Abstract
Adipose tissue is composed of lipid-filled mature adipocytes and a heterogeneous stromal vascular fraction (SVF) population of cells. Similarly, the bone marrow (BM) is composed of multiple cell types including adipocytes, hematopoietic, osteoprogenitor, and stromal cells necessary to support hematopoiesis. Both adipose and BM contain a population of mesenchymal stromal/stem cells with the potential to differentiate into multiple lineages, including adipogenic, chondrogenic, and osteogenic cells, depending on the culture conditions. In this study we have shown that human adipose-derived stem cells (ASCs) and bone marrow mesenchymal stem cells (BMSCs) populations display a common expression profile for many surface antigens, including CD29, CD49c, CD147, CD166, and HLA-abc. Nevertheless, significant differences were noted in the expression of CD34 and its related protein, PODXL, CD36, CD 49f, CD106, and CD146. Furthermore, ASCs displayed more pronounced adipogenic differentiation capability relative to BMSC based on Oil Red staining (7-fold vs. 2.85-fold induction). In contrast, no difference between the stem cell types was detected for osteogenic differentiation based on Alizarin Red staining. Analysis by RT-PCR demonstrated that both the ASC and BMSC differentiated adipocytes and osteoblast displayed a significant upregulation of lineage-specific mRNAs relative to the undifferentiated cell populations; no significant differences in fold mRNA induction was noted between ASCs and BMSCs. In conclusion, these results demonstrate human ASCs and BMSCs display distinct immunophenotypes based on surface positivity and expression intensity as well as differences in adipogenic differentiation. The findings support the use of both human ASCs and BMSCs for clinical regenerative medicine.
A stem cell is characterized by its ability to undergo self-renewal and its capacity to undergo multilineage differentiation and generate terminally differentiated cells. Ideally, a stem cell for regenerative medical applications should meet the following set of criteria: (i) should be found in abundant quantities (millions to billions of cells); (ii) can be collected and harvested by a minimally invasive procedure; (iii) can be differentiated along multiple cell lineage pathways in a reproducible manner; (iv) can be safely and effectively transplanted to either an autologous or allogeneic host (Gimble, 2003).
It was originally believed that tissue-specific adult stem cells were only capable of differentiation along cell lineages of their tissue of origin; however, multiple studies indicate that mesenchymal stem cells (MSCs) from adipose, bone marrow (BM), and other sites are capable of differentiation along mesodermal lineages other than that of their tissue of origin (Friedenstein, 1976; Pittenger et al., 1999; Bianco et al., 2001; Dawn and Bolli, 2005). Human adipose-derived stromal/stem cells (hASCs) are multipotent progenitor cells that can be readily derived from human adipose tissue in abundance (Zuk et al., 2001, 2002; Gimble and Guilak, 2003; Estes et al., 2004; Guilak et al., 2006; Bunnell et al., 2008a,b). Adipose tissue is composed of lipid containing mature adipocytes and stromal vascular fraction (SVF) cells (Zuk et al., 2002). The SVF includes various cell types, including immune cells, fibroblasts, pericytes, endothelial cells, and stromal cells, which can be isolated by collagenase digestion (Gimble et al., 2007). Upon plastic adherent selection, the SVF cells yield between 0.25 and 0.375 million hASCs from a single milliliter of human lipoaspirate (Aust et al., 2004; Mitchell et al., 2006; Yu et al., 2010). The human ASCs exhibit a distinct immunophenotypic profile that has been confirmed in multiple independent studies (Gronthos et al., 2001; Zuk et al., 2002; Aust et al., 2004; McIntosh et al., 2006; Mitchell et al., 2006). The human ASCs share similarities with the bone marrow-derived mesenchymal stromal/stem cells (BMSCs) first described by Friedenstein over four decades ago (Friedenstein, 1976). While the BMSCs have been identified over the years as mechanocytes, fibroblasts, reticuloendothelial cells, stromal cells, and Westin Bainton cells, among other names, there is a consensus that they consistently display the following properties: (i) plastic adherence; (ii) the ability to differentiate along the adipocyte, chondrocytes, and osteoblast pathways; (iii) expression of common surface antigens including CD105, CD73, and CD90; and (iv) the absence of expression of hematopoietic and myeloid surface antigens (Pittenger et al., 1999; Dominici et al., 2006).
The multipotentiality and accessibility of ASCs and BMSCs makes them promising candidates for mesodermal defect repair and disease management. Although some might conclude that all MSCs are equivalent, independent of their tissue of origin, there is evidence suggesting that ASCs and BMSCs differ with respect to their immunophenotype, differentiation ability, and utility for specific regenerative medical applications. While some investigators have reported comparable levels of adipogenesis and osteogenesis with human ASCs and BMSCs (De Ugarte et al., 2003), others have concluded that BMSCs display superior osteogenic capacity while ASCs display superior adipogenic capacity (Sakaguchi et al., 2005). The current study set out to perform a direct comparison of the differentiation potential and immunophenotypic profile of human ASCs and BMSCs obtained from cohorts (n = 12) of adult female donors. The study documents similar but distinct differentiation and immunophenotypic profiles between the two cell populations.
Materials and Methods
Study subjects and cell isolation
The ASCs and BMSCs were used at passage 1 (P1) or passage 2 (P2). All protocols were reviewed and approved by the Pennington Biomedical Research Center (adipose) or Tulane University School of Medicine (bone marrow) Institutional Review Boards prior to tissue collection. All tissue was obtained from patients undergoing elective liposuction surgery or voluntarily from BM donors with a signed informed consent agreement. The isolated cells were provided to the investigators in an anonymous manner. ASCs were isolated from adipose tissue (n = 12 female donors; 31 ± 3 years, range 27–35; body mass index (BMI) 27.1 ± 4.9, range 18.6–37.2). BMSCs were isolated from BM (n = 12 female donors, 29.1 ± 7.2 years, range 21–41; the necessary information for the calculation of the BM donor BMI was not obtained at the time of anonymous tissue collection). The ASC and BMSC were isolated according to published protocols (Dubois et al., 2005,2008; Wolfe et al., 2008). Briefly, ASC were obtained from washed lipoaspirate tissues by collagenase digestion at 37°C for 1 h, centrifugation for 5 min at 300g (room temperature), and culture of the resulting SVF cells stromal medium consisting of DMEM/F12, 10% fetal bovine serum (FBS), 1% antibiotic/antimycotic (penicillin, streptomycin, fungizone) at 37°C, 5% CO2 overnight. The following day, the ASC were rinsed with phosphate-buffered saline (PBS) warmed to 37°C and maintained until 80–90% confluent in stromal medium (Dubois et al., 2008). Similarly, BMSC were obtained from BM aspirates (iliac crest) separated over a Ficoll gradient by centrifugation for 30 min at 1,800g (room temperature) as a buffy coat, washed in Hank’s-buffered saline solution (HBSS), centrifuged at 1,000g for 10 min, and the pelleted nucleated cells cultured overnight at 37°C, 5% CO2 in complete culture medium (CCM) consisting of α-modified Eagle’s medium (αMEM), 20% FBS, 2mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). The following day, the cultures were rinsed with warm PBS and maintained in CCM until 80% confluent (Wolfe et al., 2008). The ASC and BMSC cultures were harvested by trypsin digestion and aliquots of 106 cells cryopreserved in liquid nitrogen until required for experimentation. cell culture medium, DMEM/F12 1:1 (1 ×), DMEM (high glucose)/F12, FBS, and fetal calf serum (FCS) were purchased from Thermo Fisher Scientific (Houston, TX), medium essential medium (MEM), L-glutamine, and antibiotic/antimycotic were purchased from Invitrogen (Carlsbad, CA), dexamethasone, IBMX, biotin, insulin, pantothenate, β-glycerophosphate, ascorbate-2-PO4 were purchased from Sigma (St. Louis, MO), rosiglitazone was purchased from AK Scientific (Mountain View, CA), monoclonal antibodies for flow cytometry were purchased from Becton Dickinson (BD) (Franklin Lakes, NJ) and Beckman Coulter (Brea, CA).
Cell harvest and culture
To thaw for use, each vial of ASCs and BMSCs was removed from the liquid nitrogen (−140°C) Dewar and rapidly thawed in a 37°C water bath. The vial was removed from the water bath immediately upon thawing, wiped with 70% ethanol, and opened inside a biological safety cabinet.
The thawed cells were transferred to 15 ml disposable centrifuge tube containing 10 volumes of corresponding growth medium (ASCs were cultured in DMEM/F12 1:1 supplemented with 3% FBS and BMSCs were cultured in MEM supplemented with 20% FBS and 200 mM L-glutamine) and the cells were subjected to centrifugation at 300g for 5 min at room temperature. After the supernatant was aspirated, the cell pellet was resuspended in fresh stromal medium and transferred to the culture vessel at a density of 5 × 103 cells/cm2 in either ASC stromal medium (DMEM/F12 Hams medium, 10% FBS, 1% antibiotic/antimycotic) or BMSC stromal medium (αMEM, 20% FBS, 1% antibiotic). The cells were maintained in a humidified tissue culture incubator at 37°C with 5% CO2.
The medium was replaced every second day until the cells reached 80% confluence. Cells were harvested by trypsin digestion for immunophenotypic analysis by flow cytometry or directly induced for either adipogenic or osteogenic differentiation (see below).
Immunophenotype of undifferentiated human ASC and BMSC by flow cytometry
Following the reagent manufacturer’s recommendations, the appropriate volumes of reagents were dispensed into a series of nine tubes (a protocol) as follows:
Tube 1: CD36 FITC, CD34 PE, CD19 ECD, CD11b PeCy5, CD45 PeCy7.
Tube 2: PCLP1 (podocalyxin) FITC, CD166 PE, CD90 PeCy5.
Tube 3: CD49b FITC, CD105 PE, CD184 APC, CD3 PeCy7.
Tube 4: CD147, FITC, CD49c PE, CD29 PeCy5.
Tube 5: CD59 FITC, CD146 PE, CD79a PeCy5.
Tube 6: HLA-Class I ABC FITC, CD271 PE, CD49f PeCy5, CD117 PeCy7.
Tube 7: HLA-Class II FITC, CD73a PE, CD106 PeCy5.
Tube 8: HGF (c-met) FITC, CD49d PE, CD14 ECD, CD44 APC
Tube 9: Isotype control.
A complete listing of the antibodies used, including manufacturer, clone number, and isotype identification is presented in Supplementary Table 1. The tubes containing the antibody cocktails were prepared prior to staining and maintained at 4°C in the dark until they were required for analysis. Undifferentiated ASC or BMSC cells were harvested by trypsin digestion and resuspended in PBS at a final concentration of 1 × 106 viable cells/ml. Between 2.5 × 105 and 5×105 cells were aliquoted per antibody tube. Additionally, a control tube containing only cell suspension in the absence of antibody served as a control for autofluorescence. Approximately 2–4 million cells were required to complete the antibody part. Samples were mixed by gently vortexing and then incubated in the dark for 20 min at room temperature. Following the incubation, the cell suspensions were washed to remove the excess reagents, the test tubes re-filled with PBS, recapped, and gently vortexed prior to centrifugation for 1 min at 110 g. The pelleted cells were then washed a total of three times in PBS, re-suspended in 500 μl of PBS and gently vortexed to resuspend the cells. Using a plastic transfer pipette the stained cell suspensions were transferred to 12 mm × 75 mm test tubes and analyzed using a Beckman Coulter Epics FC500 flow cytometer running CXP software for acquisition and analysis.
Differentiation of ASCs and BMSCs
To induce adipogenesis, confluent cultures of ASCs and BMSC were cultured for 3 days in adipocyte differentiation medium (DMEM (high glucose)/F-12 1:1 supplemented with 3% FBS, 1 μM dexamethasone, 500 μM IBMX (methyl isobutyl methylxanthine), 33 μM biotin, 5 μM rosiglitazone, 100nM insulin, and 17μM pantothenate) (Dubois et al., 2008). The induced cells were re-fed every 3 days with adipocyte maintenance medium (adipocyte differentiation medium without the IBMX and rosiglitazone). As controls, equivalent wells of confluent cells were maintained in stromal medium. At day 9 of differentiation, control and adipogenic wells were fixed and stained with Oil Red O (see below). Unstained wells were harvested for total RNA using TriReagent (Molecular Research Center, Cincinnati, OH).
Oil Red O Staining
At day 10 after the induction of adipogenesis, the 24-well plates containing cultured ASC or BMSC were washed with PBS three times (1 ml/well), fixed with 10% formalin (1 ml/well), sealed to prevent dehydration, and stored at 4°C Subsequently, the fixative was aspirated and the individual wells were stained with 600 μl of freshly prepared 0.3% Oil Red O staining solution for 20 min and then washed five times with water. Photomicrographs of individual wells and scans of the entire plate were taken after the final wash. Upon completion of the photographs and scans, 400 μl of 100% isopropanol was added into each well and the plates were shaken for 2 h at room temperature. Eluates from each well were transferred to individual wells on a 96-well plate for optical density readings at 500 nm. Eluate from an empty well stained with Oil Red O, washed, and eluted as described above served as a blank control that was subtracted from all experimental data points. The subtracted values were then used to determine the relative ratio of Oil Red O staining for cells cultured under adipogenic conditions compared to cells cultured in stromal medium (Halvorsen et al., 2001a).
To induce osteogenic differentiation, confluent cultures of ASCs and BMSCs were cultured in DMEM high glucose supplemented with 10% FCS, 50μg/mL L-ascorbate-2-phosphate, 10nM dexamethasone, and 10 mM glycerophosphate for 2 weeks. Wells were harvested for total RNA in TriReagent or fixed for Alizarin Red staining of mineralized deposits as outlined as follows.
Alizarin Red staining
On day 12 after the induction of osteogenesis, the 24-well plates containing cultured ASC or BMSC were washed with 0.9% NaCl a total of four times (1 ml/well) and fixed with 70% ethanol (1 ml/well). Subsequently, the fixative was removed by aspiration and plates were stained with 2% Alizarin Red for 10 min and washed with H2O six times. Photomicrographs were taken after the final wash. The plates were destained by the addition of 400 μl of 10% cetylpyridinium chloride monohydrate to each well followed by shaking for 10 min at room temperature. The optical density of the eluates was then measured at OD540 with a Bio-Rad plate reader and the relative ratio of the cells cultured in osteogenic conditions determined relative to cells cultured in stromal medium. Eluate from an empty well stained with Alizarin Red, washed, and eluted as described above served as a blank control that was subtracted from all experimental data points prior to each calculation (Halvorsen et al., 2001b).
Semi-quantitative real-time RT-PCR
Total RNA was purified from samples using TriReagent according to the manufacturer’s specifications. Approximately 2 mg of total RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (MMLV-RT; Promega, Madison, WI), with Oligo-dT at 42°C for 1 h in a 20 μl reaction volume. Primers for genes of interest (listed in Table 1) were identified using Primer Express software (Applied Biosystems, Carlsbad, CA). Real-time RT-PCR was performed on diluted cDNA samples with SYBR® Green PCR Master Mix (Applied Biosystems) using the 7900 Real-Time PCR system (Applied Biosystems) under universal cycling conditions (95°C for 10 min; 40 cycles of 95°C for 15 sec; then 60°Cfor 1 min). The RT-PCR for all primer pairs had been validated and determined to display single peaks in their dissociation curves. All results were normalized relative to a cyclophilin B expression control (Zvonic et al., 2006; Goh et al., 2007).
TABLE 1.
Semi-quantitative real-time RT-PCR: total RNA from induced (adipogenesis or osteogenesis) or non-induced human ASC (n=12) and human bone marrow (n = 12) stem cells were analyzed by RT-PCR using the primer sets outlined below, an approach that has been validated in multiple prior studies (Wu et al., 2007, 2008)
| Gene | Accession # | Forward | Reverse |
|---|---|---|---|
| Cyclophilin B | M60857 | GGAGATGGCACAGGAGGAAA | CGTAGTGCTTCAGTTTGAAGTTCTCA |
| Adiponectin | NM_004797 | GGCCGTGATGGCAGAGAT | TTTCACCGATGTCTCCCTTAGG |
| aP2 | NM_001442 | AAAGAAGTAGGAGTGGGCTTTGC | CCCCATTCACACTGATGATCAT |
| C/EBPα | NM_004364.2 | GGGTCTGAGACTCCCTTTCCTT | CTCATTGGTCCCCCAGGAT |
| Leptin | NM_000230.1 | GGTTGCAAGGCCCAAGAA | ACATAGAAAAGATAGGGCCAAAGC |
| LPL | NM_000237.1 | CAGATGCCCTACAAAGTCTTCCA | TGATTGGTATGGGTTTCACTCTCA |
| PPARγ2 | NM_015869 | AGGCGAGGGCGATCTTG | CCCATCATTAAGGAATTCATGTCATA |
| E4BP4 | NM_005384 | ACATGTTGTCTGTTTGGTGTCTTTTT | ATACA GCCTTCGCATGGACTATC |
| GILZ1 | NM_198057.2 | CTGCAACCGCAACATCGA | CGGAGGCACTGTGGAAGAAG |
| GILZ2 | NM_004089.3 | GCCATGGATCTGGTGAAGAATC | TCGGATCTGCTCCTTCAGGAT |
| Osteocalcin | NM_199173.2 | GCCCAGCGGTGCAGAGT | TAGCGCCTGGGTCTCTTCAC |
| Osteonectin | NM_003118 | GCGGGACTGGCTCAAGAAC | GATCTTCTTCACCCGCAGCTT |
| RANKL | NM_003701 | CATCCCATCTGGTTCCCATAA | GCCCAACCCCGATCATG |
Results for each mRNA were normalized relative to the expression of cyclophilin B as a control.
Statistics
Values are reported as the mean ± standard deviation and were compared based on Students’ t-test. Significance was determined as P-value <0.05.
Results
Immunophenotypic profile of ASCs and BMSCs
While it would be desirable to compare ASC and BMSC isolated from the same donors, it is technically difficult to obtain tissues in this manner. Consequently, the current study evaluated ASC and BMSC isolated from independent groups of 12 healthy human adult female donors with statistically similar age profiles (mean ages of 31 and 29, respectively). Although the donor weight and height information required to calculate the BMI was not collected on the BMSC donors, the mean BMI of the ASC donors was 27.1, with 7 out of the 12 donors showing an overweight or obese phenotype. These values are consistent with recent analyses of the US demographic, where 64% of women are overweight or obese (Flegal et al., 2010). The flow cytometric histograms were compared between ASC and BMSC isolated from 12 human adult female donors based on their expression of a panel of cell surface markers including those associated with stromal cells (CD44, CD90, CD105, CD106, CD146, and CD166), endothelial cells (CD31, CD105, CD106, and CD166), hematopoietic cells (CD14, CD31, and CD45), and pericytes (CD146) (Fig.1 and Supplementary Figs. 1 and 2). Overall, the ASC and BMSC populations displayed expression of a common set of surface antigens including the activated lymphocyte common adhesion molecule CD166 (Table 2 and Supplementary Figs. 1 and 2). Nevertheless, multiple significant differences were noted with respect to the percentage of positive surface expression. For example, while ASCs displayed surface positivity for CD34, CD36, and CD49d (8%, 34%, and 83%, respectively), the corresponding levels on BMSCs were markedly reduced (0%, 0%, and 36%, respectively). In contrast, while BMSCs were positive for CD49f, CD106, and PODXL (85%, 23%, and 57%, respectively), these antigens were only dimly positive on ASCs (6%, 7%, and 9%, respectively).
Fig. 1.
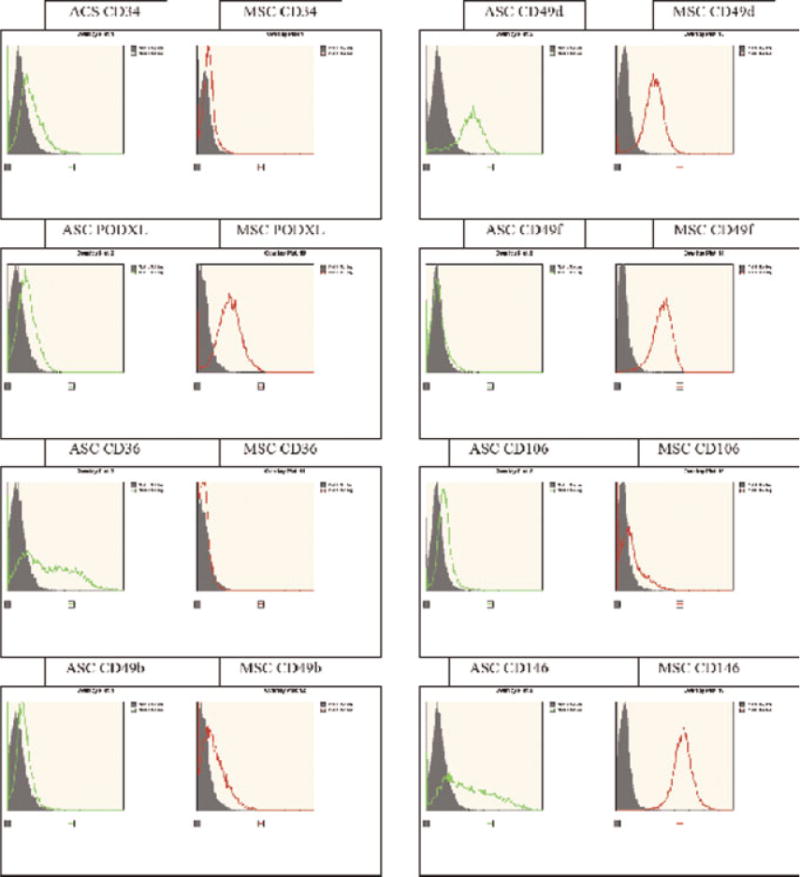
Immunophenotypic characterization of the ASC and BMSC from age-matched female donors: Flow cytometry histograms for selected surface protein antigens in representative female donors for ASC (left part, green) versus BMSC (right part, red).
TABLE 2.
Immunophenotypic profile of surface antigens on age-matched female donor ASCs and BMSCs
| Ab | ASC | BMSC | P-value |
|---|---|---|---|
| CD3 | 8 ± 3 | 0.5 ± 0.3 | <0.001 |
| CD11b | 1 ± 1 | 0 ± 0 | 0.012 |
| CD14 | 5 ± 7 | 0 ± 0 | 0.018 |
| CD19 | 1 ± 1 | 0 ± 0 | 0.188 |
| CD29 | 92 ± 5 | 99 ± 1 | <0.001 |
| CD34 | 8 ± 7 | 0 ± 0 | <0.01 |
| CD36 | 34 ± 10 | 0 ± 0 | <0.001 |
| CD44 | 86 ± 7 | 96 ± 1 | <0.001 |
| CD45 | 8 ± 3 | 0 ± 0 | <0.001 |
| CD49 | 11 ± 5 | 27 ± 15 | <0.01 |
| CD49c | 85 ± 14 | 98 ± 1 | <0.01 |
| CD49d | 83 ± 10 | 36 ± 18 | <0.001 |
| CD49f | 6 ± 3 | 85 ± 10 | <0.001 |
| CD59 | 95 ± 5 | 99 ± 0 | <0.01 |
| CD73a | 93 ± 4 | 99 ± 1 | <0.01 |
| CD79a | 6 ± 6 | 1 ± 1 | 0.034 |
| CD90 | 96 ± 3 | 91 ± 26 | 0.515 |
| CD105 | 88 ± 7 | 99 ± 1 | <0.001 |
| CD106 | 7 ± 5 | 23 ± 21 | 0.023 |
| CD117 | 2 ± 1 | 2 ± 3 | 0.875 |
| CD146 | 38 ± 24 | 99 ± 0 | <0.001 |
| CD147 | 91 ± 3 | 98 ± 1 | <0.001 |
| CD166 | 66 ± 18 | 90 ± 5 | <0.001 |
| CD184 | 5 ± 2 | 1 ± 1 | <0.001 |
| CD271 | 5 ± 2 | 6 ± 11 | 0.622 |
| HGFcmet) | 5 ± 4 | 3 ± 3 | 0.254 |
| HLA-abc | 94 ± 4 | 95 ± 2 | 0.683 |
| HLA-II | 2 ± 2 | 0 ± 0 | <0.01 |
| PODXL | 9 ± 5 | 57 ± 10 | <0.001 |
The values reflect the mean ( ± SD) percent surface positive staining for a part of surface antigens including hematopoietic, pericytic, and stromal markers (n = 12). The P-values were determined based on a paired, two tailed t-test.
Influence of culture conditions
The standard culture medium used for the expansion of human ASC and BMSC in the literature is different with respect to the medium composition and percentage of FBS (Dubois et al., 2008; Wolfe et al., 2008). Since it is conceivable that these differences in culture medium alone could account for any alterations in the expression profile of cell surface antigens, studies were conducted to compare two representative lots of ASC and BMSC expanded in parallel for one passage in either ASC stromal medium or BMSC complete culture medium (CMM) containing 10% FBS or 20% FBS, respectively (Supplementary Fig. 3). Under both culture conditions, each lot from both cell types displayed the same surface antigen expression profile for the entire part of antibodies. In all ASC and BMSC cell populations, CCM with 20% FBS increased the mean fluorescent intensity (MFI) for CD49f relative to stromal medium with 10% FBS. In three out of four cell populations, CCM increased the MFI for CD166 and decreased the MFI for CD49b. Furthermore, the presence of CCM increased the MFI for PODXL in both BMSC lots. Thus, culture medium composition had minimal effect on the percentage of surface positive cells and impacted MFI for only a subset of surface antigens.
Differentiation potential of ASCs and BMSCs: adipogenesis and osteogenesis
The adipogenic and osteogenic differentiation of human ASC and BMSC were compared based on histochemical assays and RNA expression levels (Figs. 2–5). While both cell populations displayed a robust adipogenic differentiation response based on morphological criteria, there was a statistically significant difference in the fold induction of Oil Red O staining, with the ASC (7-fold) exceeding that of the BMSC (2.85-fold) (Fig. 2). The expression of adipogenic-specific genes was examined by RT-PCR (Fig. 4). Following adipocyte differentiation, all mRNA levels increased significantly (P<0.05) and to a comparable extent in both the adipogenic-induced ASCs and BMSCs relative to their uninduced controls (Fig. 4A–F). In the uninduced cells, the mRNA expression levels of adiponectin and leptin were below levels of detection (<0.01 relative to the cyclophilin B control). While the mRNA levels in uninduced ASCs exceeded those of BMSCs for PPAR-gamma (0.83 ± 1.73 vs. 0.03 ± 0.09), C/EBP alpha (0.02 ± 0.02 vs. 0.01 ± 0), and aP2 (1.75 ± 4.09 vs. 0.02 ± 0.02), these differences were not significant.
Fig. 2.
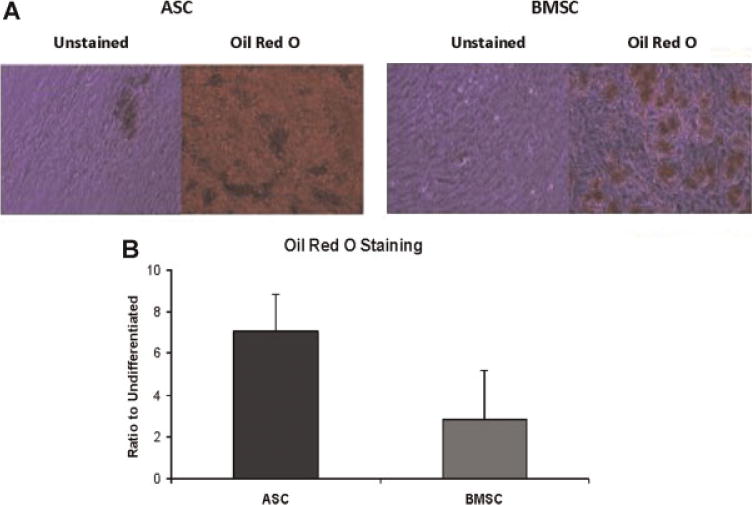
Adipogenic differentiation potential of ASC and BMSC: Histochemical stained Oil Red O photomicrograph of representative ASC and BMSC donor (A) and relative induction of Oil Red O staining (mean ± SD) under adipogenic conditions relative to untreated controls for n =12 or 11 donors for ASC and BMSC lineages, respectively (B).
Fig. 5.
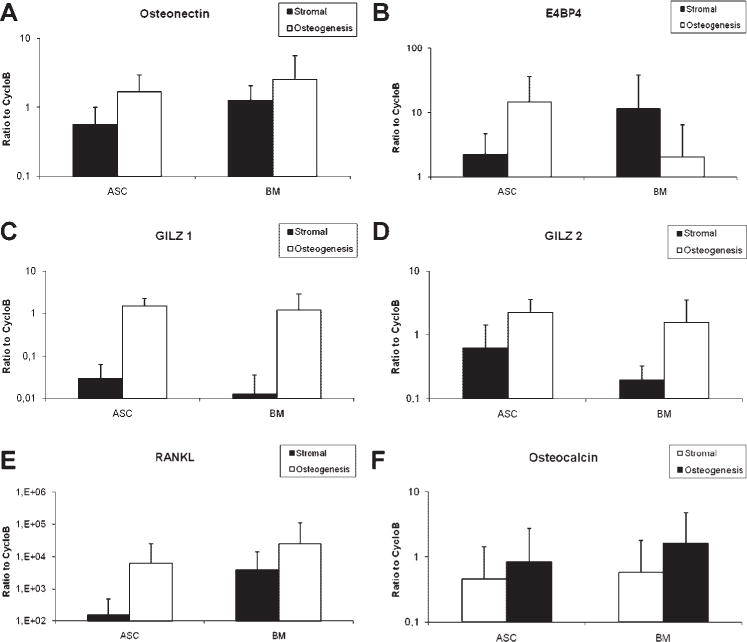
Expression of osteoblast lineage-associated mRNAs in vitro. Total RNA was isolated from ASCs (n = 12) and BMSCs (n =11) treated under pre-adipocyte (black) and adipocyte conditions (white) grown in the respective growth media. RT-PCR analysis was performed to detect expression of adipose markers, osteonectin (A), E4BP4 (B), GILZ1 (C), GILZ2 (D), RANKL (E), and osteocalcin (F). The values represent the mean ± standard error bars.
Fig. 4.

Expression of adipocyte lineage-associated mRNAs in vitro. Total RNA was isolated from ASCs (n =12) and BMSCs (n =11) treated under pre-adipocyte (black) and adipocyte conditions (white) grown in the respective growth media. RT-PCR analysis was performed to detect expression of adipose markers, PPARg2 (A), C/EBP alpha (B), adiponectin (C), leptin (D), lipoprotein lipase (LPL) (E), and adipocyte fatty acid-binding protein (AP2) (F). The values represent the mean ± standard error bars.
Both the ASC and BMSC differentiated along both the osteoblast lineage pathways based on histochemical analyses (Fig. 3); the fold induction in their Alizarin Red staining (2.16 vs. 3.25) was not significantly different. The expression of the osteoblast-associated mRNAs (E4BP4, GILZ1, GILZ2, osteocalcin, osteonectin, and RANKL) was determined by RT-PCR analysis (Fig. 5). Under osteogenic culture conditions, all mRNAs were inducedin both ASC and BMSC. There was no significant difference in the level of mRNA expression between the two cell types in either the uninduced or osteogenic differentiated state (Fig. 5A–F).
Fig. 3.
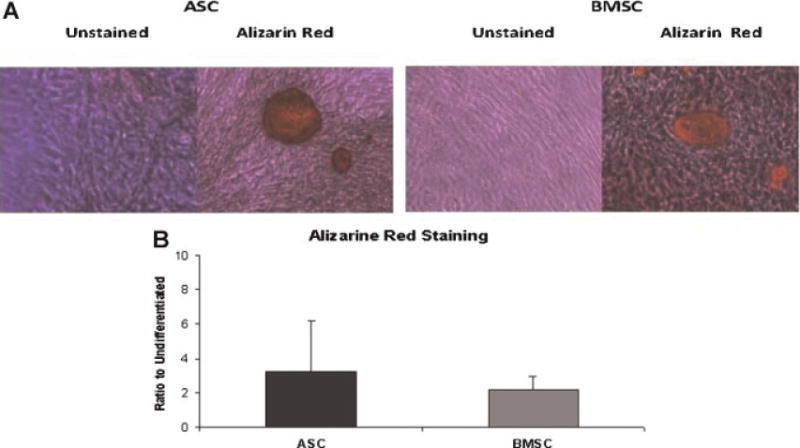
Osteogenic differentiation potential of ASC and BMSC: Histochemical stained Alizarin Red photomicrograph of representative ASC and BMSC donor (A) and relative induction of Alizarin Red staining (mean ± SD) under osteogenic conditions relative to untreated controls for n = 12 or 11 donors for ASC and BMSC lineages, respectively (B).
Discussion
Relative expression profile of surface antigens on ASCs versus BMSCs
This comparative study demonstrates that human ASC and BMSC derived from equivalent aged female subjects display a similar immunophenotypic profile for the majority of surface antigens; however, significant differences were noted in the percentage of cells positive for each antigen and/or their fluorescent intensity. While ASC were positive for the hematopoietic stem cell (HSC)-associated antigen CD34, a mucosiaoloprotein, they were only dimly positive for the CD34-related protein podocalyxin-like protein (PODXL) while lacking α6 integrin (CD49f). In contrast, BMSC-lacked CD34 but expressed high levels of both PODXL and CD49f. The combination of PODXL and CD49f has been used to select early BMSC progenitors exhibiting enhanced clonogenic and reduced aggregation properties (Lee et al., 2009). While both ASC and BMSC expressed the pericytic marker Muc-18 (CD146), the antigen was significantly more abundant on BMSC compared to ASC. In contrast, only the ASC expressed the fatty acid transporter (CD36), a surface antigen induced during adipogenesis (Abumrad et al., 1993). While some discrepancies in the detection of cell surface antigens on ASC did occur between the current findings and previous published findings, this likely reflects technical differences or the use of monoclonal antibodies detecting different epitopes and is oforms. For example, while Gronthos et al. (2001) detected V-CAM (CD106) on ASC, this was not detected in the current analysis; most likely, this reflects technical differences between the flow cytometric approach used in the current study and the immunohistochemical method used in (Gronthos et al., 2001), as well as possible differences in culture medium composition. Overall, the current observations are consistent with previous studies showing that human ASCs cells express many of the same adhesion proteins that BMSCs use to support the proliferation and differentiation of HSCs (Gronthos et al., 2001; Zuk et al., 2002; Aust et al., 2004; McIntosh et al., 2006; Mitchell et al., 2006). It should be noted that our previously published studies examining freshly isolated human ASCs have detected higher levels of CD34 positivity on the cell surface as compared to the values reported in the current manuscript (Yu et al., 2010). The fact that the current manuscript examined cryopreserved cells exclusively, rather than freshly isolated ASCs, may account for this discrepancy.
Similarities between ASCs and BMSCs extend to their secretome, which contains many of the same hematopoietic supportive cytokines (M-CSF, GM-CSF, tumor necrosis factor-α (TNFα), IL-6, IL-7, IL-8, IL-11, and stem cell factor (SCF)). In the BM microenvironment, these cytokines mediate the differentiation of CD34+ HSCs along the B-cell, T-cell, and myeloid lineages and this can be replicated with ASC or BMSC feeder layer in vitro co-cultures supporting human HSCs (Koc and Lazarus, 2001; Kilroy et al., 2007). In adipose depots, the ability of ASCs to release such pro-inflammatory cytokines may account for their contribution to the inflammatory events associated with pathophysiology of obesity and diabetes (Cousin et al., 2001; Weisberg et al., 2003; Xu et al., 2003; Wang et al., 2005; Wood et al., 2005).
Differentiation potential of ASCs and BMSC under identical inductive conditions
Adipose cell differentiation is the result of a sequential induction of specific genes leading to lipid accumulation. The two transcription factors on the part of adipocyte mRNAs, peroxisome proliferator-activated receptor γ (PPARγ), and CCAAT/enhancer-binding protein a (C/EBPα), play critical roles in adipogenic regulation (Morrison and Farmer, 1999; Rosen and Spiegelman, 2000; Wu et al., 1996). Directly or indirectly, they drive the expression of the remaining adipogenic mRNAs on the part encoding the fatty acid-binding protein, aP2, the enzyme lipoprotein lipase, and the adipokines; adiponectin, and leptin (Zuk et al., 2001, 2002; Sen et al., 2001). Consistent with prior studies, the levels of mRNA for adiponectin, aP2, and leptin increased by several log10 orders of magnitude during the differentiation process in both ASCs and BMSCs indicating that the differentiation results in changes of gene expression profiles (Sen et al., 2001; Yu et al., 2010). While some studies in the literature have reported no significant difference between human ASC and BMSC adipogenic potential in vitro (De Ugarte et al., 2003), others have suggested that the BMSC differentiation is reduced relative to ASCs (Sakaguchi et al., 2005). The current data are consistent with the latter observation. Under identical inductive conditions, human ASC adipogenesis exceeded that of BMSCs based on Oil Red O staining; however, no significant difference was observed at the level adipogenic mRNAs levels based on RT-PCR analyses.
The current study confirmed that ASCs and BMSCs, under identical inductive conditions in vitro, are capable of adipogenesis and osteogenesis based on morphology, histochemical staining, and mRNA expression profiles. While multiple previous studies have shown that ASCs and BMSCs possess the capacity to differentiate along the adipocyte and osteoblast lineage pathways including (De Ugarte etal., 2003; Gimble and Guilak, 2003; Strem et al., 2005), there is evidence suggesting that the cells retain some characteristics of their tissue of origin (Sakaguchi et al., 2005). The current study is partially consistent with the latter observations. Under adipogenic conditions, human ASCs displayed a significantly greater induction of neutral lipid accumulation based on Oil Red O staining as compared to BMSC. Furthermore, the levels of multiple adipogenic mRNAs was greater in both induced and uninduced ASCs compared to equivalent BMSCs, although these values did not reach statistical significance. These observations suggest that ASCs may be more responsive to adipogenic stimuli due to their tissue of origin. While it is possible that epigenetic programming mechanism during tissue development could account for these findings, further studies will be required to explore this possibility (Eilertsen et al., 2008).
In contrast, the in vitro osteogenic response of BMSCs was not significantly greater than that of ASCs. While further in vivo studies will be required to document whether ASCs and BMSCs perform osteogenic functions equally well, these in vitro findings lend credence to the use of human ASCs for orthopedic repair. Consistent with this are exciting recent studies in the clinical literature reporting complete repair of craniofacial defects in patients using autologous ASCs (Mesimäki et al., 2009).
The panel of osteoblast-associated markers included mRNAs encoding the secreted matrix proteins osteocalcin and osteonectin as well as the osteoclastogenic cytokine, RANKL. Of interest is the fact that osteonectin, also known as secreted protein acidic and rich in cysteine (SPARC), has been shown to inhibit adipogenesis through a β-catenin-mediated mechanism (Nie and Sage 2009). Two isoforms of the glucocorticoid-induced leucine zipper (GILZ) transcription factor and E4BP4, a transcription factor activated by PTH, were also examined (Ozkurt and Tetradis, 2003; Shi et al., 2003; Ozkurt et al., 2004); each also displays an oscillatory expression profile consistent with a circadian biological rhythm (Zvonic et al., 2006; Gimble et al., 2009). GILZ has been found to regulate adipogenic and osteogenic differentiation in a reciprocal manner. Overexpression of GILZ mRNA in the 3T3-L1 pre-adipocytes significantly inhibited lipid vacuole accumulation and expression of the C/EBPα and PPARγ2 mRNAs (Shi et al., 2003). These results demonstrated that GILZ functions as a transcriptional repressor of PPAR-γ2 (Shi et al., 2003). In gain of function experiments, overexpression of GILZ enhanced BMSC osteogenesis based on mineralization and gene expression analyses (Zhang et al., 2008). In parallel studies, GILZ transduction of BMSC inhibited adipogenesis (Zhang et al., 2008). Consistent with these observations, both GILZ1 and GILZ2 mRNA levels were increased after osteogenic differentiation in both human ASCs and BMSCs. In both cell types, the level of osteogenic GILZ1 and GILZ2 mRNAs induction was not significantly different. Together, these data suggest that, by modulating PPAR-γ expression, GILZ may serve as an important regulator of the ASC and BMSC lineage commitment along the adipocyte and osteoblast pathways. This role of GILZ may have potential clinic relevance in the context of human aging, which has been associated with an increase in marrow adipogenesis at the expense of osteoblast function (Gimble et al., 2006). Thus, GILZ may be a potential therapeutic target for a variety of conditions characterized by an altered adipocyte/osteoblast balance.
The E4BP4 factor, also know as nuclear factor IL-3 (NFIL-3), has been associated with parathyroid hormone signal transduction and the regulation of osteoblast differentiation (Ozkurt and Tetradis, 2003; Ozkurt et al., 2004). This has potential implications in light of the multi-lineage differentiation potential of adipose-derived stem cells (ASCs) and the inverse relationship between the adipocyte and osteoblast pathways (Gimble et al., 2006). Consistent with these observations, ASCs displayed a significantly higher expression of E4BP4 compare to BMSCs during the differentiation process. Future studies comparing the osteogenic function of ASCs and BMSCs should consider E4BP4 as a potential transcriptional regulator of lineage commitment.
Summary
This study provides a detailed comparison between the characteristics of ASC and BMSC derived from sizable cohorts of adult human female donors (n=12). One potential limitation of the study is the fact that adipose tissue and BM samples were not collected from the same donors. Nevertheless, the consistency between the findings suggests that there is a high degree of reproducibility between cell types without substantial biological variability. Furthermore, while a panel of 30 monoclonal antibodies was employed in the flow cytometric comparisons, more global and unbiased comparisons need to be performed between the ASC and BMSC populations. To this end, studies using proteomic and transcriptomic approaches have been performed (Izadpanah, Kheterpal, Gimble, Bunnell, manuscript in preparation). In addition, the current study examined ASC and BMSC harvested at 80–90% confluence. There is evidence in the literature that adherent stem/stromal cells alter their gene expression profile and display contact inhibition when cultured under exponential growth or confluent conditions (Sekiya et al., 2002; Larson et al., 2008). The replication status and confluence of the culture conditions may be variables that have influenced the outcomes in this study and there would be value for future studies to contrast the expression profiles between exponentially dividing, subconfluent and confluent ASC and BMSC cultures. Nevertheless, the current findings support the potential utility of both human ASCs and BMSCs for the clinical regeneration of both adipose tissue and bone.
Supplementary Material
Acknowledgments
The authors wish to thank the following individuals: Laura Dallam for administrative and secretarial assistance; Dr. James Wade and Dr. Elizabeth Clubb, their office staff, and patients for providing tissues; the staff of the Cell Culture Core at Tulane; the Pennington Biomedical Research Foundation (J.M.G.), the Clinical Nutrition Research Unit Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK (J.M.G., G.Y.), the NIH/NHLBI (P01 HL075161), Louisiana Gene Therapy Research Consortium, and Tulane University (B.A.B., AT.) for funding.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008a;2:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell BA, Estes BT, Guilak F, Gimble JM. Differentiation of adipose stem cells. Methods Mol Biol. 2008b;456:155–171. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- Cousin B, Andre M, Casteilla L, Penicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J Cell Physiol. 2001;186:380–386. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dawn B, Bolli R. Adult bone marrow-derived cells: Regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dubois S, Halvorsen YDC, Ravussin E, Gimble JM. Primary stromal cell culture from adipose tissue: From liposuction to needle biopsy. Adipocytes. 2005;1:139–144. [Google Scholar]
- Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, Halvorsen YD, Ravussin E, Gimble JM. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69–79. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- Eilertsen KJ, Floyd Z, Gimble JM. The epigenetics of adult (somatic) stem cells. Crit Rev Eukaryot Gene Expr. 2008;18:189–206. doi: 10.1615/critreveukargeneexpr.v18.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Obden CL, Curtin LR. 1999–2008 Prevalence and trends in obesity among US adults. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Friedenstein A. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Gimble JM. Adipose tissue derived therapeutics. Expert Opin Biol Ther. 2003;3:705–713. doi: 10.1517/14712598.3.5.705. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:360–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Ptitsyn AA, Goh BC, Hebert T, Yu G, Wu X, Zvonic S, Shi XM, Floyd ZE. Delta sleep-inducing peptide and glucocorticoid induced leucine zipper Potential links between circadian mechanisms and obesity? Obes Rev. 2009;10:46–51. doi: 10.1111/j.1467-789X.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- Goh BC, Wu X, Evans AE, Johnson ML, Hill MR, Gimble JM. Food entrainment of circadian gene expression altered inPPARα−/− brown fat and heart. Biochem Biophys Res Commun. 2007;360:828–833. doi: 10.1016/j.bbrc.2007.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: Biochemical, cellular, and molecular analysis. Metabolism. 2001a;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001b;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Koc ON, Lazarus HM. Mesenchymal stem cells: Heading into the clinic. Bone Marrow Transplant. 2001;27:235–239. doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- Larson BL, Ylostalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008;26:193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and α6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infracted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen YD, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunophenotype of human adipose derived cells: Temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. J Cell Biochem. 1999;32–33:59–67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. Review. [DOI] [PubMed] [Google Scholar]
- Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkurt IC, Tetradis S. Parathyroid hormone-induced E4BP4/NFIL3 down-regulates transcription in osteoblasts. J Biol Chem. 2003;278:26803–26809. doi: 10.1074/jbc.M212652200. [DOI] [PubMed] [Google Scholar]
- Ozkurt IC, Pirih FQ, Tetradis S. Parathyroid hormone induces E4bp4 messenger ribonucleic acid expression primarily through cyclic adenosine 3′,5′-monophosphate signaling in osteoblasts. Endocrinology. 2004;145:3696–3703. doi: 10.1210/en.2003-1436. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Sen A, Lea-Currie YR, Sujkowski D, Franklin DM, Wilkinson WO, Halvorsen YD, Gimble JM. The adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001;81:312–319. doi: 10.1002/1097-4644(20010501)81:2<312::aid-jcb1046>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Shi X, Shi W, Li Q, Song B, Wan M, Bai S, Cao X. A glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells. EMBO Rep. 2003;4:374–380. doi: 10.1038/sj.embor.embor805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: Integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MPR, Swaney W, Reger RL. Isolation and culture of bone marrow-derived human multipotential stromal cells (hMSCs) Methods Mol Biol. 2008;449:3–25. doi: 10.1007/978-1-60327-169-1_1. [DOI] [PubMed] [Google Scholar]
- Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun. 2005;337:422–429. doi: 10.1016/j.bbrc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, Eckel RH, Mynatt RL, Gimble JM. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity. 2007;11:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA, Pelled G, Izadpanah R, Gazit D, Bunnell BA, Gimble JM. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861–870. doi: 10.1016/j.bone.2007.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyses. Cytotherapy. 2010;12:538–546. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang N, Shi XM. Regulation of mesenchymal stem cell osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ) J Biol Chem. 2008;283:4723–4729. doi: 10.1074/jbc.M704147200. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz KP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Urgate DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–967. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


