Abstract
Nonalcoholic fatty liver disease (NAFLD) is becoming a public health problem worldwide. A subset of patients develop an inflammatory disease, nonalcoholic steatohepatitis (NASH), characterized by steatosis, hepatocellular death, macrophage and neutrophil accumulation, and varying stages of fibrosis. Hepatocyte cell death triggers the cellular inflammatory response, therefore reducing cell death may be salutary in the steatohepatitis disease process. Recently, a better understanding of hepatocyte apoptosis in NASH has been obtained and new information regarding other cell death modes such as necroptosis and pyroptosis has been reported. Hepatocyte lipotoxicity is often triggered by death receptors. In addition to causing apoptosis, death receptors have been shown to mediate proinflammatory signaling, suggesting that apoptosis in this context is not an immunologically silent process. Here, we review recent developments in our understanding of hepatocyte cell death by death receptors and its mechanistic link to inflammation in NASH. We emphasize how proapoptotic signaling by death receptors may induce the release of proinflammatory extracellular vesicles, thereby recruiting and activating macrophages and promoting the steatohepatitis process. Potential therapeutic strategies are discussed based on this evolving information.
Keywords: Apoptosis, Caspase Inhibitor, Cell Death, Death Receptors, Exosomes, Extracellular Vesicles, Fibrosis, Inflammation, Inflammasome, Microvesicles, Necroptosis, Pyroptosis
Abbreviations used in this paper: cFLIP, cellular FLICE/caspase 8-like inhibitory protein; ER, endoplasmic reticulum; FFA, free fatty acid; FADD, Fas-associated protein with death domain; FasL, Fas ligand; fasudil, 5-(1,4-diazepan-1-ylsulfonyl)isoquinoline; GS-9450, (5R)-N-[(2S,3S)-2-(fluoromethyl)-2-hydroxy-5-oxooxolan-3-yl]-3-isoquinolin-8-yl-5-propan-2-yl-4H-1,2-oxazole-5-carboxamide; HSC, hepatic stellate cells; IDN-6556, (3S)-3-[[(2S)-2-[[2-(2-tert-butylanilino)-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid; IL, interleukin; JNK, c-Jun N-terminal kinase; MCD, methionine/choline-deficient; MCP-1, monocyte chemotactic protein-1; MLKL, mixed lineage kinase domain-like protein; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor kappa B; NK, natural killer; NKT, natural killer T; NLRP3, NLR family, pyrin domain containing 3; PUMA, p53 up-regulated modulator of apoptosis; RIP, receptor-interacting protein kinase; ROCK1, Rho-associated, coiled-coil containing protein kinase 1; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; TRAIL-R, tumor necrosis factor-related apoptosis-inducing ligand receptor; VX-166, (3S)-3-[[(2S)-2-[3-(methoxycarbonylamino)-2-oxopyridin-1-yl]butanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid; VX-740, (4S,7S)-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]-7-(isoquinoline-1-carbonylamino)-6,10-dioxo-2,3,4,7,8,9-hexahydro-1H-pyridazino[1,2-a]diazepine-4-carboxamide; VX-765, (2S)-1-[(2S)-2-[(4-amino-3-chlorobenzoyl)amino]-3,3-dimethylbutanoyl]-N-[(2R)-2-ethoxy-5-oxooxolan-3-yl]pyrrolidine-2-carboxamide
Summary.
This review discusses recent developments in our understanding of hepatocyte death receptor signaling and its mechanistic link to inflammation in steatohepatitis. Tumor necrosis factor-related apoptosis-inducing ligand receptor activation in hepatocytes during lipotoxicity induces release of proinflammatory extracellular vesicles, which, in turn, promote proinflammatory macrophage activation.
Nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in the United States and other Western countries. It is estimated that up to 30% of the Western world’s population is affected by NAFLD.1 A subset of patients with NAFLD (∼10% to 25%) develop nonalcoholic steatohepatitis (NASH), a more severe form of the disease characterized by hepatocellular death, accumulation of inflammatory cells, and varying stages of fibrosis. NASH can further progress to end-stage liver disease, cirrhosis, and hepatocellular carcinoma. NASH has become a significant public health concern, confounded by the lack of effective therapies. Thus, there is an urgent and unmet need for effective treatment that would parallel patients’ lifestyle modifications. Insight regarding the molecular and cellular mechanisms underlying disease development and progression may provide the impetus for rationale therapeutic strategies.
Cell death is a cardinal feature of NASH, as is accumulation of inflammatory cells, especially macrophages and neutrophils. Although it is widely accepted that cell death promotes cellular inflammation, the mechanistic link between cell death and hepatic inflammation remains enigmatic. Recent information links death receptor signaling to cell death in NASH by processes that promote cell-based inflammation. Thus, it is both timely and topical to review this information and emphasize therapeutic opportunities.
Modes of Cell Death
Hepatocyte apoptosis is the predominant cell death pathway in NASH. In addition, other types of programmed cell death such as necroptosis and pyroptosis have been reported. These cell death modes remain largely unexplored and merit further examination. Cell death modes such as necrosis and autophagy are not discussed in this review as they are not triggered by death receptors. These cell biological processes and their respective roles in liver pathobiology and steatohepatitis are reviewed elsewhere.2, 3
Apoptosis
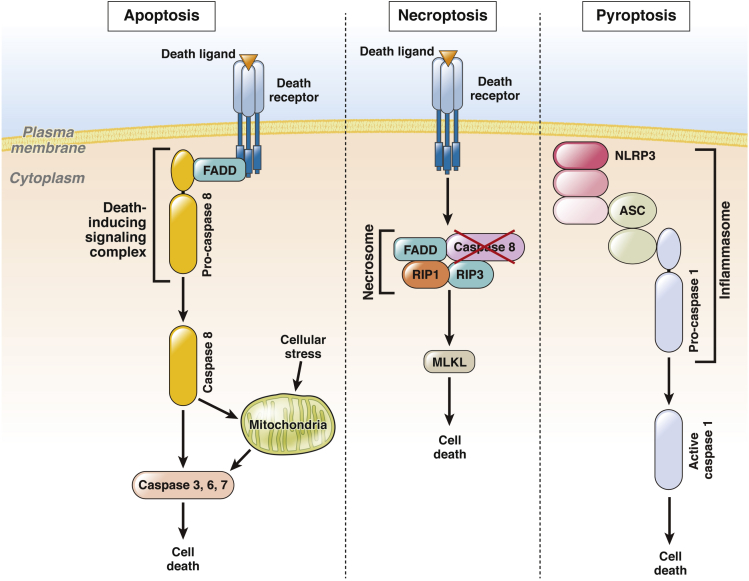
Apoptosis is highly organized cell death process that is morphologically characterized by cell shrinkage, membrane blebbing, chromatin condensation, DNA fragmentation, and formation of apoptotic bodies. Importantly, apoptotic cell death depends on the activity of caspases, a family of cysteine-dependent aspartate-specific proteases. Apoptosis can be initiated by two fundamental pathways: extrinsic and intrinsic pathways. The extrinsic pathway is initiated by death receptors that belong to tumor necrosis factor (TNF) receptor superfamily. Hepatocytes express death receptors Fas, TNF receptor 1 (TNFR1), and TNF-related apoptosis-inducing ligand (TRAIL) receptor (TRAIL-R) 1 and 2. Activation of these receptors leads to formation of a death-inducing signaling complex (DISC), which consists of caspase 8 and adaptor proteins, such as Fas-associated protein with death domain (FADD, Figure 1). In hepatocytes, activated caspase 8 cleaves Bid, generating tBid, which then induces the cytosolic egress of proapoptotic factors from the intermitochondrial space (eg, cytochrome c). Downstream of mitochondrial dysfunction, cytochrome c promotes formation of the apoptosome, which activates caspase 3, 6, and 7. These effector caspases execute cellular demolition. In hepatocytes, apoptosis is often initiated by the death receptor pathway.4
Figure 1.
Modes of cell death. Activation of apoptotic, necroptotic, or pyroptotic signaling pathways leads to cell death. Left panel: Upon binding of a death ligand, the intracellular domain of a death receptor recruits adaptor proteins such as Fas-associated protein with death domain (FADD) and pro-caspase 8 to form a signaling platform termed the death-inducing signaling complex (DISC). Caspase 8 undergoes proteolytic autoactivation, resulting in direct or indirect (via mitochondria) activation of caspases 3, 6, and 7, which execute the final step of cell death. Various stimuli, including endoplasmic reticulum stress, trigger apoptosis via the intrinsic pathway, where mitochondrial permeabilization is a central event. Release of proapoptotic factors from mitochondria into the cytosol activates caspase 3, 6, and 7 and cell death by apoptosis. Middle panel: Necroptosis is initiated by death receptors in cells where caspase 8 function is inhibited. Under these conditions, death receptor activation promotes formation of a necrosome, a signaling complex comprising FADD, receptor-interacting protein kinase 1 (RIP1), and RIP3. This complex induces cell death via mixed lineage kinase domain-like protein (MLKL), and probably other mediators. Right panel: Pyroptosis is a cell death mode resulting from activation of inflammasomes, including NLRP3 inflammasome. Upon activation, the intracellular receptor NLR family, pyrin domain containing 3 (NLRP3) recruits apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase 1. Cleavage-activated caspase 1 then induces cell death.
The intrinsic pathway of apoptosis is mediated by mitochondrial or lysosomal permeabilization. In addition, signaling between the endoplasmic reticulum (ER) and mitochondria can promote apoptosis. The intrinsic pathway of apoptosis is regulated by proapoptotic and antiapoptotic members of Bcl-2 family of proteins. Bak and Bax are proapoptotic proteins, activation of which results in outer mitochondrial membrane permeabilization and apoptosis as previously described. The antiapoptotic proteins of Bcl-2 family, such as Mcl-1 and Bcl-2, can prevent apoptosis by binding to Bak and Bax. Other proteins of this family, including p53 up-regulated modulator of apoptosis (PUMA), Bid, Bim, Bad, and Noxa, can activate proapoptotic Bax and Bak directly or indirectly via sequestration of antiapoptotic proteins. In hepatocytes, there is cross-talk between these two pathways as death receptor cell killing often relies on tBid generation to trigger the mitochondrial cell death pathway.
Necroptosis
Necroptosis refers to a recently discovered cell death mode that incorporates features of both apoptosis and necrosis. It shares some molecular machinery with apoptosis, but the final outcome resembles necrosis with organelle swelling and cellular leakage. Necroptosis occurs only under conditions when caspase 8 activation is suppressed by inhibition or genetic deletion,5 hence its physiologic context is unclear and highly controversial. Necroptosis can be triggered by activation of death receptors, Toll-like receptors, and perhaps other signals, which leads to an assembly of a large multiprotein signaling complex termed necrosome (Figure 1). The necrosome consists of caspase 8, FADD, cellular FLICE/caspase 8–like inhibitory protein (cFLIP), receptor-interacting protein (RIP) 1 and RIP3, and via RIP3 activates downstream effectors of necroptosis, such as mixed lineage kinase domain-like protein (MLKL). During necroptosis, RIP3 phosphorylates MLKL, which in turn oligomerizes and translocates to the plasma membrane, causing plasma membrane permeabilization.6, 7 In physiologic conditions, the complex caspase 8/FADD/cFLIP may act as a suppressor of necroptosis by limiting RIP3 activation.8 Given the fact that livers of caspase 8−/− mice are resistant to Fas- and TNF-α-induced cell death,9, 10 it is unlikely that significant necroptosis occurs in hepatocytes.
Pyroptosis
Pyroptosis is another form of programmed cell death, first described in immune cells during antimicrobial responses.11 Unlike apoptosis, pyroptosis requires activity of caspase 1, 4, or 5 and, similarly to necrosis, leads to cellular leakage and release of intracellular content.12, 13 Pyroptosis is initiated by pathogen-associated molecular pattern interaction with intracellular pattern recognition receptors, which leads to a formation of the inflammasome, a large intracellular signaling platform.
Several proteins recruited to inflammasomes have been identified. The best characterized inflammasome is a complex consisting of NLR family, pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), and pro-caspase 1 (Figure 1). Upon complex formation, activated caspase-1 cleaves inactive precursors of the proinflammatory cytokines pro-interleukin 1β (pro-IL-1β)and pro-IL-18 into their active forms IL-1β and IL-18, respectively. The proinflammatory signaling of the cytokines can occur both in infection and sterile inflammation. Inflammasome can also be activated in response to endogenous damage-associated molecular pattern and perhaps can be implicated in sterile liver inflammation.14 Recently, lipopolysaccharide was shown to directly activate caspase 4 and 5 and induce pyroptosis.13 Nevertheless, whether cells can die via pyroptosis in the absence of microbial infection is unclear.
Lipotoxicity
Hepatocyte lipotoxicity is a cardinal feature of NASH. Lipotoxicity originates from accumulation of lipid intermediates that cause cellular dysfunction and eventually cell death. During NASH, hepatocytes accumulate triglycerides and various lipid metabolites, including free cholesterol, ceramides, and free fatty acids (FFA).15 Hepatocytes store most fatty acids in the form of triglycerides, and recent evidence suggests that fatty acid esterification into neutral triglycerides is a protective mechanism against lipotoxicity.16, 17 In contrast, FFA cause hepatocyte injury and activate specific signaling cascades, resulting in hepatocyte apoptosis, which in this context is referred to as lipoapoptosis. Thus, FFA are considered a major mediator of hepatocyte lipotoxicity. Indeed, in NASH there is an increased hepatic influx of FFA as a consequence of enhanced lipolysis in peripheral adipose tissue due to insulin resistance.1, 18 FFA lipotoxicity in hepatocytes is mediated, in part, by their intracellular metabolite lysophosphatidyl choline, which was also found to be increased in the liver of NAFLD patients proportionally to disease severity.19, 20
Lipoapoptosis
Hepatocyte apoptosis is the most common and best-characterized cell death pathway in NASH. Indeed, hepatocyte apoptosis appears to be a cellular mechanism distinguishing NASH from simple steatosis, and excessive hepatocyte apoptosis is a pathologic hallmark of NASH.21, 22, 23 Moreover, the magnitude of hepatocyte apoptosis correlates with degree of inflammatory activity and stage of fibrosis.22
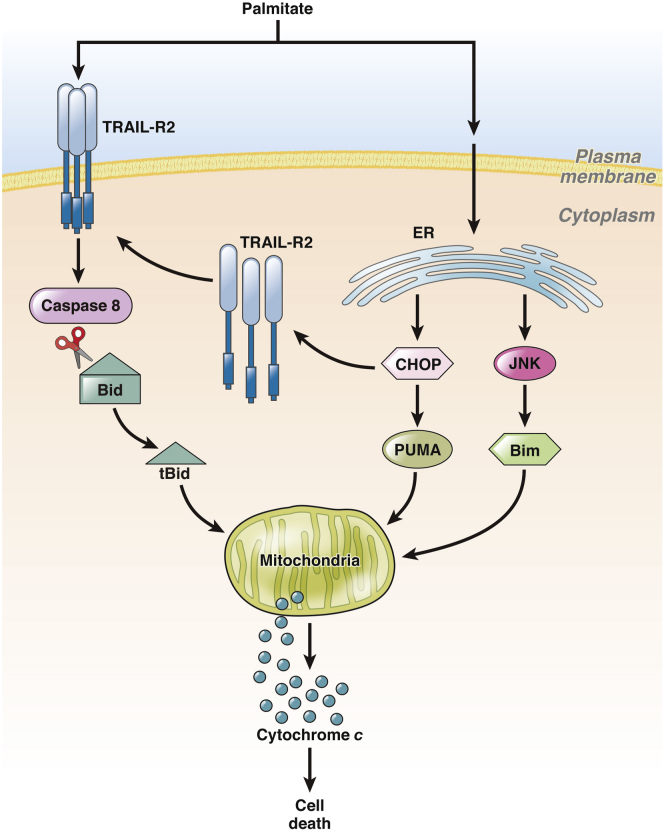
In vitro, death receptor signaling via TRAIL-R2 has emerged as a key mechanism of hepatocyte lipoapoptosis (Figure 2). Treatment of hepatocytes with the toxic saturated FFA palmitate causes reorganization of plasma membrane domains, allowing clustering and thus ligand-independent activation of TRAIL-R2, leading to hepatocyte cell death via caspase 8.24 Recently it has been demonstrated that persistent ER stress promotes apoptosis via TRAIL-R2.25 Unmitigated ER stress increases protein levels of TRAIL-R2 but not other death receptors, leading to ligand-independent activation of TRAIL-R2 and caspase 8–dependent apoptosis. Because saturated FFA induce ER stress,26, 27 this TRAIL-R2 signaling pathway may also contribute to palmitate-induced lipoapoptosis. For example, FFA also induce ER stress and expression of the transcription factor CAAT/enhancer-binding homologous protein (CHOP), which transcriptionally mediates PUMA up-regulation.27 Enhanced PUMA expression is also a feature of hepatocyte lipoapoptosis. PUMA likely cooperates with tBid to activate the mitochondrial pathway of cell death. In addition, FFA increase the expression of proapoptotic protein Bim via mechanisms dependent on c-Jun N-terminal kinase (JNK) and FoxO3a, which also may be the result of ER stress.28, 29 Taken together, these data suggest that by altering plasma and intracellular membranes, toxic lipids induce the expression and activation of TRAIL-R2, which in the context of JNK-dependent PUMA and Bim up-regulation causes cell death. This latter event is likely critical for lipoapoptosis as TRAIL-R activation is nontoxic to healthy hepatocytes but is toxic to steatotic hepatocytes.30, 31
Figure 2.
Apoptotic signaling pathways in lipotoxic hepatocyte. Hepatocyte treatment with the free fatty acid (FFA) palmitate causes ligand-independent clustering and activation of tumor necrosis factor (TNF)-related apoptosis-inducing ligand receptor 2 (TRAIL-R2), resulting in caspase-dependent cell death. Active caspase 8 cleaves Bid into its truncated form tBid, which translocates to mitochondria to promote the release of proapoptotic factors such as cytochrome c. FFA also causes endoplasmic reticulum stress, which via CAAT/enhancer binding homologous protein (CHOP) or c-Jun N-terminal kinase (JNK) up-regulates proapoptotic proteins p53 up-regulated modulator of apoptosis (PUMA) and Bim, and TRAIL-R2. Increased levels of PUMA and Bim facilitate hepatocyte cell death via the mitochondrial apoptotic pathway.
In vivo, liver expression of TRAIL-R2 is increased in both human and experimental NASH, and fatty murine livers are sensitized to TRAIL-R2-mediated hepatocyte apoptosis.32, 33 We have recently explored the role of TRAIL-R in a murine model of NASH induced by a high fat, fructose, and cholesterol diet.34 In contrast to humans, mice possess only a single ortholog for both human TRAIL-R1 and TRAIL-R2, which we here refer to as TRAIL-R. Mice deficient in TRAIL-R were remarkably protected against all features of NASH, including steatosis, hepatocyte apoptosis, macrophage-associated inflammation, and fibrosis. The protective effect against liver inflammation was associated with diminished hepatocyte apoptosis. Thus, TRAIL-R signaling plays a key role in NASH pathogenesis by promoting hepatocyte apoptosis.
Fas is abundantly expressed in the liver, and its expression is also increased in NASH.22 The steatotic liver is greatly susceptible to Fas-mediated hepatocyte apoptosis, as demonstrated in ob/ob mice or mice fed a high-carbohydrate diet that were administered anti-Fas antibody.35, 36 It has been reported that in the normal liver, hepatocyte growth factor receptor (c-Met) binds directly to Fas, precluding Fas activation. However, this inhibitory effect was absent in the steatotic liver, facilitating ligand-dependent activation of Fas and apoptosis.37 Despite these observations, there are no data implicating Fas in the pathogenesis of NASH in vivo.
TNFR1 signaling has been also studied in the context of NAFLD. Liver expression of both TNFR1 and its ligand TNF-α are increased in NASH.38, 39 In hepatocytes, FFA cause lysosomal destabilization, which results in release of cathepsin B into the cytosol and enhanced TNF-α expression. TNF-α may further promote lysosomal permeabilization, thus exacerbating liver injury in a feed-forward loop.40 TNF-α is an important mediator of insulin resistance and appears to be involved in development of steatosis. TNFR1−/− mice are protected against high-carbohydrate diet–induced steatosis and liver injury.40 Controversial results were obtained in ob/ob mice where anti-TNF-α therapy improved steatosis, but genetic deletion of both TNF receptors did not.41, 42 The precise role of TNFR1 signaling in NASH pathogenesis remains controversial.
Necroptosis in Nonalcoholic Steatohepatitis
Currently, information regarding necroptosis in NASH is very limited. RIP3 seems to be expressed at low levels in healthy human liver as compared with other organs such as the pancreas.43 On the other hand, RIP3 expression increases at both mRNA and protein levels in the liver of NASH patients.44, 45 An increased hepatocyte RIP3 expression was detected by immunohistochemistry in human NASH livers, albeit cholangiocytes stained for RIP3 even more strongly.45 The role of liver RIP3 in steatohepatitis was studied in the methionine/choline-deficient (MCD) diet where RIP3 was increased especially in mice with conditional deletion of caspase 8 in liver parenchyma (hepatocytes and cholangiocytes), which confirms the consent that caspase 8 functions as a potent suppressor of RIP3.45 RIP3−/− mice and double knockout mice caspase 8−/−/Rip3−/− were protected against MCD diet-induced macrophage liver infiltration and fibrosis. Caspase 8 deletion in liver parenchymal cells exacerbated the liver injury in this study.
The critical question arising from these studies is, do these data indicate that caspase inhibitors would be detrimental in NASH? The answer is probably not. For example, the study discussed above is in sharp contrast to a previous report by Hatting et al46 where caspase 8 genetic deletion in hepatocytes prevented MCD diet-induced liver injury, inflammation, and fibrosis. The inconsistency of these two studies is unclear and perhaps lies in the genetic models using different promoters for caspase 8 deletion in hepatocytes versus liver parenchymal cells. Similar to Hatting et al, unpublished data from our laboratory suggest that hepatocyte-specific caspase 8−/− mice are protected against nutrient excess-induced NASH. We also note that caspase inhibitors are protective in animal models of NASH.47, 48 Given the composite observations, it is unlikely that necroptosis contributes to liver injury and inflammation in NASH.
Pyroptosis in Nonalcoholic Steatohepatitis
Can hepatocytes undergo pyroptosis during NASH? In vivo studies using mice lacking the functional component of the Nlrp3 inflammasome provide an early answer to this question. Caspase 1−/− mice fed a high-fat diet display the same amount of hepatocellular cell death and no improvement of liver injury, suggesting that the inflammasome plays a marginal role in hepatocyte cell death in this model.49 In contrast, Nlrp3−/− mice were protected against hepatocellular cell death in a NASH model induced by the choline-deficient amino acid-defined diet.50 Because both studies used whole-body knockout models, their distinct outcomes may arise from different effects of the deleted gene in various liver cell types.
Another piece of evidence that inflammasome activation may lead to increased hepatocellular death by pyroptosis was provided by a study with transgenic mice expressing constitutively active Nlrp3.51 However, the whole-body Nlrp3 hyperactivation precludes physiologic assessment of inflammasome regulation in disease cell types. Although it is unlikely that pyroptosis causes cell death in the absence of a pathogen, inflammasome activation may be an important non-cell-death mediator of NASH.
Cell Death as a Driver of Inflammation
Cell death and inflammation are intricately connected and can positively regulate each other. In contrast to necrosis, cell death via apoptosis has been considered as an immunologically silent process that is intrinsically tolerogenic, based on the notion that apoptotic cells maintain their plasma membrane integrity and thus do not release their intracellular content. However, an increasing body of evidence indicates that apoptotic signaling, especially by death receptors, may be proinflammatory by releasing extracellular vesicles and various chemokines, which may potentially recruit and activate immune cells.
Another outcome of apoptotic cell death may be secondary necrosis. Failure of efferocytosis, a process by which apoptotic cells are removed by phagocytic cells, may lead to a release of intercellular contents into the extracellular milieu, eliciting an inflammatory response. Although secondary necrosis in NASH is largely an unexplored phenomenon, it may also represent a possible link between apoptosis and inflammation. Indeed, failure of efferocytosis has been implicated in the inflammatory response contributing to atherogenesis, another complication of the metabolic syndrome.52
Death Receptor Signaling and Inflammation
Death receptor signaling may activate a proinflammatory response directly or indirectly via inducing apoptosis. TNFR1 signaling results in transcription of a wide spectrum of genes involved in the inflammatory response, such as proinflammatory cytokines, via transcription factor nuclear factor kappa B (NF-κB).53 Recent observations that certain cancers and cancer cell lines are resistant to TRAIL-induced apoptosis led to a notion that TRAIL may activate nonapoptotic signaling pathways. Such signaling may drive a proinflammatory response via kinase activation.54, 55, 56 In the context of NASH, our in vitro studies with primary mouse macrophages lacking TRAIL-R have demonstrated that TRAIL-R is required for NF-κB activation and proinflammatory cytokine expression upon palmitate and lipopolysaccharide treatment.34
A number of studies in multiple cell types, including hepatocytes, demonstrate that Fas signaling leads to the production of IL-8 and monocyte chemotactic protein-1 (MCP-1), chemokines for neutrophils and macrophages, respectively. Cullen et al57 have demonstrated that primary hepatocytes secrete a variety of chemokines, including MCP-1, chemokine (C-C motif) ligand 3, and chemokine (C-X-C motif) ligand 1, while undergoing apoptosis upon treatment with an anti-Fas antibody. Indeed, MCP-1, a potent chemokine for macrophages, was implicated in NAFLD pathogenesis.58 It remains an open question as to whether Fas signaling in steatotic hepatocytes during NASH promotes cell death and secretion of chemokines, thereby stimulating proinflammatory responses. It also is not known whether TRAIL-induced cell death is associated with the same chemokine release as observed with Fas.
Extracellular Vesicles
Extracellular vesicles are membrane-defined vesicles secreted by cells to the extracellular environment in a highly regulated manner. There are three major types of extracellular vesicles categorized as exosomes, microvesicles, and apoptotic bodies. They are distinguished based on their cellular origin: exosomes are derived from the endolysosomal pathway, microvesicles bud from the plasma membrane, and apoptotic bodies are formed by blebbing of the apoptotic cell membrane.59 The size of exosomes and microvesicles ranges between 40–120 nm and 50–1000 nm, respectively. Apoptotic bodies are usually more than 500 nm in diameter.
Exosomes and microvesicles have recently emerged as a new mechanism of a cell-to-cell communication because they carry lipids, proteins, and various species of RNA that can trigger a myriad of responses in target cells. Because liver injury, including NASH, is a complex process involving interactions between multiple cell types, extracellular vesicles likely are mediators of this intercellular communication. Phagocytosis of hepatocyte-derived apoptotic bodies by Kupffer cells potently increases expression of TNF-α, TRAIL, and Fas ligand (FasL).60 These death receptor ligands induce further hepatocyte apoptosis, which can precipitate liver inflammation and fibrosis.
It has been demonstrated that palmitate-treated hepatocytes release proangiogenic microvesicles.61 The effect of these microvesicles depends on their internalization into endothelial cells via vanin-1, a protein expressed on these microvesicles. Our unpublished data indicate that lipotoxic hepatocytes release extracellular vesicles that induce macrophage migration and activation. Importantly, the release of these vesicles occurs before the onset of apoptosis, indicating that these vesicles are not apoptotic bodies. Similarly, Bretz et al62 reported that exosomes from body fluids such as liver cirrhosis ascites can induce a proinflammatory phenotype in monocytes. Collectively, these data indicate that extracellular vesicles contain functional mediators of inflammatory response.
The extracellular vesicles may carry various cytokines, chemokines, and micro-RNA, and which cargo mediates inflammation needs to be determined by future studies. Currently, our knowledge of the role of extracellular vesicles in NASH is limited, but lipotoxic hepatocyte-derived extracellular vesicles might be a potential additional link between hepatocellular injury and inflammation in NASH (Figure 3).
Figure 3.
Proinflammatory feed-forward loop between lipotoxic hepatocytes and activated macrophages. The illustration depicts the circular feed-forward relationship between hepatocyte lipotoxicity and inflammation during nonalcoholic steatohepatitis (NASH). Lipotoxicity induces the release of extracellular vesicles from hepatocytes. These can be engulfed by hepatic resident and recruited macrophages, causing macrophage activation. Activated macrophages increase the expression of death receptor ligands such as Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL), and proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which can cause further hepatocyte apoptosis and exacerbation of inflammation. This vicious circle may be interrupted by several therapeutic strategies. IL-1β, interleukin-1β; ROCK1, Rho-associated, coiled-coil containing protein kinase 1.
Nonalcoholic Steatohepatitis and Inflammation
Liver inflammation in NASH is orchestrated by multiple intricate mechanisms, and its complex nature is not completely understood. Cell death elicits inflammation by cells of the innate immune system. Indeed, these are the cell types that have been implicated in NASH pathogenesis, macrophages, and neutrophils.
Cell Types Involved
Healthy livers encompass many types of immune cells, such as resident macrophages (Kupffer cells), natural killer (NK) cells, and natural killer T (NKT) cells.63 Chronic activation of the innate immune system is a fundamental feature of NASH. Several lines of evidence suggest that Kupffer cells and infiltrating macrophages/monocytes play a cardinal role in NASH pathogenesis. Indeed, macrophage accumulation in the human liver correlates with the severity of histologic activity in NASH.64
During NASH, Kupffer cells acquire a proinflammatory phenotype and secrete multiple cytokines and chemokines. Secreted chemokines, such as IL-8 and MCP-1, which may also originate from apoptotic hepatocytes,57 recruit neutrophils and cells of monocyte/macrophage lineage to the liver, further accentuating the liver inflammation. Activated macrophages express death receptor ligands and secrete profibrogenic factors, which further exacerbates the hepatocellular injury and induces liver fibrogenesis in a feed-forward loop. The importance of macrophages in NASH pathogenesis is highlighted by studies where the depletion of Kupffer cells or inhibition of macrophage influx into the liver prevented hepatic inflammation and fibrosis in murine models of NASH.65, 66
Beside macrophages, other immune cells were studied in NASH pathogenesis. NKT cells appear to be depleted from the liver during simple steatosis, but they start to accumulate with the progression toward NASH.67, 68, 69, 70, 71 In sterile liver injury, two different subtypes of NKT cells appear to have distinct functions. Recent evidence suggests that type I NKT cells, which express semi-invariant T-cell receptor, may promote liver inflammation, whereas type II NKT cells, characterized by more diverse T-cell receptors, may have a protective role against liver injury.72 Little is known about the role of dendritic cells in NASH, but it appears that they exert anti-inflammatory and antifibrotic effects in preclinical models.73, 74
Inflammatory cells, which express ligands of the death receptors, may also enhance hepatocyte apoptosis. FasL and TRAIL can be found on NK cells, NKT cells, and macrophages. Given the fact that hepatocyte apoptotic bodies induce expression of death receptor ligands in Kupffer cells,60 hepatocyte apoptosis in NASH may be involved in a feed-forward apoptotic pathway, further exacerbating liver injury.
Inflammation as a Mediator of Fibrosis
Fibrosis is the most nefarious consequence of continuous liver injury and inflammation. Progressive fibrosis may result in cirrhosis, portal hypertension, liver failure, and hepatocellular carcinoma. It is now well recognized that chronic inflammation is the main driving force for fibrogenesis in NASH.75, 76 Hepatic stellate cells (HSC) and their production of extracellular matrix have a central role in NASH-related fibrogenesis. Activated Kupffer cells, infiltrating macrophages/monocytes, and injured hepatocytes release cytokines and various profibrogenic factors, such as platelet-derived growth factor and transforming growth factor-β1, thereby activating HSC and changing their phenotype into myofibroblast-like cells.
Interesting concepts have emerged that suggest that hepatocyte lipotoxicity and cell death can contribute to fibrogenesis via extracellular vesicles. We have demonstrated that engulfment of hepatocyte apoptotic bodies by HSC increases expression of profibrogenic genes, suggesting that the cells underwent profibrogenic activation.77 A similar effect was also observed when HSC were incubated with isolated microvesicles released from lipotoxic hepatocytes.78 Hence, cell death drives hepatic fibrosis indirectly via inflammation and perhaps directly by vesicle-mediated cell-to-cell communication (hepatocyte → stellate cell).
Therapeutic Strategies
Caspase Inhibition
Caspase activation has been well documented in both human and preclinical NASH. Mice lacking caspase 3 and caspase 8 are protected against diet-induced NASH.46, 79 Thus, inhibition of hepatocyte apoptosis via caspase inhibition is a mechanistically attractive target for pharmacotherapy for NASH. Moreover, pan-caspase inhibitors may also inhibit proinflammatory caspases such as caspase 1.
Small-molecule caspase inhibitors have been recently developed to circumvent the unfavorable pharmacokinetics of the original peptide-based inhibitors. The pan-caspase inhibitor IDN-6556 [(3S)-3-[[(2S)-2-[[2-(2-tert-butylanilino)-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid] was able to decrease fibrosis in association with decreased hepatocyte apoptosis in a model of liver injury leading to fibrosis.80 The pan-caspase inhibitors IDN-6556 and VX-166 [(3S)-3-[[(2S)-2-[3-(methoxycarbonylamino)-2-oxopyridin-1-yl]butanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid] decreased liver injury, inflammation, and fibrosis in murine models of NASH.47, 48, 81 A recent phase 2 clinical trial concluded that GS-9450 [(5R)-N-[(2S,3S)-2-(fluoromethyl)-2-hydroxy-5-oxooxolan-3-yl]-3-isoquinolin-8-yl-5-propan-2-yl-4H-1,2-oxazole-5-carboxamide] therapy improved liver injury in a dose-dependent manner in patients with NASH.82 However, the development of this drug may be hampered due to safety concerns that were raised in another clinical trial with this agent.
To date, the effect of caspase inhibitors on NASH-associated inflammation and fibrosis has not been evaluated in humans. Given the promising results, a therapeutic approach based on caspase inhibition warrants more clinical trials in human NASH.
Interleukin-1β Inhibition
IL-1β, a cytokine generated by inflammasome activation, initiates inflammatory cascades and the production of a number of other proinflammatory mediators. Increased hepatic levels of IL-1β have been detected in preclinical models of NASH.49, 83, 84 The role of IL-1β in NASH has been highlighted by studies using mice lacking genes important for IL-1β production or signaling. For example, Il-1β−/−, Il-1 receptor−/−, Nlrp3−/−, and caspase 1−/− mice were protected against diet-induced NASH.49, 84, 85, 86
IL-1β and its receptor can be targeted pharmacologically by multiple approaches. Human recombinant IL-1 receptor antagonist (anakinra), anti-IL-1β antibody (CDP-484), IL-1 trap, and soluble IL-1 receptor II are biologic agents that target IL-1β neutralization.87 The production of IL-1β can also be diminished by caspase 1 inhibition. The small-molecule caspase 1–specific inhibitors VX-765 [(2S)-1-[(2S)-2-[(4-amino-3-chlorobenzoyl)amino]-3,3-dimethylbutanoyl]-N-[(2R)-2-ethoxy-5-oxooxolan-3-yl]pyrrolidine-2-carboxamide] and VX-740 [(4S,7S)-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]-7-(isoquinoline-1-carbonylamino)-6,10-dioxo-2,3,4,7,8,9-hexahydro-1H-pyridazino[1,2-a]diazepine-4-carboxamide] are safe for use in humans. Collectively, given the encouraging preclinical data and availability of experimental therapeutics, IL-1β inhibition appears to be a potential target for NASH therapy.
Chemokine-Targeted Therapy
Chemokines have a central role in the recruitment of macrophages/monocytes to the liver during NASH. The cellular source of chemokines is not only activated Kupffer cells but also epithelial cells undergoing apoptosis or secreting chemokine-bearing extracellular vesicles. For example, MCP-1 and its receptor CCR2 (C-C chemokine receptor type 2) have emerged as potential targets for NASH.58 Indeed, a CCR2 small-molecule inhibitor remarkably prevented hepatic inflammation and fibrosis in preclinical NASH.65 Thus, targeting the chemokine signaling pathways may be a therapeutic approach for NASH.
Rho-Associated Kinase 1 Inhibition
In our recent study, we identified rho-associated kinase 1 (ROCK1) as a key kinase involved in lipotoxicity-induced extracellular vesicle release (manuscript in preparation). Genetic deletion or pharmacologic inhibition of ROCK1 significantly decreases extracellular vesicle release from hepatocyte in vitro. We also tested the effect of a ROCK1 inhibitor in a murine model of NASH induced by nutrient excess. Similarly to the in vitro data, the ROCK1 inhibitor fasudil [5-(1,4-diazepan-1-ylsulfonyl)isoquinoline] decreased diet-induced increases in serum extracellular vesicles. ROCK1 inhibition in this model also prevented hepatocellular injury and decreased macrophage-associated inflammation. Moreover, fasudil is approved for use in Japan for the treatment of cerebral vasospasm. Hence, its short-term use in humans is safe.
Summary
In this article, we examined the intimate and mechanistic relationship between cell death and proinflammatory signaling. Intriguingly, key components of cell death signaling pathways, such as caspase and death receptor activation, are also implicated in proinflammatory signaling. Over the past decade, our knowledge of cell death and proinflammatory signaling in NASH has increased. However, the precise dominant molecular and cellular mechanisms still remain relatively obscure. Very recently, apoptosis by death receptors has been revised in terms of its role in proinflammatory signaling. A better understanding of the interplay between cell death and inflammation may answer why some patients with steatosis advance to steatohepatitis and may enable us to halt the disease progression.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK41876] (to G.J.G.) and by the Mayo Clinic.
References
- 1.Fujii H., Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 2012;47:215–225. doi: 10.1007/s00535-012-0527-x. [DOI] [PubMed] [Google Scholar]
- 2.Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783.e4. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir M., Czaja M.J. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guicciardi M.E., Malhi H., Mott J.L., Gores G.J. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linkermann A., Green D.R. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Li W., Ren J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Z., Jitkaew S., Zhao J. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nature Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon C.P., Oberst A., Weinlich R. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Reports. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke C., Bangen J.M., Freimuth J. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176–2187. doi: 10.1053/j.gastro.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann T., Jost P.J., Pellegrini M. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathinam V.A., Vanaja S.K., Fitzgerald K.A. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J., Zhao Y., Wang Y., Gao W. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 14.Kubes P., Mehal W.Z. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K., Mizukoshi E., Sunagozaka H. Characteristics of hepatic fatty acid compositions in patients with nonalcoholic steatohepatitis. Liver Int. 2014 doi: 10.1111/liv.12685. http://dx.doi.org/10.1111/liv.12685 Published online. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K., Yang L., McCall S. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 17.Listenberger L.L., Han X., Lewis S.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Almeida I.T., Cortez-Pinto H., Fidalgo G. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 19.Han M.S., Park S.Y., Shinzawa K. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Kakisaka K., Cazanave S.C., Fingas C.D. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G77–G84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhouri N., Carter-Kent C., Feldstein A.E. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein A.E., Canbay A., Angulo P. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 23.Puri P., Mirshahi F., Cheung O. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 24.Cazanave S.C., Mott J.L., Bronk S.F. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286:39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu M., Lawrence D.A., Marsters S. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y., Wang D., Topczewski F., Pagliassotti M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 27.Cazanave S.C., Elmi N.A., Akazawa Y. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G236–G243. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barreyro F.J., Kobayashi S., Bronk S.F. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 29.Akazawa Y., Cazanave S., Mott J.L. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashkenazi A., Pai R.C., Fong S. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhi H., Barreyro F.J., Isomoto H. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsova P., Ibrahim S.H., Bronk S.F. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkmann X., Fischer U., Bahr M.J. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 34.Idrissova L, Malhi H, Werneburg NW, et al. Trail receptor deletion in mice suppresses the inflammation of nutrient excess. J Hepatol. Published online. http://dx.doi.org/10.1016/j.jhep.2014.11.033. [DOI] [PMC free article] [PubMed]
- 35.Feldstein A.E., Canbay A., Guicciardi M.E. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 36.Siebler J., Schuchmann M., Strand S. Enhanced sensitivity to CD95-induced apoptosis in ob/ob mice. Dig Dis Sci. 2007;52:2396–2402. doi: 10.1007/s10620-006-9148-7. [DOI] [PubMed] [Google Scholar]
- 37.Zou C., Ma J., Wang X. Lack of Fas antagonism by Met in human fatty liver disease. Nat Med. 2007;13:1078–1085. doi: 10.1038/nm1625. [DOI] [PubMed] [Google Scholar]
- 38.Crespo J., Cayon A., Fernandez-Gil P. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro P.S., Cortez-Pinto H., Sola S. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 40.Feldstein A.E., Werneburg N.W., Canbay A. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 41.Li Z., Yang S., Lin H. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 42.Memon R.A., Grunfeld C., Feingold K.R. TNF-alpha is not the cause of fatty liver disease in obese diabetic mice. Nat Med. 2001;7:2–3. doi: 10.1038/83316. [DOI] [PubMed] [Google Scholar]
- 43.Sun X., Lee J., Navas T. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 44.Csak T., Dolganiuc A., Kodys K. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917–1931. doi: 10.1002/hep.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gautheron J., Vucur M., Reisinger F. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatting M., Zhao G., Schumacher F. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–2201. doi: 10.1002/hep.26271. [DOI] [PubMed] [Google Scholar]
- 47.Barreyro F.J., Holod S., Finocchietto P.V. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2014 doi: 10.1111/liv.12570. http://dx.doi.org/10.1111/liv.12570 Published online. [DOI] [PubMed] [Google Scholar]
- 48.Witek R.P., Stone W.C., Karaca F.G. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 49.Dixon L.J., Flask C.A., Papouchado B.G. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wree A., McGeough M.D., Pena C.A. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014;92:1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wree A., Eguchi A., McGeough M.D. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvestre-Roig C., de Winther M.P., Weber C. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 53.Chu W.M. Tumor necrosis factor. Cancer Lett. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azijli K., Weyhenmeyer B., Peters G.J. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ. 2013;20:858–868. doi: 10.1038/cdd.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azijli K., Yuvaraj S., Peppelenbosch M.P. Kinome profiling of non-canonical TRAIL signaling reveals RIP1-Src-STAT3-dependent invasion in resistant non-small cell lung cancer cells. J Cell Sci. 2012;125:4651–4661. doi: 10.1242/jcs.109587. [DOI] [PubMed] [Google Scholar]
- 56.Collison A., Foster P.S., Mattes J. Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin Exp Pharmacol Physiol. 2009;36:1049–1053. doi: 10.1111/j.1440-1681.2009.05258.x. [DOI] [PubMed] [Google Scholar]
- 57.Cullen S.P., Henry C.M., Kearney C.J. Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell. 2013;49:1034–1048. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Marra F., Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594.e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 59.Andaloussi E.L., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 60.Canbay A., Feldstein A.E., Higuchi H. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 61.Povero D., Eguchi A., Niesman I.R. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bretz N.P., Ridinger J., Rupp A.K. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(Suppl):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 64.De Vito R., Alisi A., Masotti A. Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med. 2012;30:49–56. doi: 10.3892/ijmm.2012.965. [DOI] [PubMed] [Google Scholar]
- 65.Miura K., Yang L., van Rooijen N. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tosello-Trampont A.C., Landes S.G., Nguyen V. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guebre-Xabier M., Yang S., Lin H.Z. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 68.Li Z., Soloski M.J., Diehl A.M. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 69.Yang L., Jhaveri R., Huang J. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–937. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 70.Syn W.K., Oo Y.H., Pereira T.A. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolf M.J., Adili A., Piotrowitz K. Metabolic activation of intrahepatic CD8(+) T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–620. doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henning J.R., Graffeo C.S., Rehman A. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58:589–602. doi: 10.1002/hep.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tacke F., Yoneyama H. From NAFLD to NASH to fibrosis to HCC: role of dendritic cell populations in the liver. Hepatology. 2013;58:494–496. doi: 10.1002/hep.26405. [DOI] [PubMed] [Google Scholar]
- 75.Argo C.K., Northup P.G., Al-Osaimi A.M., Caldwell S.H. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 76.Canbay A., Friedman S., Gores G.J. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 77.Canbay A., Taimr P., Torok N. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 78.Povero D., Eguchi A., Feldstein A.E. Hepatocytes-derived microparticles released during lipotoxicity induce hepatic stellate cells activation and migration. Hepatology. 2013;58(Suppl):S574A–S575A. [Google Scholar]
- 79.Thapaliya S., Wree A., Povero D. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59:1197–1206. doi: 10.1007/s10620-014-3167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canbay A., Feldstein A., Baskin-Bey E. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 81.Anstee Q.M., Concas D., Kudo H. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–550. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Ratziu V., Sheikh M.Y., Sanyal A.J. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Csak T., Ganz M., Pespisa J. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dixon L.J., Berk M., Thapaliya S. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012;92:713–723. doi: 10.1038/labinvest.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamari Y., Shaish A., Vax E. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55:1086–1094. doi: 10.1016/j.jhep.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miura K., Kodama Y., Inokuchi S. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braddock M., Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]