Abstract
Human users of synthetic cannabinoids (SCBs) JWH-018 and JWH-073 typically smoke these drugs, but preclinical studies usually rely on injection for drug delivery. We used the cannabinoid tetrad and drug discrimination to compare in vivo effects of inhaled drugs with injected doses of these two SCBs, as well as with the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC). Mice inhaled various doses of Δ9-THC, JWH-018 or JWH-073, or were injected intraperitoneally (IP) with these same compounds. Rectal temperature, tail flick latency in response to radiant heat, horizontal bar catalepsy, and suppression of locomotor activity were assessed in each animal. In separate studies, mice were trained to discriminate Δ9-THC (IP) from saline, and tests were performed with inhaled or injected doses of the SCBs. Both SCBs elicited Δ9-THC-like effects across both routes of administration, and effects following inhalation were attenuated by pretreatment with the CB1 antagonist/inverse agonist rimonabant. No cataleptic effects were observed following inhalation, but all compounds induced catalepsy following injection. Injected JWH-018 and JWH-073 fully substituted for Δ9-THC, but substitution was partial (JWH-073) or required relatively higher doses (JWH-018) when drugs were inhaled. These studies demonstrate that the SCBs JWH-018 and JWH-073 elicit dose-dependent, CB1 receptor-mediated Δ9-THC-like effects in mice when delivered via inhalation or via injection. Across these routes of administration, differences in cataleptic effects and, perhaps, discriminative stimulus effects, may implicate the involvement of active metabolites of these compounds.
Keywords: Behavior, Cannabinoids, Drug discrimination, Antinociception, Hypothermia, Locomotor activity
1. Introduction
Over the past 5 years, synthetic cannabinoids (SCBs) rapidly emerged as popular drugs of abuse in Europe and the US. Commercial preparations (typically branded as “K2” in the US or as “Spice” in Europe) are readily available online and in business establishments such as convenience stores and truck stops (Vardakou et al., 2010). Most of these preparations consist of inert plant materials laced with SCBs, typically from the aminoalkylindole (AAI) family (Fattore and Fratta, 2011), and are presumed to possess pharmacological properties similar to Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive constituent of marijuana (Gaoni and Mechoulam, 1964). The widespread over-the-counter availability of these products has led to the perception that they are safe to use, and this, combined with the fact that their active constituents are not detected in standard drug screens, has spurred use of SCBs to epidemic levels on many college campuses (Vandrey et al., 2012). Similarly, one in nine high school seniors admitted using SCBs over the past year, making these compounds the 2nd most frequently used recreational drug, after marijuana, in this population (Johnston et al., 2011). State and federal scheduling of some of the more common SCBs under the Controlled Substances Act has largely failed to curtail drug availability, and commercial preparations containing these drugs remain quasi-legal and easily obtainable (Seely et al., 2012).
Although structurally distinct from Δ9-THC, the synthetic AAI cannabinoid compounds also bind and activate cannabinoid CB1 receptors (CB1Rs) (Estep et al., 1990; Eissenstat et al., 1990). The abuse liability of AAI SCBs therefore most likely results from their capability to potently and efficaciously activate these CB1Rs. While a plethora of different SCBs are reported to be present in various commercial preparations, two of the most commonly observed are JWH-018 [1-pentyl-3-(1-naphthoyl)indole] and JWH-073 [1-butyl-3-(1-naphthoyl)indole] (Logan et al., 2012; Seely et al., 2013). Previous studies revealed that these SCBs have high affinity for CB1Rs, and possess much higher efficacy at these receptors than Δ9-THC (Lindigkeit et al., 2009; Atwood et al., 2010).
In this regard, although humans typically smoke commercial preparations of SCBs (Vandrey et al., 2012), almost all preclinical studies with these compounds have involved systemic injection. Drugs administered via inhalation largely bypass first-pass metabolism, whereas systemic injection allows for significant first-pass effects (Pond and Tozer, 1984). Importantly, we have recently reported that several phase I hydroxylated metabolites of JWH-018 and JWH-073 retain biological activity (Brents et al., 2011, 2012), which could have implications for human use. As such, it may be the case that laboratory animal models employing systemic injection of SCBs maximize formation of active phase I metabolites, whereas the human condition, i.e. smoking, would be expected to minimize metabolite formation. At the time of this writing, only a single study has evaluated the effects of a single inhaled SCB, JWH-018, in mice (Wiebelhaus et al., 2012), demonstrating dose-dependent effects on all measures of the cannabinoid tetrad, and reversal of these drug effects by prior administration of the CB1R antagonist/inverse agonist rimonabant. In the present studies, utilizing a whole-body exposure system, we extend these previous observations by directly comparing the effects of multiple doses of JWH-018 to its structural analogue JWH-073 and to Δ9-THC using the cannabinoid tetrad (Martin et al., 1991) following inhaled exposure or intraperitoneal injection. The CB1R receptor antagonist/inverse agonist rimonabant was used to determine whether any observed drug effects in the tetrad assay following inhalation of cannabinoids were mediated via CB1R actions. Additional studies compared the interoceptive effects of inhaled or injected SCBs to those of intraperitoneal Δ9-THC using drug discrimination. This further evaluation of the effects of SCBs via inhalation in mice may increase our understanding of their biological effects and may provide a more translational approach to the study of these compounds, as compared to systemic injection.
2. Materials and methods
2.1. Animals
All experiments were conducted in adult male Swiss Webster mice housed in the University of Arkansas for Medical Sciences (UAMS). Mice were maintained on a 12 h light:12 h dark cycle (lights on at 0700 h, off at 1900 h) in a temperature- and humidity-controlled room within the UAMS vivarium. Food and water were available ad libitum throughout the duration of all studies. All animals in the present studies were drug-naïve prior to initiation of experimental protocols. Mice in the tetrad studies were used only once, and were sacrificed immediately after testing, but mice in drug discrimination studies were repeatedly tested, with experimental observations taking place no more frequently than once per week. Experimental protocols were approved by the UAMS Institutional Animal Care and Use Committee, and complied with principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Apparatus
Inhaled cannabinoids were administered within a cylindrical glass chamber housed within a fume hood. Three mice (which were cagemates in the animal housing room) were placed in the chamber at the same time. The glass cylinder measured 30.48 cm × 45.72 cm (volume 30 L), and closure of the cylinder was ensured by a 1/2″ edge EPDM compression seal (product number 1120A45, McMaster-Carr, Aurora, OH) and a Plexiglas lid which was tightly screwed into three aluminum supports. A four blade fan attached to an electric motor (model OM87, Dayton Electric Manufacturing Co., Chicago, IL) was fixed to the inner surface of the Plexiglas lid to distribute the volatilized drug throughout the chamber, and a metal cage was fitted beneath the fan to contain the drug-impregnated nitrocellulose paper (see Drugs section, below) prior to ignition. An igniter was inserted through a hole in the Plexiglas lid to ignite the medium; once the medium was lit, the igniter was rapidly removed and a rubber stopper was used to plug the hole to prevent any loss of vaporized drug. A rubber tube connected to the chamber from an air source in the fume hood allowed air flow (applied at approximately 5 min into the experiment) to prevent hypoxia as a result of oxygen consumption in the chamber. For studies involving systemic administration of cannabinoids, mice were injected IP and then placed in the chamber 5 min later in order to rule out any non-specific effects of the inhalation procedure on subsequent measures in the tetrad test. The chamber was tested for air leaks periodically during the course of these studies by filling the chamber with colored smoke and sealing it tight. Colored smoke rapidly filled the chamber, and visual checks did not reveal any areas where smoke could escape. After these tests, the smoke was evacuated through the fume hood, and the chamber was disassembled for cleaning and sanitizing.
For drug discrimination experiments, mice were injected IP with cannabinoids, or exposed to vaporized drugs in the chamber as described above, then rapidly transported to an adjacent laboratory for behavioral testing in operant-conditioning chambers (model ENV-307A; MED Associates, St. Albans, VT) that were individually enclosed in larger lightproof Malaguard sound-attenuating cubicles (model ENV-022MD; MED Associates) modified to include retractable response levers (model ENV-312 M) for murine subjects. The right side wall of each chamber used in these studies was equipped with a dipper, centered between the two retractable levers, through which liquid reinforcement was delivered, and stimulus lights were present above each response lever. The left wall of each chamber contained a nose-poke aperture, which was not used in these studies.
2.3. Drugs
Trans-Blot Transfer Medium Pure Nitrocellulose Membrane (0.45 μm thick, BioRad Labs, Hercules, CA) was used as a matrix to volatilize cannabinoids in the chamber. Δ9-THC, JWH-018 and JWH-073 were dissolved in 100% ethanol at a concentration of 100 mg/mL. Cannabinoid solutions were applied to nitrocellulose paper (8.0 × 8.0 cm squares), then left overnight in labeled beakers under the fume hood. During this time, ethanol evaporated off, leaving cannabinoids impregnated in the nitrocellulose paper, which was dry and ready for combustion the following morning. For all experiments with inhaled cannabinoids, doses are expressed as total mg of drug per 30 L of air in the chamber.
JWH-018, JWH-073, Δ9-THC were also prepared for intraperitoneal injection, as was the CB1 antagonist/inverse agonist rimonabant. All compounds were dissolved in a solution of Tween 80 (8% of final volume) and 0.9% saline (92% of final volume). Injections were administered in a volume of 0.01 mL/g via 28 gauge needles. Rimonabant was administered via IP injection 15 min prior to cannabinoid exposure. After rimonabant injection, mice were placed back into their home cages during this 15-min period.
2.4. Cannabinoid tetrad
Immediately upon removal from the inhalation chamber, mice were sequentially tested for (1) hypothermia, (2) analgesia, (3) catalepsy and (4) suppression of locomotor activity, in that order. Hypothermia was measured using a digital thermometer (model BAT-12, PhysiTemp, Clifton, NJ) equipped with a Ret-3 mouse probe (model 50314, Stoelting Co., Dale, IL) inserted rectally approximately 2 cm; stable temperatures were obtained within ∼6 s. Analgesia was measured as tail-flick latency using the EMDIE-TF6 radiant heat apparatus (Emdie Instrument Co., Montpelier, VA). For analgesia trials, mice were positioned on the stage of apparatus, while the tail was extended into a groove to break a photobeam. Beginning at t = 0, a button was depressed to begin a timer and illuminate a radiant heat source directed onto the dorsal surface of the tail, approximately 2 cm from its origin from the body. Movement of the tail at any point after the beginning of the trial broke the photobeam, stopped both the heat source and the timer, and ended the trial. The sensitivity of the photobeam detector was set at 150, and the light intensity was set to 369 for all trials in order to produce a tail flick latency between 2 and 4 s for untreated mice, and the maximum allowed time for each trial was10s.Catalepsy was measured by the horizontal bar test, utilizing a cylindrical steel bar (0.5 cm in diameter) that was supported 4.0 cm above and horizontal to a Plexiglas platform (which was covered with a paper towel to provide better traction). To begin the test trial, a mouse was placed into a species-atypical position with its forelimbs on the horizontal bar and its hindlimbs on the platform, in such a way that the mouse assumed a rearing posture. Upon placement on the catalepsy bar, a timer was started, and counted until the mouse removed both of its paws from the bar. The maximum time allowed on the bar was 30 s. Finally, locomotor activity was measured using plastic activity boxes (26.67 cm × 20.96 cm × 15.24 cm), and the base of each box was marked in order to divide it into four equally-sized quadrants. Each mouse was placed into a separate activity box, and quadrant crossings (defined as all of the paws crossing from one quadrant into another) were scored for 5 min using an overhead video camera. Between experimental observations, boxes were rinsed with tap water, sprayed and wiped with Coverage Plus NPD (a one-step cleaner, disinfectant and deodorizer concentrate) (Steris Healthcare, Mentor, OH), and dried with paper towels. In this manner, all four measures were sequentially obtained from each mouse used in these studies.
2.5. Drug-discrimination
Mice were shaped to stably respond under an FR10 schedule reinforced by presentation of evaporated milk in daily sessions using procedures similar to those previously described (Murnane et al., 2009; Fantegrossi et al., 2009). When responding became stable and reliable, subjects were trained to discriminate 10 mg/kg Δ9-THC from saline, as previously described (Brents et al., 2013). During each training session we tracked overall response rate, overall distribution of responses on the injection-appropriate response device, and the distribution of responses on the injection-appropriate response device prior to delivery of the first reinforcer. Pre-session injections were chosen in a pseudorandom order with the constraint that no animal could receive the same injection for more than 3 consecutive sessions. When animals reliably achieved >85% injection-appropriate responding prior to delivery of the first reinforcer for 3 consecutive sessions, a substitution test occurred the following day. During a substitution test, mice were placed in the inhalation chamber (as described above) and a dose of JWH-018 or JWH-073 was ignited. After 10 min of exposure, mice were immediately placed in operant chambers for discrimination testing, and sessions started 5 min later. For injection control studies, mice were administered JWH-018 or JWH-073 IP, then immediately placed in the operant chambers; sessions started 10 min later. In all cases, testing sessions ended as soon as 10 responses were recorded on one response option or the other. Overall response rate was recorded, along with the percent of responses emitted on the training drug-associated response option.
2.6. Data analysis
All data are presented as individual mean ± SEM for groups where n = 6. Data points without error bars indicate that the SEM was smaller than the symbol used to denote the mean. For tetrad experiments involving volatilization and inhalation of cannabinoids, statistical analyses were conducted using one-way ANOVA and post hoc pairwise multiple comparisons on significant effects and interactions were accomplished using Tukey's HSD. Antagonist studies were analyzed by one-way ANOVA and Dunn's test (comparing each group to vehicle-treated controls). For tetrad experiments involving IP injection of cannabinoids, one-way ANOVA was performed, and each dose of each drug was again compared to vehicle controls using Dunn's method. Since drug discrimination data were not normally distributed, a Kruskal–Wallis one-way ANOVA on ranks was performed for data gathered following inhalation and following injection, and Tukey's HSD was then performed to test significance of all pairwise comparisons. All statistical tests were executed using commercially available software, and significance was judged at P < 0.05.
3. Results
3.1. Cannabinoid tetrad
3.1.1. Hypothermia
Mean rectal temperatures in animals subjected to vehicle injection (Fig. 1, left, open circle) or either of the two inhalation control conditions (Fig. 1, middle, open circles) were all approximately 37 °C, which is species-typical for mice. All three cannabinoids, whether injected (Fig. 1, left) or volatilized and inhaled (Fig. 1, middle), elicited dose-dependent hypothermic effects. For inhaled Δ9-THC, exposure to 100 mg/30 L produced rectal temperatures which were significantly different from those induced by exposure to 30 mg/30 L (q = 9.035, P < 0.05), and both of these doses elicited hypothermic effects which were significantly different from EtOH controls (q = 2.300 for 30 mg/30 L, q = 11.334 for 100 mg/30 L, P < 0.05 in both cases). As observed with Δ9-THC, exposure to 30 mg/30 L JWH-018 resulted in rectal temperatures which were significantly different than those measured following 10 mg/30 L (q = 6.817, P < 0.05), and temperatures following exposure to 100 mg/30 L were significantly different than following 30 mg/30 L (q = 15.112, P < 0.05). Moreover, rectal temperatures observed after 10 (q = 6.078, P < 0.05), 30 (q = 12.895, P < 0.05) and 100 mg/30 L JWH-018 (q = 28.007, P < 0.05) were all significantly different from EtOH controls. A similar pattern of dose-dependent hypothermic effects was observed for volatilized JWH-073, where exposure to 30 mg/30 L JWH-073 resulted in significantly different rectal temperatures than following 10 mg/30 L (q = 6.078, P < 0.05), and temperatures following exposure to 100 mg/30 L were significantly different than those measured following 30 mg/30 L (q = 12.238, P < 0.05). Observed rectal temperatures following exposure to 30 (q = 8.542, P < 0.05) and 100 mg/30 L JWH-073 (q = 20.780, P < 0.05) were both significantly different than EtOH control values. Similarly, IP injection of these three cannabinoids also elicited dose-dependent hypothermic effects, with the highest dose of each compound significantly different from vehicle controls (Q = 3.955 for Δ9-THC, Q = 4.246 for JWH-018, Q = 2.951 for JWH-073, and P < 0.05 in all cases). Maximal hypothermic effects for Δ9-THC differed as a function of route of administration, but were similar for JWH-018 and JWH-073. For Δ9-THC, mean rectal temperatures of 34.13 ± 0.05 °C and 32.10 ± 0.37 °C were observed after inhalation of 100 mg/30 L or injection of 100 mg/kg, respectively. For JWH-018, mean rectal temperatures of 30.75 ± 0.30 °C and 30.90 ± 0.33 °C were observed after inhalation of 100 mg/30 L or injection of 10 mg/kg, respectively. For JWH-073, mean rectal temperatures of 32.22 ± 0.20 °C and 32.87 ± 0.58 °C were observed after inhalation of 100 mg/30 L or injection of 30 mg/kg, respectively. Importantly, hypothermic effects of similarly-effective doses of volatilized and inhaled Δ9-THC, JWH-018 and JWH-073 were all significantly attenuated by pretreatment with rimonabant (Fig. 1, right).
Fig. 1.

Effects of JWH-018 (filled circles), JWH-073 (open squares), and Δ9-THC (filled triangles) on rectal temperature after intraperitoneal injection (left) or inhalation (center), and comparison of effect-scaled doses of inhaled Δ9-THC (100 mg/30 L), JWH-018 (10 mg/30 L) and JWH-073 (30 mg/30 L) on rectal temperature with (open bars) and without (filled bars) 3 mg/kg rimonabant pretreatment (right). “Air” indicates data obtained from mice placed in the inhalation chamber and exposed to the fan and room air only, while “EtOH” indicates data obtained from mice placed in the inhalation chamber and exposed to combustion of nitrocellulose paper previously treated with the 100% EtOH solution used to dissolve the cannabinoids. Data points in dose-effect curves represent mean ± SEM, and absence of error bars indicate instances where the variability is contained within the point See Results section for statistical comparisons. Bars indicate mean ± SEM, and asterisks indicate significant differences from vehicle-treated EtOH controls by one-way ANOVA and Dunn's test.
3.1.2. Locomotor activity
Quadrant crossings in mice subjected to vehicle injection (Fig. 2, left, open circle) or either of the two inhalation control conditions (Fig. 2, middle, open circles) were similar to one another, and to control conditions routinely observed in the laboratory. All three cannabinoids, whether injected (Fig. 2, left) or volatilized and inhaled (Fig. 3, middle), suppressed locomotor activity. For inhaled Δ9-THC, exposure to 100 mg/30 L produced quadrant crossings which were significantly different than those observed after exposure to EtOH control conditions (q = 6.969, P < 0.05). Exposure to JWH-018 via this route of administration induced dose-dependent effects on locomotor activity, with30and 100 mg/30LJWH-018 resulting in significantly different quadrant crossings than following 10 mg/30 L (q = 6.247 and q = 6.211, respectively, P < 0.05 in both cases). Moreover, locomotor counts quantified after 10(q= 5.850, P < 0.05), 30 (q= 12.096, P < 0.05) and 100 mg/30 L JWH-018 (q = 12.060, P < 0.05) were all significantly different from EtOH controls.A similar pattern of dose-dependent locomotor suppression was observed for volatilized JWH-073, where exposure to 100 mg/30 L JWH-073 resulted in significantly different quadrant crossings than following 10 mg/30 L (q = 7.836, P < 0.05). Locomotor activity counts following exposure to 30 (q = 5.814, P < 0.05) and 100 mg/30 L JWH-073 (q = 11.988, P < 0.05) were both significantly different than EtOH control values. Likewise, IP injection of these three cannabinoids also elicited dose-dependent locomotor suppression, with the highest dose of each compound significantly different from vehicle controls (Q = 3.506 for Δ9-THC, Q = 3.748 for JWH-018, Q = 2.952 for JWH-073, and P < 0.05 in all cases). Maximal locomotor suppression for Δ9-THC differed as a function of route of administration, but was similar for JWH-018 and JWH-073. For Δ9-THC, mean quadrant crossings were 32.00 ± 4.46 and 5.20 ± 2.58 following inhalation of 100 mg/30 L or injection of 100 mg/kg, respectively. For JWH-018, mean quadrant crossings were 8.50 ± 2.22 and 1.50 ± 0.96 following inhalation of 100 mg/30 L or injection of 10 mg/kg, respectively. For JWH-073, mean quadrant crossings were 8.83 ± 3.59 and 11.80 ± 4.33 following inhalation of 100 mg/30 L or injection of 30 mg/kg, respectively. Importantly, doses of volatilized and inhaled Δ9-THC, JWH-018 and JWH-073 which similarly reduced locomotor activity were all significantly attenuated by pretreatment with rimonabant (Fig. 2, right).
Fig. 2.

Effects of JWH-018 (filled circles), JWH-073 (open squares), and Δ9-THC (filled triangles) on locomotor activity after intraperitoneal injection (left) or inhalation (center), and comparison of effect-scaled doses of inhaled Δ9-THC, JWH-018 and JWH-073 on motor activity with (open bars) and without (filled bars) rimonabant pretreatment (right). All other graph properties as described in Fig. 1.
Fig. 3.

Effects of JWH-018 (filled circles), JWH-073 (open squares), and Δ9-THC (filled triangles) on tail flick latency after intraperitoneal injection (left) or inhalation (center), and comparison of effect-scaled doses of inhaled Δ9-THC, JWH-018 and JWH-073 on motor activity with (open bars) and without (filled bars) rimonabant pretreatment (right). All other graph properties as described in Fig. 1.
3.1.3. Analgesia
Tail flick latencies in mice subjected to vehicle injection (Fig. 3, left, open circle) or either of the two inhalation control conditions (Fig. 3, middle, open circles) were similar to one another, and to control conditions routinely observed in the laboratory. All three cannabinoids, whether injected (Fig. 3, left) or volatilized and inhaled (Fig. 3, middle), induced analgesic effects following at least one dose. For inhaled Δ9-THC, exposure to 100 mg/30 L induced significantly different tail flick latencies as compared to EtOH controls (q = 4.862, P < 0.05). For inhaled JWH-018, exposure to both 30 and 100 mg/30 L resulted in significantly different tail flick latencies than that observed in EtOH controls (q = 5.049 and 6.358, respectively, P < 0.05 in both cases). Inhalation of volatilized JWH-073 at a dose of 100 mg/30L produced a significantly different tail flick latency as compared to EtOH controls (q = 5.587, P < 0.05). Analgesic effects were more pronounced following IP injection for all three cannabinoids, with the highest doses of all three compounds engendering significantly different tail-flick latencies than vehicle controls (Q = 3.179 for Δ9-THC, Q = 3.816 for JWH-018, Q = 2.971 for JWH-073, and P < 0.05 in all cases). Maximal analgesic effects differed as a function of route of administration for all three cannabinoids. For Δ9-THC, mean tail-flick latencies were 4.98 ± 1.16 s and 7.48 ± 1.49 s following inhalation of 100 mg/30 L or injection of 100 mg/kg, respectively. For JWH-018, mean tail-flick latencies were 5.10 ± 0.29 s and 10.00 ± 0.00 s (all animals exhibited the maximum allowed latency) following inhalation of 100 mg/30 L or injection of 10 mg/kg, respectively. For JWH-073, mean tail-flick latencies were 4.93 ± 1.03 s and 6.66 ±1.06 s following inhalation of 100 mg/30 L or injection of 30 mg/kg, respectively. Rimonabant pretreatment blunted the analgesic effects of all three volatilized and inhaled cannabinoids, but none of these effects were statistically significant (Fig. 3, right).
3.1.4. Catalepsy
No significant cataleptic effects were observed in mice subjected to vehicle injection (Fig. 4, left, open circle) or either of the two inhalation control conditions (Fig. 4, right, open circles). Interestingly, none of the three cannabinoids induced significant cataleptic effects following inhalation in any animals, at any dose (Fig. 4, right), despite eliciting apparent CB1-mediated effects in all other measures of the tetrad. Nevertheless, profound catalepsy was observed following injection of all three cannabinoids (Fig. 4, left). For Δ9-THC, injection of 30 (Q = 2.846) and 100 mg/kg (Q = 3.287) induced significant cataleptic effects (P < 0.05 for both doses). Similar results were obtained with JWH-018 at 3 (Q = 2.849) and 10 mg/kg (Q = 3.555), and with JWH-073 at 30 mg/kg (Q = 2.868, P < 0.05 for all of these dose conditions). Another noteworthy finding here was that the cataleptic effects of maximal injected doses of Δ9-THC and JWH-018 differed qualitatively, despite being very similar quantitatively. Mice injected with 100 mg/kg Δ9-THC maintained the species-atypical rearing posture, but otherwise displayed relatively normal muscle tone (Fig. 5, left). In contrast, subjects injected with 10 mg/kg JWH-018 exhibited pronounced rigidity and profound leg splay (Fig. 5, middle), as well as intermittent tonic-clonic jerking movements which progressed to full-body convulsions (including barrel-rolling and “popping”) during handling. These convulsant effects were not observed at lower doses of JWH-018, nor were they seen in any subjects injected with any dose of Δ9-THC or JWH-073. Importantly, pretreatment with rimonabant blocked the leg splay (Fig. 5, right) and convulsant effects of 10 mg/kg JWH-018 (see Table 1), suggesting that these effects are CB1-mediated.
Fig. 4.

Effects of JWH-018 (filled circles), JWH-073 (open squares), and Δ9-THC (filled triangles) on horizontal bar catalepsy after intraperitoneal injection (left) or inhalation (right). Since no cataleptic effects were observed after inhaled cannabinoids, no tests were performed in the presence of rimonabant pretreatment. All other graph properties as described in Fig. 1.
Fig. 5.
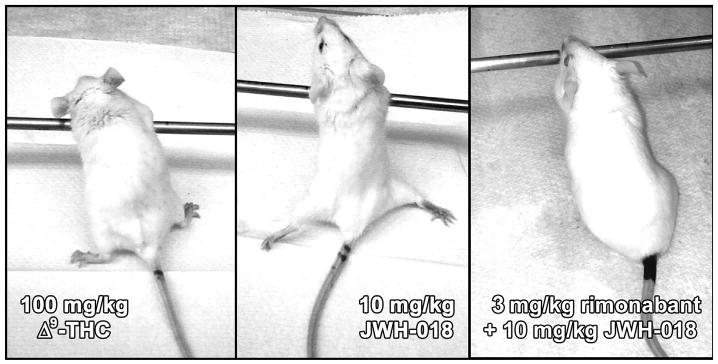
Cataleptic effects of maximal doses of injected Δ9-THC (left) and JWH-018 (middle) yielded similar quantitative data, but the appearances of animals in each condition were qualitatively distinct. All mice injected with JWH-018 exhibited rigid posture and profound leg splay in the hindlimbs, full-body twitches, and handling-induced convulsions, but these effects were not observed following injection of Δ9-THC, even in animals displaying the maximum possible catalepsy score of 30s. Leg splay, twitches and convulsions were blocked in mice treated with 3 mg/kg rimonabant prior to JWH-018 (right, and see Table 1).
Table. 1.
Convulsant-like effects of 10 mg/kg JWH-018 include hindlimb splay (see Fig. 5), full-body twitches, and profound handling-induced convulsions (including barrel-rolling and loss of righting reflex) in all animals studied. All of these effects were blocked by prior administration of 3 mg/kg rimonabant.
| Endpoint | Vehicle + JWH-018 | Rimonabant + JWH-018 |
|---|---|---|
| Rigid posture and leg splay | 6/6 | 1/6 |
| Full-body twitches | 6/6 | 0/6 |
| Convulsions | 6/6 | 0/6 |
3.2. Drug discrimination
Mice reliably learned to discriminate 10 mg/kg Δ9-THC (Fig. 6, black triangles) from saline (Fig. 6, white squares). When saline was administered in training sessions, mice primarily responded on the saline lever. Similarly, when the training dose was administered, mice responded almost exclusively on the Δ9-THC lever. During substitution tests with injected doses of JWH-018 and JWH-073, both drugs dose-dependently and fully generalized to the Δ9-THC training dose (Fig. 6, left). Injected doses of 1 mg/kg JWH-018 (q = 6.809, P < 0.05) and 10 mg/kg JWH-073(q= 6.220,P< 0.05) were both significantly different from discriminative responding elicited by saline, but neither of these doses was statistically different from responding engendered by the Δ9-THC training dose. Dose-dependent substitution for the Δ9-THC training dose was also observed when cannabinoids were volatilized and inhaled, with ∼50% and ∼80% of the total responses emitted on the drug lever following maximal doses of JWH-073 (Fig. 6, white circles, right) and JWH-018 (Fig. 6, black circles, right), respectively, although rate-decreasing effects were evident at all inhaled doses, and complete response suppression was obtained in all mice at a dose of 100 mg/30 L JWH-073 (Table 2). The highest tested inhaled doses that did not suppress responding of these synthetic cannabinoids were each significantly different from discriminative responding elicited by saline (q = 4.157 for JWH-018, q = 3.244 for JWH-073; P < 0.05 in both instances), and the highest tested dose of JWH-073 was also significantly different from the training dose of Δ9-THC (q = 3.125, P < 0.05). Thus, JWH-073 only partially substituted for Δ9-THC, while the training dose fully generalized to JWH-018.
Fig. 6.

Δ9-THC-like discriminative stimulus effects of JWH-018 (filled circles) and JWH-073 (open circles), after inhalation (left) or intraperitoneal injection (right). All other graph properties as described in Fig. 1.
Table. 2.
Response rates obtained during discrimination testing with substitution doses of JWH-018 and JWH-073 via intraperitoneal or inhaled routes of administration.
| Condition | Rate (response/s) | SEM |
|---|---|---|
| JWH-018 — 0.1 mg/kg, IP | 1.92 | 0.41 |
| JWH-018 — 0.3 mg/kg, IP | 2.09 | 0.32 |
| JWH-018 — 1.0 mg/kg, IP | 1.60 | 0.53 |
| JWH-073 — 1.0 mg/kg, IP | 2.14 | 0.19 |
| JWH-073 — 3.0 mg/kg, IP | 1.88 | 0.30 |
| JWH-073 — 10.0 mg/kg, IP | 1.54 | 0.42 |
| JWH-018 — 3.0 mg/30 L, inhaled | 0.84 | 0.31 |
| JWH-018 — 30.0 mg/30 L, inhaled | 0.63 | 0.33 |
| JWH-018 — 100.0 mg/30 L, inhaled | 0.21 | 0.22 |
| JWH-073 — 3.0 mg/30 L, inhaled | 0.79 | 0.35 |
| JWH-073 — 30.0 mg/30 L, inhaled | 0.35 | 0.16 |
| JWH-073 — 100.0 mg/30 L, inhaled | 0.00 | 0.00 |
4. Discussion
The studies reported here include the first direct comparisons of in vivo effects of synthetic cannabinoids JWH-018 and JWH-073 with those of Δ9-THC as a function of route of administration. For the most part, both of these compounds elicited effects which were similar to those of Δ9-THC across both routes of administration, although the synthetics were always more potent. Importantly, the data obtained in these studies validates our method of delivering volatilized drug for inhalation in mice. In this regard, we observed clear dose-dependent Δ9-THC-like effects in the cannabinoid tetrad following exposure to volatilized JWH-018 and JWH-073, and these effects were attenuated by prior administration of the CB1 receptor antagonist/inverse agonist rimonabant. Furthermore, inhalation of these synthetic cannabinoids elicited significant Δ9-THC-like responding in the drug discrimination task. This pattern of results suggests that the present method used to engender inhalation of volatilized cannabinoids resulted in CB1 receptor mediated biological effects which were dose-dependent and generally similar to those observed following drug injection.
Although it is not possible to directly equate doses of volatilized and inhaled cannabinoids with injected doses, principles of “effect scaling” (eg., Sharma and McNeill, 2009) suggest that drug doses which produce similar pharmacological effects may imply similar drug concentrations reaching the site of action, despite the different routes of drug delivery. In this regard, it is important to note that similar degrees of hypothermia, locomotor suppression, and analgesia were observed at maximal volatilized, inhaled and injected doses of JWH-018 and JWH-073. Thus, comparing the effects observed after these maximal doses across routes of administration would seem to be appropriate. The most striking finding in this regard is that none of the cannabinoids presently studied elicited any cataleptic effects following volatilization and inhalation, despite inducing profound catalepsy when these drugs were injected IP. One explanation for this could simply be that the doses administered in the inhalation chamber were too low to observe cataleptic effects. This seems unlikely, however, since an injected dose of JWH-018 induced maximal cataleptic effects in all subjects at a dose (10 mg/kg) that dropped rectal temperatures to 30.9 °C, but elicited no cataleptic effects whatsoever at an inhaled dose of 100 mg/30 L, despite reducing rectal temperatures to an almost identical degree. A similar pattern of results was also obtained with JWH-073, but it is likely that the highest injected dose of Δ9-THC was not comparable to the maximal dose delivered via inhalation, as more extreme hypothermia and locomotor suppression were observed following injection for this compound. Another possible reason for this large discrepancy in cataleptic effects as a function of route of administration focuses on the specific method used to assess this effect. We used a horizontal bar test, but the “ring test” might be a more common method of assessing cataleptic effects in mice. In this procedure, subjects are placed on a vertical tube and catalepsy is calculated as a percentage of time that animals spend motionless during the predetermined duration of the test session. Our procedure was perhaps more stringent, as small amounts of movement were usually enough to terminate the test as animals would remove their paws from the bar. In fact, the previous study utilizing inhaled JWH-018 (Wiebelhaus et al., 2012) demonstrated significant, dose-dependent cataleptic effects in mice using the ring test, while we saw no such effects in our inhalation procedure. A more interesting possibility, however, is that cataleptic effects of JWH-018 and JWH-073 are not induced by the parent drugs, but rather by active metabolites. We have previously described several hydroxylated phase I metabolites of JWH-018 and JWH-073 which maintain CB1 receptor affinity and efficacy, as well as Δ9-THC-like effects in some measures of the cannabinoid tetrad (Brents et al., 2011, 2012). It is perhaps to be expected that injection of JWH-018 or JWH-073 would maximize formation of these metabolites, while inhalation would minimize metabolite formation (Pond and Tozer, 1984). Future studies will be conducted to quantify brain levels of these parent drugs and their biologically-active metabolites after administration via inhalation or injection.
Apparent differences in Δ9-THC-like discriminative stimulus effects as a function of route of administration are also noteworthy. Before discussing these data in detail, it should be noted that this procedure was technically challenging, and numerous habituation sessions were required for all subjects. Following the first few exposures to the inhalation chamber and combustion of EtOH control media (and the momentary auditory and visual stimuli of this combustion, the prolonged auditory and tactile stimuli from the fan, possible stress effects of being transported from the colony room, to the fume hood, to the behavioral testing room, etc.), mice responded at very low rates, if they responded at all. It thus seems likely that the volatilization and inhalation procedure imposes significant confounds on subsequent behavioral testing. Nevertheless, animals eventually habituated to the procedure enough to allow some collection of data. In this regard, it may not be the case that the partial generalization of the Δ9-THC training dose to inhaled JWH-073 is the maximal possible effect for this compound. Rather, it may simply be that the interaction of inhaled JWH-073 at 100 mg/30 L and the cumulative stress effects involved with accomplishing these studies precluded assessment of discriminative responding, as no mice subjected to that dose emitted a single response during the 10 min substitution test. More habituation training will likely be needed if future experiments along these lines are to be conducted. On the other hand, volatilized and inhaled JWH-018 fully substituted for the Δ9-THC training dose, although the dose required to engender this effect (100 mg/30 L) also induced profound hypothermia and locomotor suppression, as well as some analgesic effects. In contrast, full substitution was observed at an injected dose of 1 mg/kg JWH-018, which had only minimal, non-significant effects on all four measures of the cannabinoid tetrad. This finding would seem to imply that active metabolites may also be involved in the discriminative stimulus effects of JWH-018.
Despite consistent recreational use and abuse of cannabinoids throughout human history, the reinforcing effects of cannabinoids have not been widely investigated in laboratory animals. To date, Δ9-THC has not been reported to maintain reliable self-administration behavior in rodents (Justinova et al., 2005), although the higher efficacy cannabinoids WIN 55,212 and HU-210 have been reported to maintain intravenous self-administration behavior in mice and rats (Martellotta et al., 1998; Fattore et al., 2001; Navarro et al., 2001). These data may suggest that other high efficacy cannabinoids, such as those present in K2/Spice products, might also display reinforcing effects in rodent self-administration procedures, but thus far, no published reports bolster this supposition. Importantly, just as route of administration can dramatically impact the in vivo effects of drugs, so can the contingency of drug administration (Jacobs et al., 2003). The fact that essentially all of our useful rodent models of cannabinoid exposure involve non-contingent experimenter-administered drug is a weakness in current methodology that likely limits our understanding of this class of compounds.
In summary, we report that whole-body exposure to volatilized and inhaled cannabinoids Δ9-THC, JWH-018, and JWH-073 elicits dose-dependent effects in three of the four measures of the cannabinoid tetrad in mice, and that such exposure to JWH-018 and JWH-073 engenders Δ9-THC-like responding in mice trained to discriminate an injected dose of 10 mg/kg Δ9-THC from saline. Both routes of administration revealed the same potency relationships across measures, with JWH-018 > JWH-073 > Δ9-THC. Inhaled doses of all three cannabinoids which induced comparable hypothermic and hypolocomotor effects were similarly attenuated by prior administration of the CB1 receptor antagonist/inverse agonist rimonabant. No cataleptic effects were observed following exposure to volatilized and inhaled cannabinoids at any dose, but injected doses of JWH-018, JWH-073 and Δ9-THC all induced significant catalepsy, perhaps implicating the involvement of active metabolites in this effect. Similarly, the fact that volatilized and inhaled doses of JWH-018 which elicited significant effects in the cannabinoid tetrad were required to elicit full Δ9-THC-like responding, while injected doses of JWH-018 which fully substituted for Δ9-THC had no significant effects on any of the tetrad measures, may also imply that active metabolites contribute to the discriminative stimulus effects of cannabimimetic AAIs. As recreational use and abuse of these compounds continues to spread, it will become critical to understand the role of route of administration on biological effects of these drugs in order to ensure that our laboratory models possess predictive validity.
Acknowledgments
We thank Sarah M. Zimmerman for her helpful technical assistance, and the UAMS Division of Laboratory Animal Medicine for expert husbandry services. This research was generously supported by the UAMS Center for Translational Neuroscience (RR020146) and the UAMS Translational Research Institute (RR029884). The views expressed herein are those of the authors and do not necessarily represent the views of the University of Arkansas for Medical Sciences.
References
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160:585–93. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83(7):952–61. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE. Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther. 2013;346(3):350–61. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenstat MA, Bell MR, D'Ambra TE, Estep KG, Haycock DA, Olefirowicz EM, et al. Aminoalkylindoles (AAIs): structurally novel cannabinoid-mimetics. NIDA Res Monogr. 1990;105:427–8. [PubMed] [Google Scholar]
- Estep KG, D'Ambra TE, Olefirowicz EM, Bell MR, Eissenstat MA, Haycock DA, et al. Conformationally restrained aminoalkylindoles: potent, stereoselective ligands at the cannabinoid binding site. NIDA Res Monogr. 1990;105:300–1. [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathúna BO, Pizarro N, de la Torre R. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: pharmacokinetic considerations. J Pharmacol Exp Ther. 2009;329(3):1006–15. doi: 10.1124/jpet.109.150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:1–12. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berlin) 2001;156:410–6. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24(11):566–73. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Marijuana use continues to rise among US teens, while alcohol use hits historic lows. Ann Arbor, MI: University of Michigan News Service; 2011. [Accessed 16 Dec 2011]. at http://www.monitoringthefuture.org. [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005;81(2):285–99. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, et al. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57(5):1168–80. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–30. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40(3):471–8. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther. 2009;331(2):717–23. doi: 10.1124/jpet.109.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond SM, Tozer TN. First-pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet. 1984;9(1):1–25. doi: 10.2165/00003088-198409010-00001. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):234–43. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, et al. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233(1–3):416–22. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157(6):907–21. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2012;120(1–3):238–41. doi: 10.1016/j.drugalcdep.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou Ch. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157–62. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126(3):316–22. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


