Abstract
Human immunodeficiency virus-1 (HIV) is a public health issue and a major complication of the disease is NeuroAIDS. In vivo, microglia/macrophages are the main cells infected. However, a low but significant number of HIV infected astrocytes also has been detected, but their role in the pathogenesis of NeuroAIDS is not well understood. Our previous data indicates that gap junction channels amplify toxicity from few HIV infected into uninfected astrocytes. Now, we demonstrated that HIV infection of astrocytes results in opening of connexin43 hemichannels (Cx43 HCs). HIV induced opening of Cx43 HCs resulted in dysregulated secretion of dickkopf-1 protein (DKK1, a soluble wnt pathway inhibitor). Treatment of mixed cultures of neurons and astrocytes with DKK1, in the absence of HIV infection, resulted in collapse of neuronal processes. HIV infection of mixed cultures of human neurons and astrocytes also resulted in collapse of neuronal processes by a DKK1 dependent mechanism. In addition, dysregulated DKK1 expression in astrocytes was observed in human brain tissue sections of individuals with HIV encephalitis as compared to tissue sections from uninfected individuals. Thus, we demonstrated that HIV infection of astrocytes induces dysregulation of DKK1 by a HC-dependent mechanism that contributes to the brain pathogenesis observed in HIV infected individuals.
Introduction
Despite effective combination antiretroviral treatment (cART), normal CD4+ T cell counts, and low to undetectable viral load, human immunodeficiency virus type 1 (HIV) results in neurological complications in 40–60% of individuals (Letendre 2011, Boisse et al. 2008). These HIV-associated neurocognitive disorders include HIV associated dementia and the less severe mild neurocognitive disorder. Importantly, even in the current cART era, a significant number of HIV infected cells may remain in the central nervous system (CNS), including microglia/macrophages (Cosenza et al. 2002) and astrocytes (An et al. 1999, Conant et al. 1994, Eugenin & Berman 2007b, Eugenin et al. 2011b, Tornatore et al. 1994). Although the evidence indicates that only a small fraction of astrocytes are infected with HIV, they are the most abundant cell type in the brain, and may constitute a significant viral reservoir.
Normally astrocytes participate in multiple functions within the CNS (Giaume 2010), but their role in NeuroAIDS disorders is still not well understood and only recently has been revisited (Hazleton et al. 2010). Previous studies by our group showed that despite the small fraction of astrocytes that become infected with HIV and the minimal to undetectable viral replication, significant bystander apoptosis, blood brain barrier (BBB) disruption, and cellular dysfunction are observed by a mechanism involving gap junction (GJ) channels (Eugenin et al. 2011a, Eugenin & Berman 2007a), but the role of hemichannels (HCs) has not been examined.
Gap junctions (GJs) are aggregates of channels connecting the cytoplasmic compartments of the coupled cells and providing direct cytoplasmic continuity between the cells allowing electrical and metabolic coordination (Saez et al. 2003). A GJ channel is formed by the docking of two HCs (one contributed by each of the joined cells), and each HC is composed of six protein subunits termed connexins (Cxs). These proteins belong to a highly conserved protein family encoded by 21 genes in humans and 20 in mice with orthologs in other vertebrate species as well as ascidians. In addition, a glycoprotein family of 3 members in humans and rodents, unrelated to Cxs but with a similar membrane topology, termed pannexins (Panxs), has been shown to form HCs on the surface of vertebrate cells (Bennett et al. 2012). Recent evidence indicates that HCs composed of Cxs or Panxs in non-junctional membranes can open to the extracellular space under appropriate conditions and allow diffusional exchange between the cytoplasmic compartment and extracellular milieu (Saez et al. 2010).
Here, we show that HIV infection of human astrocytes increases the opening of Cx43, but not Panx1 HCs, which results in increased expression and secretion of Dickkopf-1 (DKK1), a soluble inhibitor of Wnt signaling. Addition of DKK1, in the absence of HIV infection, to mixed cultures of human neurons and astrocytes results in collapse of neuronal processes. HIV injection of mixed cultures of neurons and astrocytes also resulted in collapse of neuronal processes by a DKK1 mechanism. Upregulation of DDK1 expression is also observed in astrocytes present in brain sections from HIVE subjects. Our results provide the basis of a novel mechanism for the spread of damage mediated by astroglial Cx43 HCs in response to HIV infection.
Materials and methods
Reagents and antibodies
DMEM medium, fetal bovine serum, penicillin/streptomycin (P/S), and trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA). The HIV isolates, HIVADA, HIVJR-CSF and HIVBal, were from the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health (Germantown, MD). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise designated. Antibodies to Cx43 and GFAP were obtained from Cell Signaling (Boston, MA). Gap26, 10panx1 and scrambled peptides were obtained from Peprotech (Rocky Hill, NJ). DKK1 blocking and immune staining antibodies were obtained from R and D systems (Minneapolis, MN). Cx43E2, a Cx43 HC antibody to the second extracellular loop was kindly provided by Dr. Jean Jiang, Department of Biochemistry, University of San Antonio, USA (Orellana et al. 2013).
Cell culture
Cortical tissue from human fetuses was obtained as part of an ongoing research protocol approved by the Albert Einstein College of Medicine and Rutgers University (protocol, 02-21-12-01A1 and Pro2012001303). The preparation of astrocyte and neuronal cultures was performed as described previously (Eugenin & Berman 2003, Eugenin et al. 2011b, Eugenin et al. 2003, Eugenin et al. 2007). U87, an astrocytoma cell line transfected with CD4 and CCR5, was used as a model of HIV infected astrocytes. Upon infection these cells maintain Cx43 expression, gap junctional communication and HC on the surface of the cells, like primary astrocytes. In addition, these cells are highly susceptible to HIV infection (~80–90 %) in comparison to the lower prevalence (5–8 %) in primary astrocytes. Thus, we infected U87CD4CCR5 cells with HIV for two days and microarray was performed using a Human whole genome onearray™ system (n=3, Phalanx, Hsinchu, Taiwan, www.phalanxbiotech.com).
HIV-infection of primary cultures of human astrocytes
Confluent cultures of human fetal astrocytes were infected by incubation with viral stocks (20–50 ng p24/ml/1×106 cells) of HIVADA, HIVJR-CSF or HIVBal, using a previously described protocol (Ohagen et al. 1999, Eugenin et al. 2003). Briefly, astrocyte cultures were exposed to the virus for 24 h. The medium was removed and astrocytes were washed extensively to eliminate the unbound virus before addition of fresh medium. Immunofluorescence analyses for GFAP and HIV-p24 indicated that cells infected with HIV were astrocytes, and that cultures showed no contamination with CD68 positive microglial cells (data not shown). Our published data indicated that infection with HIVADA, HIVJR-CSF or HIVBal always resulted in ~5% infectivity despite the amount of virus used (Eugenin et al. 2011a, Eugenin & Berman 2007a).
Dye uptake and time-lapse fluorescence imaging
For “snapshot” experiments, control and treated cells were exposed to 5 μM ethidium (Etd) bromide and TOPRO for 10 min at 37°C in culture medium. Then they were washed five times for 2 min each with Hank’s balanced salt solution (in mM: 137 NaCl, 5.4 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 1.2 CaCl2 at pH 7.4), and fixed at room temperature with 2% paraformaldehyde for 30 min, mounted in antifade reagent conjugated with DAPI and examined in a confocal laser-scanning microscope. Stacks of consecutive confocal images taken with a 63X objective at 500 nm intervals were acquired sequentially with two lasers (argon 488 nm and helium/neon 543 nm), and Z projections were reconstructed using Leica confocal software. For time-lapse fluorescence imaging, cells were plated on dishes in Locke’s solution (containing (in mM) 154 NaCl, 5.4 KCl, 2.3 CaCl2, 5 HEPES and pH 7.4) with 5 μM Etd and TOPRO. Phase-contrast and fluorescence microscopy with time-lapse imaging were used to record cell appearance and fluorescence intensity changes in each condition as described previously. No differences in dye uptake were found before the addition of the virus. Images of Etd uptake were analyzed with the Image J program (NIH software). For data representation, the average of four independent background fluorescence intensity measurements (FB, expressed as arbitrary units, AU) was subtracted from the fluorescence intensity in each cell (F1). Results of this calculation (F1-FB) [or F – F0] for 20 cells were averaged and plotted vs. time. Slopes of dye uptake were calculated using Microsoft Excel software and mean values of slopes were compared using GraphPad Prism software.
Immunofluorescence and apoptosis assays
Cultures of human fetal astrocytes and mixed cultures of neurons and astrocytes were grown on coverslips and fixed and permeabilized in cold 70% ethanol for 20 min at −20°C. Cells were incubated in TUNEL reaction mixture (Roche, Germany) at 37°C for 1 h, washed three times in PBS and incubated in blocking solution for 30 min at room temperature. Cells were incubated in diluted primary antibody (anti-GFAP, anti-neuron specific beta III tubulin and DDK1; 1:800, 1:2,000 and 1:300, respectively) overnight at 4°C. Cells were washed several times with PBS at room temperature and incubated with the appropriate secondary antibodies conjugated to FITC or Cy3 for 1 h at room temperature, followed by another wash in PBS for 1 h. Coverslips were then mounted using antifade reagent with DAPI, and cells were examined by confocal microscopy as described above. Antibody specificity was confirmed by replacing the primary antibody with a non-specific myeloma protein of the same isotype or non-immune serum.
Human tissue sections
Autopsy brain tissue was collected as part of the IRB approved protocols of the Manhattan HIV Brain Bank (U01MH083501). We analyzed tissues from 5 uninfected donors as controls and from 5 donors with HIV encephalitis. All human brains were subjected to postmortem ischemic events, due to time of tissue processing. Sections of 10 μm thickness were processed for immunofluorescence and confocal microscopy as described above.
ELISA for DKK1 and HIV-p24
DKK1 and HIV-p24 levels were determined by ELISA according to the manufacture’s protocols (R&D Systems, Minneapolis, MN and Perkin Elmer, Boston, MA, respectively).
Statistical Analysis
Paired t tests were used to calculate significance. A value of p<0.05 was considered significant.
Results
HIV induces opening of Cx43 hemichannels in primary cultures of human astrocytes
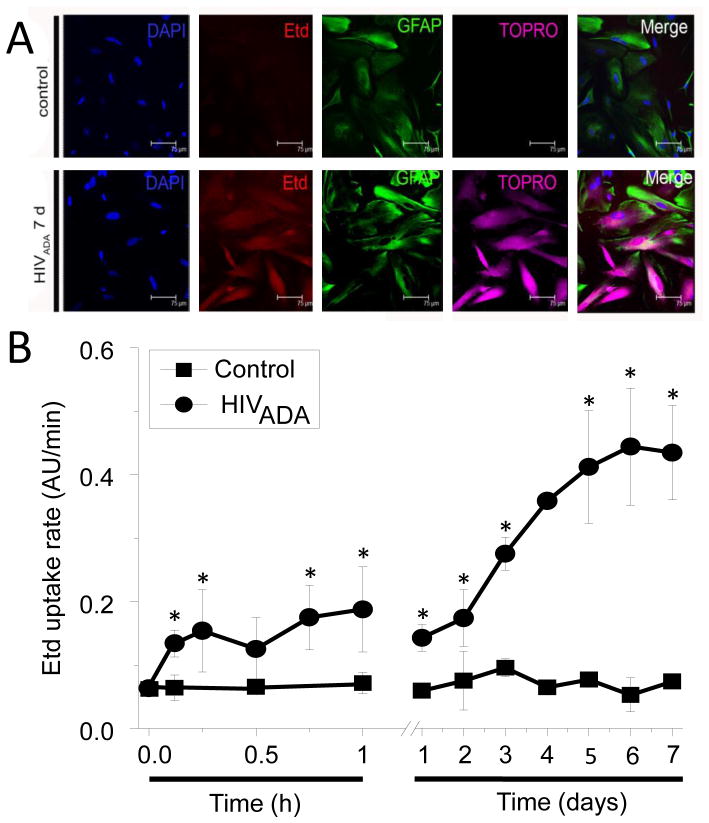
To examine whether HC opening is modulated by HIV, we infected human primary astrocytes with HIV using three different viral strains. Astrocytes were exposed to HIVADA, HIVJR-CSF or HIVBal (20–50 ng/ml) for 24 h and then evaluated for HC activity by Etd uptake (charge +1) at different times after infection. Etd only crosses the plasma membrane in healthy cells by passing through relatively non-specific channels, such as Cx and Panx HCs, and becomes fluorescent upon binding to intracellular nucleotides (Orellana et al. 2011). Time-lapse fluorescence imaging revealed that similar to rodent astrocytes (Orellana et al. 2010), human astrocytes cultured under control conditions exhibited a basal Etd uptake (Fig. 1A and B). After HIVADA infection, the uptake of Etd (Fig. 1) and TOPRO (Fig. 1A) gradually increased, approaching a plateau at 5–7 days post-exposure, to ~650% of the basal rate (Fig. 1B). Similar results were obtained after stimulation with HIVBal or HIVJR-CSF (data not shown). These findings were corroborated using “snapshot” experiments of TOPRO (charge +2) uptake (Fig. 1A), another dye employed to measure HC activity (Sandilos et al. 2012). Despite the fact that only 5–8% of the cells became productively infected with HIV (Eugenin & Berman 2007b, Eugenin et al. 2011b), all astrocytes exhibited increased Etd and TOPRO uptake after HIV stimulation (Fig. 1A). No differences were found in dye uptake induced by the three HIV strains tested (not shown). In addition, we observed an increase in GFAP staining in HIV expose cultures (Fig. 1A). Increased GFAP has been observed in vitro and in vivo as a marker of astroglial activation in HIV infected individuals (Petito et al. 2001, Vanzani et al. 2006, Anderson et al. 2003).
Figure 1. HIV increased Etd uptake in human primary astrocytes.
(A) Representative snapshot of the time lapse described in (B) of Etd and TOPRO uptake in control conditions or 168 h after HIVADA infection (HIVADA 7 d). Astrocytes were identified by GFAP staining. Bar: 75 μm. (B) Time course of Etd uptake rate, 0.12 to 168 h (7 days), obtained from human primary astrocyte cultures under control conditions (lines with squares) or after HIVADA infection (lines with circles). Each value corresponds to the mean ± SD of the Etd intracellular intensity present in at least 20 cells per time point, *p<0.005, n=4.
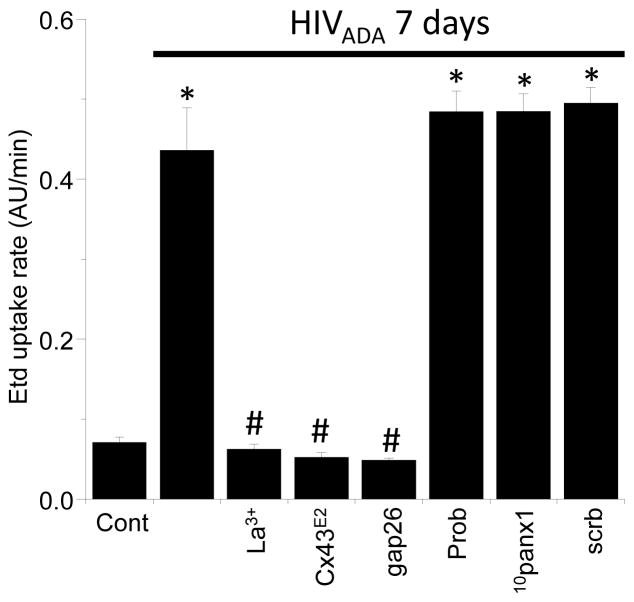
The possible role of Cx43 HCs in the HIV-induced Etd uptake was examined using an Cx43E2 antibody (Orellana et al. 2011) and the mimetic peptide Gap26 with an amino acid sequence identical to a segment of the second extracellular loop of Cx43 (Evans et al. 2006). Cx43E2 (1:500) and Gap26 (200 μM) reduced Etd uptake rate to control values; La3+, a general blocker of Cx HCs, caused a similar block in Etd uptake (Fig. 2). Pretreatment with the preimmune antibodies (1:500, not shown) and a scrambled peptide of Gap26 did not affect the rate of HIV-induced Etd uptake (Fig. 2), supporting the specificity of Cx43E2 and Gap26. In contrast, 10panx1 (200 μM) and probenecid (200 μM), both Panx1 HC blockers (Pelegrin & Surprenant 2006, Silverman et al. 2008), failed to reduce the rate of HIV-induced Etd uptake, ruling out the possible involvement of Panx1 HCs in this phenomenon. Taken together, these data indicate that HIV infection induces Cx43 HC opening in primary human astrocytes.
Figure 2. Astroglial Cx43 hemichannels increases their opening upon HIV infection.
Etd uptake rate for human primary astrocytes exposed to HIVADA for 7 days is shown. Control cells have minimal Etd uptake (Cont). HIV infection resulted in significant increase in Etd uptake (*p<0.005). Addition of Cx43 HC blockers, lanthanum ions (La3+, 200 μM), Cx43E2 antibody (1:500 dilution) or peptide gap26 (200 μM) abolished the increase of Etd uptake induced by HIV infection. In contrast, blockers of Panx1 HCs, such as Probenecid (Prob, 500 μM), and 10panx1 (200 μM) did not alter the Etd uptake induced by the virus. The scrambled peptide for gap26 and 10panx1 (scrb, 200 μM) did not affect Etd uptake induced by the virus. Each value corresponds to the mean ± SD, (* represents significance of p<0.005 as compared to control conditions (cont), # represent significant difference as compared to HIV infection alone, n=4).
Chemokine receptors involved in HIV entry do not induce hemichannel opening in cultured human astrocytes
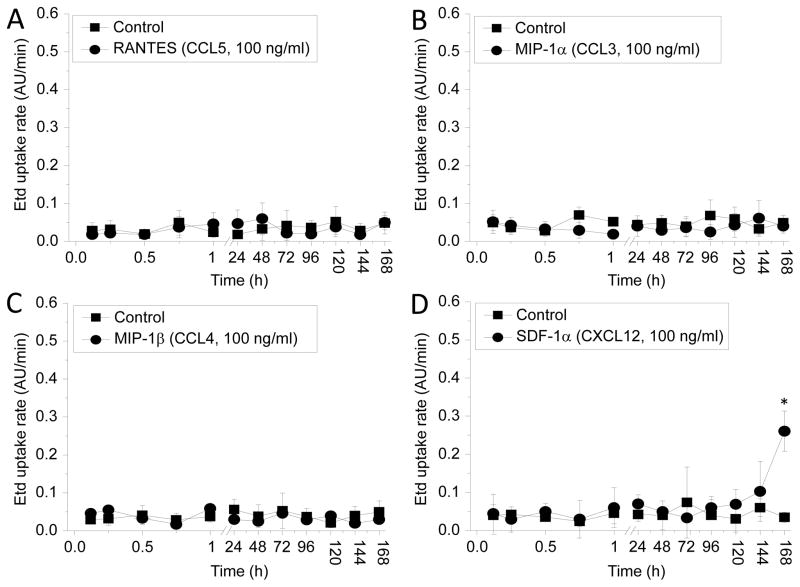
Recent findings of our laboratory indicate that different HIV strains and chemokines that bind receptors associated with HIV entry (CCR5 or CXCR4) increase the opening of Panx1 HCs in human CD4+ T lymphocytes (Orellana et al. 2013). Thus, to examine whether chemokines that bind CCR5 or CXCR4 participate in the opening of Cx43 HCs in human astrocytes, a time course of Etd uptake, as described in figure 1B, was performed. RANTES/CCL5 (100 ng/ml), MIP-1α/CCL3 (100 ng/ml) and MIP-1β/CCL4 (100 ng/ml), all chemokine ligands for CCR5, failed into increase rates of Etd uptake (Fig. 3A–C). Increasing concentrations (to 300 or 500 ng/ml, n = 5) also did not affect rates of Etd uptake (not shown). Moreover short periods of stimulation with SDF-1/CXCL12 (100 ng/ml), a physiological ligand for CXCR4, did not affect the rates of Etd uptake, except for 7 days (168 h) post treatment. However, after 7 days no further increase in Etd uptake rate was detected (Fig. 3D). No chemokine treatment reproduced the HIV induced time course of Etd uptake (compare to Fig. 1), ruling out mediation by CCR5 or CXCR4 and differentiating the mechanism of Panx-1 HC opening in CD4+ T lymphocytes in response to HIV infection (Orellana et al. 2013).
Figure 3. Chemokines involved in HIV infection do not affect hemichannel opening on human primary astrocytes.
Etd uptake measurements were perform in human primary cultures of astrocytes under control conditions (lines with squares) or after several times of incubation with the following chemokines (lines with circles): RANTES (CCL5, A), MIP-1α (CCL3, B), MIP-1β (CCL4, C) and SDF-1α (CXCL12, D). No differences were obtained using higher concentrations (300 and 500 ng/ml) of these chemokines (*p<0.003, n=4), except by SDF-1α at 7 days post treatment only.
To further identify the mechanism of Cx43 HC opening, we characterized whether infection with (VSV)-HIVBAL, a pseudotyped HIV with a vesicular stomatitis viral envelope that does not require viral membrane interaction to enter the cells (Superti et al. 1987), results in HC opening. Infection with this pseudotyped virus did not increase the Etd uptake rate as compared to HIV treatment, suggesting that virus-plasma membrane interaction is required for the HIV-induced Cx43 HC activity (data not shown). Previous reports indicated that the mannose receptor is involved in HIV entry into astrocytes (Liu et al. 2004). However, ligands for this receptor such as albumin, dextran or insulin did not increase Etd uptake in primary astrocytes (data not shown). Altogether these results indicate that the opening of Cx43 HCs does not depend on chemokine-chemokine receptor or mannose receptors interaction, but results from HIV interaction with the plasma membrane. Thus differs from the mechanism recently described for HIV induced opening of Panx1 HCs in CD4+ T lymphocytes (Orellana et al. 2013).
Opening of Cx43 hemichannels modulates DKK1 secretion induced by HIV infection of astrocytes
Previous studies indicated that HIV infection of astrocytes results in bystander apoptosis of neighboring uninfected astrocytes by a GJ dependent mechanism (Eugenin et al. 2012, Eugenin & Berman 2007b, Eugenin et al. 2011b), but none of our previous studies examined the role of HCs. Despite the low rate of infection and the low number of infected astrocytes, significant changes in the secretion of inflammatory factors (CCL2/MCP-1) and neurotransmitters (glutamate) occurred (Eugenin et al. 2012, Eugenin & Berman 2007b, Eugenin et al. 2011b). Blockade of Cx43 HCs did not alter the number of HIV infected astrocytes (7.3±2.1% versus 6.9±3.5% in the presence of La+3, Cx43E2 or gap 26). In addition, HIV viral replication and bystander apoptosis (HIV infected astrocytes affect neighboring uninfected cells) were not altered by the HC blockers (data not shown). Thus, to identify additional HIV factors altering neuronal and glial functions, cDNA microarray analysis was performed using HIV infected astrocytes. We used the astrocytoma cell line, U87, transfected with CD4 and CCR5 (80–90% of the cells become infected, instead of primary cells when only 5–8 % of the cells become infected) to perform the microarray. Three independent cDNA microarrays consistently indicated that DKK1 mRNA was the main upregulated gene (7.2±2.8 folds, p=6.1×10−5, n=3).
DKK1 is a secreted protein member of the dickkopf family and is involved in embryonic development through the inhibition of the Wnt signaling pathway (Zorn 2001). DKK-1 interacts with LRP5/6 and the co-receptor Kremen 1/2 (Krm, green). This triggers LRP5/6 endocytosis, thereby preventing formation of the LRP5/6–Wnt–Frizzled complex and reducing cellular differentiation induced by the Wnt signaling pathway (Kawano & Kypta 2003). Dysregulation of DKK1 secretion has been associated with cancer and Alzheimer’s disease (Rosi et al. 2010, Purro et al. 2012). Coordination of the Wnt pathway is mediated by GJ channels (Ai et al. 2000, Du et al. 2008, van der Heyden et al. 1998, Xu et al. 2001). However, the role of HC or GJ channels in HIV infection and Wnt signaling is unknown. Recent studies in HIV infection have associated DKK1 expression and β-catenin pathways with changes in viral replication (Li et al. 2011). However, the mechanisms by which DKK1 is dysregulated during HIV infection in glial cells remain to be elucidated. For all studies shown HIVADA was used, however, similar data was obtained for HIVJR-CSF and HIVBal (data not shown). Interestingly, during the time period analyzed (0 to 7 days post HIV stimulation), cultures of human astrocytes not treated with virus release low and stable levels of DKK1 into the medium as determined by ELISA (Fig. 4A). Blockade of Cx43 HC by using Cx43E2 and Gap 26, or inhibition of Panx1 HCs with 10panx1 did not alter secretion of DKK1 in these conditions (Fig. 4A). Thus, in the absence of virus Cx43 HCs do not play a role in secretion of DKK1.
Figure 4. The opening of Cx43 hemichannels enhances secretion of DKK1 in response to HIV infection of astrocytes.
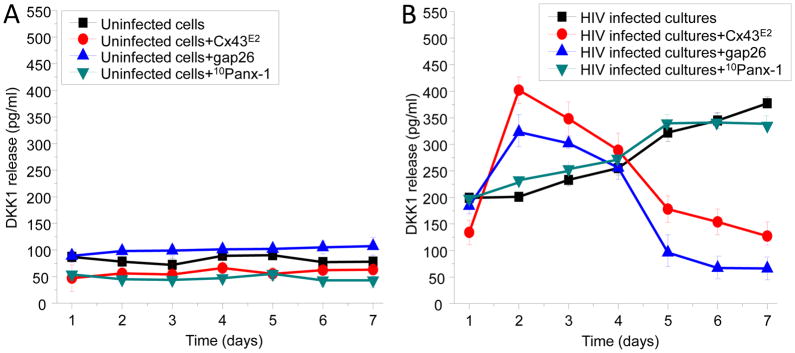
(A) In control conditions, minimal secretion of DKK1 was detected during the time course analyzed (1 to 7 days). Blockers of Cx43 HCs did not alter the secretion of DKK1 during the time course examined in uninfected cells. The blockers used were Cx43E2 (1:500), gap 26 (200 μM), and 10panx-1 (200 μM). All highly effective blockers of these channels as described in figure 2. (B) HIV infection of human primary astrocytes increased DKK1 release in a time-dependent manner (black line with squares) as compared to uninfected conditions (see A, black line with squares). Blockade of Cx43 HCs with Cx43E2 (red line with circles) or gap26 (blue line with upright triangles) after HIV infection results in increased secretion of DKK1 as compared to HIV infection alone during the first three days post-infection. After 5 days post-infection blockade of Cx43 HCs reduces secretion of DKK1, whereas blockade of Panx1 HCs with 10Panx-1 peptide did not block secretion of DKK1 in response to HIV infection (purple line with triangles) (n=6, all numbers are significant as compared to uninfected cells, except days 5–7 of HIV infected cultures+gap26).
In contrast to untreated cultures, extracellular levels of DKK1 increased in correlation with the time of post HIV infection (Fig. 4B). Notably, the Cx43 HC blockers, Cx43E2 or Gap26, increased the HIV-induced DKK1 secretion at 1–4 days post HIV infection, but later on (5–7 days post HIV) DKK1 release returned to the level at or below that observed after 1 day (Fig. 4B). 10panx-1 did not alter secretion of DKK1 during the time period analyzed (Fig. 4B), indicating that Panx1 HCs do not participate in the HIV-induced DKK1 secretion in human astrocytes. Our data indicate that opening of Cx43 HCs in response to HIV infection (Fig. 1B, 2 – 4 days) maintain a low level of DKK1 secretion, because blockade of these channels enhanced DKK1 secretion. After 4 days, Cx43 HC opening participates in the increased secretion of DKK1 (5–7 days), because blockade of these channels reduced DKK1 secretion (Fig. 1B, 5–7 days). In all these experiments no viral replication was detected, thus, changes in secretion of DKK1 were not associated with enhanced viral replication as determined by HIV-p24 ELISA (data not shown).
DKK1 is upregulated in human brain from subjects with HIV encephalitis
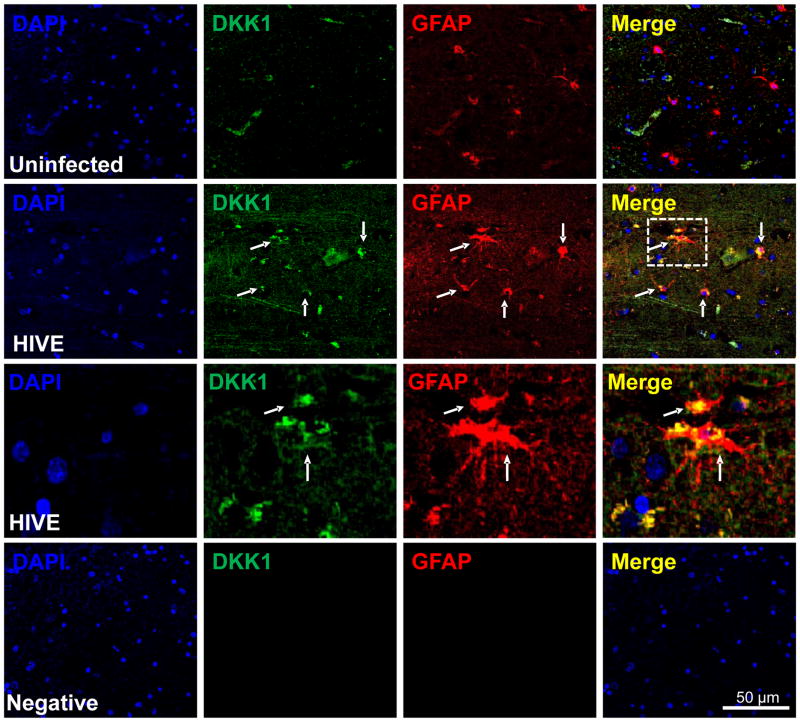
To examine where DKK1 is normally expressed in the CNS and to identify the cell type involved, uninfected and HIV encephalitic brain sections were analyzed by immunofluorescence and subsequent confocal microscopy. Sections obtained from adult uninfected individuals showed minimal DKK1 reactivity and did not colocalize with GFAP positive cells, suggesting that under control conditions, astrocytes do not express DKK1 (Fig. 5, uninfected). Confocal analysis of brain sections obtained from patients with HIV encephalitis showed increased DKK1 staining compared to normal controls in reactive astrocytes as well as in other cells (Fig. 5, HIVE). The inset in the HIVE merge is shown in the subsequent panel, demonstrating that astrocytes express a significant amount of DKK1 (Fig. 5, HIVE, third row). Negative controls using IgGs and control sera did not show any staining (Fig. 5, Negative). In addition, DKK1 expression was also associated with other cell types, in addition to astrocytes, such as endothelial cells (data not shown). Thus, DKK1 expression is upregulated in astrocytes and other brain cells in HIVE, supporting the idea that HIV infection increases the secretion of DKK1 in the CNS during HIV invasion of the brain.
Figure 5. DKK1 expression is increased in human brain tissue sections obtained from individuals with HIV encephalitis.
Brain sections from 5 uninfected individuals and from 5 with HIV encephalitis (HIVE) were evaluated by immunohistochemistry and confocal microscopy. Astrocyte expression of DKK1 (FITC-green) was evaluated using astroglial fibrillary acid protein (GFAP, an astrocyte marker, red staining). In uninfected tissue sections, minimal detection of DKK1 was observed and minimal co-localization with GFAP positive cells was detected (uninfected row). In contrast, in HIVE tissue sections, DKK1 staining was increased and co-localization with GFAP positive cells was increased (HIVE row). The inset in the HIVE row, Merge, was magnified to demonstrate colocalization of DKK1 with GFAP positive cells (HIVE third row). Negative control for DKK1 or GFAP antibodies did not show staining (negative row). DAPI staining was used in counter staining. Arrows represent colocalization of GFAP and DKK1. Thus, DKK1 was consistently elevated in all encephalitic tissue examined (n=5 cases by condition).
DKK1 participates in HIV-induced neuronal damage by a mechanism depending on astroglial Cx43 hemichannels
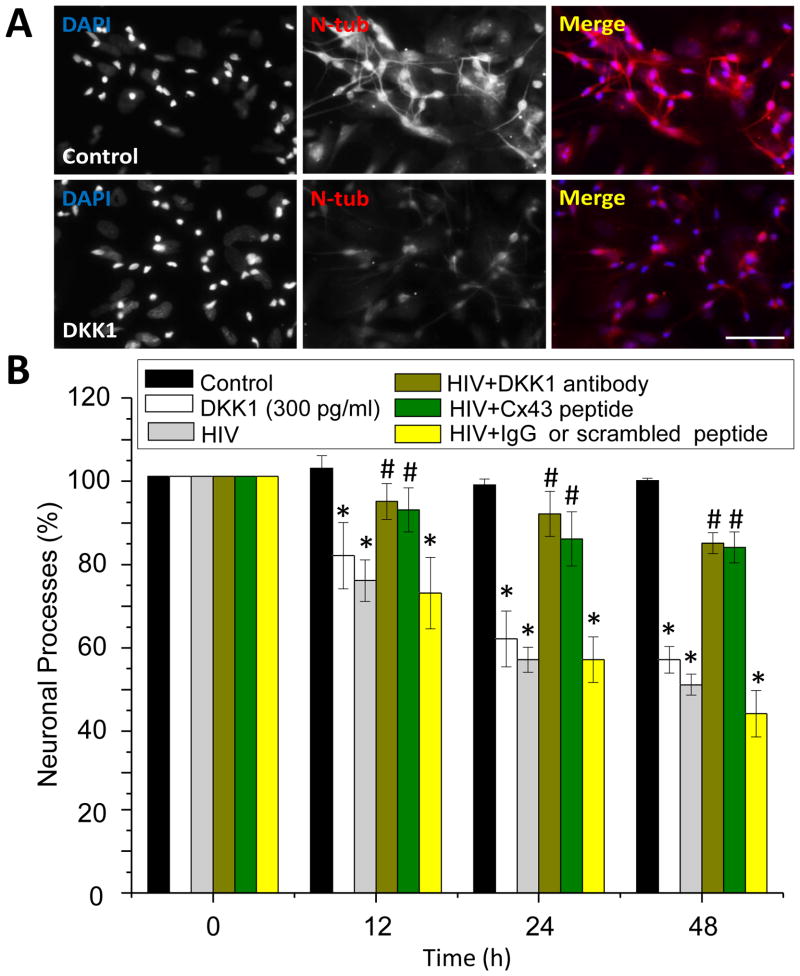
To fully demonstrate the direct participation of DKK1 in neuronal processes collapse, we examine whether concentrations of DKK1 mimicking the secretion curve induced by HIV (Fig. 4B) could recapitulate the HIV-induced collapse of neuronal processes. DKK1 treatment (300 or 500 pg/ml) of uninfected mixed cultures of neurons and astrocytes did not alter neuronal apoptosis or HIV infectivity after 0.5, 1, 2, 7 and 14 days as assayed by TUNEL, ELISA, or immunofluorescence for p24 (not shown). However, DKK1 (300 or 500 pg/ml) treatment for 12 and 48 h resulted in significant collapse of neuronal processes in uninfected HIV mixed cultures of neurons and astrocytes (Fig. 6, white bars). A similar morphology of collapsed neuronal processes has been observed in the brain of HIV infected individuals, but the mechanism of synaptic compromise is unknown (Adamson et al. 1996, Epstein & Gelbard 1999, Kim et al. 2008, Kolson 2002). In addition, DKK1 treatment of mixed cultures of neurons and astrocytes did not open Cx43 HCs (data not shown), suggesting that the opening of HC induced by the virus regulates expression and subsequent secretion of DKK1.
Figure 6. DKK1 is critical for the HIV-induced collapse of neuronal.
(A) Examples of neuronal processes in control conditions (control) and after 24 h of DKK1 (300 ng/ml) treatment as analyzed by confocal microscope and imaging software. Neuronal cultures were stained for neuronal specific beta III tubulin (N-tub, red staining) and DAPI to identify nuclei (DAPI). Note that that the treatment of neuronal cultures with DKK1 (300 ng/ml, the same concentration released in response to HIV infection) reduced the length and numbers of neuronal processes. Bar: 300 μm. (B) Quantification of the length of the neuronal processes in control and DKK1 treated conditions using an imaging program. DKK1 decreased significantly in 40% the number neuronal processes (white bars) as compared to control conditions (black bars), without altering the total number of neurons during the time course analyzed (data not shown). Similar results were found using 500 pg/ml of DKK1 (data not shown). HIV infection of these mixed cultures of neurons and astrocytes resulted in similar decay in neuronal processes as compared to DKK1 treatment (gray bars). Preincubation of cultures with a neutralizing antibody to DKK1 (DKK1 antibody) or gap26 (Cx43 HC mimetic peptide, Cx43 peptide), abolished the damage induced by HIV infection, indicating that DKK1 and opening of Cx43 HCs is essential to trigger neuronal damage. The use of IgG control or scrambled peptide did not alter the time course of neuronal damage observed by HIV infection. *p<0.007 as compared to control untreated/uninfected conditions, #p<0.005 as compared to HIV infection alone, n=5.
To further determine the effect of DKK1 on neuronal function in the context of HIV infection, we treated HIV-infected mixed primary cultures of neurons and astrocytes with a neutralizing antibody for DKK1 or blocking opening of Cx43 HC and then the length of neuronal processes was determined. In these cultures, HIV induced in a time dependent manner an increased collapse of neuronal processes, as determined by staining and quantification of the length of neuronal processes using the imaging program NIS Elements Advance Research (Fig. 6, grey bars). Pre-treatment with the neutralizing antibody for DKK1 completely prevented this response (Fig. 6, brown bars), suggesting that DKK1 is critical for the HIV-induced neuronal processes collapse. Because here we show that DKK1 is released from HIV infected astrocytes by a mechanism involving Cx43 HCs and given that neurons do not express Cx43 HCs, we investigate whether the blockade of these channels could affect the HIV-induced neuronal processes collapse. As expected, blockade of astroglial Cx43 HCs with the mimetic peptide gap26 (Fig. 6, green bars), reduced the neuronal damage associated with HIV infection, indicating that DKK1 as well as opening of Cx43 HCs are essential for the neuronal processes collapse. Importantly, pre-treatment with non-immune IgG or scrambled peptide was not protective against HIV-induced neuronal processes collapse (Fig. 6, yellow bars).
Discussion
Here, we demonstrate that HIV stimulation of human primary astrocytes results in increased secretion of DKK1, in vitro and in vivo. Moreover, our results show that opening of astroglial Cx43 HCs participates in the upregulation and mechanism of secretion of DKK1 in response to HIV infection. This altered secretion of DKK1 for HIV infection of astrocytes may contribute to the neuronal compromise often observed in HIV infected subjects.
Astrocytes accomplish critical functions within the CNS, but their role in several CNS diseases, especially in HIV infection, has been largely ignored. The fraction of astrocytes that are infected by HIV in vivo and in vitro is low, and viral production is low to undetectable. Previous studies performed in our laboratory indicate that HIV infects only 5–8% of primary human astrocytes, and viral replication is minimal to undetectable (Eugenin et al. 2012, Eugenin & Berman 2007b, Eugenin et al. 2011b). Despite the low rates of infection and viral production, HIV toxicity appears to spread to neighboring uninfected cells by a mechanism mediated by GJs (Eugenin et al. 2012, Eugenin & Berman 2007b, Eugenin et al. 2011b). Thus, the few infected astrocytes generate unknown intracellular toxic mediators that diffuse through GJs into uninfected cells (astrocytes and endothelial cells) resulting in apoptosis (Eugenin & Berman 2007b, Eugenin et al. 2011b). However, our early work did not characterize the contribution of HCs to HIV CNS damage. HC opening results in the release of several metabolites into the extracellular space including ATP, glutamate, nicotinamide adenine dinucleotide (NADH) and prostaglandins (Orellana et al. 2012), which contribute to cellular and immune activation (Eugenin et al. 2012, Orellana et al. 2012). In the context of HIV infection, we and others have described that extracellular ATP as well as particular ATP receptors, P2X1, P2X7, P2Y1 and P2Y2, are required for efficient infection and viral replication in immune cells (Hazleton et al. 2012, Seror et al. 2011). However, it was unknown whether HIV modulates the HC opening in astrocytes, and whether HCs have a role in the pathogenesis of NeuroAIDS disorders. In the current work, we demonstrated that HIV exposure results in the opening of Cx43 HCs which participate in the mechanism of DKK1 expression and secretion. Our results indicate that DKK1 secretion is modulated by the opening of Cx43 but not Panx1 HCs. Indeed, blockade of Cx43 HCs revealed that they can play a dual role in DKK1 secretion.
DKK1 is a soluble factor that inhibits the Wnt pathway by binding to LRP5/6 and kremen and causing internalization of the Wnt receptor, LRP5/6 (Kawano & Kypta 2003). DKK1 dysregulation contributes to neuronal death in several CNS diseases, such as stress and Alzheimer’s disease (Matrisciano et al. 2011, Zorn 2001, Purro et al. 2012, Rosi et al. 2010), but no data is available on infectious diseases and DKK1 expression in astrocytes. The Wnt pathway participates in HIV replication in astrocytes (Al-Harthi 2012, Li et al. 2011), and here we showed that application of DKK1 to primary cultures of human neurons and astrocytes did not alter apoptosis, viral infectivity or replication. Importantly, confocal analysis of human brain sections indicates that DKK1 is upregulated in vivo in NeuroAIDS as compared to uninfected subjects. These results are in agreement with our in vitro data, suggesting that HIV infection of the CNS results in expression and upregulation of DKK1, especially in astrocytes and potentially other GFAP negative cells. Most of the astrocytes expressing DKK1 were hyper reactive, a common feature of glial cells in HIV infected individuals (Petito et al. 2001, Vanzani et al. 2006, Anderson et al. 2003). Experiments in rodents indicate that neurons in distress secrete DKK1 (Busceti et al. 2007, Mastroiacovo et al. 2009, Matrisciano et al. 2011, Munji et al. 2011, Purro et al. 2012, Rosi et al. 2010). In these reports it is clear that DKK1 expression is mostly in neurons. However, our data in vitro and in vivo indicate that human astrocytes also express and secrete high amounts of DKK1 resulting in neuronal synaptic compromise. Here, we show that DKK1 expression and secretion in response to HIV resulted in significant decrease in number and length of neuronal processes. Neuronal processes are important players in the formation and stability of synapses (Chen et al. 2012, Knobel et al. 1999, Moreno-Lopez & Gonzalez-Forero 2006). Decrease in neuronal processes results in synaptic impairment and subsequently cognitive disease. Importantly, mixed cultures of neurons and astrocytes treated with agonists of NMDA receptors induce a fast secretion of DKK1 in association with a decrease in nuclear β-catenin, which is indicative of inhibition of the Wnt pathway (Cappuccio et al. 2005). Previous studies indicate that HIV infection of astrocytes dysregulates glutamate metabolism (Eugenin & Berman 2007b), and most of the HIV toxicity observed in neurons is mediated by activation of NMDA receptors (Eugenin et al. 2003, Eugenin et al. 2007, King et al. 2006). Thus, alterations in glutamate metabolism and DKK1 secretion may be involved in neuronal/synaptic compromise in response to HIV infection.
Recently we demonstrated that HIV infection results in opening of Panx1 HCs in CD4+ T lymphocytes, depending on the viral isolate used and the associated-chemokine receptors involved in HIV entry (Orellana et al. 2013). It is widely known that binding of the HIV envelope protein gp120 to the host CD4, CCR5 and/or CXCR4 receptors in CD4+ T lymphocytes depends on the viral isolate used (Choe et al. 1996, Dragic et al. 1996, Wu et al. 1996, Wu et al. 1997). However, HIV infection of astrocytes is CD4-independent (Liu et al. 2004, Schweighardt et al. 2001). Although only 5–8% of astrocytes become infected, all astrocytes exhibit increased Cx43 HC activity in response to HIV infection. Infection of astrocytes with VSV-HIV which does not require virus receptor interaction (CD4 and CCR5 and/or CXCR4, (Superti et al. 1987)) does not change HC activity in human astrocytes, again suggesting that neither chemokine nor CD4 receptors are required for the opening of these channels. In agreement, chemokine stimulation of astrocytes did not cause opening of Cx43 HCs. Another receptor in astrocytes involved in HIV entry is the mannose receptor (CD206, MRs) (Liu et al. 2004), however, ligands for this receptor did not open Cx43 HCs and in addition only 50% of the cells express this receptor, but 100 % of the cells respond to HIV exposure. Thus, viral replication or infection is not required for HC opening, but interaction of the envelope with the membrane is essential. Importantly, the mechanism of HIV-induced HC opening in human astrocytes is different from that in human CD4+ T lymphocytes. Thus, further experiments will be required to identify the mechanism(s) underlying HIV-induced opening of Cx43 HCs.
Our studies demonstrated that HIV infection of astrocytes induces opening of Cx43 HCs, and we propose that the relatively non-specific increase in permeability participates in the CNS dysfunction observed in HIV infected individuals. These data provide a novel mechanism of damage in NeuroAIDS and indicate potential new targets for therapeutic interventions, such as DKK1 and GSK-3β inhibitors, to reduce the ongoing CNS effects of HIV.
Acknowledgments
This work was supported by the National Institutes of Mental Health grants MH096625 to E.A.E. Chilean funding was supported by the following grants: MECESUP-PUC0708 (to J.A.O), FONDECYT 11121133 (to J.A.O), Anillo ATC71 (to J.C.S) and Instituto Milenio de Neurociencias (to J.C.S). Human tissues were supplied by the Manhattan HIV Brain Bank (U01MH083501), member of the National NeuroAIDS Tissue Consortium.
Footnotes
Conflict of interest: None
References
- Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Molecular medicine. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harthi L. Interplay Between Wnt/beta-Catenin Signaling and HIV: Virologic and Biologic Consequences in the CNS. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012 doi: 10.1007/s11481-012-9411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Groves M, Giometto B, Beckett AA, Scaravilli F. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta neuropathologica. 1999;98:481–487. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, Simmonds P, Bell JE. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol. 2003;29:378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Busceti CL, Biagioni F, Aronica E, et al. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Cappuccio I, Calderone A, Busceti CL, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Conant K, Tornatore C, Atwood W, Meyers K, Traub R, Major EO. In vivo and in vitro infection of the astrocyte by HIV-1. Advances in neuroimmunology. 1994;4:287–289. doi: 10.1016/s0960-5428(06)80269-x. [DOI] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain pathology. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium chloride regulates connexin43 in skeletal myoblasts in vitro: possible involvement in Wnt/beta-catenin signaling. Cell Commun Adhes. 2008;15:261–271. doi: 10.1080/15419060802198587. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Gelbard HA. HIV-1-induced neuronal injury in the developing brain. J Leukoc Biol. 1999;65:453–457. doi: 10.1002/jlb.65.4.453. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Basilio D, Saez JC, Orellana JA, Raine CS, Bukauskas F, Bennett MV, Berman JW. The Role of Gap Junction Channels During Physiologic and Pathologic Conditions of the Human Central Nervous System. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007a;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007b;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011a;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011b;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C. Astroglial Wiring is Adding Complexity to Neuroglial Networking. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazleton JE, Berman JW, Eugenin EA. Novel mechanisms of central nervous system damage in HIV infection. HIV/AIDS. 2010;2:39–49. doi: 10.2147/hiv.s9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazleton JE, Berman JW, Eugenin EA. Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol. 2012;188:4488–4495. doi: 10.4049/jimmunol.1102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Knobel KM, Jorgensen EM, Bastiani MJ. Growth cones stall and collapse during axon outgrowth in Caenorhabditis elegans. Development. 1999;126:4489–4498. doi: 10.1242/dev.126.20.4489. [DOI] [PubMed] [Google Scholar]
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med. 2002;22:703–717. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. Journal of immunology. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroiacovo F, Busceti CL, Biagioni F, Moyanova SG, Meisler MH, Battaglia G, Caricasole A, Bruno V, Nicoletti F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Busceti CL, Bucci D, et al. Induction of the Wnt antagonist Dickkopf-1 is involved in stress-induced hippocampal damage. PloS one. 2011;6:e16447. doi: 10.1371/journal.pone.0016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez B, Gonzalez-Forero D. Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects. Rev Neurosci. 2006;17:309–357. doi: 10.1515/revneuro.2006.17.3.309. [DOI] [PubMed] [Google Scholar]
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:1676–1687. doi: 10.1523/JNEUROSCI.5404-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohagen A, Ghosh S, He J, et al. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. Journal of virology. 1999;73:897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Diaz E, Schalper KA, Vargas AA, Bennett MV, Saez JC. Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun. 2011;409:603–609. doi: 10.1016/j.bbrc.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Hernandez DE, Ezan P, Velarde V, Bennett MV, Giaume C, Saez JC. Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia. 2010;58:329–343. doi: 10.1002/glia.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Velasquez S, Williams DW, Saez JC, Berman JW, Eugenin EA. Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.0512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, von Bernhardi R, Giaume C, Saez JC. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev Neurosci. 2012;23:163–177. doi: 10.1515/revneuro-2011-0065. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001;60:377–385. doi: 10.1093/jnen/60.4.377. [DOI] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi MC, Luccarini I, Grossi C, et al. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. Journal of neurochemistry. 2010;112:1539–1551. doi: 10.1111/j.1471-4159.2009.06566.x. [DOI] [PubMed] [Google Scholar]
- Saez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem. 2012;287:11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighardt B, Shieh JT, Atwood WJ. CD4/CXCR4-independent infection of human astrocytes by a T-tropic strain of HIV-1. J Neurovirol. 2001;7:155–162. doi: 10.1080/13550280152058816. [DOI] [PubMed] [Google Scholar]
- Seror C, Melki MT, Subra F, et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208:1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti F, Seganti L, Ruggeri FM, Tinari A, Donelli G, Orsi N. Entry pathway of vesicular stomatitis virus into different host cells. J Gen Virol. 1987;68(Pt 2):387–399. doi: 10.1099/0022-1317-68-2-387. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111(Pt 12):1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- Vanzani MC, Iacono RF, Caccuri RL, Troncoso AR, Berria MI. Regional differences in astrocyte activation in HIV-associated dementia. Medicina (B Aires) 2006;66:108–112. [PubMed] [Google Scholar]
- Wu L, Gerard NP, Wyatt R, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. N-cadherin and Cx43alpha1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adhes. 2001;8:321–324. doi: 10.3109/15419060109080746. [DOI] [PubMed] [Google Scholar]
- Zorn AM. Wnt signalling: antagonistic Dickkopfs. Curr Biol. 2001;11:R592–595. doi: 10.1016/s0960-9822(01)00360-8. [DOI] [PubMed] [Google Scholar]