Abstract
Purpose
To determine whether motor outcomes of an exercise intervention beginning at 2 months corrected age (CA) in children with periventricular brain injury (PBI) are correlated with fractional anisotropy (FA) measures derived from diffusion tensor imaging (DTI) at 12 months CA.
Materials and Methods
DTI was performed in eight infants with PBI who were randomly assigned to kicking and treadmill stepping exercise or a no-training condition. Development was assessed using the Alberta Infant Motor Scale (AIMS) and the Gross Motor Function Classification System (GMFCS). FA values were derived from regions of interest (ROI) in the middle third of the posterior limb of the internal capsule (PLIC) and the posterior thalamic radiation (PTR).
Results
Significant correlations were observed between motor development and FA measures. For PLIC, the correlation coefficients were 0.82 between FA and AIMS, and -0.92 between FA and GMFCS, while for PTR the corresponding correlation coefficients were 0.73 and -0.80, respectively.
Conclusion
Results of this study suggest that quantitative evaluation of white matter tracts using DTI at 12 months CA may be useful for assessment of brain plasticity in children.
Keywords: DTI, fractional anisotropy (FA), periventricular brain injury, treadmill exercise, infants
Introduction
Infants born preterm and with a very low birthweight demonstrate, on average, significant motor impairment persisting throughout childhood (1). The presence of additional perinatal complications, such as periventricular brain injury (PBI) or chronic lung disease, increases the risk for disability. Periventricular brain injury includes conditions such as intraventricular hemorrhage (IVH), periventricular hemorrhagic infarction, periventricular leukomalacia (PVL), and diffuse or focal noncystic injury(1-4) in which both sensory and motor white matter structures may be affected (5). The rate of cerebral palsy (CP) ranges from 50-85% in children with severe white matter injury (WMI) compared with rates of 5-10% in all premature infants (4).
In addition to an increased risk of CP, children with PBI resulting in WMI have a high incidence of atypical motor development (6-10). Walking is delayed in children born preterm, occurring on average at 14 months corrected age (CA; defined here as the chronologic age minus the number of weeks born preterm)(11), and reduction of movement complexity and variability is a characteristic result of perinatal WMI (12). Barbosa and colleagues (13-15) reported both delayed motor development and regressions in leg movements in infants who were later diagnosed with CP. Antigravity leg movements in supine position and spontaneous kicking movements regressed over time (15). Based on these movement impairments in preterm infants with WMI and CP, an innovative exercise program was designed with a focus on leg movements and development of locomotor activity which was anticipated to improve motor developmental outcomes, lower the age at walking, and facilitate development of white matter pathways to enhance recovery of infants from PBI (16).
Despite the high risk for poor motor outcome of children with PBI, few studies have asked the question of whether intervention can alter outcomes. In fact, studies of early intervention for infants at high risk for motor dysfunction tend to show no or very limited treatment effects (17-19). Questions that need to be addressed include whether it is possible to reduce delay in motor development or the severity of CP in those who fail to recover from brain injury without significant impairment, and whether intervention effects include adaptations in brain development that can be captured with neuro-imaging.
Several studies have utilized diffusion tensor imaging (DTI) to measure the development of white matter in children with CP (20-25). Results have uniformly demonstrated delayed or deficient maturation of white matter pathways as assessed with fractional anisotropy (FA) from the posterior limb of the internal capsule (PLIC) through which the corticospinal tracts from the motor cortex descend to eventually control the activation of skeletal muscles. These tracts are concentrated in the middle third of the PLIC with descending fibers to the legs and trunk found more anteriorly in the PLIC and fibers from upper parts of the body found more posteriorly (25). Murakami and colleagues (22) reported that PLIC FA measures < 0.5 were correlated with a diagnosis of CP in infants with PVL, but other reports do not concur with this value as a threshold for predicting permanent neurologic involvement (26). Decreased FA in the posterior thalamic radiation (PTR) also suggests impaired sensory pathway development in children with CP (26). Numerous studies have suggested that knowledge of white matter integrity and connectivity might be useful in determining the need for intervention in children who sustained early brain injury (21-23).
Given the widespread belief in plasticity of the infant brain, we are interested in whether measures of white matter structural integrity, such as FA in the PLIC and PTR, can be used as imaging markers to demonstrate improved recovery from perinatal brain injury as a result of exercise interventions. The purpose of this communication is to present results of a DTI study to assess white matter development at 12 months CA in a subgroup of 8 infants from a study of an innovative exercise intervention beginning at 2 months CA.
Materials and Methods
Subjects
The protocol for this study was approved by the Institutional Review Boards at each participating institution and parental assent was obtained for enrollment of subjects in the study. Subjects with PBI were recruited from three NICUs. Inclusion criteria included the diagnosis of Grade III or IV IVH or PVL as visualized on ultrasound scan during the perinatal period. Subjects were randomly assigned to experimental groups (exercise or control). The parental assent document at one recruitment site included permission to perform brain MRI at 12 months CA.
Intervention
Details of the intervention can be found in reference (16). In brief, children assigned to the exercise group received monthly visits from an exercise physical therapist who provided them with a series of 4 toys to facilitate kicking. At 4 months CA stepping practice with the parent suspending the child over a portable treadmill1 was added. Children in both groups were allowed to also participate in any intervention prescribed by their personal caregivers, but only half of the subjects had physical therapy outside of the study protocol. Only 4 children (1 in the exercise group, 3 in the control group) had early (i.e., before 4 months CA) additional physical therapy.
Assessment
Infants in both groups were visited 5 times during the course of the study for testing of motor development by one of three testing physical therapists blinded to experimental group assignment. Motor development was tested at 2 (study entry), 4, 6, 10, and 12 months CA with the Alberta Infant Motor Scale (AIMS) (27).
At 12 months CA all children were assessed by a pediatric rehabilitation medicine physician blinded to group assignment and to AIMS performance until after the examination was completed. After administering a standard protocol to assess reflexes, postural tone, and movement quality, the physician judged whether the child did or did not have CP, and was then given information on the child's AIMS performance. At the same time (12 months CA), the physician also assigned a functional level to those with CP based on the Gross Motor Function Classification System (GMFCS) categories typically used for children before the 2nd birthday (28) [www.canchild.ca]. Levels on the GMFCS are based on mobility skills of the child and vary from level I (infants can pull to stand and take steps holding on to furniture and are expected walk independently between 18 months and 2 years of age) to Level V (unable to maintain antigravity head and trunk postures in prone and sitting and require adult assistance to roll over).
Magnetic Resonance Imaging (MRI)
At 12 months CA, infants underwent brain MRI on a General Electric 3T Signa HDx scanner (General Electric Health Care, Waukesha, WI) using an 8-channel adult knee coil as a head coil for the infants. All infants were scanned under sedation to reduce motion artifacts. In order to ensure the safety of the infants a pediatric anesthesiologist evaluated them prior to the sedation, administered the sedative and monitored the child during the exam and recovery period.
Our sedative of choice was chloral hydrate administered orally because of its proven safety record, ease of administration and minimal interference with MRI signal characteristics. The infants were NPO (nil per os/nothing per mouth) on the day of the exam and initially received 50 mg/kg of chloral hydrate administered orally. If after 30 minutes an adequate level of sedation was not achieved an additional dose of 25 mg/kg of chloral hydrate was administered. All infants achieved adequate levels of sedation. The child was brought to the MRI suite and American Society of Anesthesiologists-approved standard monitors were applied (EKG, non-invasive blood pressure, pulse oximeter). Additionally we inserted ear plugs to protect the child from the acoustic noise of the MRI scan. The pediatric anesthesiologist remained in the MRI suite throughout the procedure and monitored the vital signs continuously. Total sedation time was approximately 1.5 hours. After the conclusion of the exam we watched the child until full recovery was assured prior to discharge home. A follow up phone call was made in the afternoon to inquire about the well being of the child. All children were successfully sedated for the exam and there were no adverse events.
The MR imaging protocol consisted of a 3-plane localizer, a calibration scan for parallel imaging, high-resolution 3D T1-weighted imaging using a 3D Inversion-recovery-prepared (IR-Prep) FSPGR (fast spoiled gradient echo) pulse sequence with parallel imaging, and a DTI acquisition using a single-shot echo planar imaging (EPI) sequence. For the DTI scan, the following data acquisition parameters were used: TR = 5325 ms, TE = 75.5 ms, b values = 0, 750 s/mm2, diffusion gradient directions = 27, FOV = 18 cm x 18cm, k-space matrix = 136 x 136; image matrix = 256x256, NEX = 2, slice thickness = 5 mm, slice gap = 0 mm, slice number = 20-22 (depending on the size of the child's head with full brain coverage), and scan time = 5 min and 9 sec. To reduce the magnetic susceptibility-induced image distortion while maintaining a relatively high spatial resolution, parallel imaging (ASSET) was used with an acceleration factor of 2.
The set of raw DTI images was processed to produce FA maps using customized software implemented on a FuncTool workstation (software version 4.2; General Electric Health Care, Waukesha, Wisconsin). A full brain FA map color-coded by the eigenvector directions (red: right-left; green: anterior-posterior; blue: superior-inferior direction) was obtained from the DTI dataset, followed by an ROI analysis on the middle third of the posterior limb of the internal capsule (PLIC) and the posterior thalamic radiation (PTR) bilaterally. The PLIC ROI was selected from the level of the anterior commissure to the base of the corona radiata for each subject with reference to color FA images and the MRI Atlas of Human White Matter (29). Our second ROI in the PTR was delineated from the retrolenticular part of the internal capsule to the thalamus, with reference to the fiber bundles lateral to the corpus callosum (26). The typical size of the ROI's was about ~50 pixels on each tract. Quantitative FA values were recorded from the selected ROIs for each subject in the study. Representative examples from two subjects showing location of ROIs are shown on the middle image (gray-scale FA image) in Figure 1.
Figure 1.
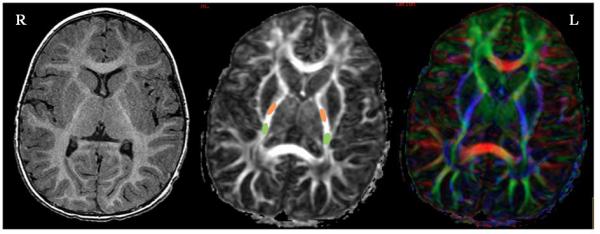
Representative images from two children showing varying degree of brain injuries. The top row was taken from a subject who had a high symmetry of FA and normal development, while the bottom row corresponded to another subject who had cerebral palsy and a greater asymmetry of FA. Three images are shown from each subject: axial T1-weighted FSPGR image (left), axial FA image in gray scale (middle) and color coded FA image (right), all matched to the same location at the level of the internal capsule. The ROIs used in the FA analysis are schematically indicated in the middle images with PLIC ROI denoted in orange and the PTR ROI in green. The color-coded FA images on the right show the fiber orientations (red: right-left; green: anterior-posterior; blue: superior-inferior direction), which were used to aid ROI selection.
Statistical Analysis
The raw scores from AIMS testing were transformed into Z scores based on means and SDs for performance of the AIMS normative sample of Canadian infants within the same age group (28). FA scores for the two sides of the brain were averaged for both the PLIC and PTR measures for correlation with both AIMS Z scores (interval level data) and GMFCS classification levels (ordinal level data). A GMFCS score of 0 was assigned to children without CP. An asymmetry index was calculated to reflect the comparison between FA results on the two sides of the brain in each child using a method derived from that of Stinear and colleagues (30) for measuring asymmetry in patients following stroke using the following formula: FA asymmetry = (FAless affected-FAmore affected)/ (FAless affected+FAmore affected). This yields a value between 0 and +1 in which a value near 0 indicates symmetry of white matter development and higher values demonstrate increasing levels of asymmetric structure.
Results
Subjects
Ten subjects with perinatal WMI were recruited from one of the NICUs and randomly assigned to the experimental groups (6 exercise, 4 control). No attrition occurred after any child began the exercise or testing program, but 2 of the 10 subjects did not complete brain MRI scans, one because she was deemed too medically fragile to be submitted to sedation for scanning after a recent hospitalization for failure to thrive, and the other because, despite several attempts to schedule the scan, the mother was unable to arrange transportation to the MRI center. As a result the brains of 5 exercise group and 3 control group subjects were imaged.
The 8 infants were diverse in terms of gender and ethnicity. There were 5 males and 3 females, all born prematurely. The range of gestational age at birth was from 23-32 weeks with a mean of 25.6 weeks (SD=2.88 weeks) for the exercise group and 29.67 weeks (SD=3.21) for control infants. Four of the 8 subjects had bronchopulmonary dysplasia (BPD) or chronic lung disease, a common problem in children born very prematurely that also increases the risk for disability. Five subjects were African-American, 2 white, and 1 Hispanic. Table I presents information comparing the equivalence of the experimental groups on conditions that might affect the outcomes of the study. There were no statistically significant differences between groups in gestational age at birth, type of brain injury, frequency of BPD, or motor development at study entry.
Table I.
Comparison of Exercise and Control Groups in Medical Conditions and Development on the AIMS at Study Entry
| Condition | Exercise Group (n=5) | Control Group (n=3) | Probability of between group differences |
|---|---|---|---|
| Mean GA at Birth (SD) | 25.6 weeks (2.88) | 29.67 weeks (3.21) | 0.112a |
| Frequency of IVH Grade III or IV | 5 | 1 | 0.1071b |
| Frequency of PVL | 0 | 2 | 0.1071b |
| Frequency of BPD | 4 | 0 | 0.1429b |
| Mean AIMS Z score at Study Entry (SD) | −0.142 (1.745) | −0.34 (0.906) | 0.6748 a |
Abbreviations: Standard deviation (SD), Gestational age (GA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD, chronic lung disease), Alberta Infant Motor Scale (AIMS)
t test
Fisher exact test
Disability Outcomes at 12 Months CA
A wide range of development and disability was demonstrated in the subjects (Table II); as anticipated our recruitment process resulted in 3 of the 8 subjects showing impaired motor performance at 12 months CA.
Table II.
Description of Subjects Arranged by Experimental Group Assignment and in Order of AIMS Outcome
| Subject Number & Group | 104E | 110E | 108E | 103E | 111E | 106C | 107C | 109C |
|---|---|---|---|---|---|---|---|---|
| IVH grade or PVL | III | III | III | III R; IV L | IV R; III L | PVL | PVL | IV |
| BPD | No | Yes | Yes | Yes | Yes | No | No | No |
| Birth weight (grams) | 1680 | 1120 | 690 | 637 | 530 | 2052 | 1820 | 680 |
| GA at birth | 30 | 27 | 23 | 24 | 24 | 32 | 31 | 26 |
| 2 mo AIMS Z | 2.40 | 0.08 | −1.98 | 0.36 | −1.57 | 0.87 | 0.87 | −0.70 |
| 12 mo AIMS Z | 0.80 | 0.53 | 0.24 | −3.42 | −5.39 | 0.24 | 0.10 | −3.70 |
| Highest motor skill at 12 mo CA | Walks alone | Walks alone | Walks with one hand held | Sits, stands with support | Does not roll or sit alone | Creeps, cruises along furniture | Cruises, takes steps with trunk support | Rolls, sits alone |
|---|---|---|---|---|---|---|---|---|
| GMFCS | --- | --- | --- | II; L hemiplegic | V | --- | --- | II |
| FA R PLIC | .599 | .630 | .604 | .500 | .370 | .611 | .607 | .490 |
| FA L PLIC | .628 | .620 | .679 | .680 | .530 | .568 | .622 | .664 |
| Asymmetry Index-PLIC | .024 | .008 | .058 | .153 | .178 | .036 | .012 | .151 |
| FA R PTR | .467 | .409 | .514 | .227 | .256 | .395 | .469 | .417 |
| FA L PTR | .499 | .379 | .526 | .396 | .261 | .445 | .569 | .488 |
| Asymmetry Index-PTR | .033 | .038 | .008 | .271 | .010 | .060 | .096 | .078 |
E, exercise group; C, control group; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; R, right; L, left; BPD, bronchopulmonary dysplasia; GA, gestational age in weeks; CA, corrected age; AIMS, Alberta Infant Motor Scale; Z, score in standard deviations units; GMFCS, Gross Motor Function Classification System; FA, fractional anisotropy; PLIC, posterior limb of the internal capsule (middle third); PTR, posterior thalamic radiation
The exercise group (n=5) had 3 children with typical development (AIMS scores at or above the 50th percentile and no abnormal neurologic findings) and 2 with CP, one at GMFCS level II (no other physical therapy until 11 months of age) and one at level V who began physical therapy at 2 months CA. The control group (n=3) had 2 children with typical development, one of whom began physical therapy at 3 months CA, and one with CP, GMFCS level II who had no physical therapy until after the study ended. All 3 children from the exercise group who scored at or above average on the AIMS were walking alone or with one hand held at 12 months CA while both children in the control group with typical development were only able to walk using furniture for support or with both hands held. Because there were no group differences in performance between exercise and control groups (reference), the groups are combined in the results reported here.
Rater Reliability
ROI-based FA of the middle one third of the PLIC and of the PTR was independently assessed by two of the investigators using unique identification numbers for the scans that did not reveal any information about subject characteristics. The rater reliability for measures of PLIC FA was an ICC of 0.83 and for the PTR was an ICC of 0.90, indicating good to excellent agreement between independent raters.
DTI findings at 12 Months CA
The corrected age at the time of scan ranged from 11 months and 15 days to 12 months and 10 days. MR imaging at 3.0 T was successfully done in 8 infants whose parents gave their assent for their child's participation and all images were of sufficiently high quality (free of eddy-current-induced distortion, minimal gross distortion, ghosting level of <1.7%, etc.) to allow extraction of FA measures. Two sets of representative images are shown in Figure 1, where the first column contains T1-weighted images selected from the 3D FSPGR dataset to match the gray-scale FA (second column) and the color-coded FA images (third column) produced from the DTI dataset. FA of the middle third of the PLIC ranged from a low of .370 (SD 0.06) on the right side of the brain in a child with CP, GMFCS level V, and an AIMS Z score of -5.39, to a high of .680 (SD 0.09) on the left side of the brain in a child with left hemiplegia, GMFCS level II, and an AIMS Z score of -3.42 (Table II). For children who were typically developing at 12 months CA, FA values for the middle third of the PLIC ranged from .568 (SD 0.088) to .679 (SD 0.0678). These children with normal development also had highly symmetrical development of PLIC white matter, ranging from .008 to .058. By contrast the children with CP had asymmetry indices of .151, .153, and .178.
FA of the PTR ranged from a low of .227 (SD 0.06) on the right side of the brain in a child with hemiplegic CP, GMFCS level II, and an AIMS Z score of -3.42 to a high of .569 (SD 0.05) on the left side of the brain in a child with typical development (AIMS Z score of 0.10, Table II). For children who were typically developing at 12 months CA, FA values for the PTR ranged from .379 (SD 0.08) to .569 (SD 0.05). These children with normal development also had highly symmetrical development of the PTR, ranging from .008 to .096. The children with CP had asymmetry indices of .010, .078, and .271. The greatest degree of asymmetry was found in the child with a left hemiplegia whose right PTR FA was .227 and left PTR FA was .396, corresponding to an asymmetry index of 0.271. The child with CP, GMFCS level V (severe impairment) showed small asymmetry (.010), but both of the FA values were exceptionally low (.256 and .261).
Relationship between FA Values and Motor Performance at 12 Months CA
The correlation between the average of the PLIC FAs on the two sides of the brain and AIMS z score at 12 months CA was 0.82 (p=.0127) and between FA and GMFCS level was -0.94 (p=.0005), indicating that better motor development and no CP or less severe CP was related to more mature white matter structure. For the PTR the correlations were lower but still statistically significant: 0.73 (p=0.0398) and -0.80 (p=0.0171), respectively. The scatterplots demonstrating these relationships are shown in Figures 2 and 3.
Figure 2.
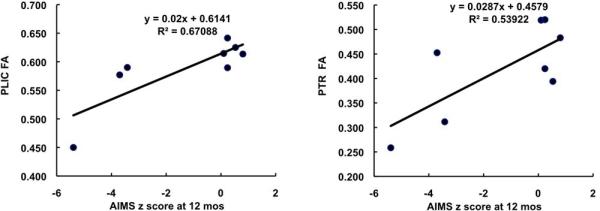
Correlation between AIMS score and average FA at the PLIC (left) and average FA at the PTR (right).
Figure 3.
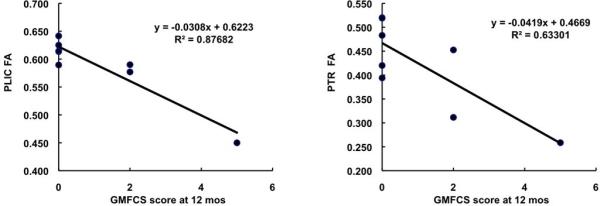
Correlation between GMFCS score and average FA at the PLIC (left) and average FA at the PTR (right).
Discussion
The present study was undertaken to understand the relationship between white matter tract integrity and sensorimotor activation deficits in these infants who participated in a kicking and stepping training program.
The study showed that reduced white matter integrity (i.e., greater white matter damage), as reflected by the decreased FA values, in the middle third of the PLIC where most descending white matter motor pathways converge was highly indicative of poor motor function and the diagnosis of CP at 12 months CA (left graphs in Figures 2 and 3). Reduced structural integrity in the PTR which hosts white matter fibers relating to sensory perception was more modestly related to motor dysfunction. To our knowledge only two research groups have utilized DTI as an outcome measure in intervention studies and both imaged the infant's brain after only a few weeks of intervention involving protection from excessive handling and stimulation in the neonatal intensive care unit (NICU). Als and colleagues employed neuroimaging as an outcome measure in an intervention study with prematurely born infants (31). At 2 weeks CA infants who were managed under the guidelines of the Newborn Individualized Developmental Care Program had higher relative anisotropy (RA) in the left internal capsule than a control group. Behavioral function at 9 months CA also favored the experimental group. Milgrom and colleagues studied the effects of training parents to reduce stress on their infants born at less than 30 weeks gestational age while in the NICU; DTI measures taken at term-equivalent age demonstrated significantly higher FA values in a variety of brain regions for infants in the intervention group (32). To our knowledge, no studies have yet followed subjects of exercise intervention to later ages using DTI as an imaging marker of outcome.
Because of the abundant descriptions of impaired leg movements in the population of infants with perinatal WMI and the paucity of research on the possible benefits of early physical therapy to improve outcomes, our study was designed as a randomized, multi-institutional pilot investigation of the effects of an home exercise intervention on motor function and brain development of infants with PBI. The study was conducted on a group of 16 infants recruited from three perinatal centers in a large metropolitan area and randomly assigned to a control group or to an innovative exercise intervention lasting 10 months and focused on development of leg movements. Results indicated that exercise group infants outperformed control group infants in motor development on the Alberta Infant Motor Scale (AIMS) after 2 and 4 months of exercise, but the reverse was true at 10 and 12 months CA; the differences between groups were not statistically significant at any age (16). At 12 months CA, however, more children in the exercise group walked alone or with one hand held (43%) than in the control group (11%). Results suggested a possible intervention effect on age at walking of self-produced kicking exercise and supported treadmill stepping designed to facilitate the development of locomotion, but it was concluded that improved compliance with the exercise protocol was needed to maximize the effects on overall motor development. The results reported here were from a subgroup of 8 children from the larger study who had imaging results.
Yoshida and colleagues (2010) reported correlations between GMFCS and corticospinal tract FAs of -0.616 in children with CP at an average age of 2 years and also reported that correlations with PTR FAs were lower at -0.366 (26), but the present study is the first, to our knowledge, to describe a quantitative relationship at 12 months CA between the extent of structural damage to the PLIC and PTR and motor and neurologic function in preterm-born children with PBI.
The range of PLIC FAs in children in our study who attained normal levels of motor development at 12 months CA was from .568 to .679; the values for the PTR ranged from .379 to .569. These values compare favorably with average FAs from 21 normal children of 0.63-0.65 in the PLIC and 0.49-0.52 in the PTR at a mean of 2 years of age documented by Yoshida and colleagues (2010) (26).
Recent studies using DTI in children with WMI have reported correlations between corticospinal tract injury and thalamocortical projections (including posterior thalamic radiation) in the pathophysiology of motor impairments (23,26,33,34). Because of safety considerations due to the need for sedation, most of these reports are either of newborns imaged without sedation or are of children older than 2 years across a wide age range and with considerable variability in the findings. It is critical that children requiring intervention are identified as early as possible to maximize developmental outcome and it is likely that MRI will increasingly be used in the perinatal period to clarify the nature and extent of perinatal brain injury. We performed DTI on a relatively homogenous sample of children born preterm with periventricular brain injury at 12 months CA. Our results support previous findings suggesting that ROI-based FA analysis comprising the PLIC and PTR is strongly correlated with motor outcomes in children with WMI. Similar to Yoshida et al. (2010), this is one of the few studies that has examined both the motor and sensory component of the white matter tracts in the same group (26).
Similar to previous reports, children who were diagnosed with CP had low PLIC FA values as compared to infants with normal development (26,33). In agreement with the results of Yoshida and colleagues (2010) (26), we did not find that an FA threshold of .50 discriminates between those with and without CP. Each of our children with CP had FA values of .50 or greater on one or both sides of the brain. It was observed, however, that all children with normal development at 12 months CA had PLIC FA measures of .599 or greater. A more prominent feature distinguishing between infants with and without CP was the asymmetry index. The highest asymmetry index for the PLIC in a child with normal development was .058 while the lowest degree of asymmetry for children with CP was .151. Studies of children with hemiplegic involvement have demonstrated that clinical severity of hemiparesis is correlated with asymmetry in PLIC FA (20), and that FA is increased on the unaffected side of the brain. This finding is hypothesized to be the result of retention of ipsilateral corticospinal pathways that in normal development should regress (24) such that motor control of one side of the body is exerted from the opposite side of the motor cortex. One infant in our study was diagnosed with left hemiplegic CP at the 12-month outcome assessment and our FA findings agree with published results (20): FA of the PLIC on the left side of the brain was the largest recorded in our study at .680 while the FA of the PLIC on the right side of the brain was .500 (Figure 4). The latter value was the highest recorded for any of the children with CP in keeping with this child's GMFCS level II and ability to stand with support at 12 months CA, but it was lower than any child with normal development at 12 months CA. Similarly, FA of the left PTR was .396 while the value for the right side of the brain was .227. The related asymmetry indices were high at .153 and .271, respectively.
Figure 4.
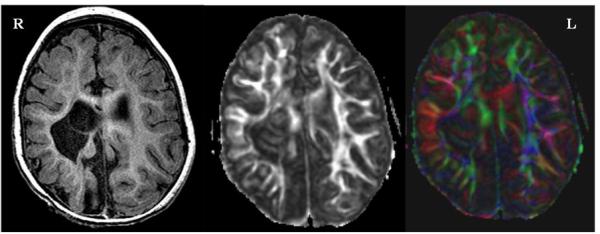
An FSPGR T1-weighted anatomical image (left), gray-scale FA image (middle) and color-coded FA image ( right ) from a subject with hemiplegic cerebral palsy. This subject had a high FA asymmetry of 0.153 in the PLIC and 0.271 in the PTR. Note the remarkable disruption of corticospinal tracts at the level of the internal capsule on the right side of the brain.
Compression of fiber tracts by surrounding infarctions was observed in all the children who participated in this study. Although the degree of compression may not be identical across children, we believe that this effect of compression on FA scores was at least partially balanced out and the FA values most likely reflected the effect of motor development.
Myelination of the central nervous system increases the ability of the axons to conduct repetitive impulses (35). The corticospinal tract is visible upon DTI already at 13 weeks gestational age (36); the PLIC demonstrates mature myelin in 50% of infants at 44 weeks postconceptional age and rapidly attains mature myelination in 75% by a year of age (35). Adult-level values of isotropic diffusion coefficients derived from DTI in the corticospinal tracts are reached by 36-48 months (37). Because myelination may be activity dependent (38) and the period with the most rapid rate of myelination is considered the most vulnerable period (35), an exercise program beginning early in infancy and continuing through the first year could be expected to have a major impact on motor development in infants with PBI. The visual and auditory systems myelinate earlier than motor pathways, but other sensory systems vary in onset and rate of myelination. (35) Reports of PTR involvement in children with CP (26,33) suggest that early intervention may be critical for maturation of these pathways in response to injury, too.
There are several limitations to our study. We acknowledge that our sample population, although relatively homogenous at birth, is small and the applicability of these findings to a larger more variable population is unknown. Because MRI scans were not routinely done in any of the NICUs participating in our study, we were unable to obtain early scans of the brain to provide a better documentation of the extent of PBI and also could not assess possible changes in the FA measures as a result of training. Because the number of children in each group (exercise vs. intervention) was small and unequal, we were unable to perform analysis of statistical differences between the groups. The random assignment of subjects also resulted in different distributions of IVH and PVL, as well as BPD, between exercise and control groups, but the sample sizes do not allow for analysis of the possible effects of different types of lesions. Especially given that the higher frequencies of IVH and BPD and lower frequency of PVL were seen in the exercise group than control group, there may be a possibility of intrinsically lower FA in the presence of PVL than IVH and BPD. If this speculation were true, then it would suggest that the differences in FA between groups might be independent from training. The current data will serve as preliminary data for a future study with a larger cohort of preterm children with PBI. We also did not attempt to correlate clinical measures of sensory function with FA of the PTR because objective assessment of sensory function in infants is difficult. Another potential limitation of this study is that in some brain images, the same location of the ROI could not be chosen for both hemispheres because of the distortion caused by the infarction. In these cases, adjustment was made to ensure that the chosen ROI was in the specific tract, guided by a neuro-anatomy expert. Additionally, the resolution of the DTI data set was too low to provide an accurate volumetric measurement. With a larger sample size and an optimized sequence, the infarction volume should be investigated in future studies.
In conclusion, results of this pilot study suggest that quantitative evaluation of white matter tracts using DTI at 12 months CA may be useful for assessment of brain plasticity in children participating in exercise programs to promote locomotion and reduce disability. Structural integrity, as revealed by FA measurements of the PLIC and to a lesser extent the PTR, was correlated with motor dysfunction as assessed by the AIMS and GMFCS.
Acknowledgment
We appreciate advice on the exercise protocol provided by Gay Girolami, George Hornby, and Dale Ulrich, referral of patients by Dr. Rama Bhat, assistance in image acquisition and analysis provided by Hagai Ganin and Michael Flannery, technical assistance with equipment design of Jim Boynewicz, and data management provided by Kristin Rankin, Andrew Cooper, and Nour Sayes.
Grant Support
This project was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the U.S. National Institutes of Health. The study sponsor played no role in the study design; collection, analysis and interpretation of the data; nor the writing or decision to submit the manuscript.
Footnotes
Carlin's Creations, 27366 Oak Drive, Sturgis, MI 49091, USA.
REFERENCES
- 1.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor Development in Very Preterm and Very Low-Birth-Weight Children From Birth to Adolescence A Meta-analysis. Jama-Journal of the American Medical Association. 2009;302(20):2235–2242. doi: 10.1001/jama.2009.1708. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Kostova FV, Ferriero DM, et al. Injury to the Preterm Brain and Cerebral Palsy: Clinical Aspects, Molecular Mechanisms, Unanswered Questions, and Future Research Directions. Journal of Child Neurology. 2009;24(9):1064–1084. doi: 10.1177/0883073809338957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back SA. Perinatal white matter injury: The changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12(2):129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 4.Roze E, Van Braeckel KNJA, van der Veere CN, Maathuis CGB, Martijn A, Bos AF. Functional Outcome at School Age of Preterm Infants With Periventricular Hemorrhagic Infarction. Pediatrics. 2009;123(6):1493–1500. doi: 10.1542/peds.2008-1919. [DOI] [PubMed] [Google Scholar]
- 5.Hoon AH, Lawrie WT, Melhem ER, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology. 2002;59(5):752–756. doi: 10.1212/wnl.59.5.752. [DOI] [PubMed] [Google Scholar]
- 6.Chen YP, Fetters L, Holt KG, Saltzman E. Making the mobile move: Constraining task and environment. Infant Behavior & Development. 2002;25(2):195–220. [Google Scholar]
- 7.Droit S, Boldrini A, Cioni G. Rhythmical leg movements in low-risk and brain-damaged preterm infants. Early Human Development. 1996;44(3):201–213. doi: 10.1016/0378-3782(95)01709-7. [DOI] [PubMed] [Google Scholar]
- 8.Fetters L, Chen YP, Jonsdottir J, Tronick EZ. Kicking coordination captures differences between full-term and premature infants with white matter disorder. Human Movement Science. 2004;22(6):729–748. doi: 10.1016/j.humov.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Vaal J, van Soest AJ, Hopkins B, Sie LTL, van der Knaap MS. Development of spontaneous leg movements in infants with and without periventricular leukomalacia. Experimental Brain Research. 2000;135(1):94–105. doi: 10.1007/s002210000508. [DOI] [PubMed] [Google Scholar]
- 10.Jeng SF, Chen LC, Tsou KI, Chen WJ, Luo HJ. Relationship between spontaneous kicking and age of walking attainment in preterm infants with very low birth weight and full-term infants. Physical Therapy. 2004;84(2):159–172. [PubMed] [Google Scholar]
- 11.Jeng SF, Yau KIT, Liao HF, Chen LC, Chen PS. Prognostic factors for walking attainment in very low-birthweight preterm infants. Early Human Development. 2000;59(3):159–173. doi: 10.1016/s0378-3782(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 12.Hadders-Algra M. Reduced variability in motor behaviour: An indicator of impaired cerebral connectivity? Early Human Development. 2008;84(12):787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa VM, Campbell SK, Berbaum M. Discriminating infants from different developmental outcome groups using the Test of Infant Motor Performance (TIMP) item responses. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2007;19(1):28–39. doi: 10.1097/PEP.0b013e31802f65f9. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa VM, Campbell SK, Sheftel D, Singh J, Beligere N. Longitudinal performance of infants with cerebral palsy on the Test of Infant Motor Performance and on the Alberta Infant Motor Scale. Physical & occupational therapy in pediatrics. 2003;23(3):7–29. [PubMed] [Google Scholar]
- 15.Barbosa VM, Campbell SK, Smith E, Berbaum M. Comparison of Test of Infant Motor Performance (TIMP) item responses among children with cerebral palsy, developmental delay, and typical development. American Journal of Occupational Therapy. 2005;59(4):446–456. doi: 10.5014/ajot.59.4.446. [DOI] [PubMed] [Google Scholar]
- 16.Campbell SK, Gaebler-Spira D, Zawacki L, et al. Effects on Motor Development of Kicking and Stepping Exercise in Preterm Infants with Periventricular Brain Injury: A Pilot Study. Journal of Pediatric Rehabilitation Medicine. 2011 doi: 10.3233/PRM-2011-0185. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badr LK, Garg M, Kamath M. Intervention for infants with brain injury: Results of a randomized controlled study. Infant Behavior & Development. 2006;29(1):80–90. doi: 10.1016/j.infbeh.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blauw-Hospers CH, Hadders-Algra M. A systematic review of the effects of early intervention on motor development. Developmental Medicine and Child Neurology. 2005;47(6):421–432. doi: 10.1017/s0012162205000824. [DOI] [PubMed] [Google Scholar]
- 19.Piper MC, Kunos VI, Willis DM, Mazer BL, Ramsay M, Silver KM. Early physical therapy effects on the high-risk infant - a randomized controlled trial. Pediatrics. 1986;78(2):216–224. [PubMed] [Google Scholar]
- 20.Glenn OA, Ludeman NA, Berman JI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. American Journal of Neuroradiology. 2007;28(9):1796–1802. doi: 10.3174/ajnr.A0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludeman NA, Berman JI, Wu YW, et al. Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology. 2008;71(21):1676–1682. doi: 10.1212/01.wnl.0000304084.59964.e2. [DOI] [PubMed] [Google Scholar]
- 22.Murakami A, Morimoto M, Yamada K, et al. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122(3):500–506. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- 23.Nagae LM, Hoon AH, Jr., Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007;28(7):1213–1222. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas B, Eyssen M, Peeters R, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- 25.Zarei M, Johansen-Berg H, Jenkinson M, Ciccarelli O, Thompson AJ, Matthews PM. Two-dimensional population map of cortical connections in the human internal capsule. Journal of Magnetic Resonance Imaging. 2007;25(1):48–54. doi: 10.1002/jmri.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida S, Hayakawa K, Yamamoto A, et al. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Developmental Medicine and Child Neurology. 2010;52(10):935–940. doi: 10.1111/j.1469-8749.2010.03669.x. [DOI] [PubMed] [Google Scholar]
- 27.Piper M, Darrah J. Motor Assessment of the Developing Infant. Philadelphia, PA: p. 1994. [Google Scholar]
- 28.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 29.Mori S, Wakana S, Nagae-Poetscher L, van Zijl P. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 30.Stinear CM, Barber PA, Smale P, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Stroke. 2007;38(2):466–466. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 31.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 32.Milgrom J, Newnham C, Anderson PJ, et al. Early Sensitivity Training for Parents of Preterm Infants: Impact on the Developing Brain. Pediatric Research. 2010;67(3):330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- 33.Hoon AH, Jr., Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son SM, Park SH, Moon HK, et al. Diffusion tensor tractography can predict hemiparesis in infants with high risk factors. Neurosci Lett. 2009;451(1):94–97. doi: 10.1016/j.neulet.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 35.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. 2. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Huang H. Structure of the Fetal Brain: What We Are Learning from Diffusion Tensor Imaging. Neuroscientist. 2010;16(6):634–649. doi: 10.1177/1073858409356711. [DOI] [PubMed] [Google Scholar]
- 37.Schneider JFL, Il'yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- 38.Fields RD. Volume transmission in activity-dependent regulation of myelinating glia. Neurochemistry International. 2004;45(4):503–509. doi: 10.1016/j.neuint.2003.11.015. [DOI] [PubMed] [Google Scholar]


