 (28%) | 55 (27%) | 29 (28%) | |
| Group A streptococcal infection status unknown (count [%]) | 4 (1%) | 3 (1%) | 1 (1%) | |
p value comparing distribution between males and females was not significant. Two sample t test was used to compare the means from continuous measures and Fisher's exact test was used for comparison of the distribution of categorical measures between males and females.
OCD, obsessive-compulsive disorder.
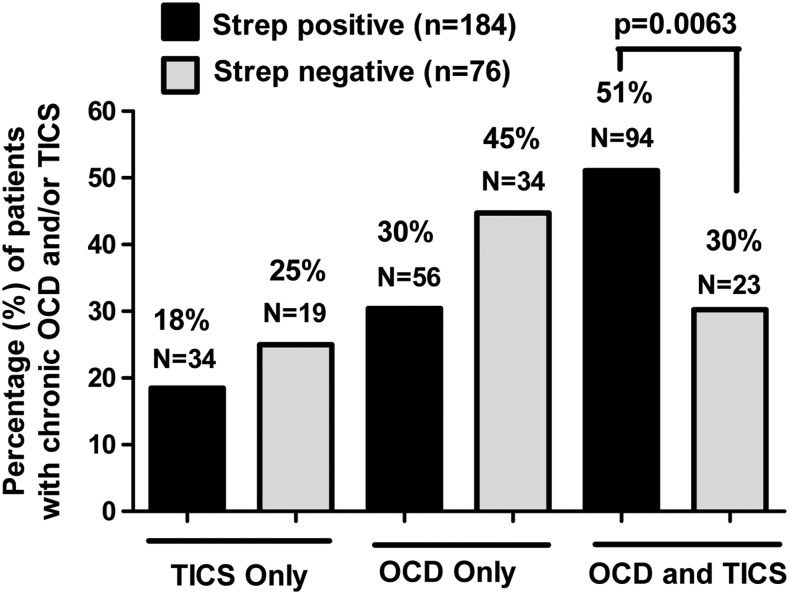
A total of 261 subjects had a documented history (>1 year) of actual OCD and/or tics. The remaining 50 had a variety of other neuropsychiatric disturbances, or no confirmable history of tics and/or OCD (Fig. 1). The presence of OCD and/or tics was significantly associated with streptococcal infection status (overall χ2 test p=0.0087) (Fig. 2). Subjects who were positive for streptococcal infection (n=184, based on numbers in Fig. 2) were more likely to have both tics and OCD (51%, 94/184) than those who were negative for or did not report positive ASO or anti-DNAse B titers or positive throat cultures for streptococcal infection/exposure (n=76, based on numbers in Fig. 2) (30%, 23/76, adjusted p=0.0063). There was no difference between the percentage of subjects with only tics (18%, 34/184, vs. 25%, 19/76, adjusted p=0.72) and the percentage with only OCD (30%, 56/184, vs. 45%, 34/76, adjusted p=0.083) when comparing participants who were positive with those who were negative for streptococcal infection. Subjects with indeterminate streptococcal infections (n=4) were not included in the analysis, which brought the total number of subjects who reported either positive or negative streptococcal histories to 307.
FIG. 2.
The presence of obsessive-compulsive disorder (OCD) and tics was significantly associated with streptococcal infection status (overall χ2 test p=0.0087). After adjustment for multiple pairwise comparisons, subjects who were positive for streptococcus were more likely to have both tics and OCD (51%) than those who were negative for streptococcus (30%) (adjusted p=0.0063). Percent of 311 minus 4 indeterminate=307 (see Fig. 1 and Table 1). Of the 307/311 patients, 222 were streptococcus positive – 72% – (See Table 1) and 85 were streptococcus negative (28%). The four who were indeterminate (1%) were not considered in the streptococcal evaluated cohort. See Table 1 under “group A streptococcal infection.” However, only 184 streptococcus positive and 76 streptococcus negative (from tics/OCD group, n=261) are compared here.
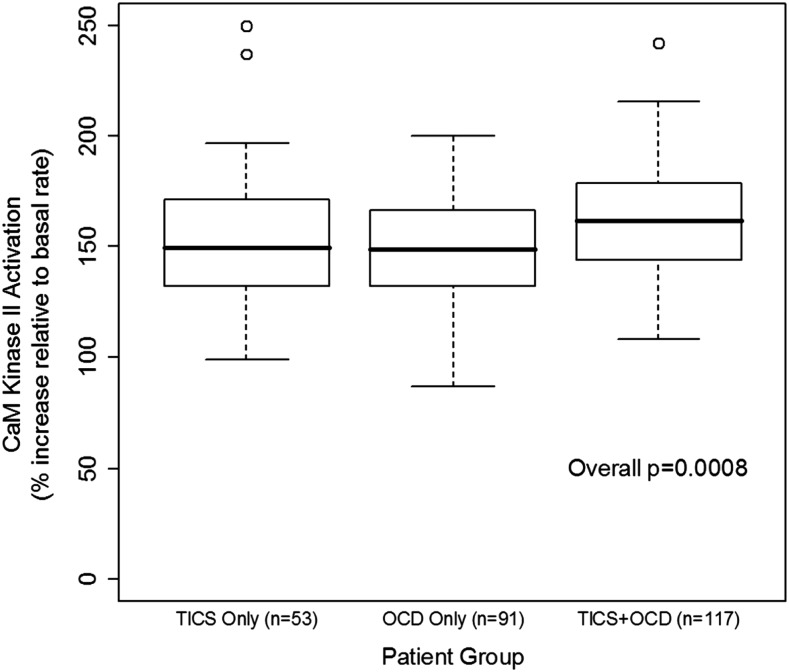
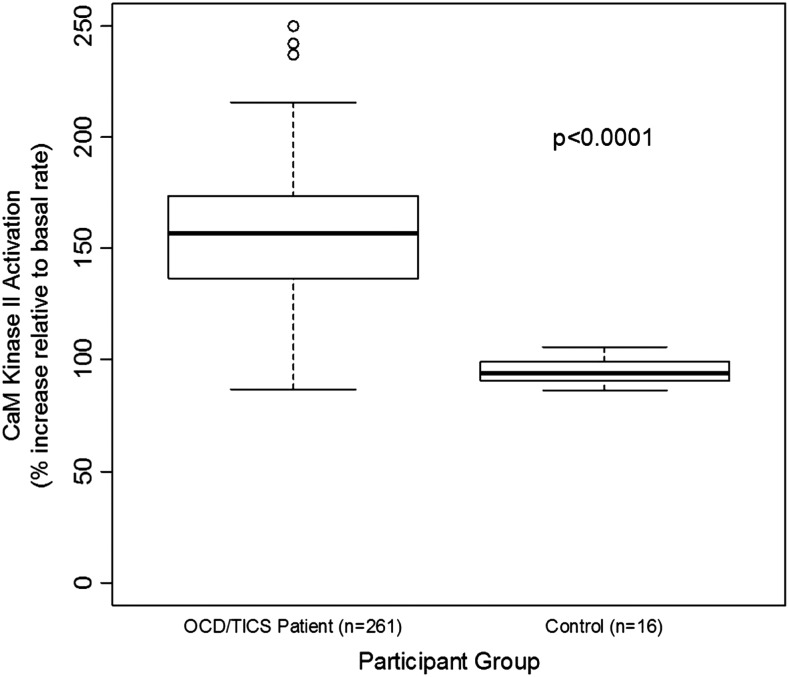
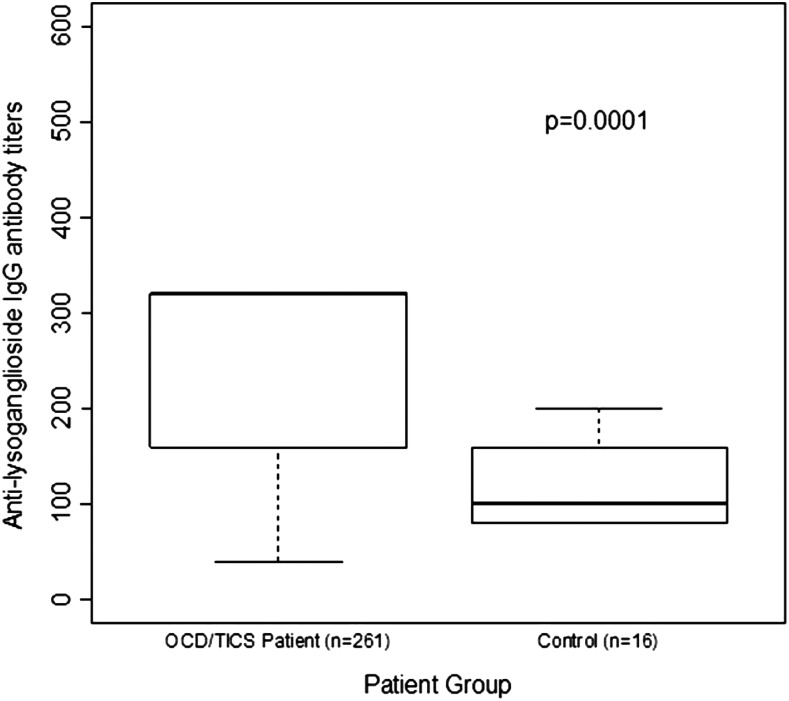
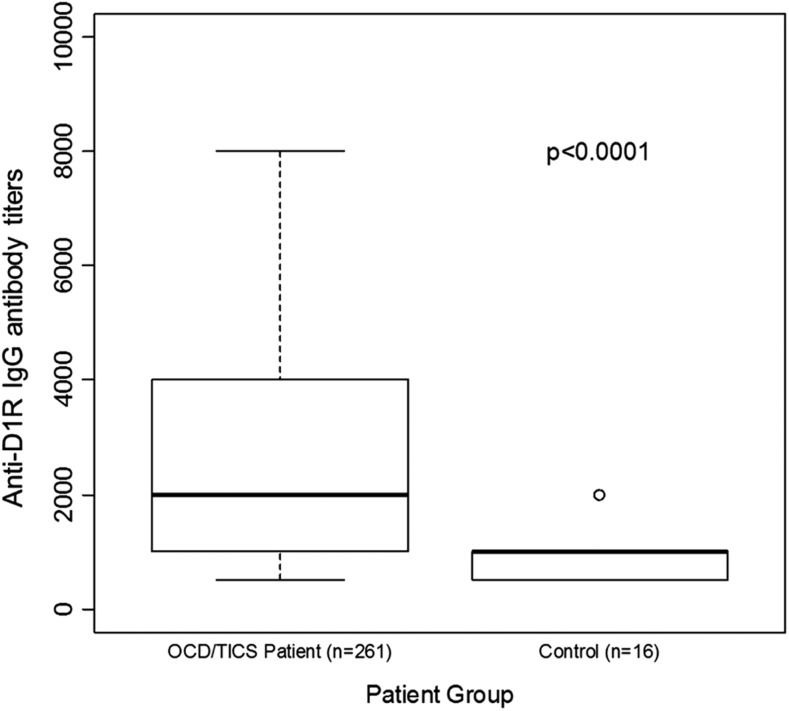
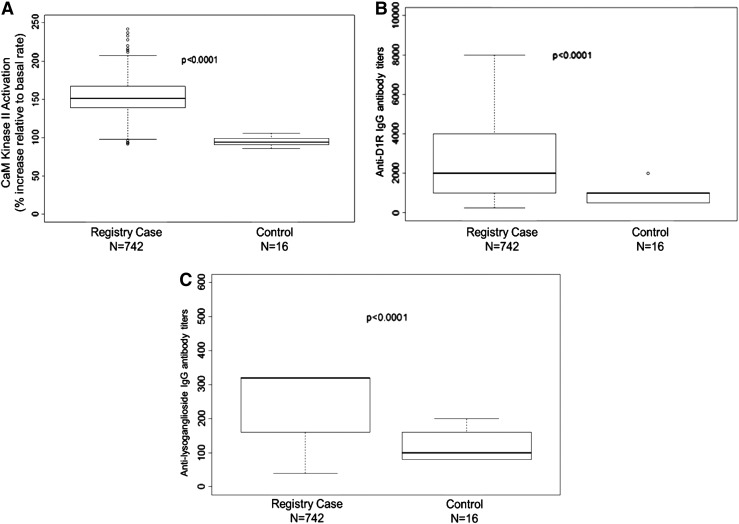
Figure 3 is a graphic summary showing that antibody-mediated activation of CaMKII is associated with OCD and tics (overall p=0.0008). The 261 subjects with OCD and/or tics were found to have significantly elevated antibody-mediated neuronal cell signaling (CaMKII) compared with healthy controls (Fig. 4 and Table 2). Anti-D1R and antilysoganglioside IgG antibody titers (n=16) were also significantly elevated when compared with those of healthy controls (Table 2, Figs. 5 and 6). Figure 7 (A–C) shows analysis of sera from the complete registry (742 individuals), and demonstrates significantly elevated (A) neuronal cell signaling (CaMKII), (B) anti-D1R, and (C) antilysoganglioside IgG antibody titers.
FIG. 3.
CaM kinase II (CaMKII) activation is associated with obsessive-compulsive disorder (OCD) and tics (p=0.0008). CaMKII enzyme activation levels shown are percent above basal level plus the basal level at 100%. Normal % CamKII activation mean: 94–100. All CaMKII activity is calculated as enzyme activity in pmol/min/μg protein (p=0.0008). (p value refers to comparison overall among the three groups – tics only, OCD only, and tics-OCD – not comparison with healthy control subjects.)
FIG. 4.
Analysis of 261/307 individuals demonstrates significantly elevated neuronal cell signaling (CaM kinase II [CaMKII]) among patients with obsessive-compulsive disorders and/or tics compared with healthy controls. CaMKII enzyme activation levels shown are percent above basal level plus the basal level at 100%. All CaMKII activity is calculated as enzyme activity in pmol/min/μg protein. Normal % CamKII activation mean: 94–100.
Table 2.
Antineuronal Antibody Titers in Participants with Tics and/or Chronic Obsessive-Compulsive Disorder versus Healthy Control Participants
| Patients with tics and/or OCD (n=261) | Healthy control subjects (n=16) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Median | P25 | P75 | Median | P25 | P75 | p value* |
| CaM kinase II activation | 157 | 137 | 173.5 | 94 | 90 | 99 | <0.0001 |
| Anti-D1R IgG titer | 2000 | 1000 | 4000 | 1000 | 500 | 1000 | <0.0001 |
| Anti-D2R IgG titer | 4000 | 2000 | 8000 | 4000 | 2000 | 8000 | 0.66 |
| Antilysoganglioside IgG titer | 320 | 160 | 320 | 100 | 80 | 160 | 0.0001 |
| Antitubulin IgG titer | 500 | 500 | 1000 | 500 | 500 | 1000 | 0.11 |
Titers were determined as described in the Methods section.
p value comparing distribution between patients with tics/OCD and healthy controls using the Wilcoxon rank sum test.
OCD, obsessive-compulsive disorder; P25, 25th percentile; P75, 75th percentile; D1R, dopamine D1 receptor antigen; D2R, dopamine D2L receptor antigen (L refers to long isoform of dopamine D2 receptor); IgG, immunoglobulin G titers specific for each antigen listed.
FIG. 5.
Analysis of 261/307 individuals demonstrates significantly elevated antilysoganglioside immunoglobulin G (IgG) antibody titers among patients with obsessive-compulsive disorders and/or tics compared with healthy controls.
FIG. 6.
Analysis of 261/307 individuals demonstrates significantly elevated anti-D1R antibody titers among patients with obsessive-compulsive disorders and/or tics compared with healthy controls.
FIG. 7.
Analysis of sera from the complete registry (742 individuals) demonstrates significantly elevated (A) neuronal cell signaling (CaM kinase II), (B) anti-D1R, and (C) antilysoganglioside immunoglobulin G (IgG) titers, p<0.0001.
Discussion
We found that subjects with chronic tics and/or OCD had elevated serum levels of several antineuronal antibodies, and increased induction of CaMKII, similar to those with SC and PANDAS. In this group, subjects with a positive history of GABHS infection were also more likely to have both tics and OCD.
Therefore, it may be that several categories of movement and behavioral disorders are triggered by infection, and are associated with elevated antineuronal antibodies. Our previous work has supported this idea by demonstrating antistreptococcal/antineuronal antibody binding, including human monoclonal antibodies, to human neuronal cells and activation of CaMKII by sera IgG from SC and PANDAS patients (Kirvan et al. 2003, 2006a,b, 2007; Brimberg et al. 2012; Ben Pazi et al. 2013; Cox et al. 2013). We previously reported that antibody titers against neuronal antigens, lysoganglioside, and tubulin were significantly elevated in acute SC sera compared with convalescent sera. Lysoganglioside competitively inhibited antigen–antibody interaction of SC acute sera with GlcNAc, the dominant epitope of the GABHS carbohydrate (Kirvan et al. 2003; Kirvan et al. 2006b). Additionally, SC acute sera were found to have higher tubulin-specific Ab titers than matched convalescent sera or sera from ARF without chorea (Kirvan et al. 2007). In 2006, sera IgG from acute PANDAS cases were tested in a competitive inhibition ELISA, and, similarly to SC sera, it was found that lysoganglioside specifically inhibited antigen-antibody interaction of SC acute sera with GlcNAc, the dominant epitope of the GABHS carbohydrate (Kirvan et al. 2006b). When the sera of 261 patients diagnosed with OCD, tics, or both were found to react in a direct ELISA with lysoganglioside as the antigen, sera IgG had statistically significantly higher titers than those from healthy controls (median values of 320 vs. 100, respectively, p=0.0001) (Table 2, Fig. 5). The direct ELISA with tubulin as the antigen did not show a statistically significant difference between sera and tics, OCD, or both, versus the sera from healthy controls (Table 2).
In 2012, Brimberg et al. reported that the sera from GABHS immunized rats reacted more significantly with human D1 and D2 receptor antigens than the sera from control rats in direct ELISA and Western blot. To draw a comparison to human disease, reactivity of acute PANDAS and SC sera IgG was tested in direct ELISA, and SC sera reacted more significantly with the dopamine D2 receptor membrane antigen compared with the sera from healthy controls, and the PANDAS sera reacted more significantly with both the D1 and D2 receptor antigens when compared with the sera from healthy controls. Our current study shows that sera IgG from subjects with more chronic OCD, tics, or both reacted more significantly with human D1 receptor antigen compared with the sera from healthy controls in direct ELISA (median value of 2000 vs. 1000, respectively, p≤0.0001) (Table 2, Fig. 6). However, these subjects did not have significant antibody levels against the D2 receptor, in contrast with our previous findings in PANDAS. As our current group consisted of subjects who had been reporting tics and OCD for >1 year, with many reporting tics and OCD for several years, they may represent a different phenotype than the original PANDAS cohort, which exhibited acute onset OCD and/or tics along with fine piano-playing choreiform movements. It is possible that different phenotypes may map with different neuronal receptor antibodies.
We also demonstrated increased CaMKII signaling in subjects with OCD and/or tics, particularly in those with both OCD and tics. CaMKII is a multifunctional enzyme highly concentrated in the brain, which mediates many different learning, memory, and developmental cell pathways, and has broad substrate specificity dependent on concentration, intracellular localization, and intracellular calcium levels (De Koninck and Schulman 1998; Bejar et al. 2002; Menegon et al. 2002; Tsui et al. 2005). In preliminary testing of acute SC sera (n=5) and matched convalescent sera (n=3), there was an increase in CaMKII activation in the acute sera (Kirvan et al. 2003). In a subsequent investigation that included PANDAS sera, 75% of acute PANDAS sera induced antibody-mediated activation of CaMKII to significantly higher levels than did matched convalescent sera (p=0.001) (Kirvan et al. 2006b). The present study reveals two important correlations involving CaMKII activation by serum: 1) The presence of OCD and/or tics was positively associated with antibody-mediated CaMKII activation (n=261, p=0.0008); and 2) antibody-mediated CaMKII activation was elevated for patients with OCD and/or tics (n=261), with median percentile increase values ranging from 149 to 162, whereas it remained unaffected in healthy controls, with a median of the 94th percentile (n=16, p<0.0001). The difference in the median value for CaMKII activation between patient samples and healthy controls is similar to what was found for PANDAS sera and non-PANDAS sera in previous studies (Kirvan et al. 2006b).
In addition to these immunological findings, we found that the presence of OCD and/or tics was associated with positive streptococcal infection history (p=0.0087) (Fig. 2). There have been both negative and positive reports on the association of streptococcal infections with tics or OCD (Murphy and Pichichero 2002; Luo et al. 2004; Murphy et al. 2004, 2007; Kurlan et al. 2008; Leckman et al. 2011; Martino et al. 2011; Murphy et al. 2012). It is sometimes difficult to detect the streptococcal infection associated with exacerbations of tics and OCD, because infections can precede symptoms by several months, or can be representative of a chronic carrier state. It has been suggested in previous studies that to accurately determine an association with streptococcal infection, longitudinal samples are required rather than a single time point (Leckman et al. 2011). This is the first report finding that streptococcal infections may be more prevalent in subjects who have combined tics and OCD.
We also found that subjects who had a positive history of streptococcal infection were more likely to have both OCD and tics (51%) than were those who were negative for streptococcal infections (30%), whereas there was no significant association with infection history when tics or OCD were considered alone. Therefore, it is possible that patients who present with both OCD and tics are more likely to have had streptococcal infections in their history. As there may be various etiologies for obsessive-compulsive symptoms and tics, it may be that a streptococcal etiology may represent a more “virulent” cause that disrupts the basal ganglia in a more widespread fashion, leading to multiple neuropsychiatric symptoms. Presentations of OCD and tics alone may be less likely to be manifestations of disorders associated with GABHS, but still may have similar pathogenic mechanisms.
Study strengths include a generalized community sample and the use of factual medical records when available. However, a diagnosis of a tic disorder, OCD, or both was based on the parents' report when a physician's report was not available. The parents' report may not have been accurate, and may have resulted in misclassification of cases. Given the nature of data reporting from multiple sources, not all variables (e.g., age, ethnicity) are complete. Missing data may result in information bias if those with available data are not representative of the target population of youth and young adults with a tic disorder, OCD, or both. Limitations of the study included lack of severity measures (e.g., Yale–Brown Obsessive Compulsive Scale [YBOCS], TGSS), the small sample size of the control cohort, and the self-report nature of neuropsychiatric symptoms. Furthermore, we do not have reports of associated neuropsychiatric symptoms that the patients may have experienced, such as anxiety, depression, irritability, or cognitive impairment, all of which have been reported in youth with PANDAS.
Conclusions
Our study suggested a significant correlation of streptococcal-associated tics and OCD with elevated anti-D1R and antilysoganglioside antineuronal antibodies in serum concomitant with higher activation of CaMKII in human neuronal cells. Youth and young adults in OCD and/or tics appeared to have higher rates of GABHS infection history than those without OCD and/or tics. Analysis of sera from the complete registry of individuals (742) demonstrated significantly elevated neuronal cell signaling through CaMKII, as well as anti-D1R and antilysoganglioside IgG titers. Youth and young adults with chronic tics and OCD may have underlying infectious/immunologic etiology.
Clinical Significance
The statistically significant correlation between a history of tics and OCD with antineuronal antibodies against D1R and lysoganglioside and functional activation of CaMKII suggests that pediatric neuropsychiatric disorders outside of PANDAS may also be associated with autoimmunity against the brain. The functional activity of the autoantibodies to signal CaMKII in human neuronal cells suggests that antibodies could target receptors in the brain and alter dopamine neurotransmission, leading to neuropsychiatric symptoms of tics and/or OCD. Furthermore, youth presenting with OCD and/or tics, regardless of acuity of onset, should be screened for GABHS infection. Treatment of these youth, however, extends beyond the scope of these findings. Clearly, additional studies are needed in youth with chronic tics and OCD to determine best evaluation and treatment approaches. By understanding the immunological and physiological factors associated with GABHS infection related chronic OCD and tic disorders, clinicians will eventually be able to more accurately identify, diagnose, and target treatment to better manage chronic symptoms to improve outcomes in this population.
Acknowledgment
We thank Dr. Tanya Murphy for critical review of this manuscript.
Disclosures
Dr. Amir Zuccolo, Dr. Julie Stoner, and Erica Edwards have no conflict of interest to declare. Dr. Kiki Chang has received research funding from GlaxoSmithKline and Merck, is on the Data and Safety Monitoring Board (DSMB) for Sunovion, and is an unpaid consultant for Bristol Myers-Squibb, GlaxoSmithKline, and Lilly. Dr. Carol Cox, Adita Mascaro-Blanco, and Kathy Alvarez declare financial interest in Moleculera Labs, a commercial laboratory for diagnostic testing of autoantibodies against the heart and brain. Dr. Madeleine Cunningham is chief scientific officer and co-founder with financial interest in Moleculera Labs.
References
- Allen AJ, Leonard HL, Swedo SE: Case-Study - A new infection-triggered, autoimmune subtype of pediatric OCD and Tourettes-syndrome. J Am Acad Child Adolesc Psychiatry 34:307–311, 1995 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Bejar R, Yasuda R, Krugers H, Hood K, Mayford M: Transgenic calmodulin-dependent protein kinase II activation: Dose-dependent effects on synaptic plasticity, learning, and memory. J Neurosci 22:5719–5726, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Pazi H, Stoner JA, Cunningham MW: Dopamine receptor autoantibodies correlate with symptoms in Sydenham's Chorea. PLoS One 8:1–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JL, Lamke GT, Walikonis JE: Serologic survey of adult patients with obsessive-compulsive disorder for neuron-specific and other autoantibodies. Psychiatry Res 81:371–380, 1998 [DOI] [PubMed] [Google Scholar]
- Brilot F, Merheb V, Ding A, Murphy T, Dale RC: Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 76:1508–1513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimberg L, Benhar I, Mascaro–Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D: Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: A novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 37:2076–2087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G: Anti-basal ganglia antibodies in acute and persistent Sydenham's chorea. Neurology 59:227–231, 2002 [DOI] [PubMed] [Google Scholar]
- Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM: Tourette's syndrome: A cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry 74:602–607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW: Brain human monoclonal autoantibody from Sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: Implications in human disease. J Immunol 191:5524–5541, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G: Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol 172:187–197, 2006 [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F: Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 135:3453–3468, 2012 [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H: Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279:227–230, 1998 [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI: A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci 24:1780–1791, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC: Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med 144:1094–1110, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Rothermel CD, Johnson DR: Antistreptolysin O and anti-deoxyribonuclease B titers: Normal values for children ages 2 to 12 in the United States. Pediatrics 101:86–88, 1998 [DOI] [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L: Anti-neuronal antibodies in movement disorders. Pediatrics 92:39–43, 1993 [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L: Antineuronal antibodies – Tics and obsessive-compulsive symptoms. J Dev Behav Pediatr 15:421–425, 1994 [PubMed] [Google Scholar]
- Kirvan CA, Cox CJ, Swedo SE, Cunningham MW: Tubulin is a neuronal target of autoantibodies in Sydenham's chorea. Journal of Immunology 178(11):7412–7421, 2007 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW: Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 9:914–920, 2003 [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW: Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity 39:21–29, 2006a [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW: Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 179:173–179, 2006b [DOI] [PubMed] [Google Scholar]
- Kondo K, Kabasawa T: Improvement in Gilles de La Tourette Syndrome after corticosteroid-therapy. Ann Neurol 4:387, 1978 [DOI] [PubMed] [Google Scholar]
- Kurlan R, Johnson D, Kaplan EL, Tourette Syndrome Study Group: Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: A prospective blinded cohort study. Pediatrics 121:1188–1197, 2008 [DOI] [PubMed] [Google Scholar]
- Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, Dure LS, Grantz H, Katsovich L, Lin H, Lombroso PJ, Kawikova I, Johnson DR, Kurlan RM, Kaplan EL: Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: A prospective longitudinal study. J Am Acad Child Adolesc Psychiatry 50:108–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Leckman JF, Katsovich L, Findley D, Grantz H, Tucker DM, Lombroso PJ, King RA, Bessen DE: Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: Relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics 113:e587–585, 2004 [DOI] [PubMed] [Google Scholar]
- Marques–Dias MJ, Mercadante MT, Tucker D, Lombroso P: Sydenham's chorea. Psychiatr Clin North Am 20:809–820, 1997 [DOI] [PubMed] [Google Scholar]
- Martino D, Chiarotti F, Buttiglione M, Cardona F, Creti R, Nardocci N, Orefici G, Veneselli E, Rizzo R, Gr ITSS: The relationship between group A streptococcal infections and Tourette syndrome: A study on a large service-based cohort. Dev Med Child Neurol 53:951–957, 2011 [DOI] [PubMed] [Google Scholar]
- Matarazzo E: Tourette's syndrome treated with ACTH and prednisone: Teport of two cases. J. Child Adolesc Psychopharmacol 2:215–226, 1992 [DOI] [PubMed] [Google Scholar]
- Menegon A, Verderio C, Leoni C, Benfenati F, Czernik AJ, Greengard P, Matteoli M, Valtorta F: Spatial and temporal regulation of Ca2+/calmodulin-dependent protein kinase II activity in developing neurons. J Neurosci 22:7016–7026, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad SS, Ramanathan S, Brilot F, Dale RC: Autoantibody-associated movement disorders. Neuropediatrics 44:336–345, 2013 [DOI] [PubMed] [Google Scholar]
- Morris CM, Pardo–Villamizar C, Gause CD, Singer HS: Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls. J Neurol Sci 276:45–48, 2009 [DOI] [PubMed] [Google Scholar]
- Murphy M, Pichichero ME: Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med 156:356–361, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Sajid M, Soto O, Shapira N, Edge P, Yang M, Lewis MH, Goodman WK: Detecting pediatric autoimmune neuropsychiatric disorders associated with streptococcus in children with obsessive-compulsive disorder and tics. Biol Psychiatry 55:61–68, 2004 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Snider LA, Mutch PJ, Harden E, Zaytoun A, Edge PJ, Storch EA, Yang MC, Mann G, Goodman WK, Swedo SE: Relationship of movements and behaviors to Group A Streptococcus infections in elementary school children. Biol Psychiatry 61:279–284, 2007 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman W: Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr 160:314–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathmanandavel K, Starling J, Dale RC, Brilot F: Autoantibodies and the immune hypothesis in psychotic brain diseases: Challenges and perspectives. Clin Dev Immunol 2013:257184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Parano E, Rizzo R, Trifiletti RR: Autoimmune neuropsychiatric disorders associated with streptococcal infection: Sydenham chorea, PANDAS, and PANDAS variants. J Child Neurol 21:727–736, 2006 [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE: Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 354:1153–1158, 1999 [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Mohammad SS, Brilot F, Dale RC: Autoimmune encephalitis: Recent updates and emerging challenges. J Clin Neurosci 21:722–730, 2013 [DOI] [PubMed] [Google Scholar]
- Singer HS, Giuliano JD, Hansen BH, Hallett JJ, Laurino JP, Benson M, Kiessling LS: Antibodies aginst human putamen in children with Tourette syndrome. Neurology 50:1618–1624, 1998 [DOI] [PubMed] [Google Scholar]
- Singer HS, Hong JJ, Yoon DY, Williams PN: Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology 65:1701–1707, 2005 [DOI] [PubMed] [Google Scholar]
- Swedo SE: Sydenham's chorea: A model for childhood autoimmune neuropsychiatric disorders. JAMA 272:1788–1791, 1994 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR: From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther 2:1–8, 2012 [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998 [DOI] [PubMed] [Google Scholar]
- Tsui J, Inagaki M, Schulman H: Calcium/calmodulin-dependent protein kinase II ( CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J Biol Chem 280:9210–9216, 2005 [DOI] [PubMed] [Google Scholar]
- Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, Lipkin WI: Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry 15:712–726, 2010 [DOI] [PubMed] [Google Scholar]