Abstract
The Transient Receptor Potential (TRP) channel family is comprised of a large group of cation-permeable channels, which display an extraordinary diversity of roles in sensory signaling. TRPs allow animals to detect chemicals, mechanical force, light, and changes in temperature. Consequently, these channels control a plethora of animal behaviors. Moreover, their functions are not limited to the classical senses, as they are cellular sensors, which are critical for ionic homeostasis and metabolism. Two genetically tractable invertebrate model organisms, Caenorhabditis elegans and Drosophila melanogaster, have led the way in revealing a wide array of sensory roles and behaviors that depend on TRP channels. Two overriding themes have emerged from these studies. First, TRPs are multitasking proteins, and second, many functions and modes of activation of these channels are evolutionarily conserved, including some that were formerly thought to be unique to invertebrates, such as phototransduction. Thus, worms and flies offer the potential to decipher roles for mammalian TRPs, which would otherwise not be suspected.
Keywords: Drosophila TRP channels, Sensory transduction, Invertebrate TRP channels, Vision, Chemosensation, Thermosensation, Nociception, Mechanosensation, Courtship, Disease models
1 Introduction
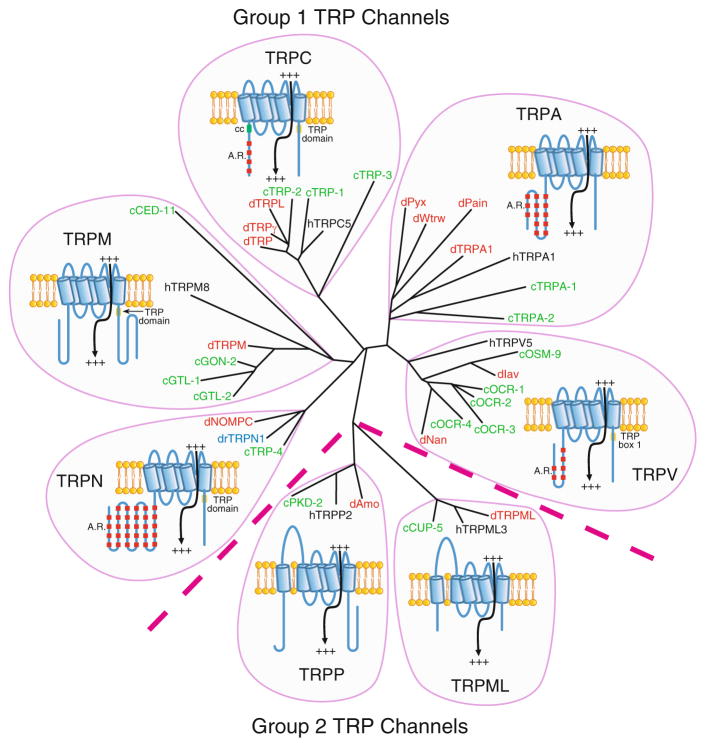
TRP channels include six transmembrane segments and are subdivided into seven subfamilies on the basis of amino acid homology (Fig. 1) (Venkatachalam and Montell 2007). The channels that are the most homologous to the original TRP protein (Montell and Rubin 1989), which functions in Drosophila phototransduction, belong to the TRPC subfamily. The TRPCs as well as four other subfamilies (TRPV, TRPM, TRPA, and TRPN) constitute the “group 1 TRPs” (Fig. 1) (Montell 2005). Two other subfamilies (TRPP and TRPML) that are relatively similar to each other in sequence, but are more distantly related group 1 TRPs, are referred to as “group 2 TRPs.”
Fig. 1.
Phylogenetic tree and cartoons of TRP channels from C. elegans (c; green), Drosophila melanogaster (d; red), and Homo sapiens (h; black). The TRPN channel from Danio rerio (dr; zebrafish; blue) is also included. To generate the tree, we used the predicted transmembrane segments of each TRP channel (http://www.cbs.dtu.dk/services/TMHMM/) in combination with the following online program: http://www.genome.jp/tools/clustalw/. Indicated on the TRP cartoons are ankyrin repeats (A.R.), coiled coil domains (cc), and the TRP domains, which include TRP boxes 1 and 2 (Montell 2001, 2005). TRPV proteins include only TRP box 1, rather than the full TRP domain. The N- and C-termini are the left and right ends of the TRP structures. The TRP termini are situated on the cytoplasmic side of the lipid bilayer
Due to the broad roles of TRPs in sensory signaling, they are critically important in allowing animals to sense a changing environment. As such, it is not surprising that these channels are ancient, evolutionarily conserved proteins that function in a wide range of metazoan organisms. These include invertebrates, such as worms (Kahn-Kirby and Bargmann 2006; Xiao and Xu 2009), arachnids (e.g., ticks), insects (e.g., flies, mosquitoes and bees) (Matsuura et al. 2009; Wolstenholme et al. 2011), and vertebrates such as zebrafish, mice, and humans (Venkatachalam and Montell 2007; Wu et al. 2010). However, the range of TRP channels in some ancient organisms, such as protozoa, is limited to group 2 TRPs (Dong et al. 2010; Wolstenholme et al. 2011).
In invertebrates, the functions of TRP channels have been studied most extensively in two genetically tractable model organisms—the fruit fly, Drosophila melanogaster (Fowler and Montell 2013), and the roundworm, C. elegans (Xiao and Xu 2011). All seven subfamilies are represented in flies and worms, although these organisms have fewer TRP channels than humans. Nevertheless, the TRPA subfamily is the largest in insects, even though only one representative exists in mice and humans. This notable difference might reflect a particularly important role for TRPA channels in sensing environmental chemicals and changes in temperature, since poikilothermic animals such as insects are particularly sensitive to heat and cold and are subjected to a very complex repertoire of compounds in their surroundings.
Characterization of TRPs in worms and flies underscore the theme that individual TRP channels do not respond to one type of sensory stimuli. Rather, a single TRP channel is capable of sensing a surprisingly broad range of sensory input. In this regard, Drosophila TRPA1 is a particularly notable polymodal sensor, as it functions in the avoidance of noxious volatile and nonvolatile chemicals, intense light, excessively warm temperatures, and small temperature differences in the comfortable range.
2 Sensory Transduction
The peripheral nervous system in Drosophila is composed of four general types of sensory elements. These include (1) external sense organs, such as chemosensory and mechanosensory bristles (sensilla); (2) chordotonal organs, which serve in part as stretch receptors; (3) multidendritic neurons; and (4) photoreceptor cells. C. elegans senses the external world through sensillar organs and a variety of isolated sensory neurons.
2.1 Light Sensation
2.1.1 Role of TRPs in Image Formation in Drosophila
In 1969, Cosens and Manning described a Drosophila mutant characterized by a loss of a sustained light response (Cosens and Manning 1969). Using a simple field recording, the electroretinogram (ERG), the flies displayed only a transient response to light. The basis for the transient receptor potential (trp) phenotype was enigmatic, despite a series of studies over the next two decades (Montell 2011). The first indication as to the function of TRP emerged from the cloning of the trp gene, and the observation that the TRP protein had a predicted transmembrane topology similar to the limited number of ion channels and transporters known at the time (Montell and Rubin 1989). A subsequent report demonstrated that loss of trp resulted in the rapid decrease the light-activated Ca2+ conductance (Hardie and Minke 1992). Together, these findings supported the model that trp encodes a Ca2+ permeable channel.
In vitro biophysical analyses of TRP, and a related channel identified in 1992 (TRP-Like; TRPL) (Harteneck et al. 1995; Hu et al. 1994; Phillips et al. 1992; Vaca et al. 1994; Xu et al. 1997), indicated that both TRP and TRPL were cation channels. Direct in vivo evidence demonstrating that TRP is a Ca2+ permeable channel was obtained by manipulation of the selectivity filter, resulting in a dramatic decrease in the light-induced Ca2+ conductance (Liu et al. 2007a).
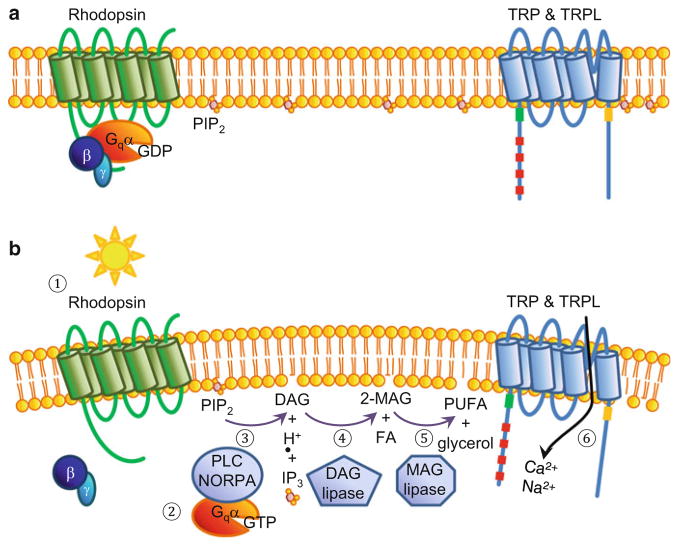
TRP and TRPL are activated via a signaling pathway that couples light stimulation of rhodopsin with a heterotrimeric G protein (Gq) that engages a phospholipase Cβ (PLCβ) encoded by the norpA locus (Bloomquist et al. 1988; Inoue et al. 1985) (Fig. 2). PLCβ catalyzes the hydrolysis of phosphoinositide-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3), diacylglycerol (DAG), and a proton (H+) (Huang et al. 2010). The enzymatic activity of NORPA is required for gating of TRP and TRPL since replacement of a single residue that is critical for phospho-lipase C activity eliminates the light response (Wang et al. 2008).
Fig. 2.
Model for activation of Drosophila TRP and TRPL. (a) The fly phototransduction cascade is not active, and the TRP and TRPL channels are closed in photoreceptor cells maintained in the dark. (b) The light-activated phototransduction cascade. (1) Light activation of rhodopsin. (2) GTP is exchanged for GDP on the Gα subunit (Gqα; also known as Gα49B) and the βγ subunit dissociates from the α subunit. (3) Activation of PLC (phospholipase Cβ) catalyzes the hydrolysis of PIP2 (phosphoinositide-4,5-bisphosphate (PIP2) into DAG (diacylglycerol), IP3 (inositol-1,4,5-trisphosphate), and H+ (proton). (4) The major products of the DAG lipase (encoded by the inaE locus) are 2-MAG (2-monoacylglycerol) and a saturated FA (fatty acid). Not shown are the minor products: 1-MAG and a polyunsaturated FA (PUFA). (5) Activity of an unknown MAG lipase catalyzes the production of a PUFA and glycerol. (6) TRP and TRPL are activated, leading to influx of Ca2+ and Na+. The cleavage of PIP2 (step 3) is proposed to contribute to activation of TRP and TRPL through a change in curvature of the plasma membrane, thereby creating mechanical force
The activation mechanism of TRP and TRPL has been scrutinized extensively and is still not fully resolved. Nevertheless, it is clear that neither IP3 nor the IP3-receptor is important for channel activation (Acharya et al. 1997; Raghu et al. 2000). According to one model, TRP and TRPL are activated by polyunsaturated fatty acids (PUFAs) such as arachidonic acid and linoleic acid, which are derived from metabolism of DAG (Chyb et al. 1999). Another proposal is that a decline in PIP2, which is inhibitory, and a decrease in pH, due to the production of H+, are the two signals necessary for channel activation (Huang et al. 2010).
The question then arises as to why PIP2 depletion leads to channel activation. A provocative concept is that light stimulation activates TRP and TRPL through mechanical gating (Hardie and Franze 2012) (Fig. 2b). PIP2 hydrolysis appears to cause minute mechanical contractions of the photoreceptor cell membrane due to removal of the bulky head group of the PIP2, and these membrane contractions may activate the TRP/TRPL channels (Hardie and Franze 2012). In further support of the concept that TRP and TRPL are mechanically gated, incorporation of a mechanically activated monovalent cation channel (gramicidin) into the membranes of photoreceptor cells dissociated from the trpl;trp double mutant is sufficient to induce a light conductance (Hardie and Franze 2012).
The mechanisms of phototransduction in the Drosophila photoreceptors are in stark contrast to those occurring in vertebrate rods and cones. Whereas Drosophila phototransduction involves depolarization of the photoreceptors, light hyperpolarizes vertebrate rods and cones (Fu and Yau 2007). On the other hand, the intrinsically photosensitive retinal ganglion cells (ipRGCs) in the mammalian retina, which participate in circadian entrainment and the pupillary light response (Berson et al. 2002; Provencio et al. 2000; Schmidt et al. 2011), use a phototransduction cascade that employs TRPC6 and TRPC7 and bears a striking resemblance to the Drosophila phototransduction cascade (Xue et al. 2011).
2.1.2 Non-image Light Sensing in Worms and Flies
Drosophila larvae also sense light and do so through two types of phototransduction cascades, both of which depend on TRP channels. However, in contrast to the adult visual system, which functions in image detection, larval phototransduction participates in phototaxis only. The two discrete larval phototransduction cascades take place in separate body parts and are activated by different light intensities. Low to moderate light is received by the Bolwig’s organ, which appears to use a cascade that is initiated by rhodopsin and culminates with activation of the TRP and TRPL channels, since these signaling proteins are expressed in this tissue (Petersen and Stowers 2011; Sprecher and Desplan 2008).
Intense bright light is detected by class IV multidendritic sensory neurons that tile the Drosophila larval body wall (Xiang et al. 2010). This light avoidance requires TRPA1, but remarkably, it appears to be independent of rhodopsin, PLC, and the flavin-based light sensor, cryptochrome (Xiang et al. 2010). Rather, the photoresponse of the class IV neurons requires a member of the gustatory receptor family (GR28b) (Xiang et al. 2010), which is the Drosophila homolog of LITE-1—a protein that functions in light detection in C. elegans (Liu et al. 2010; Ward et al. 2008). Whether Gr28b homozygous mutant flies have a defect in light avoidance behavior has not been reported. Other open questions are whether GR28b binds a chromophore, the spectral sensitivity of this light receptor, and how activity of GR28b is coupled to TRPA1. One possibility is that GR28b engages a heterotrimeric G protein. However, at least some members of the insect GR family have a topology opposite to classical G protein-coupled receptors (Zhang et al. 2011).
2.2 Chemical Senses
Volatile chemicals are detected through the sense of smell, while nonvolatile chemicals are sensed through contact chemosensation, which includes the sense of taste.
2.2.1 Odor and CO2 Detection (Non-contact Chemosensation)
The first TRP channel shown to have a role in invertebrate chemosensation is OSM-9. This TRPV protein couples to an odorant receptor, ODR-10, and plays a regulatory role in C. elegans olfaction (Colbert et al. 1997). The osm-9 mutants are defective in chemotaxis towards a subset of olfactory cues that are mediated by the AWA neurons. These mutants also exhibit reduced avoidance to benzaldehyde, which depends on ASH neurons, and display diminished olfactory adaptation to some odorants detected by AWC neurons (Colbert et al. 1997).
Drosophila use two primary organs to sense volatile chemical, the third antennal segment and the maxillary palp (Vosshall and Stocker 2007). The olfactory receptor neurons (ORNs) that extend from these organs terminate in the antennal lobe, which send projection neurons into higher brain centers (Vosshall and Stocker 2007). The two main classes of channels that are critical for Drosophila olfaction are seven-transmembrane ionotropic receptors, referred to “olfactory receptors” (ORs) (Clyne et al. 1999; Gao and Chess 1999; Robertson et al. 2003; Vosshall et al. 1999), and ionotropic receptors (IRs) (Benton et al. 2009), which are distantly related to glutamate receptors. Several Drosophila TRP channels also participate in olfaction, although they are not the primary olfactory detectors. For instance, the classical TRP plays a role in olfactory adaptation (Störtkuhl et al. 1999). In addition, TRPA1 is expressed in ORNs and is required for avoiding the naturally occurring insect repellent, citronellal (Kwon et al. 2010a). Fly TRPA1 is not effectively activated by citronellal directly. Rather, it is activated through a Gq and PLC (NORPA)-dependent signaling cascade. However, the TRPA1 expressed by the mosquito, Anopheles gambiae, which is an insect vector that spreads malaria, is directly and potently activated by citronellal (Kwon et al. 2010a). Both TRP and TRPL are co-expressed in CO2-sensitive olfactory receptor neurons (ORNs) in the antennae and contribute to CO2 avoidance, possibly through a Gq/PLC signaling cascade (Badsha et al. 2012). Another TRPA channel, Painless (Tracey et al. 2003), functions in projection neurons emanating from the olfactory glomeruli, where it participates in an olfactory circuit that inhibits male-male courtship (Wang et al. 2011).
2.2.2 Taste (Contact Chemosensation)
The sense of taste in flies is mediated through gustatory receptor neurons (GRNs) housed in hairlike projections referred to as sensilla. Gustatory sensilla are distributed on multiple body parts, including the main gustatory organ (labellum), the legs, wing margins, and ovipositor. Taste in flies depends predominately on a large family of gustatory receptors (GRs), which are related to ORs, and at least one IR (Montell 2009; Zhang et al. 2013b). This is in contrast to mammalian taste, which is mediated largely through a TRP channel (Pérez et al. 2002; Zhang et al. 2003). Nevertheless, at least three TRP channels participate in Drosophila taste sensation (TRPA1, Painless, and TRPL) as described below.
TRPA1 is expressed in the main taste organ, the labellum, and is critical for sensing the naturally occurring plant compound, aristolochic acid (Kim et al. 2010). Activation of TRPA1 through this chemical depends on the same Gq and PLC that is required for phototransduction in the compound eye and for activating TRPA1 in ORNs. TRPA1 is also expressed in internal mouthparts where it is employed to detect a subset of aversive chemicals prior to ingestion (Kang et al. 2010a). As with mammalian TRPA1, Drosophila TRPA1 is a receptor for electrophilic chemicals, such as allyl isothiocyanate (AITC) (Bandell et al. 2004; Hinman et al. 2006; Jordt et al. 2004; Kang et al. 2010a; Macpherson et al. 2007). Avoidance of AITC—the pungent ingredient of wasabi—also appears to depend on Painless (Al-Anzi et al. 2009). However, whether Painless is required in GRNs or postsynaptic to these afferent neurons is unclear. In C. elegans, the TRPV channel, OSM-9, mediates avoidance behavior towards the bitter chemical quinine (Hilliard et al. 2004).
The TRPL channel functions in diet-induced changes in taste preference through a mechanism that employs reversible changes in GRNs (Zhang et al. 2013c). Animals including flies tend to avoid some unpalatable compounds, if more appealing options are available in the diet. Indeed, flies will reject the bitter tasting compound, camphor. However, after long-term exposure to camphor, the animals reduce their distaste to this food ingredient. TRPL is expressed in GRNs that mediate an avoidance response and is directly activated by camphor. Persistent exposure to TRPL causes ubiquitination and downregulation of TRPL, thereby diminishing camphor rejection (Zhang et al. 2013c). If camphor is removed from the diet for a long time, the concentration of TRPL gradually increases, thereby restoring the original aversion to this food additive. Thus, the reversible changes in TRPL levels provide a mechanism whereby an animal can change their food preferences as a result of long-term alterations in the diet.
2.3 Thermal Sensation
Animals avoid noxious heat and cold for survival and seek out the ideal temperature zone to maximize comfort. Responding to small differences in temperature is especially important for poikilothermic organisms, since their body temperature equilibrates with the environment. Consequently, in invertebrate animals, changes in temperature affect the rate of growth and development, and aging. In addition, since it is warmer during the day than at night, daily temperature fluctuations can set circadian rhythms. Seminal work on mammalian somatosensation established that channels such as TRPV1 are thermal sensors and can be directly activated by changes in temperature (Caterina et al. 1999; Dhaka et al. 2006). We now know that roles for TRPs as thermosensors are evolutionarily conserved, and invertebrate thermoTRPs sense changes in temperature for multiple purposes, beyond the avoidance of noxious heat and cold.
2.3.1 Nociception
2.3.1.1 Responding to Warm and Hot Temperatures
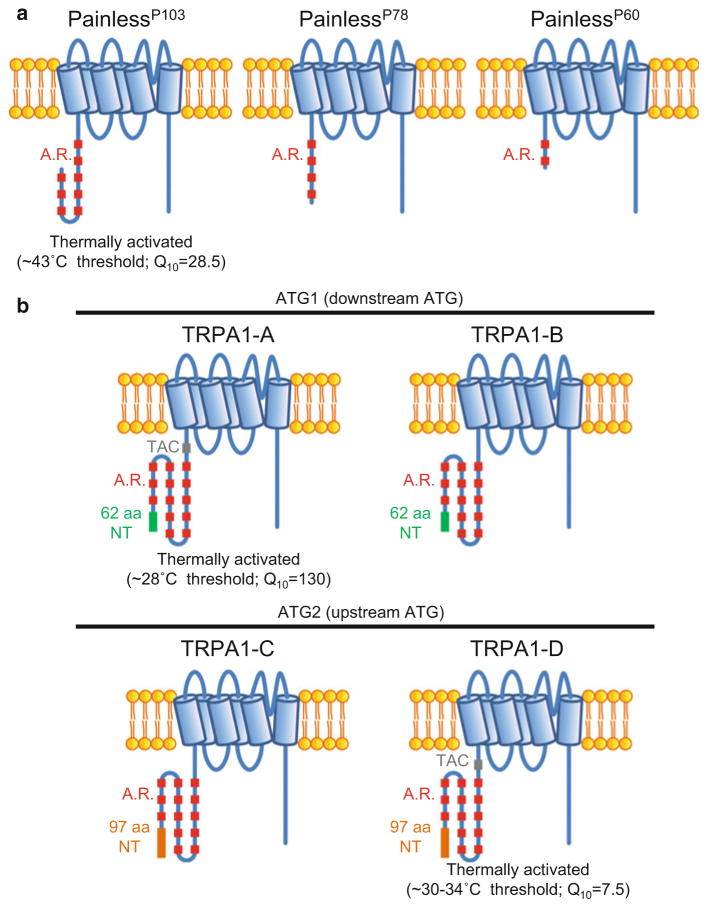
Three channels, all within the TRPA subfamily, contribute to nociceptive responses of Drosophila larvae to excessive heat. Normally, wild-type larvae move along a surface parallel to their body axis. However, if they are exposed to a heat probe with a threshold around 40 °C, they quickly roll in a corkscrew fashion around their body axis. This writhing response depends on Painless expression in multidendritic neurons in the body wall (Tracey et al. 2003). There are three Painless isoforms, one of which includes a long N-terminus with eight ankyrin repeats (PainlessP103) and another with a very short N-terminus (PainlessP60) (Fig. 3a). Expression of PainlessP103 rescues the pain mutant phenotype, but not PainlessP60 (Hwang et al. 2012). PainlessP103 appears to be a direct thermosensor, as it is activated with a threshold (≥42 °C) (Sokabe et al. 2008) similar to that needed to initiate the rolling response (Tracey et al. 2003).
Fig. 3.
Protein isoforms of Painless and Drosophila TRPA1. (a) Painless. (b) TRPA1. A.R. ankyrin repeats, NT N-terminal domain, TAC TRP ankyrin cap domain
Another TRPA channel, Pyrexia, is activated directly by warm temperatures (≥40 °C) and enable larvae to manage the thermal stress of relatively high temperatures (Lee et al. 2005). In the absence of Pyrexia, the larvae are paralyzed by exposure to 40 °C for several minutes (Lee et al. 2005).
TRPA1 participates in the response to high temperatures in both larvae and adult flies (Neely et al. 2011). The channel is activated with a threshold of ~25–29 °C (Viswanath et al. 2003) and is used as a sensor in larvae and adults to avoid temperature above this threshold, which are uncomfortable but not acutely dangerous (Hamada et al. 2008; Kwon et al. 2008; Rosenzweig et al. 2005). In adults, TRPA1-mediated temperature sensation in this range (≥25° C) is detected by sensory neurons in the brain (anterior cell neurons) (Hamada et al. 2008). There are four TRPA1 isoforms (Kwon et al. 2010a; Zhong et al. 2012), two of which include a 37 amino acid domain between the N-terminal 13 ankyrin repeat and the transmembrane segments (Fig. 3b). These two isoforms, TRPA1-A and TRPA1-D, are directly activated by increases in temperature, and the 37 amino acid “TRP ankyrin cap” (TAC) domain appears to be essential for thermal activation (Kang et al. 2012; Zhong et al. 2012) (note that the TRPA1-A and TRPA1-D isoforms are referred to as TRPA1-B and TRPA1-A, respectively, in one study)(Kang et al. 2012). The TRPA1-A isoform is remarkably sensitive to temperature activation, as it has a Q10 of ~130 (Kang et al. 2012). TRPA1-C does not appear to be a direct thermosensor. Yet, expression of this isoform also rescues the trpA1 nociceptive phenotype (Zhong et al. 2012). These findings indicate that there are both direct and indirect mechanisms for thermal activation of TRPA1 (see below).
The mosquito vector for malaria, Anopheles gambiae, expresses a TRPA1 homolog in small coeloconic sensilla in the antenna, which is heat activated (≥25 °C) (Wang et al. 2009). These sensilla house thermosensitive neurons that are activated as the temperature rises above ~25–40 °C. Thus, A. gambiae appear to use peripheral neurons in the antenna to detect warm temperatures (Wang et al. 2009).
C. elegans employ two parallel pathways for noxious heat avoidance (≥25 °C). The first uses the neuropeptide receptor, NPR-1, and the second depends on the two TRPV proteins, OSM-9 and OCR-2 (Glauser et al. 2011). Whether OSM-9 and OCR-2 are heat activated is not known.
2.3.1.2 Responding to Excessively Cool Temperatures
Temperatures slightly below 18 °C are avoided by Drosophila larvae and adults. When given a choice between 18 °C and lower temperatures, the animals will migrate towards 18 °C. In larvae, this behavior depends in part on the TRPL channel (Rosenzweig et al. 2008) in chordotonal neurons (Kwon et al. 2010b), but not the TRP channel (Kwon et al. 2010b). The fly genome encodes two TRPV channels, Inactive and Nanchung, and at least one of these channels, Inactive, contributes to the avoidance of cool temperatures (Kwon et al. 2010b). Whether Nanchung also functions in this behavior is unclear due to the severe defect in locomotion, which is also associated with this mutation.
In adult flies, cool sensation is mediated via thermosensory neurons in the antenna (arista and the sacculus) and requires three proteins, referred to as Brivido1–3 (Gallio et al. 2011). These proteins contain either eight (Brv1) or ten predicted transmembrane domains (Brv2 and Brv3) (Gallio et al. 2011) and are most akin to mammalian polycystin1 proteins, which are part of the TRPP channel complex and thought to regulate the activity of the channels. The last six trans-membrane segments are related to TRPP channels. Since it is not known whether the individual Brv proteins are cation channels or whether all three proteins are subunits of a cool-sensing channel, these proteins are not currently listed as TRPP proteins.
2.3.2 Temperature Control in the Comfortable Range
Animals including Drosophila are capable of discriminating small temperature differences in the comfortable range (≤1°C). Although temperatures from 18 to 24 °C are comfortable for Drosophila larvae, 18 °C is preferred, and they will select 18 °C over 19–24 °C. TRPA1 is required for fine temperature discrimination in this range (Kwon et al. 2008). However, activation of TRPA1 is indirect since the comfortable temperatures (18–24 °C) are below the threshold for direct thermal activation of the channel (24–29 °C) (Viswanath et al. 2003). Rather, TRPA1 is activated downstream of phototransduction-like signaling cascade that depends on a rhodopsin (Rh1) (Shen et al. 2011) and the same heterotrimeric G protein and PLC that function in phototransduction (Kwon et al. 2008). Although thermal discrimination in the comfortable range depends on a rhodopsin, the concentration of rhodopsin in the thermosensory neurons is extremely low, precluding efficient photon capture. Consequently, light exposure does not impact on selection of 18° over 19–24 °C. This thermosensory signaling cascade may facilitate amplification of small temperature differences in the comfortable range and allow for thermal adaptation to 19–24 °C, if the larvae are unable to identify an area that is 18 °C within their thermal landscape.
2.3.3 Temperature Control of Circadian Rhythm
Circadian rhythms in most animals are entrained principally by day/night cycles (Allada and Chung 2010; Hardin 2011; Kwon et al. 2011; Peschel and Helfrich-Forster 2011; Reppert and Weaver 2002). In some poikilothermic organisms, such as Drosophila, circadian rhythms can also be set by higher and lower relative temperatures that simulate day/night periods (Glaser and Stanewsky 2007). TRPA1 is one molecular sensor that contributes to the temperature control of circadian rhythms in flies (Lee and Montell 2013). TRPA1 is expressed and functions in a subset of pacemaker neurons in the adult brain, and loss of this channel alters, but does not eliminate entrainment.
Recently, Pyrexia has also been reported to contribute to temperature synchronization of circadian rhythm through expression in peripheral sensory neurons in chordotonal organs (Wolfgang et al. 2013). Pyrexia affected temperature synchronization to relatively low temperatures (16–20 °C) but not at higher temperatures (21–29 °C). Since Pyrexia is activated by temperatures with a threshold near 40 °C (Lee et al. 2005), it may function in temperature control of clock synchronization via a thermosensory signaling cascade.
2.3.4 Temperature Control of Aging
Lower body temperature can increase animal lifespan (Conti 2008), and it was formerly assumed that this was due to a general reduction in metabolism. However, this is not the case, at least in C. elegans. Worm TRPA-1 is a cold-activated channel, as is mammalian TRPA1, and functions in the intestine to sense a decrease in temperature (Xiao et al. 2013). Cold activation of TRPA-1 leads to stimulation of a Ca2+-sensitive protein kinase C (PKC-2) and nuclear entry of the transcription factor DAF-16/FOXO, which promotes an increase in longevity. Given that expression of TRPA-1 in the intestine is sufficient for cold-induced longevity, this finding demonstrates that a non-excitable tissue functions in thermosensation and lifespan extension (Xiao et al. 2013).
2.4 Mechanosensation
Mechanosensation contributes to gentle and noxious touch, proprioception, sensing gravity, and hearing, and invertebrate TRP channels are important for each of these functions.
2.4.1 Touch, Proprioception, and Gravity Sensation
In C. elegans several TRP channels contribute to the response induced by nose touch. These include TRPA-1, which is activated in vitro by applying negative pressure to cells (Kindt et al. 2007), and both TRPV proteins, OSM-9 and OCR-2 (Colbert et al. 1997; Tobin et al. 2002). Since OSM-9 and OCR-2 are required for sensing volatile and nonvolatile compounds, mechanosensation, as well as osmosensation, they are polymodal sensors (Colbert et al. 1997; Tobin et al. 2002). The diversity of roles for these two proteins may reflect their expression in several types of sensory neurons. The two proteins may be subunits of a single channel since they form a complex and mutually affect the spatial distribution of the other protein (Tobin et al. 2002). However, it is not known if TRPA-1, OSM-9, or OCR-2 is responding directly or indirectly to mechanical stimulation.
Drosophila larvae depend on the TRPA channel, Painless, for responding to noxious touch, and this function is mediated through the same nociceptors that sense noxious heat (Tracey et al. 2003). A role for Painless in mechanosensation extends to the Drosophila heart (Senatore et al. 2010). Painless is expressed in the heart where it senses mechanical stress, which in turn regulates heart activity (Senatore et al. 2010). Painless, as well as a second TRPA channel, Pyrexia, and two TRPV channels, Inactive and Nanchung, are also expressed in the Johnston’s organ of the second antennal segment, where they participate in gravity sensing (Sun et al. 2009).
An important advance in dissecting the roles of TRP channels in mechanosensation is the demonstration that the C. elegans TRPN protein, TRP-4, is a direct mechanosensor (Kang et al. 2010b). Unlike the other six TRP subfamilies, TRPN proteins are not expressed in mammals. However, TRPN proteins are conserved from worms to zebrafish (Sidi et al. 2003; Walker et al. 2000). One of the intriguing features of TRPN channels is the large number of N-terminal ankyrin repeats (29) in flies and worms (Walker et al. 2000), which are proposed to comprise a gating spring (Howard and Bechstedt 2004). The Drosophila TRPN protein, NOMPC, is necessary for touch transduction in adult flies (Walker et al. 2000) and in two of the four classes of multidendritic neurons (II and III) for light touch transduction in larvae (Tsubouchi et al. 2012; Yan et al. 2013). Fly TRPN is also required for larval locomotion and normal proprioception (Cheng et al. 2010). The C. elegans TRP-4 protein plays a role in proprioception (Li et al. 2006) and harsh touch (Kang et al. 2010b).
The quintessential hallmark of a mechanotransducer is very rapid activation kinetics (Christensen and Corey 2007; Sharif-Naeini et al. 2008), and the TRP-4-dependent conductance is activated in vivo in less than 1 ms (Kang et al. 2010b). Thus, worm TRP-4 is the first TRP shown to be a direct mechanosensor in vivo. Fly NOMPC also appears to be a mechanosensor, since ectopic expression of this protein in non-mechanosensitive neurons confers touch sensitivity, and this channel is activated rapidly in vitro by applying negative pressure (latency <2 mS) (Yan et al. 2013). Currently, it is not known if any mammalian TRP channel is a direct mechanosensor.
2.4.2 Hearing
In flies hearing plays critical roles in courtship and mating, in addition to the avoidance of predators (Kamikouchi 2013). The mechanotransduction that is essential for audition occurs in the Johnston’s organ, which contains chordotonal sensilla (Kernan 2007). Hearing requires both the detection and amplification of sound-evoked signaling, and at least three TRP channels (NOMPC, Iav and Nan) contribute to hearing in adult (Eberl et al. 2000; Effertz et al. 2011, 2012; Gong et al. 2004; Göpfert et al. 2006; Kamikouchi et al. 2009; Kim et al. 2003; Lehnert et al. 2013) and in larval chordotonal organs (Zhang et al. 2013a). Primary sound sensation and sound amplification appear to be distinct processes. Nan and Iav mediate the initial detection of mechanical vibrations, while NOMPC plays a role in sound amplification (Lehnert et al. 2013).
2.4.3 Sensing Moist and Dry Environments
Hygrosensation refers to the ability of an organism to sense moisture or humidity in the environment. In flies, hygrosensation requires a region of the antenna known as the arista (Sayeed and Benzer 1996). The mechanisms for detecting moist and dry air are distinct because the TRPA channel, Waterwitch (Wtrw), is involved in sensing the transition to moist air, whereas Nan mediates the response to dry air (Liu et al. 2007b). However, neurons expressing both channels project to the region of the fly CNS associated with mechanotransduction (Liu et al. 2007b). Thus, hygrosensation may involve humidity-dependent alterations in mechanical stretch in the hygrosensitive neurons.
Drosophila larvae avoid dry surfaces through nocifensive behavior that depends on class IV multidendritic neurons in the body wall, and the TRPA channel, Painless (Johnson and Carder 2012). The larval cuticle is rich in charged glycoproteins (Silvert et al. 1984) that are likely to have an incredibly high affinity towards dry surfaces, thereby causing the cuticle to adhere to such surfaces. Thus, larval propensity to avoid dry surfaces may arise from noxious mechanical stretch as the larval cuticle adheres to a dry substrate during peristalsis.
2.5 Complex Behaviors
2.5.1 Feeding Behavior and Social Influences
Feeding behavior is a product of specialized interactions between chemosensory and somatosensory modalities and involves sensing of the internal metabolic state followed by state-dependent regulation of food seeking and feeding. In vertebrates, neuropeptide Y (NPY) signaling in the arcuate nucleus of the hypothalamus is intimately linked to feeding and energy expenditure (Gruninger et al. 2007; Stanley et al. 1985; Tomaszuk et al. 1996). The NPY receptors in both C. elegans and Drosophila larvae play critical roles in determining the selection of social or solitary feeding (de Bono and Bargmann 1998; Wu et al. 2003). Some worm strains exhibit social feeding, which involves avoidance of noxious chemical stimuli in a process requiring the TRP channels, OCR-2 and OSM-9 (de Bono et al. 2002). Additionally, OCR-2 impacts on the biosynthesis of serotonin (Sokolchik et al. 2005; Zhang et al. 2004)—a neurotransmitter with conserved roles in regulating feeding behavior (Magalhaes et al. 2010; Neckameyer 2010; Sze et al. 2000). OCR-2 also functions in a small subset of peripheral sensory neurons in C. elegans larvae that mediate the detection of nutrient availability and neuropeptide release (Lee and Ashrafi 2008).
Social feeding in worms involves aggregation, and this causes a drop in O2 levels. Worms have developed a mechanism for detecting changes in O2 levels as a part of their strategy to promote aggregation, and both OSM-9 and OCR-2 are required for sensing O2 (Rogers et al. 2006). Mammals also use O2-sensing TRP channels, as mouse TRPA1 can be directly activated by O2 under hyperoxic conditions, through a mechanism involving cysteine modification (Takahashi et al. 2011). It is proposed that TRPA1 may contribute to detecting O2 toxicity in the mouse (Takahashi et al. 2011).
In Drosophila larvae, an NPY family member (known as NPF in flies) mediates the developmental switch from food-seeking behavior in younger larvae to food-avoiding behavior in late third instar larvae (Xu et al. 2008). In response to food, late-stage larvae also display social behavior as they aggregate in order to dig cooperatively through hard food (Wu et al. 2003). Both the food avoidance and aggregation behavior are impaired in painless mutant late-stage larvae (Xu et al. 2008). NPF exerts its effects by inhibiting the Painless conductance in larval sensory neurons (Xu et al. 2008). The normal role for Painless in food aversion is consistent with the general function of invertebrate TRPA channels and mammalian TRPA1 in mediating avoidance behavior in response to noxious stimuli.
2.5.2 Nicotine-Dependent Behavior
Exposure of worms to nicotine, which is the addictive ingredient in tobacco, causes a variety of behaviors that are reminiscent of those induced in mammals (Dwoskin et al. 1999). These include increased activity to acute exposure, sensitization to intermittent administration of nicotine, and withdrawal upon removal of chronic nicotine (Feng et al. 2006). These behaviors are greatly diminished in worms missing either of two TRPC channels, TRP-1 or TRP-2. Expression of these channels is required in command neurons, which is consistent with their role in locomotion (Feng et al. 2006). Roles for TRP channels in nicotine sensitivity may be conserved throughout animal phylogeny since mouse TRPA1 is activated by nicotine and contributes in vivo to the irritant effects of nicotine (Talavera et al. 2009).
2.5.3 Courtship and Mating
Courtship and mating involves a complex interplay between multiple senses, including hearing, touch, chemosensation, and vision. Drosophila Painless contributes to mating, as the mutant females display enhanced sexual receptivity (Sakai et al. 2009). The mutant females copulate with a shorter latency than do wild-type females, once the males initiate courtship behavior. A role for Painless in sexual receptivity requires expression of the channel in GABAergic and/or cholinergic neurons (Sakai et al. 2009). Thus, it is unclear if Painless modulates female receptivity by acting in inhibitory or excitatory neurons. Painless also functions in courtship in males, as expression of the channel in olfactory projection neurons in the brain suppresses male-male courtship (Wang et al. 2011).
C. elegans display mating behavior, which involves males seeking out the vulva of the hermaphrodite, followed by spicule insertion and sperm transfer (Liu and Sternberg 1995). Male C. elegans lacking the TRPP2 homolog, pkd2, or its interacting protein, PKD1 (TRPP1), are defective in vulva location (Barr et al. 2001; Barr and Sternberg 1999).
2.6 Sperm and Fertilization
A C. elegans TRPC homolog, trp-3, functions in sperm and is critical for fertility. Upon sperm activation, TRP-3 translocates from an intracellular compartment to the plasma membrane. TRP-3-dependent Ca2+ influx is proposed to promote gamete fusion (Xu and Sternberg 2003). Roles for mammalian TRPs in sperm function, including TRPC2, have been suggested (Darszon et al. 2012; Jungnickel et al. 2001). However, because human TRPC2 is a pseudogene (Wes et al. 1995), it does not have a role in human sperm.
Loss of the Drosophila TRPP2 homolog, Amo, affects sperm function (Gao et al. 2003; Watnick et al. 2003). Normally, the beat frequency of sperm increases after they exit the uterus (Köttgen et al. 2011). The sperm then enters the storage organs, which serves to supply sperm for fertilization over an extended time. Amo is localized to the posterior end of mature sperm and is required for beating hyperactivity and for the backward entry of the sperm into the storage organs (Köttgen et al. 2011).
While the roles of the fly and worm TRPPs are distinct, they both function in ciliated cells. Human TRPP1 and TRPP2, which are disrupted in autosomal poly-cystic kidney disease (ADPKD), are expressed in monocilia (Gallagher et al. 2010). Thus, TRPPs have evolutionarily conserved roles in ciliated cells.
2.7 Metabolism
2.7.1 Ionic Homeostasis
Mg2+ homeostasis is essential for animal survival, as low or high intracellular Mg2+ impairs a wide range of essential cellular events. Mg2+ is absorbed by the intestines and secreted by the kidneys. In humans, mutations in the Mg2+ and Ca2+ permeable channel, TRPM6, cause familial hypomagnesemia with secondary hypocalcemia (HSH), which leads to seizures and muscle spasms (Schlingmann et al. 2002; Walder et al. 2002).
Roles for TRPM channels for Mg2+ homeostasis are conserved in worms and flies. Two C. elegans TRPMs (GON-2 and GTL-1) are required for Mg2+ uptake by intestinal cells (Teramoto et al. 2005). Worms missing GON-2 and GTL-1 grow poorly under low Mg2+ conditions. GON-2 and GTL-1 may form distinct channels, since mutation of just gon-2 or gtl-1 result in different phenotypes, and the two TRPMs have different Mg2+ sensitivities. GON-2 is inactive when the Mg2+ concentration is <1 mM, while GTL-1 is not inhibited by Mg2+ to a significant extent and may be constitutively active. Thus, GON-2 only comes into play when the Mg2+ concentration is high, thereby maintaining Mg2+ homeostasis. A third worm TRPM (GTL-2) is expressed in the excretory cell and is required for Mg2+ excretion (Teramoto et al. 2010).
The sole Drosophila TRPM functions in the fly kidney equivalent, the Malpighian tubules, where it serves to remove Mg2+ from the hemolymph (Hofmann et al. 2010). The trpm-deficient animals are characterized by hypermagnesemia in the hemolymph. Loss of trpm also affects intracellular Zn2+ homeostasis (Georgiev et al. 2010). Unlike all other fly TRPs, the TRPM channel is absolutely required for viability. The mutant animals die as prepupae or pupae (Georgiev et al. 2010; Hofmann et al. 2010). Supplementation of the food with high Mg2+ exacerbated the phenotype, resulting in larval lethality (Hofmann et al. 2010).
2.7.2 Lysosomal Function and a Model for MLIV
Mutations in the human TRPML1 cause a lysosomal storage disorder, mucolipidosis type IV (MLIV), which is characterized by motor impairments, severe cognitive deficits, and blindness (Bargal et al. 2000; Bassi et al. 2000; Sun et al. 2000). The C. elegans TRPML homolog, CUP-5, is localized to lysosomes, and elimination of this protein results in defective lysosomal biogenesis and maternal-effect embryonic lethality (Fares and Greenwald 2001; Hersh et al. 2002; Treusch et al. 2004). Due to nutrient deprivation, there appears to be an upregulation of autophagy in cup-5 mutant cells (Schaheen et al. 2006). However, the autophagy is ineffective since there is a decrease in lysosomal biogenesis (Schaheen et al. 2006).
In Drosophila, mutation of trpml results in severe neurodegeneration (Venkatachalam et al. 2008). The neurons die due to a requirement for TRPML for autophagic removal of damaged mitochondria. TRPML is localized to late endosomes and lysosomes and is essential in a variety of cell types for releasing luminal Ca2+ and promoting Ca2+-dependent fusion of late endosomes and lysosomes (Venkatachalam et al. 2008, 2013; Wong et al. 2012). The widespread neuronal cell death is a consequence of a role for TRPML in two cell types. First, trpml functions in neurons to promote cell survival. Second, trpml acts in phagocytic cells such as glia and macrophages to remove early apoptotic neurons (Venkatachalam et al. 2008). In the absence of TRPML activity, the early apoptotic neurons are not removed effectively. This results in accumulation of late apoptotic neurons, release of cytotoxic agents, and magnification of cell death due to a bystander effect (Venkatachalam et al. 2008).
Concluding Remarks
There are two recurring themes that apply to the worm and fly TRP channels. First, these channels are multitaskers. There are many examples that illustrate this concept. The fly TRPL channel contributes to light detection, cool sensation, taste adaptation, and sensitivity to CO2 (Badsha et al. 2012; Kwon et al. 2010b; Niemeyer et al. 1996; Phillips et al. 1992; Rosenzweig et al. 2008; Zhang et al. 2013c). Painless serves in the detection of noxious heat, noxioius mechanical stimuli, gravity, wasabi, food avoidance stimuli in late-stage larvae, dry surfaces, and in signals that control female receptivity and male-male courtship (Al-Anzi et al. 2006; Johnson and Carder 2012; Sakai et al. 2009; Sun et al. 2009; Tracey et al. 2003; Wang et al. 2011; Xu et al. 2008). The worm TRPV channel OSM-9 functions in olfaction, contact chemosensation, mechanosensation, thermosensation, O2 sensation, and social feeding (Colbert et al. 1997; de Bono et al. 2002; Rogers et al. 2006). Drosophila TRPA1 is a particularly notable example of a polymodal sensor as it either directly or indirectly senses suboptimal or excessively warm temperature, noxious olfactory and gustatory cues, and light (Kang et al. 2010a, 2012; Kim et al. 2010; Kwon et al. 2008, 2010a; Lee and Montell 2013; Rosenzweig et al. 2005; Viswanath et al. 2003).
Second, the repertoire of functions of worm, fly, and vertebrate TRP channels is far more similar than initially envisioned. Roles for PLC signaling and TRP channels in phototransduction are not peculiar to flies but are conserved in mammalian ipRGCs (Berson et al. 2002; Provencio et al. 2000; Schmidt et al. 2011). Thermally activated TRPs are also employed in vertebrates and invertebrates. However, some TRP channels in flies and mammals exhibit opposite thermal activities. TRPA1 is heat activated in flies (Viswanath et al. 2003), but cold activated in mice (Bandell et al. 2004; Karashima et al. 2009; Kwan et al. 2006; Story et al. 2003). Nevertheless, in some snakes (Gracheva et al. 2010), lizards, and frogs (Saito et al. 2012), TRPA1 is heat activated. The threshold for heat activation for rattlesnake TRPA1 (~28 °C) is similar to fly TRPA1 (~24–29 °C) (Gracheva et al. 2010; Viswanath et al. 2003), rather than mammalian TRPA1. A similar scenario emerges with thermally activated TRPV channels, which are heat activated in mammals (Caterina 2007). A Drosophila TRPV channel, Inactive, functions in the discrimination of 17.5 °C and slightly cooler temperatures (14–16 °C) (Kwon et al. 2010b). TRPV3 in western clawed frogs is cool activated with a threshold around 16 °C (Saito et al. 2011). The mechanisms for detecting some noxious chemicals are also conserved between several vertebrate and invertebrate TRP channels, such as reactive electrophiles (e.g., allyl isothiocyanate), which activate mammalian and Drosophila TRPA1 (Bandell et al. 2004; Hinman et al. 2006; Jordt et al. 2004; Kang et al. 2010a; Macpherson et al. 2007). Evolutionarily conserved functions for TRP channels are not limited to sensory physiology, as worms, flies, and vertebrates use TRPMs in ionic homeostasis: TRPPs in cilia and TRPMLs in lysosomes.
In view of the many functional similarities between invertebrate and vertebrate TRPs, it is intriguing to speculate that some roles that are currently known specifically in worms or flies will turn out to be conserved in mammals. Among the many examples are gravity sensation, hygrosensation, and fertility.
Contributor Information
Kartik Venkatachalam, Email: kartik.venkatachalam@uth.tmc.edu, Department of Integrative Biology and Pharmacology, University of Texas School of Medicine, Houston, TX 77030, USA.
Junjie Luo, Department of Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA. Department of Molecular, Cellular, and Developmental Biology, Neuroscience Research Institute, University of California at Santa Barbara, Santa Barbara, CA 93106, USA.
Craig Montell, Email: craig.montell@lifesci.ucsb.edu, Department of Molecular, Cellular, and Developmental Biology, Neuroscience Research Institute, University of California at Santa Barbara, Santa Barbara, CA 93106, USA.
References
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Al-Anzi B, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S. Obesity-blocking neurons in Drosophila. Neuron. 2009;63:329–341. doi: 10.1016/j.neuron.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badsha F, Kain P, Prabhakar S, Sundaram S, Padinjat R, Rodrigues V, Hasan G. Mutants in Drosophila TRPC channels reduce olfactory sensitivity to carbon dioxide. PLoS One. 2012;7:e49848. doi: 10.1371/journal.pone.0049848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin- and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Darszon A, Sanchez-Cardenas C, Orta G, Sanchez-Tusie AA, Beltran C, Lopez-Gonzalez I, Granados-Gonzalez G, Trevino CL. Are TRP channels involved in sperm development and function? Cell Tissue Res. 2012;349:749–764. doi: 10.1007/s00441-012-1397-5. [DOI] [PubMed] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berlin) 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz T, Wiek R, Gopfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Gopfert MC. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci. 2012;15:1198–1200. doi: 10.1038/nn.3175. [DOI] [PubMed] [Google Scholar]
- Fares H, Greenwald I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat Genet. 2001;28:64–68. doi: 10.1038/ng0501-64. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2013;92:394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant poly-cystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol. 2003;13:2175–2178. doi: 10.1016/j.cub.2003.11.053. [DOI] [PubMed] [Google Scholar]
- Georgiev P, Okkenhaug H, Drews A, Wright D, Lambert S, Flick M, Carta V, Martel C, Oberwinkler J, Raghu P. TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab. 2010;12:386–397. doi: 10.1016/j.cmet.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Glaser FT, Stanewsky R. Synchronization of the Drosophila circadian clock by temperature cycles. Cold Spring Harb Symp Quant Biol. 2007;72:233–242. doi: 10.1101/sqb.2007.72.046. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Chen WC, Agin R, Macinnis BL, Hellman AB, Garrity PA, Tan MW, Goodman MB. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger TR, LeBoeuf B, Liu Y, Garcia LR. Molecular signaling involved in regulating feeding and other motivated behaviors. Mol Neurobiol. 2007;35:1–20. doi: 10.1007/BF02700621. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338:260–263. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C, Obukhov AG, Zobel A, Kalkbrenner F, Schultz G. The Drosophila cation channel trpl expressed in Sf9 cells is stimulated by agonists of G-protein-coupled receptors. FEBS Lett. 1995;358:297–300. doi: 10.1016/0014-5793(94)01455-a. [DOI] [PubMed] [Google Scholar]
- Hersh BM, Hartwieg E, Horvitz HR. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc Natl Acad Sci U S A. 2002;99:4355–4360. doi: 10.1073/pnas.062065399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 2004;23:1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Chen X, Dietz AS, Gudermann T, Montell C. Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLoS One. 2010;5:e10519. doi: 10.1371/journal.pone.0010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein Painless is important for thermal nociception but not mechanical nociception. PLoS One. 2012;7:e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yoshioka T, Hotta Y. A genetic study of inositol trisphosphate involvement in phototransduction using Drosophila mutants. Biochem Biophys Res Commun. 1985;132:513–519. doi: 10.1016/0006-291x(85)91163-5. [DOI] [PubMed] [Google Scholar]
- Johnson WA, Carder JW. Drosophila nociceptors mediate larval aversion to dry surface environments utilizing both the Painless TRP channel and the DEG/ENaC subunit, PPK1. PLoS One. 2012;7:e32878. doi: 10.1371/journal.pone.0032878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A. Auditory neuroscience in fruit flies. Neurosci Res. 2013;76:113–118. doi: 10.1016/j.neures.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010a;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010b;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, Kernan MJ, Kim C. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, Watnick T. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One. 2011;6:e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010a;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010b;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Choe HK, Son GH, Kim K. Mammalian molecular clocks. Exp Neurobiol. 2011;20:18–28. doi: 10.5607/en.2011.20.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4:e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Montell C. Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons. J Neurosci. 2013;33:6716–6725. doi: 10.1523/JNEUROSCI.4237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 2013;77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, Hardie RC. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J Neurosci. 2007a;27:604–615. doi: 10.1523/JNEUROSCI.4099-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007b;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, Kang L, Yu Y, Ma D, Xu T, Mori I, Xie Z, Xu XZ. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Magalhaes CP, de Freitas MF, Nogueira MI, Campina RC, Takase LF, de Souza SL, de Castro RM. Modulatory role of serotonin on feeding behavior. Nutr Neurosci. 2010;13:246–255. doi: 10.1179/147683010X12611460764723. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. Evolutionary conservation and changes in insect TRP channels. BMC Evol Biol. 2009;9:228. doi: 10.1186/1471-2148-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001(90):re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The history of TRP channels, a commentary and reflection. Pflugers Arch. 2011;461:499–506. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. A trophic role for serotonin in the development of a simple feeding circuit. Dev Neurosci. 2010;32:217–237. doi: 10.1159/000304888. [DOI] [PubMed] [Google Scholar]
- Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, Penninger JM. TrpA1 regulates thermal nociception in Drosophila. PLoS One. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Peschel N, Helfrich-Förster C. Setting the clock–by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Petersen LK, Stowers RS. A Gateway MultiSite recombination cloning toolkit. PLoS One. 2011;6:e24531. doi: 10.1371/journal.pone.0024531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Colley NJ, Webel R, James T, Hasan G, Danin M, Selinger Z, Hardie RC. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol Cell Neurosci. 2000;15:429–445. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brenman KM, Taylor TD, Phelps P, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Fukuta N, Shingai R, Tominaga M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011;7:e1002041. doi: 10.1371/journal.pgen.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem. 2012;287:30743–30754. doi: 10.1074/jbc.M112.362194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kasuya J, Kitamoto T, Aigaki T. The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes Brain Behav. 2009;8:546–557. doi: 10.1111/j.1601-183X.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci U S A. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaheen L, Dang H, Fares H. Basis of lethality in C. elegans lacking CUP-5, the Mucolipidosis Type IV orthologue. Dev Biol. 2006;293:382–391. doi: 10.1016/j.ydbio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore S, Rami Reddy V, Semeriva M, Perrin L, Lalevee N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2010;6:e1001088. doi: 10.1371/journal.pgen.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Dedman A, Folgering JH, Duprat F, Patel A, Nilius B, Honore E. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflugers Arch. 2008;456:529–540. doi: 10.1007/s00424-007-0432-y. [DOI] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- Silvert DJ, Doctor J, Quesada L, Fristrom JW. Pupal and larval cuticle proteins of Drosophila melanogaster. Biochemistry. 1984;23:5767–5774. doi: 10.1021/bi00319a015. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolchik I, Tanabe T, Baldi PF, Sze JY. Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins. J Neurosci. 2005;25:1015–1023. doi: 10.1523/JNEUROSCI.3107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF, Eppel N, St-Pierre S, Hoebel BG. Suppression of norepinephrine-elicited feeding by neurotensin: evidence for behavioral, anatomical and pharmacological specificity. Brain Res. 1985;343:297–304. doi: 10.1016/0006-8993(85)90747-4. [DOI] [PubMed] [Google Scholar]
- Störtkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc Natl Acad Sci U S A. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Lambie EJ, Iwasaki K. Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metab. 2005;1:343–354. doi: 10.1016/j.cmet.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto T, Sternick LA, Kage-Nakadai E, Sajjadi S, Siembida J, Mitani S, Iwasaki K, Lambie EJ. Magnesium excretion in C. elegans requires the activity of the GTL-2 TRPM channel. PLoS One. 2010;5:e9589. doi: 10.1371/journal.pone.0009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tomaszuk A, Simpson C, Williams G. Neuropeptide Y, the hypothalamus and the regulation of energy homeostasis. Horm Res. 1996;46:53–58. doi: 10.1159/000184996. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci U S A. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi A, Caldwell JC, Tracey WD. Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr Biol. 2012;22:2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;266:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Long A, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Wong CO, Montell C. Feast or famine: role of TRPML in preventing cellular amino acid starvation. Autophagy. 2013;9:98–100. doi: 10.4161/auto.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang X, Xie Q, Montell C. The SOCS box protein STOPS Is required for phototransduction through its effects on phospholipase C. Neuron. 2008;57:56–68. doi: 10.1016/j.neuron.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Qiu YT, Lu T, Kwon HW, Pitts RJ, Van Loon JJ, Takken W, Zwiebel LJ. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci. 2009;30:967–974. doi: 10.1111/j.1460-9568.2009.06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Guo Y, Wang F, Wang Z. Drosophila TRPA channel painless inhibits male-male courtship behavior through modulating olfactory sensation. PLoS One. 2011;6:e25890. doi: 10.1371/journal.pone.0025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci. 2008;11:916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang W, Simoni A, Gentile C, Stanewsky R. The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc Biol Sci. 2013;280:20130959. doi: 10.1098/rspb.2013.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme AJ, Williamson SM, Reaves BJ. TRP channels in parasites. Adv Exp Med Biol. 2011;704:359–371. doi: 10.1007/978-94-007-0265-3_20. [DOI] [PubMed] [Google Scholar]
- Wong CO, Li R, Montell C, Venkatachalam K. Drosophila TRPML is required for TORC1 activation. Curr Biol. 2012;22:1616–1621. doi: 10.1016/j.cub.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZ. Function and regulation of TRP family channels in C. elegans. Pflugers Arch. 2009;458:851–860. doi: 10.1007/s00424-009-0678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Xu XZ. C. elegans TRP channels. Adv Exp Med Biol. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZ. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Sternberg PW. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114:285–297. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat Neurosci. 2008;11:676–682. doi: 10.1038/nn.2119. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sokolchik I, Blanco G, Sze JY. Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons. Development. 2004;131:1629–1638. doi: 10.1242/dev.01047. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Anderson AR, Trowell SC, Luo AR, Xiang ZH, Xia QY. Topological and functional characterization of an insect gustatory receptor. PLoS One. 2011;6:e24111. doi: 10.1371/journal.pone.0024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Jan LY, Jan YN. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci U S A. 2013a;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013b;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Raghuwanshi RP, Shen WL, Montell C. Food-experience induced taste desensitization modulated by the Drosophila TRPL channel. Nat Neurosci. 2013c;16:1468–1476. doi: 10.1038/nn.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, Tracey WD. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]