To The Editor:
Kleine–Levin syndrome (KLS) is a sleep disorder of unknown etiology characterized by episodic hypersomnia, hyperphagia, cognitive and mood abnormalities, and abnormal behaviors. The average age of onset is 15 years, and males are more frequently affected than females (Gadoth et al. 2001; Arnulf et al. 2005, 2008). Active episodes have an average duration of 10–13 days with reoccurrences averaging every 3.5 months, with patients typically asymptomatic between episodes (Arnulf et al. 2005, 2008). Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain are typically normal in patients with KLS (Servan et al. 1993; Poryazova et al. 2007; Huang et al. 2012b), and polysomnography tends to display impairment of slow wave sleep and rapid eye movement sleep at symptom onset (Huang et al. 2008). Studies of the long-term effects of KLS are limited, but short-term memory dysfunction, academic decline, and auditory-verbal attention deficits have been observed even during periods of remission (Fontenelle et al. 2000; Landtblom et al. 2003).
Pediatric autoimmune neuropsychiatric disorder associated with streptococcus (PANDAS) is a disorder characterized by an acute, sudden onset of obsessive-compulsive disorder (OCD) and/or tics, often accompanied by attention deficit/hyperactivity, separation anxiety, oppositional behaviors, and emotional lability (Swedo et al. 1998). Personality changes, cognitive disturbances, motor abnormalities, sensory sensitivity, behavioral regression, and, occasionally, psychosis are also often present (Bernstein et al. 2010; Murphy et al. 2012). The pathogenesis of PANDAS is hypothesized to be related to group A streptococcal (GAS) infections via molecular mimicry, in that streptococcal antibodies target brain proteins of similar epitopic structure to that of GAS (Cunningham 2012). PANDAS exhibits immunological similarities to Sydenham's chorea (SC), a classic infection-triggered autoimmune disorder that also presents with a high degree of OCD comorbidity (Swedo et al. 1989). However, unlike SC, definitive evidence for autoantibody-mediated reactions in PANDAS has been less conclusive (Brilot et al. 2011; Morris-Berry et al. 2013). A newer iteration of acute-onset neuropsychiatric symptoms not dependent upon a GAS association is termed “pediatric acute-onset neuropsychiatric syndrome” (PANS) (Swedo et al. 2012). Given the dramatic phenotype and the potential for long-standing neuropsychiatric sequelae, PANS is perhaps a subtype of autoimmune encephalopathy affecting primarily psychiatric symptom domains rather than the neurological symptom domains typical of other encephalopathies. For a complete review of the clinical presentation, assessments, and proposed pathophysiology of PANDAS/PANS, please see our referenced reviews (Chang et al. forthcoming, Murphy et al. 2014).
Our aim is to report a case of a 9-year-old boy who exhibited symptoms of both KLS and PANDAS. This case is the second that calls attention to two uncommon syndromes that can occur concurrently (Das and Radhakrishnan 2012). We also provide a brief literature review to discuss symptom overlap and similar hypothesized pathogeneses.
Case Report
The patient was a 9-year-old boy with no premorbid psychiatric history, who presented with acute-onset OCD and tics that had begun 4 months prior to evaluation. Medical history was significant for frequent upper respiratory infections (URIs), allergies, hemihypertrophy, psoriasis, multiple croup infections as an infant, and mild obesity. His hemihypertrophy had been monitored closely from early childhood with abdominal ultrasounds, which had been negative for tumor growth. Developmental history was noncontributory, but notable for meeting developmental milestones relatively early. His intellect was above average and he excelled in academics. Maternal family history was significant for heart murmur, rheumatoid arthritis, unspecified drug abuse, generalized anxiety disorder, and panic disorder. Paternal family history was significant for adult-onset diabetes, unspecified drug abuse, and a history of seizures in male family members, including febrile, absence, and tonic–clonic. Notably, the patient's sister had frequent GAS infections, attention-deficit/hyperactivity disorder, and dyslexia.
The patient's symptoms began when he awoke one morning extremely fatigued, and proceeded to experience three panic attacks that day. Over the following 2 days, he began experiencing contamination fears, vocal and motor tics, emotionality, nonsensical speech, severe separation anxiety, and staring spells. Two days post-onset, his mother called his pediatrician, as the family was on vacation, and per the pediatrician's advice, the family took the boy to the emergency department (ED), where he tested negative for a GAS infection. Notably, because his sister was experiencing a sore throat, she was also examined in the ED, and tested positive for a GAS infection. Complete blood count (CBC) was within normal limits except for increased white blood cell (WBC) count at 12.5×103/μL, suggestive of recent infection. Comprehensive metabolic panel (CMP) was normal. Urinalysis for common drugs of abuse was negative. ED physicians believed him to have a brain tumor, and instructed the family to return home to see a neurologist as soon as possible.
Six days after initial onset, all symptoms remitted. However, after 2 asymptomatic days, the patient began experiencing increased appetite, hypersomnia, and cognitive abnormalities, including not recognizing his father. OCD symptoms continued to evolve, with persisting contamination fears and emerging aggressive obsessions and intrusive thoughts, worries about “bad things” happening to his family, fear of losing or misplacing his belongings, and checking compulsions.
An episodic pattern continued, which over time was noted to consist of 7–10 days without symptoms, followed by a 2 day prodromal period of increased agitation and irritability, hypersomnia, asocial behavior, and “saying random words to himself.” The active phase was characterized by full-blown OCD; motor tics, including mouth movements, biting fingers and toenails to the point of bleeding; and vocal tics of throat clearing and uncontrollable singing. The patient began sleeping 12 or more hours during the day and would insist that his mother or father sleep in his room with him at night. His total sleep averaged 18–20 hours per 24 hour period, and during one episode, he slept for 72 hours consecutively. He experienced diurnal restlessness, such as “walking in patterns” and pacing. He progressively became withdrawn, and eventually would not speak even to his family. Hyperphagia characterized by almost constant eating also developed, leading to a weight gain of 4.5 kg over a course of 1 month. As the patient would forget that he had eaten, his mother began taking photographs of him eating to later assure him. Paradoxically, he would refuse to drink most fluids, except, occasionally, for water. Neurological abnormalities were also present, including dysgraphia, dyscalculia, and dysphasia. The longest symptom-free interval was 2 weeks, during which the patient was “talkative and social” and without OCD; sleep, cognitive, or mood disorders; or disordered eating symptoms. During his symptom-free intervals, some motor tics were present, although not of the intensity or frequency as during active episodes.
At the neurology consultation 2 weeks post-onset, secondary to concerns of a seizure disorder, an MRI of the brain was performed and was reported as normal, as well as a 24 hour video electroencephalogram (VEEG). During the VEEG, the patient experienced intermittent facial twitching and staring, but no changes in EEG background activity were observed. At the neurology follow-up 6 weeks post-onset, he was diagnosed with OCD and tics of PANDAS/PANS subtype and prescribed a 10 day course of amoxicillin 875 mg twice daily, without symptom alleviation. After completing the course of amoxicillin, the patient was prescribed valproate 250 mg twice daily with some benefit to his agitation. With the episodic course of symptoms persisting, including occasional staring spells, neurology performed a 48 hour VEEG that was also normal. During the course of 4 months, the patient frequented the ED multiple times secondary to “seizures.” Because of concerns of PANDAS/PANS not resolving with amoxicillin treatment, the Neurology Department referred the patient to the Child Psychiatry Department.
At the time of the evaluation, the patient was displaying vocal and motor tics, as well as expressing delusions, in that he believed the doctors, whom he described as “murderers,” were watching him through cameras and videotaping him. He appeared restless, as he was pacing the floor in squares throughout the evaluation.
Physical examination was normal, except for a papular rash on the legs and arms, as well as mild livedo reticularis. The patient had left-sided hemihypertrophy without functional impairment. His tympanic membranes were scarred, and he had one erythematous palatoglossal arch without tonsillopharyngitis. Rapid GAS test was negative. Anti-DNase B titer was elevated at 672 U/mL and antistreptolysin O (ASO) titer was elevated at 322 IU/mL. Mycoplasma immunoglobulin (Ig) G was elevated (1666 U/mL) as was IgM (797 U/mL), suggesting a possible recent infection. CBC was notable for elevated WBC at 11.3×103/uL, absolute neutrophils (7.2×10e3/uL), and absolute monocytes (1.1×10e3/uL). Testing for serum N-methyl-d-aspartate receptor antibody was negative (Mayo Clinic). Baseline urinalysis revealed trace ketones and calcium oxalate crystals (repeat urinalysis 2 weeks later when symptoms were remitted was normal).
He was diagnosed with PANDAS/PANS and rule out KLS. He was enrolled in a study in which he received either azithromycin or placebo for 4 weeks. During this time, he experienced an episode that his parents characterized as the most severe to date. He was walking in circles, was mostly nonverbal, and kept bouncing a ball. When his mother asked him to stop, he became aggressive. He was eating almost constantly (which he did not remember). He took naps during the day and could not sleep at night. He exhibited facial tics, jaw popping, and threw his head back constantly. He also had separation anxiety in that he wanted his parents with him at all times. At the time of 4 week follow-up, he was completely asymptomatic aside from intermittent mild tics, and he began open-label azithromycin treatment.
Over the weeks following the initial evaluation, he received ongoing azithromycin and two intravenous immunoglobulin (IVIG) treatments. His asymptomatic intervals are continuing to lengthen over time (maximum interval=20 days), and the severity of his active episodes has attenuated (symptom severity ratings from baseline to week 12 demonstrated a 79% decrease in OCD severity and 68% decrease in tic severity). At the 12 week follow-up, the patient stated that his mood, OCD, and tics were much improved. Laboratory results yielded an elevated mycoplasma pneumonia IgG titer (1008 U/mL), but IgM titer had normalized (<770 U/mL), although it is unclear if this would have spontaneously occurred or if it was secondary to IVIG therapy. ASO titer was elevated at 395.1 IU/mL and anti-DNase B was elevated at 458 U/mL.
Discussion
This case demonstrates how clinical presentation can be complicated by comorbid conditions. Our patient exhibited various symptoms congruent with those of KLS, including an episodic course of hypersomnia, changes in appetite, cognitive disturbances, agitation, and irritability. Staring spells were also present, but VEEG was normal, decreasing the likelihood of a seizure disorder. It is also noteworthy that our patient's episodes were occurring every 7–10 days, which is much more frequent than the average published frequency of every 3 months (Arnulf et al. 2005). There is one other case report to our knowledge that discusses a rapid cycling of KLS (De Gusmao et al. 2014). For our patient, PANDAS diagnostic criteria were also met, in that the patient experienced acute-onset OCD and tics, as well as separation anxiety, delusions, and sensory sensitivity. Similar to other cases of PANDAS/PANS as well as KLS, this patient had experienced a sore throat and GAS exposure prior to onset, and displayed elevated GAS and mycoplasma pneumonia titers at evaluation 4 months post-onset, suggesting recent infections. His presentation differs from that of typical PANDAS/PANS, in that youth with PANDAS/PANS often have severe insomnia and food refusal. It is noteworthy that our patient had a history of congenital hemihypertrophy, which is a rare condition with an unknown etiology hypothesized to be secondary to vascular or lymphatic abnormalities, endocrine malfunctions, chromosome abnormalities, or abnormal embryonic growth (Ballock et al. 1997; Clericuzio and Martin 2009). Of particular interest is that one cause of hemihypertrophy, Beckwith–Wiedemann syndrome, has been associated with chromosomal area 11p15.5 (Ko 2013), the same area involved in DRD4 polymorphisms that have been implicated in psychiatric disorders (Docherty et al. 2012), as well as perhaps in tic disorders (Toufexis et al. 2013). However, this association is of unknown relevance to our patient's clinical presentation.
Viral infections, including various febrile illnesses (Gillberg 1987; Gadoth et al. 2001), URIs (Arnulf et al. 2005; Huang et al. 2012b), encephalitis (Merriam 1986; Fenzi et al. 1993; Huang et al. 2012b), and Epstein–Barr and varicella-zoster (Salter and White 1993) have been reported as precipitating factors in KLS. PANDAS exacerbations have been correlated with GAS infections (Swedo et al. 1998; Mell et al. 2005; Murphy et al. 2012). For PANS, infectious triggers other than GAS have been suggested, including the common cold (Hoekstra et al. 2005), mycoplasma pneumonia (Müller et al. 2000, 2004; Yiş et al. 2008), and influenza (Allen et al. 1995). Notably, our patient's medical history was significant for frequent URIs as well as croup infections as an infant. At evaluation, he displayed titer elevations for both GAS and mycoplasma pneumonia, which may have, theoretically, accounted for the dual presentation of KLS and PANDAS. Both KLS and PANDAS/PANS are notable for an early age of onset, recurrent episodic symptoms, and infections prior to onset, which may collectively be suggestive of a postinfectious autoimmune etiology. Autoimmunity has been purported as a potential etiology for KLS, with human leukocyte antigen DQB1*0201 allele frequency being significantly increased in patients with KLS (Dauvilliers et al. 2002; Huang et al. 2012a). Higher rates of maternal autoimmunity have been reported in youth with PANDAS compared with community rates (Murphy et al. 2010). In addition, OCD and/or tics or other neurological disorders are often reported in the families of youth with PANDAS (Lougee et al. 2000; Falcini et al. 2014).
Mixed evidence suggests hypothalamic dysfunction in KLS (Koerber et al. 1984; Gadoth et al. 1987; Fernábdez et al. 1990; Chesson et al. 1991; Malhotra et al. 1997; Mayer et al. 1998; Kas et al. 2014). Evidence for thalamic involvement also exists; Tc-99m ethyl cysteinate dimer (ECD) single-photon emission CT analysis during symptomatic periods of KLS has demonstrated thalamic hypoperfusion (Huang et al. 2005; Hong et al. 2006; Huang et al. 2012b; Kas et al. 2014). MRI assessment of PANDAS youth has demonstrated enlarged basal ganglia (Giedd et al. 1996, 2000), and positron emission tomography has demonstrated increased activated microglial cells, which are suggestive of neuroinflammation, in bilateral caudate nuclei and bilateral lentiform in youth with PANDAS (Kumar et al. 2014). The basal ganglia have also been implicated in pediatric OCD (Rosenberg et al. 1997; Szeszko et al. 2004) and tic disorders (Moriarty et al. 1997; Peterson et al. 2003; Amat et al. 2006).
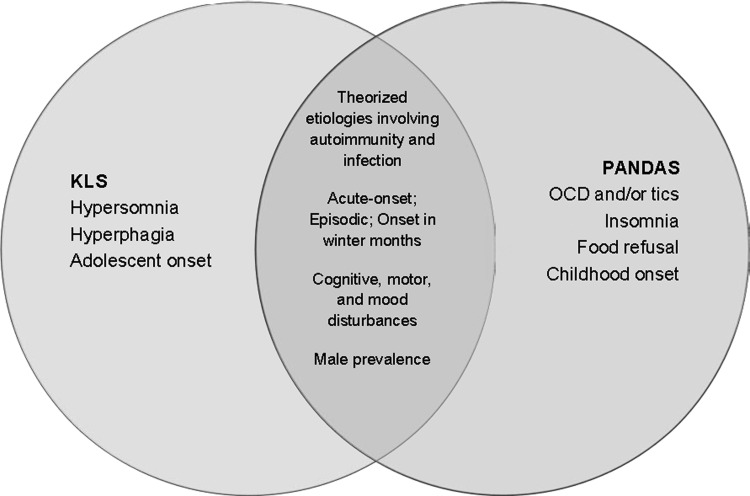
Symptomatic overlap is present between KLS and PANDAS/PANS, in that compulsive behaviors, such as singing, body rocking, and lip chewing, may be present in PANDAS and have also been reported in KLS patients (Thacore et al. 1969; Papacostas and Hadjivasilis 2000; Muratori et al. 2002). Irritability, oppositional behaviors, depersonalization and derealization, and mania may also present in both KLS (Reynolds et al. 1980; Gillberg 1987; Sagar et al. 1990; Arnulf et al. 2005, 2008) and PANDAS (Swedo et al. 1998; Bernstein et al. 2010; Murphy et al. 2012). (See Fig. 1 for a visual representation of symptom overlap.) Comorbidity between KLS and OCD has been reported, with a case report noting acute-onset OCD in an adolescent male who was also being treated for KLS (Chakraborty and Chatterjee 2007). Although it is not certain that modafinil treatment did not trigger the OCD, the KLS symptoms, which were preceded by a febrile viral illness, appeared when the OCD symptoms remitted, and OCD symptoms reappeared when KLS symptoms were treated. The researchers postulated that the two disorders may share a common pathophysiology that was expressed differently episodically.
FIG. 1.
Features of Kleine–Levin syndrome (KLS) and pediatric autoimmune neuropsychiatric disorder associated with streptococcus (PANDAS).
There has been one reported case of PANDAS and KLS (Das and Radhakrishnan 2012), with the patient, an 11-year-old female, experiencing eight episodes of 6–12 days' duration consisting of hypersomnia, facial tics, clumsy movements, inattention, poor speech comprehension, derealization, diurnal fatigue, irritability, confusion, alternating hyperphagia and anorexia, and disorientation, in which ∼30% of the episodes were preceded by a URI. At evaluation, the patient had elevated ASO and anti-DNase B titers. Treatments with various antipsychotics and antiepileptics were ineffective. MRI of the brain, overnight VEEG, polysomnography, metabolic parameters, antibody panel, and cerebrospinal fluid studies were normal. She was successfully treated with oral penicillin V 500 mg/day, with symptoms remitting within 1 month and her ASO titer gradually normalizing within 1 year.
Treatments for KLS and PANDAS differ in terms of effective therapy. KLS treatments are symptom targeted, in that various therapies may be implemented concurrently. KLS relapses have been effectively treated with lithium carbonate, whereas other antiepileptics, including valproate and carbamazepine, have not shown efficacy in systematic reviews (Arnulf et al. 2005, 2008). Somnolence has been successfully treated with stimulants. Antipsychotics have generally not demonstrated efficacy in behavioral abnormalities and derealization, but some benefit has been seen with risperidone (Arnulf et al. 2008). Interestingly, IVIG has shown partial benefit (Arnulf et al. 2005, 2008). Treatment for PANDAS has been somewhat effective with antibiotics during active infection (Murphy and Pichichero 2002; Murphy et al. 2014), IVIG (Perlmutter et al. 1999; Kovacevic et al. 2014,), and plasmapheresis (Perlmutter et al. 1999; Latimer et al. 2014). Studies using psychotropic medications, such as selective serotonin reuptake inhibitors, are lacking in number, but patients with PANDAS may be prone to behavioral activation (Murphy et al. 2006). In this case, treatment with IVIG and azithromycin appeared to improve overall symptom severity, but at the time of last follow-up, KLS episodes had not been eliminated.
Our case underscores the importance of a thorough diagnostic workup, including history (personal, family medical, and psychiatric), comprehensive neurological examination, and laboratory analyses, including tests to assess for autoimmunity and infectious triggers, in children with symptoms congruent with PANDAS/PANS or KLS. As GAS infections and/or viral infections are often precipitating factors in both of these disorders, the PANDAS/PANS or KLS diagnosis should be considered in a child with acute-onset neuropsychiatric symptoms with risk factors such as recent infections, recent exposures, or family history of autoimmunity. Although they are clinically dissimilar, the co-occurrence of these conditions in a pediatric patient provides an intriguing clinical presentation that may shed light on the pathogenesis of these conditions.
Disclosures
Tanya K. Murphy has received research support from All Children's Hospital Research Foundation, AstraZeneca Neuroscience iMED, Centers for Disease Control, the International OCD Foundation (IOCDF), the National Institutes of Health, Ortho McNeil Scientific Affairs, Otsuka, Pfizer Pharmaceuticals, Roche Pharmaceuticals, Shire, Sunovion Pharmaceuticals Inc., Tourette Syndrome Association, and Transcept Pharmaceuticals, Inc. Dr. Murphy is on the Medical Advisory Board for Tourette Syndrome Association and on the Scientific Advisory Boards for IOCDF and for PANDAS Network. She receives a textbook honorarium from Lawrence Erlbaum. The other authors have nothing to disclose.
References
- Allen AJ, Leonard HL, Swedo SE: Case study: A new infection-triggered, autoimmune subtype of pediatric OCD and Tourette's syndrome. J Am Acad Child Adolesc Psychiatry 34:307–311, 1995 [DOI] [PubMed] [Google Scholar]
- Amat JA, Bronen RA, Saluja S, Sato N, Zhu H, Gorman DA, Royal J, Peterson BS: Increased number of subcortical hyperintensities on MRI in children and adolescents with Tourette's syndrome, obsessive-compulsive disorder, and attention deficit hyperactivity disorder. Am J Psychiatry 163:1106–1108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnulf I, Lin L, Gadoth N, File J, Lecendreux M, Franco P, Zeitzer J, Lo B, Faraco JH, Mignot E: Kleine–Levin syndrome: a systematic study of 108 patients. Ann Neurol 63:482–493, 2008 [DOI] [PubMed] [Google Scholar]
- Arnulf I, Zeitzer J, File J, Farber N, Mignot E: Kleine–Levin syndrome: A systematic review of 186 cases in the literature. Brain 128:2763–2776, 2005 [DOI] [PubMed] [Google Scholar]
- Ballock RT, Wiesner GL, Myers MT, Thompson GH: Hemihypertrophy: concepts and controversies. J Bone Joint Surg Am 79:1731–1738, 1997 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Victor AM, Pipal AJ, Williams KA: Comparison of clinical characteristics of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 20:333–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilot F, Merheb V, Ding A, Murphy T, Dale R: Antibody binding to neuronal surface in Sydenham chorea, but not in PANDAS or Tourette syndrome. Neurology 76:1508–1513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Chatterjee A: Comorbid obsessive-compulsive disorder in a patient with Kleine–Levin syndrome. Ger J Psychiatry 10:1–2, 2007 [Google Scholar]
- Chang K, Frankovich J, Cooperstock M, Latimer ME, Murphy T, Pasternack M, Thienemann M, Williams KA, Walter J, Swedo SE: Clinical evaluation of youth with pediatric acute onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS consensus conference. J Child Adolesc Psychopharmacol, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson A, Jr, Levine S, Kong L-S, Lee S: Neuroendocrine evaluation in Kleine–Levin syndrome: Evidence of reduced dopaminergic tone during periods of hypersomnolence. Sleep 14:226–232, 1991 [PubMed] [Google Scholar]
- Clericuzio CL, Martin RA: Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 11:220–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW: Streptococcus and rheumatic fever. Curr Opin Rheumatol 24:408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Radhakrishnan A: A case of PANDAS with Kleine–Levin type periodic hypersomnia. Sleep Med 13:319–320, 2012 [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Mayer G, Lecendreux M, Neidhart E, Peraita–Adrados R, Sonka K, Billiard M, Tafti M: Kleine–Levin syndrome: An autoimmune hypothesis based on clinical and genetic analyses. Neurology 59:1739–1745, 2002 [DOI] [PubMed] [Google Scholar]
- De Gusmao C, Maski K, Urion D: Kleine–Levin Syndrome with rapid cycling: case report and review of the literature. Neurology 82:P5.294–P295, 2014 [Google Scholar]
- Docherty SJ, Davis O, Haworth C, Plomin R, D'Souza U, Mill J: A genetic association study of DNA methylation levels in the DRD4 gene region finds associations with nearby SNPs. Behav Brain Funct 8:31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcini F, Lepri G, Bertini F, Cerinic MM, Rigante D: From PANDAS to PANS: A nosographic entity in evolution throughout a descriptive analysis of a cohort of 103 Italian children and adolescents. Pediatric Rheumatol 12(Suppl 1):300, 2014 [Google Scholar]
- Fenzi F, Simonati A, Crosato F, Ghersini L, Rizzuto N: Clinical features of Kleine–Levin syndrome with localized encephalitis. Neuropediatrics 24:292–295, 1993 [DOI] [PubMed] [Google Scholar]
- Fernábdez JM, Lara I, Gila L, O'Neill A, Tovar J, Gimeno A: Disturbed hypothalamic‐pituitary axis in idiopathic recurring hypersomnia syndrome. Acta Neurol Scand 82:361–363, 1990 [DOI] [PubMed] [Google Scholar]
- Fontenelle L, Mendlowicz MV, Gillin JC, Mattos P, Versiani M: Neuropsychological sequelae in Kleine–Levin syndrome: Case report. Arq Neuropsiquiatr 58:531–534, 2000 [DOI] [PubMed] [Google Scholar]
- Gadoth N, Dickerman Z, Bechar M, Laron Z, Lavie P: Episodic hormone secretion during sleep in Kleine–Levin syndrome: Evidence for hypothalamic dysfunction. Brain Dev 9:309–315, 1987 [DOI] [PubMed] [Google Scholar]
- Gadoth N, Kesler A, Vainstein G, Peled R, Lavie P: Clinical and polysomnographic characteristics of 34 patients with Kleine–Levin syndrome. J Sleep Res 10:337–341, 2001 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE: MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry 157:281–283, 2000 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE: Case study: acute basal ganglia enlargment and obsessive-compulsive symptoms in an adolescent boy. J Am Acad Child Adolesc Psychiatry 7:913–915, 1996 [DOI] [PubMed] [Google Scholar]
- Gillberg C: Kleine–Levin syndrome: Unrecognized diagnosis in adolescent psychiatry. J Am Acad Child Adolesc Psychiatry 26:793–794, 1987 [DOI] [PubMed] [Google Scholar]
- Hoekstra PJ, Manson WL, Steenhuis M-P, Kallenberg CGM, Minderaa RB: Association of common cold with exacerbations in pediatric but not adult patients with tic disorder: A prospective longitudinal study. J Child Adolesc Psychopharmacol 15:285–292, 2005 [DOI] [PubMed] [Google Scholar]
- Hong SB, Joo EY, Tae WS, Lee J, Han SJ, Lee HW: Episodic diencephalic hypoperfusion in Kleine–Levin syndrome. Sleep 29:1091–1093, 2006 [DOI] [PubMed] [Google Scholar]
- Huang C-J, Liao H-T, Yeh G-C, Hung K-L: Distribution of HLA-DQB1 alleles in patients with Kleine–Levin syndrome. J Clin Neurosci 19:628–630, 2012a [DOI] [PubMed] [Google Scholar]
- Huang Y, Guilleminault C, Kao P, Liu F: SPECT findings in the Kleine–Levin syndrome. Sleep 28:955–960, 2005 [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Guilleminault C, Lin K-L, Hwang F-M, Liu F-Y, Kung Y-P: Relationship between Kleine–Levin syndrome and upper respiratory infection in Taiwan. Sleep 35:123–129, 2012b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-S, Lin Y-H, Guilleminault C: Polysomnography in Kleine–Levin syndrome. Neurology 70:795–801, 2008 [DOI] [PubMed] [Google Scholar]
- Kas A, Lavault S, Habert M-O, Arnulf I: Feeling unreal: A functional imaging study in patients with Kleine–Levin syndrome. Brain 137:2077–2087, 2014 [DOI] [PubMed] [Google Scholar]
- Ko JM: Genetic syndromes associated with overgrowth in childhood. Ann Pediatr Endocrinol Metab 18:101–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber RK, Torkelson R, Haven G, Donaldson J, Cohen SM, Case M: Increased cerebrospinal fluid 5‐hydroxytryptamine and 5‐hydroxyindoleacetic acid in Kleine‐Levin syndrome. Neurology 34:1597–1597, 1984 [DOI] [PubMed] [Google Scholar]
- Kovacevic M, Grant P, Swedo SE: Use of intravenous immunoglobulin in the treatment of twelve youth with PANDAS. J Child Adolesc Psychopharmacol, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Williams M, Chugani HT: Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol, 2014August12. pii: . [Epub ahead of print] PubMed PMID: [DOI] [PubMed] [Google Scholar]
- Landtblom A, Dige N, Schwerdt K, Säfström P, Granerus G: Short‐term memory dysfunction in Kleine–Levin syndrome. Acta Neurol Scand 108:363–367, 2003 [DOI] [PubMed] [Google Scholar]
- Latimer ME, L'Etoile N, Seidlitz J, Swedo SE: Therapeutic plasmapheresis as a treatment for 32 severely ill children with PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections). J Child Adolesc Psychopharmacol, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougee L, Perlmutter SJ, Nicolson R, Garvey MA, Swedo SE: Psychiatric disorders in first-degree relatives of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). J Am Acad Child Adolesc Psychiatry 39:1120–1126, 2000 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Kumar Das M, Gupta N, Muralidharan R: A clinical study of Kleine–Levin syndrome with evidence for hypothalamic–pituitary axis dysfunction. Biol Psychiatry 42:299–301, 1997 [DOI] [PubMed] [Google Scholar]
- Mayer G, Leonhard E, Krieg J, Meier–Ewert K: Endocrinological and polysomnographic findings in Kleine–Levin syndrome: No evidence for hypothalamic and circadian dysfunction. Sleep 21:278–284, 1998 [DOI] [PubMed] [Google Scholar]
- Mell LK, Davis RL, Owens D: Association between streptococcal infection and obsessive-compulsive disorder, Tourette's syndrome, and tic disorder. Pediatrics 116:56–60, 2005 [DOI] [PubMed] [Google Scholar]
- Merriam AE: Kleine–Levin syndrome following acute viral encephalitis. Biol Psychiatry 21:1301–1304, 1986 [DOI] [PubMed] [Google Scholar]
- Moriarty J, Vama AR, Stevens J, Fish M, Trimble MR, Robertson MM: A volumetric MRI study of Gilles de la Tourette's syndrome. Neurology 49:410–415, 1997 [DOI] [PubMed] [Google Scholar]
- Morris–Berry C, Pollard M, Gao S, Thompson C, Singer H: Anti-streptococcal, tubulin, and dopamine receptor 2 antibodies in children with PANDAS and Tourette syndrome: Single-point and longitudinal assessments. J Neuroimmunol 264:106–113, 2013 [DOI] [PubMed] [Google Scholar]
- Müller N, Abele–Horn M, Riedel M: Infection with Mycoplasma pneumoniae and Tourette's syndrome (TS): increased anti-mycoplasmal antibody titers in TS. Psychiatry Res 129:119–125, 2004 [DOI] [PubMed] [Google Scholar]
- Müller N, Riedel M, Blendinger C, Forderreuther S, Abele–Horn M: Childhood Tourette's syndrome and infection with mycoplasma pneumoniae. Am J Psychiatry 157:481–482, 2000 [DOI] [PubMed] [Google Scholar]
- Muratori F, Bertini N, Masi G: Efficacy of lithium treatment in Kleine–Levin syndrome. Eur Psychiatry 17:232–233, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME: Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med 156:356, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Gerardi DM, Leckman J: Pediatric acute-onset neuropsychiatric syndrome. Psychiatr Clin North Am 37:353–374, 2014 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Parker–Athill EC, Lewin AB, Storch EA, Mutch PJ: Cefdinir for recent onset pediatric neuropsychiatric disorders: A pilot randomized trial. J Child Adolesc Psychopharmacol. DOI: 10.1089/cap.2014.0010 Epub ahead of print October8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK: Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatrics 160:314–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Strawser MS: Selective serotonin reuptake inhibitor-induced behavioral activation in the PANDAS subtype. Prim Psychiatry 13:87–89, 2006 [Google Scholar]
- Murphy TK, Storch EA, Turner A, Reid JM, Tan J, Lewin AB: Maternal history of autoimmune disease in children presenting with tics and/or obsessive-compulsive disorder. J Neuroimmunol 229:243–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papacostas S, Hadjivasilis V: The Kleine–Levin syndrome: Report of a case and review of the literature. Eur Psychiatry 15:231–235, 2000 [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE: Therapeutic plasma exchange and intravenous immunoglobulin for obsessive–compulsive disorder and tic disorders in childhood. Lancet 354:1153–1158, 1999 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L: Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry 60:415–424, 2003 [DOI] [PubMed] [Google Scholar]
- Poryazova R, Schnepf B, Boesiger P, Bassetti CL: Magnetic resonance spectroscopy in a patient with Kleine–Levin syndrome. J Neurol 254:1445–1446, 2007 [DOI] [PubMed] [Google Scholar]
- Reynolds C, Black R, Coble P, Holzer B, Kupfer D: Similarities in EEG sleep findings for Kleine–Levin syndrome and unipolar depression. Am J Psychiatry 137:116–118, 1980 [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB, Montrose DM, Pierri JN, Birmaher B: Frontostriatal measurement in treatment-naïve children with obsessive-compulsive disorder. Arch Gen Psychiatry 54:824–830, 1997 [DOI] [PubMed] [Google Scholar]
- Sagar R, Khandelwal S, Gupta S: Interepisodic morbidity in Kleine–Levin syndrome. Br J Psychiatry 157:139–141, 1990 [DOI] [PubMed] [Google Scholar]
- Salter MS, White PD: A variant of the Kleine–Levin syndrome precipitated by both Epstein–Barr and varicella-zoster virus infections. Biol Psychiatry 33:388–390, 1993 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman J, Rose N: From research subgroup to clinical syndrome: Modifying the PANDAS criteria to describe PANS. Pediatr Therapeut 2:113. doi: 10.4172/2161-0665.1000113 (open access journal, article covers pages 1–8), 2012 [DOI] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Dow S, Zamkoff J, Dubbert BK, Lougee L: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998 [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Cheslow D, Hosier DM, Wald ER: High prevalence of obsessive-compulsive symptoms in patients with Sydenham's chorea. Am J Psychiatry 146:246–249, 1989 [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR: Brain structural abnormalities in psychotropic drug-naïve pediatric patients with obsessive-compulsive disorder. Am J Psychiatry 161:1049–1056, 2004 [DOI] [PubMed] [Google Scholar]
- Thacore V, Ahmed M, Oswald I: The EEG in a case of periodic hypersomnia. Electroencephalogr Clin Neurophysiol 27:605–606, 1969 [DOI] [PubMed] [Google Scholar]
- Toufexis M, Lewin A, Storch EA, DeOleo C, Murphy TK: A possible link between tic disorders associated with beta thalassemia minor and sickle cell disease. J Clin Case Rep 3244. doi: 10.4172/2165-7920.1000244, 2013 [DOI] [Google Scholar]
- Yiş U, Kurul SH, Çakmakçı H, Dirik E: Mycoplasma pneumoniae: nervous system complications in childhood and review of the literature. Eur J Pediatr 167:973–978, 2008 [DOI] [PubMed] [Google Scholar]