Abstract
Background
During recovery from alcoholism, other behavior likely increases. The development of alternative behavior may reduce attention to alcohol-associated stimuli. This could result in greater persistence of the alternative behavior when individuals again encounter alcohol-associated stimuli that might precipitate relapse. Developing animal models of this process could facilitate a better understanding of the mechanisms involved in relapse and recovery. However, current pre-clinical models of recovery and relapse rarely measure alternative behavior. Thus, our objective was to establish a procedure in rats in which an increase in alternative behavior (responding for food) reduced responding for ethanol. The amount of responding for food and ethanol was then assessed after re-exposure to the alcohol-associated stimulus after varying the number of preceding sessions of increased responding for food and reduced responding for ethanol. These results were compared with those from a parallel group responding for saccharin solution instead of ethanol.
Methods
The solution (ethanol or saccharin) was always available following 5 responses. Presentation of flashing stimulus lights indicated food delivery followed 150 responses and resulted in responding predominately for the solution (84 – 86% of total responses). Presentation of solid stimulus lights indicated food delivery followed 5 responses and resulted in responding predominately for food (1 – 3% of total responses were for the solution). Rats were exposed to solid light conditions for 0, 1, 2, 4, or 16 consecutive sessions before being re-exposed to the flashing stimulus lights in extinction.
Results
Responding for either solution resumed when rats were re-exposed to the flashing stimulus lights (associated with solution-predominate responding). However, more responses occurred on the food lever with longer recent histories of responding for food instead of the solution.
Conclusions
These results suggest that the longer alternative behavior replaces drinking, the more that attention to stimuli associated with drinking decreases. These results are consistent with the notion that the risk of relapse declines with longer periods of recovery because alternative behavior comes to predominate even in the presence of stimuli associated with drinking.
Keywords: alcohol, reinstatement, resurgence, rat, attentional bias
Introduction
During recovery from alcoholism, drinking declines. This decline is often accompanied by increases in alternative behavior. Increases in alternative behavior likely contribute to successful recovery and may protect against relapse. However, even after many years of recovery, relapse remains a constant threat (O’Brien, 2008). The hope of reducing relapse among those in recovery has led to the development of animals models of relapse (Epstein et al., 2006).
Reinstatement is the most widely used animal model of relapse. In this procedure, animals are trained to respond for drug or ethanol in the presence of a stimulus cue. Once this behavior is established, it is reduced by withholding access to the drug or ethanol (Ciccocioppo et al., 2003; de Wit and Stewart, 1981). This is accomplished in two different ways. One way is called extinction where responses no longer produce access to the drug or ethanol. This is typically done in the presence of a different stimulus cue. The other is by removing animals from the experimental apparatus for extended periods (Epstein et al., 2006). Subsequent re-exposure to the apparatus or stimuli in the apparatus that had previously predicted drug or ethanol availability results in a resumption of responding for the drug or ethanol. This is called cue-induced reinstatement (Crombag et al., 2008). Such reinstatement is thought to be related to relapse; particularly relapse caused by drug- or alcohol-associated cues. Cue-induced reinstatement has been demonstrated with many different substances with various routes of administration, caloric content, or palatability including: orally administered ethanol, sucrose, and saccharin as well as intravenously delivered drugs of abuse such as cocaine and heroin. This indicates that the processes involved in cue-induced reinstatement do not depend on the particular substance that maintains the behavior.
Cue-induced reinstatement is assessed in animals in the absence of any measured alternative behavior, even though alternative behavior is likely critical to initiating and maintaining recovery and may protect against relapse in humans (Vuchinich and Tucker, 1988). Clearly, there is a need for a procedure which shares the relapse-related features of the reinstatement procedure but that also includes a measured alternative behavior. Such a procedure could improve our understanding of the processes involved in the initiation and maintenance of recovery.
The ability of an alternative to reduce alcohol use depends on the relationship between the alternative behavior and its outcome. For example, responding for ethanol decreases and responding for sucrose solution increases as a function of increasing concentrations of sucrose when both are concurrently available at the same response requirement in calorie-restricted rats (Samson et al., 1982). Increasing the response requirement for the sucrose solution shifted responding back to ethanol.
Our goal was thus to establish an alternative model of relapse in which ethanol use is reduced when an alternative (food) is concurrently available. In this procedure, the number of responses required for ethanol remains constant. Two distinct lighting conditions indicated whether the number of responses required for food was relatively high or low. When the response requirement for food was relatively high, rats responded predominately for ethanol; when the response requirement for food was relatively low, rats responded predominately for food and responding for ethanol was almost abolished. With this procedure, we determined whether re-exposure to stimuli associated with high levels of ethanol use resulted in resumption of responding for ethanol after a history of responding for food rather than ethanol. Further, we evaluated whether the extent of resumed responding for ethanol varied as a function of the length of this history. Simultaneous responding for food was also measured and expressed as a function of the number of preceding sessions of food-predominant responding. We repeated this experiment in rats responding for saccharin solution instead of ethanol to determine whether the observed results were specific to ethanol.
Materials and Methods
Subjects
Male Lewis rats weighing approximately 250g upon arrival (Harlan, Inc., Frederick, MD) served as subjects. Rats habituated to the vivarium for two weeks with ad libitum access to food and water. Then, food was restricted to maintain body weights at 280–330g for the duration of the study (approximately 12–15g/d). Rats had ad libitum access to water at all times in their home cage. Rats were singly housed in a room maintained on a 14/10 light/dark cycle adjacent to the procedure room. Behavioral procedures occurred during the first two hours of the light period. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the institution’s Animal Care and Use Committee.
Test apparatus
Studies were conducted in commercially available testing chambers (ENV008, Med Associates, St. Albans, VT). Chambers were enclosed in light- and sound- attenuating cubicles (ENV018MD, Med Associates, St. Albans, VT) ventilated by a small fan. Pink noise was present in the procedure room to mask ambient noise. Each chamber had two levers arranged along one wall with stimulus lights above each lever. Food pellets (45 mg chow-flavored pellets, BioServ, Frenchtown, NJ) and 0.1 ml of the solutions were accessible in a receptacle equidistant between the two levers. An additional light was in the center of the opposite wall of the chamber and a houselight was positioned a few inches above this stimulus light.
Training
Rats were separated into two groups and were initially trained to respond for either an 8% sucrose or 0.03% saccharin solution (w/v). During 12-hour overnight session, the illumination of the light above the left lever indicated that the solution was available. A single response on the left lever turned the light above the lever off, illuminated the house light, and raised a lever with a 0.1 ml cup on the end into an accessible position for 15-sec. After this presentation, the cup was withdrawn, the house light was turned off, the light above the lever was again illuminated, and a response on the left lever again resulted in solution presentation and associated stimulus changes. Once rats earned over 80 presentations per session (1–5 sessions, depending on the rat), sessions were moved to the morning and the session length was reduced to 30-min. The response requirement was then increased to 5 over 1 – 5 sessions. Subsequently, ethanol was added to the solution (10% w/v) provided to the ethanol group. Over the next 20 – 30 sessions, the sucrose in this solution was gradually removed until rats were responding for a 10% ethanol solution in tap water alone.
Rats in both groups were trained to respond for food in a subsequent 30-min session. In this session, the light above the right lever was illuminated, and a single response on the right lever resulted in the stimulus light above the lever turning off, the light on the opposite wall turning on, and delivery of one 45mg food pellet. After 15-sec, the light on the opposite wall was turned off, the light above the right lever was turned on, and the lever was active again. Once rats earned at least 80 pellets in a session, the response requirement was increased to 5 over the next 3 – 5 sessions. Rats then began training under the multiple concurrent schedule.
Under the multiple concurrent schedule, stimulus conditions during each component were presented in random order (by sampling without replacement from the two stimulus conditions) during a 30-min session. In the “flashing light” condition, lights above both levers flashed (on for 0.1 sec, off for 0.1sec), and either ethanol or saccharin solution was available after 5 responses on the left lever and food was available after 150 responses on the right lever. In the “solid light” condition, lights above both levers were solidly illuminated, and ethanol or saccharin solution and food were both available after 5 responses on the appropriate lever. Rats were exposed to one multiple concurrent schedule session each weekday until responding on the solution lever in the presence of flashing lights was >50% and responding on the solution lever in the presence of solid lights was <50%. Once rats met this criteria, testing commenced.
Testing
The experimental design included four phases as shown in Figure 1. These phases were repeated sequentially in each subject such that every intervention condition in Phase III was assessed in all rats. In Phase I, rats were exposed to at least five consecutive sessions under the multiple concurrent schedule. Then, in Phase II, rats were exposed to 10 consecutive sessions under the “flashing lights” condition (where flashing lights were presented and the solution was available after 5 responses and food after 150 responses). Subsequently, in Phase III, rats were exposed to an intervention period of 0, 1, 2, 4, or 16 consecutive sessions in which only the “solid light” condition was presented (where only solid lights were presented and both the solution and food were available after 5 responses). The order of intervention length was mixed across subjects as shown in Table 1. Finally, in Phase IV, rats were re-exposed to the flashing lights during a single test session. During this session, responses turned off the stimulus lights and illuminated either the house or opposite wall light, but no food or solution was delivered.
Figure 1.
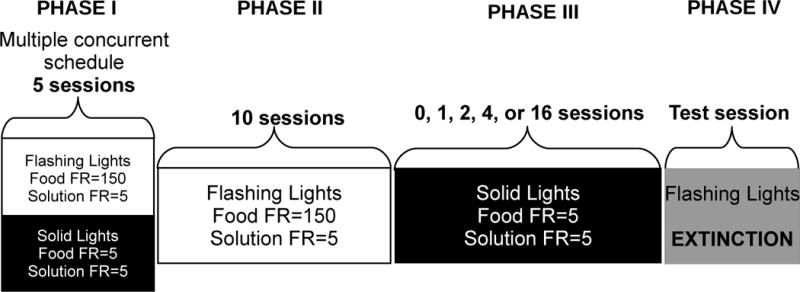
Experimental design. The design of the study included at least 5 days of training under the multiple concurrent schedule (Phase I) in which conditions alternated within the session between a response requirement for food of 150 and the associated flashing stimulus lights and a response requirement for food of 5 and the associated solid stimulus lights. The response requirement for ethanol or saccharin solution was always 5. Phase I was followed by Phase II in which only flashing lights signaling a response requirement of 150 for food were presented for 10 consecutive sessions. This was followed Phase III in which only solid lights signaling a response requirement for food of 5 were present for varying numbers of sessions. Finally, during the Phase IV session, flashing stimulus lights were presented, but responses did not result in delivery of food or the solution. These four phases were repeated for each subject so that each subject was exposed to each number of intervening sessions listed for Phase III.
Table 1.
Order of testing of each intervention condition for each subject.
| Ethanol group | Saccharin group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Order Subject |
1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 1 | 0 | 4 | 1 | 2 | 16 | 4 | 2 | 16 | 0 | 1 | |
| 2 | 0 | 16 | 1 | 4 | 2 | 4 | 2 | 1 | 16 | 0 | |
| 3 | 0 | 16 | 4 | 1 | 2 | 4 | 1 | 16 | 2 | 0 | |
| 4 | 16 | 0 | 1 | 4 | 2 | 4 | 1 | 2 | 0 | 16 | |
| 5 | 16 | 0 | 1 | 4 | 2 | 4 | 1 | 16 | 2 | 0 | |
| 6 | 2 | 0 | 4 | 16 | 1 | 4 | 0 | 1 | 2 | 16 | |
| 7 | 1 | 0 | 4 | 2 | 16 | 1 | 4 | 16 | 2 | 0 | |
| 8 | 1 | 16 | 0 | 4 | 2 | ||||||
Analysis
To determine whether re-exposure to the flashing lights resulted in resumed responding for the solution, a repeated-measures ANOVA was performed on the number of responses on the solution lever with solution type (ethanol or saccharin), intervention condition (0, 1, 2, 4, or 16 sessions), and session (preceding or test session) as factors. Because an interaction was present for intervention condition x session, separate t-tests were performed on the number of responses on the solution lever in either session for each intervention condition.
The effect of intervention condition on the number of responses for the solution was assessed using a repeated measures ANOVA with intervention condition (0,1,2,4, or 16 sessions) and solution type (ethanol or saccharin) as factors. A parallel analysis was performed on the total number of responses on the food lever. Once the first 5 responses on the solution lever were completed, the rats experienced extinction (lack of reward for responding). This may have influenced subsequent responding. In order to determine effects that were not influenced by the extinction conditions present during the test session, another, parallel analysis with intervention condition and solution type as factors was performed on the number of responses on the food lever that occurred before the first 5 responses on the solution lever were completed. F-values for which p<0.05 were considered significant.
Solutions
Ethanol solutions were made by mixing ethanol (95%, Decon Labs, Inc., King of Prussia, PA) with tap water to achieve a 10% w/v solution. The solution was allowed to reach room temperature before being provided to rats. Saccharin (Sigma, St. Lous, MO) was dissolved in tap water to achieve a 0.03% w/v solution. Separate trays were used for ethanol and saccharin solutions and dippers were wiped clean prior to introducing a new solution to minimize cross contamination.
Ethanol Consumption
Blood ethanol concentration (BEC) was estimated from breath ethanol concentrations using a rat breathalyzer to confirm that rats were consuming ethanol earned in the session (Javors et al., 2005). With this apparatus, we estimate blood ethanol levels by measuring expired ethanol collected in a head chamber using gas chromatography. With this procedure, we determined BEC in each subject immediately after a session similar to those in Phase II, in which only the “flashing light” condition was present in rats responding for the ethanol solution.
Results
During sessions in which only “flashing light” conditions were presented and BEC was assessed, rats consumed substantial amounts of ethanol. Rats earned a dose of 0.72 ± 0.09 g/kg (mean ± S.E.M.) during the 30-min session. The estimated BEC after this session was 0.10 ± 0.01 g/dL. This represents a pharmacologically active level of ethanol.
During the five multiple concurrent sessions of Phase I, responding was controlled by the stimulus light conditions. During these sessions, presentation of flashing stimulus lights (that indicated a response requirement of 150 for food) resulted in responding predominantly for the solution [Ethanol: 76.1% ± 4.7; Saccharin: 72.7% ± 3.3]. Presentation of solid lights (that indicated a response requirement of 5 for food) resulted in responding primarily on the food lever [Ethanol: 2.2% ± 0.8; Saccharin: 1.7% ± 0.8]. It is important to remember that stimulus conditions randomly alternated during these sessions, so responding during a stimulus condition could not be guided by the preceding stimulus condition. Thus the stimulus lights were associated with solution- or food-predominant responding.
During the 10 sessions of Phase II, where only flashing lights were presented, rats responded predominately on the lever associated with solution delivery (Figures 2 and 3, open symbols). Rats completed 136 ± 2.4 responses for ethanol per session, or 152 ± 4.0 responses for saccharin per session. In contrast, rats completed only 27 ± 1.7 or 31 ± 2.5 responses for food per session in the ethanol and saccharin groups, respectively. Prior to completing the first 5 responses for solution across these 10 sessions, rats completed 4 ± 0.2 and 5 ± 0.2 responses on the food lever in the ethanol and saccharin groups, respectively.
Figure 2.
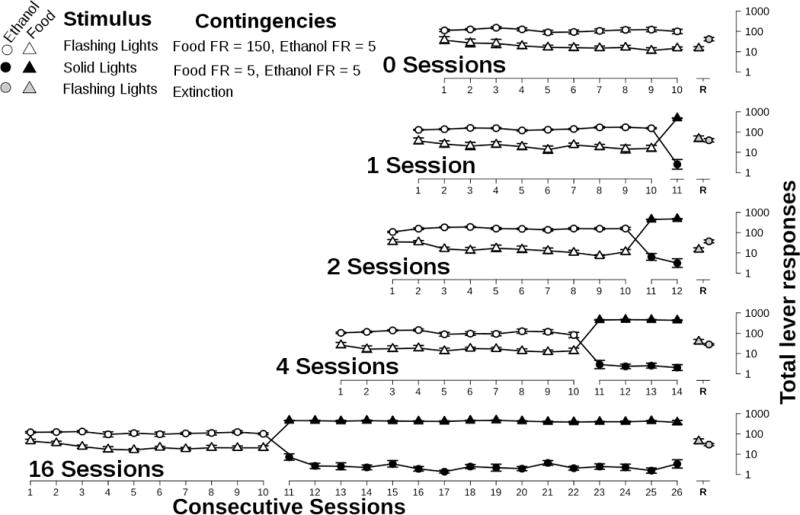
Total responses for ethanol (circles) and food (triangles) during each phase of the experiment. Points represent mean ± S.E.M. for 8 rats plotted on a log scale. White symbols represent Phase II. Black symbols represent Phase III. Grey symbols (offset for clarity) represent Phase IV. Phase I data are not plotted.
Figure 3.
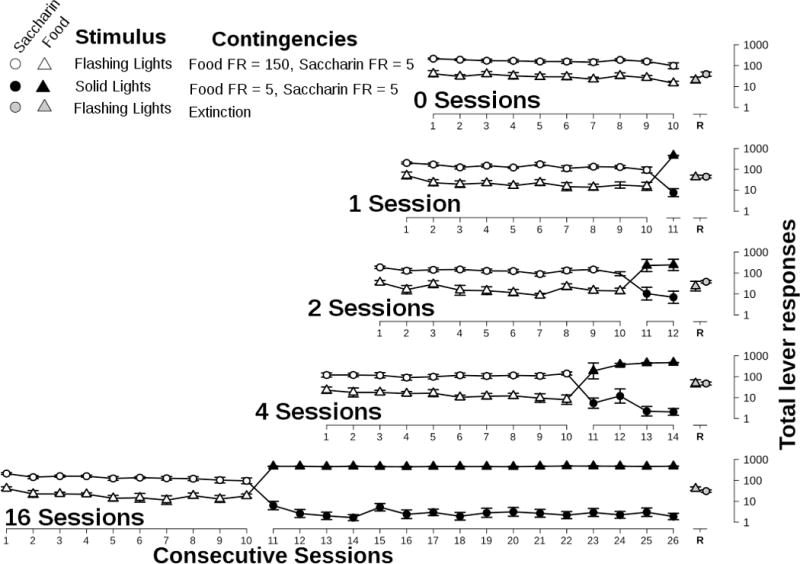
Total responses for saccharin (circles) and food (triangles) during each phase of the experiment. Points represent mean ± S.E.M. for 7 rats plotted on a log scale. White symbols represent Phase II. Black symbols represent Phase III. Grey symbols (offset for clarity) represent Phase IV. Phase I data are not plotted.
During Phase III, when only solid lights were presented, responding for food predominated across these 1, 2, 4, or 16 sessions (Figures 2 and 3, closed symbols). Responses on the solution lever were almost abolished with only 4 ± 0.6 and 9 ± 1.8 total ethanol or saccharin responses per session, respectively. In Phase III, rats completed 438 ± 4.8 or 452 ± 6.0 responses for food per session in the ethanol and saccharin groups, respectively.
In Phase IV, the effects of re-exposure to the flashing stimulus lights (which was associated with solution-predominant responding) were examined after each of the various histories established in Phase III. Of interest was whether responding for the solution resumed, and whether the recent history from Phase III affected the number of responses for food before the first 5 responses for the solution were completed, the total number of responses for food, or the total number of responses for either solution.
First, to determine if re-exposure to the flashing lights resulted in a resumption of responding for the solution, an ANOVA was performed on the number of solution-lever responses with the session (test or preceding session), solution (ethanol or saccharin), and intervention condition (0, 1, 2, 4, or 16) as factors. An interaction between intervention condition and session was present (F[4, 117] = 7.7, p<0.0001) and main effects of session (F[1, 117] = 19.7, p<0.0001) and intervention condition (F[4, 117] = 18.7, p<0.0001) were also significant. No other interactions were significant, nor was there a main effect of solution type. The interaction between intervention condition and day was due to a difference between the 0-session intervention condition and the other intervention conditions. Responding on the solution lever decreased from the preceding session to the test session in the 0-session intervention condition, but increased in all other intervention conditions (t= −3.3, 5.0, 3.3, 7.4, and 4.2 for 0, 1, 2, 4, and 16 session intervention conditions, respectively). Each of these changes was significant (p<0.05 after Bonferroni correction for multiple comparisons).
In Phase IV, there was a main effect (F[4, 52] = 7.9, p<0.001) of the length of the history of food-predominant responding established in Phase III on the number food responses completed before the first 5 solution lever responses (Figure 4, top panel). There was no main effect of solution type (F[1, 13] = 0.02, p>0.05) nor was an interaction present (F[4, 52] = 1.2, p>0.05). Further examination revealed that prior to the first 5 solution responses, food responses were significantly elevated after 4 or 16 preceding sessions of reduced responding for ethanol compared with the 0-session intervention condition (p<0.05 after Bonferroni correction for multiple comparisons).
Figure 4.
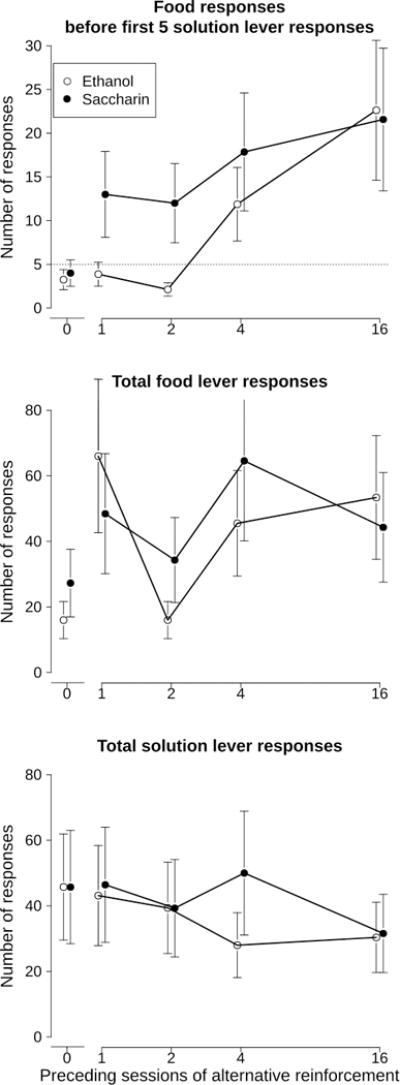
Effects of varying the number of sessions during Phase III on food responses before the first 5 responses on the solution lever were completed during the test session (top panel); total number of food responses during the test session (middle panel), and total number of responses on the solution lever (bottom panel). The dotted line in the top panel represents 5 responses on the lever. Points above this line would have resulted in food delivery during Phase I, and represent more responding for food than for the solution prior to the first completion of the response requirement. Points represent mean ± S.E.M. For n=8 (ethanol, open symbols) or n=7 (saccharin, closed symbols) rats and have been offset for clarity. Note the abscissa is plotted on a log scale.
Similarly, there was a main effect (F[4, 52] = 5.3, p<0.005) of the length of the history of food-predominant responding established in Phase III on the total number of food responses in Phase IV (Figure 4, middle panel). There was no main effect of the solution type (F[1, 13] = 1.5, p>0.05) or an interaction between solution type and preceding number of sessions (F[4, 52] = 1.8, p>0.05). Post-hoc analysis revealed that total responses for food were elevated after 1, 4, or 16 preceding sessions of reduced responding for food compared with the 0-session intervention condition collapsed across both groups (p<0.05 after Bonferroni correction).
In contrast with effects on food responding, there was no main effect (F[4, 52] = 2.0, p>0.05) of the length of the history of reduced responding for the solution established in Phase III on the total number of solution responses during Phase IV (Figure 4, bottom panel). There was also no main effect of the solution type (F[1, 13]=0.00, p>0.05) on the total number of solution-associated lever responses during the test session. An interaction was not present, either (F[4, 52]=1.4, p>0.05).
To ensure that the effects observed were not simply due to the difference between the 0 and 1 session intervention conditions, we reanalyzed the same data, excluding 0-session data. These results were consistent with the global analysis. A main effect (F[3, 39] = 8.1, p<0.05) of the length of the history established in Phase III was still present on the number of responses for food before the first 5 responses for solution and on the total number of food responses during the session (F[3, 39] = 4.0, p<0.05). No main effect of the length of Phase III was present for responses for the solution. No effects of solution type were present for any measure, nor were any interactions significant.
Discussion
In the present study, providing concurrent access to food reduced responding for ethanol and saccharin, consistent with results from similar studies (Nader and Woolverton, 1992; Samson et al., 1982). Re-exposure to the stimulus previously associated with responding for each solution resulted in the re-emergence responding for the solution, similar to other models of cue-induced relapse. However, upon re-exposure to this stimulus (which had previously resulted in almost no responding for food), a longer recent history of responding for food instead of ethanol or saccharin resulted in more responding for food. This effect was present before the first 5 responses on the solution lever were completed (i.e., before extinction is encountered) as well as across the entire session. These data suggest an opportunity for relapse prevention by reinforcing alternative behaviors during recovery. A longer history of such alternative reinforcement may lead to the alternative behavior persisting even when ethanol-associated cures are encountered. However, once responding for the solution resurged, the history of alternative behavior did not affect the persistence of responding for the solution during extinction. This might reflect a limitation of behavioral treatments for relapse. If the alternative behavior is not rewarded, alcohol-associated cues may again prompt seeking, and the persistence of this resumed seeking may not be affected by the length of recovery. Because similar results were observed in rats responding for either ethanol or saccharin, these effects appear to be due to a general behavioral phenomena rather anything specific to ethanol. Thus, this strategy might be applicable to other, similar disorders.
Others have shown that providing alternative reinforcement can reduce a targeted behavior, and this strategy has been applied to the treatment of several behavioral disorders (Athens and Vollmer, 2010), including drug (Iguchi et al., 1997) and alcohol use (Hunt and Azrin, 1973). However, the lasting impact of such an intervention when the individual is frequently re-exposed to conditions where the target behavior (e.g. drinking) had typically occurred remains largely untested (but see Alessi et al., 2004). Here, we demonstrate that a longer history of alternative behavior in the same context where drinking occurred can shift attention away from the stimuli that occasion drinking. Further, longer (or perhaps more) exposure to the intervention results in more alternative behavior before ethanol- or saccharin-seeking resurge.
A transition from goal-directed to habitual behavior has been proposed as a mechanism by which drug dependence can develop (Everitt et al., 2001), and it follows that a similar process might be responsible for the transition from dependence to lasting recovery. The transition from goal-directed to habitual behavior has been described by Robinson and Berridge (1993) as incentive-sensitization; drugs and associated stimuli produce an effect to which the individual sensitizes over repeated use. This sensitization results in the associated stimuli assuming increasing salience that eventually overwhelms the salience of other, competing stimuli in the environment and may lead to habitual substance use guided more by a Pavlovian stimulus-response process than an operant goal-directed response-outcome process (Everitt et al., 2001). One application of incentive-sensitization theory has been in the area of attentional bias (Field and Cox, 2008). Typically attentional bias is measured by assessing the latency to identify a target in an image or series of words in the presence or absence of competing drug- or alcohol-related stimuli. Latencies are greater in alcohol dependent individuals as they attend more to stimuli associated with alcohol or drinking than controls (Franken, 2003). Further, heavier drinkers show greater attentional bias than social drinkers (Townshend and Duka, 2001). Additionally, the amount of attentional bias is inversely related to treatment outcome for alcohol and other substance dependencies (Cox et al., 2007; Field and Cox, 2008). Finally, longer periods of recovery may result in reduced attentional bias to alcohol-related stimuli (Field and Cox, 2008).
The effect observed in the present study might therefore be due to decreased attention to the stimulus presented and more habitual responding for food, though additional studies are required to confirm this. Such an effect would be consistent with results of human studies in which longer periods of recovery result in reduced likelihood of relapse and reduced attention to stimuli associated with drinking (Field and Cox, 2008; Finney and Moos, 1991). Identifying treatment strategies that facilitate this decrease in attention to ethanol-associated stimuli may improve treatment outcomes, and may also provide a strategy for other, similar behavioral disorders.
Because test sessions were conducted under extinction in our study, responding for food decreased as the session proceeded and responding for the solution eventually resurged. Others have also shown that discontinuation of alternative reinforcement can result in a resurgence of responding for ethanol in a context where both had been available (Podlesnik et al., 2006). Once ethanol- or saccharin-seeking resurge, the number of preceding intervention sessions (where responding for the solution was reduced) did not affect the amount of extinction responding for the solution. This contrasts with the responding for food which was affected by recent history. The reason for this difference is not entirely clear. However, there appear to be two different types of extinction occurring during Phase IV sessions. Responding for food increased as a function of the number of sessions in Phase III. However, rats never completed 150 responses on the food lever during Phase IV, and thus never experienced a stimulus change coupled with non-delivery of food. In contrast, rats always completed 5 responses on the solution lever during Phase IV, resulting in a stimulus change coupled with non-delivery of the solution. After about 5 such stimulus changes, rats ceased responding for the solution entirely. Thus, extinction of responding on the food lever (in the absence of any programmed consequence) was affected by recent history, while extinction of responding on the solution lever (which was signaled by stimulus changes) was not.
The similar results observed in the ethanol and saccharin groups suggest that the changes seen with longer recovery periods are due to general behavioral mechanisms and are not specific to ethanol. Indeed, attentional bias appears to reflect a general behavioral process that has been observed for substance use disorders, eating disorders, anxiety disorders, and other emotional disorders (Browning et al., 2010; Dobson and Dozois, 2004; Field and Cox, 2008; Roy et al., 2008). This suggests that the transition from goal-directed to habitual behavior may play a role in a variety of psychiatric disorders. Thus, it is not surprising that our results are similar for behaviors maintained both by ethanol and saccharin. Developing therapeutic strategies that interfere with habitual pathological behavior and that develop new, adaptive habitual behavior could improve outcomes for a wide variety of compulsive disorders.
The present study shows that longer periods or more experience engaging in alternative behavior in the same context where drinking had occurred can reduce attention to the cues that had previously occasioned drinking. This effect is revealed by an effect of a longer recent history of alternative behavior on the amount of responding for food when rats are re-exposed to the stimulus that had occasioned ethanol- or saccharin-maintained responding. This effect appears to be due to a general behavioral process, shared by ethanol- and saccharin-maintained behaviors. Better understanding how to develop new habits that interfere with existing, pathological habits could result in improved behavioral therapies for a variety of psychiatric disorders, including alcohol abuse and dependence.
Acknowledgments
The authors with to thank Olivia Dominguez and Gerardo Martinez for the expert technical assistance and Dr. John Roache for his help editing the manuscript. This work was supported by PHS grant AA016987.
Supported by PHS grant AA016987
References
- Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Exp Clin Psychopharmacol. 2004;12:276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- Athens ES, Vollmer TR. An investigation of differential reinforcement of alternative behavior without extinction. J Appl Behav Anal. 2010;43:569–589. doi: 10.1901/jaba.2010.43-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Harmer CJ. The modification of attentional bias to emotional information: A review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci. 2010;10:8–20. doi: 10.3758/CABN.10.1.8. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology. 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Dozois DJA. Attentional biases in eating disorders: a meta-analytic review of Stroop performance. Clin Psychol Rev. 2004;23:1001–1022. doi: 10.1016/j.cpr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Finney JW, Moos RH. The long-term course of treated alcoholism: I. Mortality, relapse and remission rates and comparisons with community controls. J Stud Alcohol. 1991;52:44–54. doi: 10.15288/jsa.1991.52.44. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Hunt GM, Azrin NH. A community-reinforcement approach to alcoholism. Behav Res Ther. 1973;11:91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. J Consult Clin Psychol. 1997;65:421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Javors MA, Ginsburg BC, Friesenhahn G, Delallo L, Lamb RJ. Rat breathalyzer. Alcohol Clin Exp Res. 2005;29:1853–1857. doi: 10.1097/01.alc.0000183228.07510.a2. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol. 1992;3:635–638. [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 1996. [PubMed] [Google Scholar]
- O’Brien CP. Evidence-based treatments of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3277–3286. doi: 10.1098/rstb.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, Bergman RL, McClure-Tone EB, Pine DS. Attention bias toward threat in pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Roehrs TA, Tolliver GA. Ethanol reinforced responding in the rat: a concurrent analysis using sucrose as the alternate choice. Pharmacol Biochem Behav. 1982;17:333–339. doi: 10.1016/0091-3057(82)90088-0. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol abuse. J Abnorm Psychol. 1988;97:181–195. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]


