Abstract
Prognostic effects of Mitosis-Karyorrhexis Index (MKI) used in the International Neuroblastoma Pathology Classification (INPC) are age-dependent. A total of 4,282 neuroblastomas reviewed at the Children’s Oncology Group Neuroblastoma Pathology Reference Laboratory (8/1/2001–3/31/2012) included 2,365 low-MKI (L-MKI), 1,068 intermediate-MKI (I-MKI), and 849 high-MKI (H-MKI) tumors. Cox proportional hazards models were fit to determine age cut-offs at which the relative risk of event/death was maximized in each MKI class. Backward-selected Cox models were fit to determine the prognostic strength of the age cut-offs for survival in the presence of other prognostic factors. The age cut-offs used in the INPC for L-MKI tumors (<60 months, n = 2,710, 84.0% ± 1.0% event-free survival [EFS], 93.8 ± 0.7% overall survival [OS] vs ≥60 months, n = 195, 49.8% ± 4.6% EFS, 71.7% ± 4.1% OS; P < 0.0001) and I-MKI tumors (<18 months, n = 568, 83.8% ± 2% EFS, 93.7% ± 1.3% OS vs ≥18 months, n = 500, 51.4% ± 2.9% EFS, 66.7% ± 2.7% OS; P < 0.0001) were within the effective range for distinguishing prognostic groups. As for H-MKI tumors (no cut-off age in the INPC, 51.0% ± 2.2% EFS, 64.4% ± 2.1% OS), a new cut-off of 3–4 months was suggested (<4 months, n = 38, 82.3% ± 8.4% EFS, 81.8% ± 8.5% OS vs ≥4 months, n = 811, 49.6% ± 2.2% EFS, 63.7% ± 2.1% OS, P = 0.0034 and 0.0437, respectively). Multivariate analyses revealed that cut-offs of 60 and 18 months for L-MKI and I-MKI tumors, respectively, were independently prognostic. However, the cut-off of 4 months for H-MKI tumors did not reach statistical significance in the presence of other factors. The age cut-offs for MKI classes (60 months for L-MKI, 18 months for I-MKI, no cut-off for H-MKI) in the current INPC are reasonable and effective for distinguishing prognostic groups with increased risk of event/death for older patients.
Keywords: age cut-off, International Neuroblastoma Pathology Classification, mitosis-karyorrhexis index, neuroblastoma, prognosis
INTRODUCTION
Peripheral neuroblastic tumors (pNTs, including neuroblastoma, ganglioneuroblastoma, and ganglioneuroma) are among the most common solid tumors in childhood [1]. These tumors were once described as enigmatic because of their unpredictable clinical behaviors, such as spontaneous regression, tumor maturation, and aggressive progression even with intensive chemotherapy/irradiation therapy. Recent progress in clinical, translational, and basic research of pNTs has provided several clues for understanding the biological characteristics directly related to the various clinical behaviors of this disease. International efforts have focused on defining risk groupings for patients with pNTs based on the combination of so-called prognostic factors [2], ie, clinical stage, age at diagnosis, histologic features, and molecular/ biologic properties. For example, the Children’s Oncology Group (COG) has been utilizing the International Neuroblastoma Staging System (INSS) [3–6], age at diagnosis [7], International Neuroblastoma Pathology Classification (INPC) [8–10], MYCN oncogene status [11,12], DNA index [13,14], 1p loss of heterogeneity (LOH) [15,16], and 11q LOH [16].
The INPC, established in 1999 [8,9] and modified in 2003 [17], is a prognostically significant and biologically relevant classification system. According to the INPC, tumors in this group are placed in 1 of 4 different categories: (1) neuroblastoma (Schwannian stroma–poor); (2) ganglioneuroblastoma, intermixed (Schwannian stroma–rich); (3) ganglioneuroma (Schwannian stroma–dominant); and (4) ganglioneuroblastoma, nodular (composite, Schwannian stroma–rich/stroma–dominant and stroma–poor). In this system, tumors in the neuroblastoma and ganglioneuroblastoma, nodular categories, respectively, have 2 histologic indicators: grade of neuroblastic differentiation and mitosis-karyorrhexis index (MKI), with prognostic impacts that differ based on the age of the patients at diagnosis. Three prognostic subtypes are distinguished by grade: (1) undifferentiated (indicating a poor prognosis in any age group); (2) poorly differentiated (indicating a better prognosis in patients <18 months and a poor prognosis in patients ≥18 months); and (3) differentiating (indicating a better prognosis in patients < 60 months and a poor prognosis in patients ≥60 months). Three prognostic classes are also distinguished by the mitotic and karyorrhectic activities of the neuroblastic cells [9,10]: (1) low MKI (L-MKI) (<100/5000 cells, indicating a better prognosis in patients <60 months and a poor prognosis in patients ≥60 months); (2) intermediate MKI (I-MKI) (100–200/5000 cells, indicating a better prognosis in patients <18 months and a poor prognosis in patients ≥18 months); and (3) high MKI (H-MKI) (≥200/ 5000 cells; indicating a poor prognosis in any age group).
The significance of age-dependent prognostic effects by different grades of neuroblastic differentiation was previously reported, with data indicating a biologically critical relationship between NTRK1 (TrkA) (a high-affinity nerve growth factor receptor) expression and the potential of age-appropriate cellular differentiation in neuroblastoma tumors [18,19]. The significant association between MYCN amplification and increased mitotic and karyorrhectic activities has also been previously reported [12,20]. In this study, age-dependent prognostic effects by different MKI classes were tested utilizing a large number of cases reviewed and filed in the COG Neuroblastoma Pathology Reference Laboratory, and their prognostic impacts were analyzed with other known prognostic indicators.
MATERIALS AND METHODS
Patient cohort
A total of 5,929 cases of pNTs were reviewed at the COG Neuroblastoma Pathology Reference Laboratory, Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles, Los Angeles, California, between August 1, 2001, and March 31, 2012, and 4680 of them were classified as neuroblastoma (Schwannian stroma–poor) tumors including undifferentiated subtype (177 cases), poorly differentiated subtype (4081 cases), and differentiated subtype (422 cases) based on the review of pathology specimens obtained before starting chemotherapy/irradiation therapy.
Among these neuroblastoma cases, 4282 patients who had MKI class data and survival information were included in this study. The median number of slides reviewed was 2.0 (range, 1 to 44) per case. Other prognostic factors, such as age at diagnosis, INSS (non–stage 4 vs stage 4), MYCN status (nonamplified vs amplified), and DNA index (hyperdiploid vs diploid), were also collected and analyzed. Informed consent approved by the institutional review board was obtained for all patients at the time of enrollment in the COG biological or therapeutic study.
MKI determination
Mitotic and karyorrhectic activities were evaluated by 2 or 3 reviewers (HS, LLW, and/or a research fellow at the COG Neuroblastoma Pathology Reference Laboratory) (Fig. 1). First, the activities were evaluated individually, and then the final MKI class was determined by the director (HS) after discussion and re-review of the cases together with other reviewer(s) with multi-head microscopy. MKI class was assigned by averaging the numbers of mitotic and karyorrhectic cells from multiple representative microscopic fields of all the sections available, but not based on the evaluation of “hot spots” (a single or a few areas of the highest activities). Activities could vary from area to area, so as the cellularity of the tumor cells (denominator) could do in the same tumor tissues. The denominator (the number of background neuroblastoma cells) of each field selected for counting the mitotic and karyorrhectic cells was determined by estimation. For example, a single high-power microscopic field (×400 with a conventional microscope) of densely packed neuroblasts easily contained more than 1500 denominator cells. Necrotic areas were avoided during MKI evaluation. One of 3 MKI classes, i.e., L-MKI (<100/5000 cells), I-MKI (100–200/5000 cells), or H-MKI (>200/ 5000 cells), was assigned to the individual neuroblastoma tumors after the number of mitotic and karyorrhectic cells per 5000 denominator cells was calculated [8]. Mitotic figures could be bipolar or multipolar, while karyorrhectic cells had nuclear fragmentation and were often associated with deeply eosinophilic and degenerative cytoplasm. Those cells with nuclei that were simply hyperchromatic were not included in the counts. Both primary and metastatic tumor samples were eligible for MKI determination, as long as they were submitted before chemotherapy/irradiation therapy was started. One exception was metastatic neuroblastoma in the bone marrow, where mitotic and karyorrhectic activities were not necessarily the same as those in the primary tumor and were not useful for prognostic evaluation (unpublished data).
Figure 1.
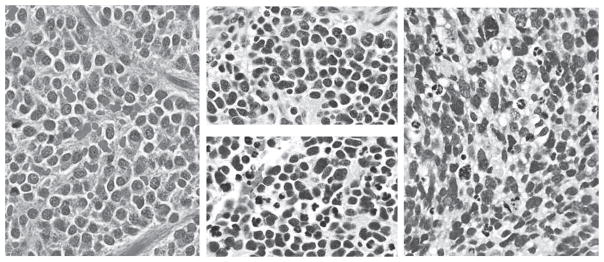
Mitotic and karyorrhectic activities in neuroblastoma tumors. Left: Low-MKI (<100/5000 cells) tumors; middle: intermediate-MKI (100–200/5000 cells) tumor typically showing markedly different areas of activity, from lower (upper) to higher (lower); and right: high-MKI (≥200/5000 cells) tumor. MKI indicates mitosis-karyorrhexis index. Hematoxylineosin, original magnification ×400. A color version of this figure is available online.
Statistical analyses
First, the relationship between MKI class and MYCN status was explored via a chi-square test. The method of Kaplan and Meier [21] was used to generate survival curves, with standard errors per Peto and coworkers [22]. For event-free survival (EFS), an event was defined as the first occurrence of relapse, progression, secondary malignancy, or death from any cause. For overall survival (OS), death was the only event considered. Time to event or death was calculated from the date of diagnosis. In the absence of an event, the survival time was censored at the last known time. Two-sided log-rank tests were used to test for differences between survival curves. For all analyses, P values less than 5% were considered statistically significant.
Cox proportional hazards (PH) models for EFS and OS, with the Efron method of handling tied events, were fit within each MKI class to determine the optimal age cut-off (in months) at which the relative risk (RR) of an event was maximized. Age cut-offs corresponding to statistically significant RR ratios greater than 3.5 (3.0 for H-MKI tumors) were considered to be able to effectively differentiate between the 2 groups. After the age cut-offs that reasonably distinguished prognostic groups in each MKI class were determined, the relationships between these age groups and other prognostic factors (INSS, MYCN status, DNA index, and grade) within each MKI class were explored via a 2-sided chi-square test. Finally, to determine the independent prognostic strength for survival of age at diagnosis, adjusting for other prognostic factors (already listed), Cox PH models with the Efron method of handling tied event times were fit. Backward selection was used to determine the most parsimonious model, with the least statistically significant term dropping out at each step. For the backward selection, only cases with all prognostic data available were included in the analysis (L-MKI, n = 2273; I-MKI, n = 1009; H-MKI, n = 809). Note that the INPC (favorable histology vs unfavorable histology) was not included in the models because it is determined from MKI, grade, and age at diagnosis, and hence is confounded with these variables.
RESULTS
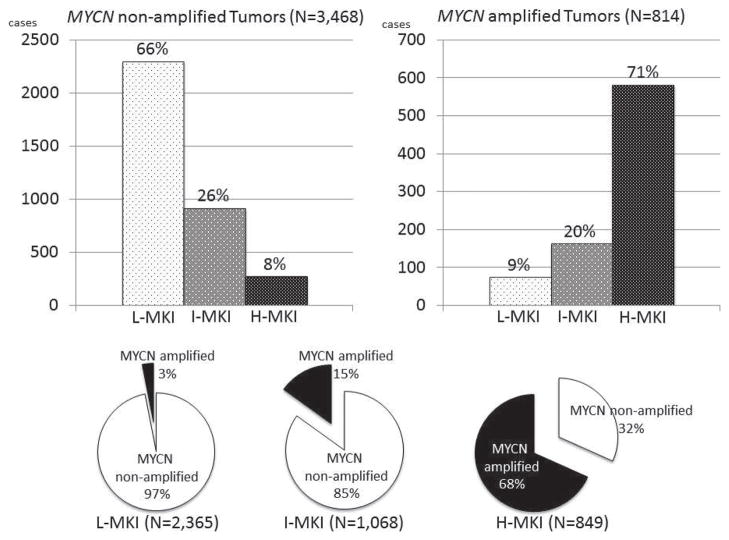
In this series of cases, there were 2365 L-MKI, 1068 I-MKI, and 849 H-MKI tumors. Of the tumors, 3468 had nonamplified MYCN and 814 had amplified MYCN. As shown in Figure 2, a statistically significant association was found between increasing MKI class and MYCN amplification (P < 0.0001). Patients with MYCN amplification were more likely to have an H-MKI tumor, while those with nonamplified tumors tended to have a L-MKI or I-MKI tumor. Overall 3-year EFS and OS were 72.1% ± 0.9% and 83.8% ± 0.7%, respectively, and patient outcome was significantly worse as MKI increased (3-year EFS: 81.2% ±1.0% for L-MKI, 68.6% ± 1.8% for I-MKI, and 51.0%± 2.2% for H-MKI; P < 0.0001; 3-year OS: 92.0% ± 0.7% for L-MKI, 81.0% ± 1.6% for I-MKI, and 64.4% ± 2.1% for H-MKI; P < 0.0001).
Figure 2.
Upper bar graphs: Distribution of MKI classes in MYCN nonamplified neuroblastomas (left) and in MYCN amplified neuroblastomas (right). Lower pie graphs: Distribution of MYCN nonamplified and MYCN amplified neuroblastomas in each MKI class. There is a significant association between increasing MKI class and MYCN amplification (P < 0.0001). L-MKI indicates low-MKI tumors; I-MKI, intermediate-MKI tumors; H-MKI, high-MKI tumors; MKI, mitosis-karyorrhexis index.
The following is a summary of the prognostic impact by each MKI class.
Patients with L-MKI tumors
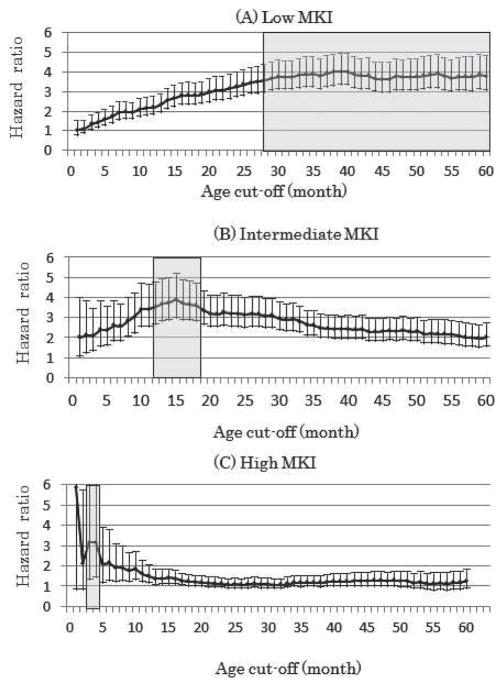
As shown in Figure 3A, any age over 28 months, including the standard INPC age of 60 months, could be considered as a cut-off point, since these all effectively differentiated between 2 groups of patients with the L-MKI tumors in terms of increased risk of events for older patients. RR ratio for the cut-off at 28 months was 3.6 and that for the cut-off at 60 months was 3.8.
Figure 3.
Cox proportional hazards model for event-free survival with age ≥ cut-off (in months) for patients with low-MKI (A, n = 2365), intermediate-MKI (B, n = 1068), and high-MKI (C, n = 849) tumors. The hazard ratio (with 95% confidence intervals) is the increased risk of event in the group of patients with age ≥ cut-off in comparison to the group with age < cut-off. Shaded areas (between 28 and 60 months for low MKI, 12 and 18 months for intermediate MKI, and 3 and 4 months for high MKI) indicate age cut-off points, effectively differentiating 2 groups of patients in terms of increased risk of event for older children in each MKI class. MKI indicates mitosis-karyorrhexis index.
Among the patients diagnosed between 28 and 60 months of age, there were 21 cases in which risk stratification and protocol assignment were significantly affected based on the different age cut-offs. These were patients with stage 3 disease and differentiating subtype, and they were classified as having favorable histology using the current age cut-off of 60 months and placed in the intermediate-risk group. When the cut-off of 28 months was used, however, the same patients would have been placed in the high-risk group due to unfavorable histology classification for intensive chemotherapy with/without bone marrow transplantation. In this age group, risk grouping of no other patients was significantly affected depending on cut-off points (there were no stage 4S patients, most of the stage 1 and 2 patients were in low/intermediate-risk groups, all stage 4 patients were in the high-risk group, and the patients with undifferentiated/poorly differentiated subtype displayed unfavorable histology regardless of MKI class). It was noted that these 21 patients with stage 3 disease and differentiating subtype had an excellent prognosis, and their 3-year EFS and OS were 87.4% ± 9.4% and 100.0%, respectively. The results clearly indicated the advantage of a current cut-off of 60 months for the L-MKI tumor, since this cut-off could avoid unnecessarily intensive treatment without reducing survival rates.
Accordingly, the age cut-off of 60 months was used for further analysis. There were 2170 L-MKI tumors (84.0% ± 1.0% EFS, 93.8% ± 0.7% OS) diagnosed in patients younger than 60 months of age and 195 L-MKI tumors diagnosed in patients older than 60 months of age (49.8% ± 4.6% EFS, 71.7% ± 4.1% OS) (P < 0.0001 for EFS and OS). As shown in Table 1a, a significant association was found between age group and other prognostic factors for patients with L-MKI tumors. Patients <60 months of age were more likely to have non–stage 4 disease, nonamplified MYCN, and hyperdiploid tumors. In contrast, patients ≥60 months of age were more likely to have stage 4 disease, amplified MYCN, and diploid tumors.
Table 1a.
Cross-tabulation of age group with prognostic factors for patients with low MKI
| Prognostic factor | Age group
|
Chi-square test P value | |
|---|---|---|---|
| <60 months | ≥60 months | ||
| INSS stagea | < 0.0001 | ||
| Not stage 4 | 1639 | 72 | |
| Stage 4 | 524 | 122 | |
| MYCN status | 0.0025 | ||
| Not amplified | 2110 | 182 | |
| Amplified | 60 | 13 | |
| DNA Indexa | < 0.0001 | ||
| Hyperdiploid | 1647 | 107 | |
| Diploid | 448 | 79 | |
Eight patients were missing INSS stage and 84 were missing DNA index data.
Full backward-selected models in terms of EFS and OS were fit for patients with L-MKI that included all prognostic variables, allowing any covariate to drop out of the models. In the final backward-selected EFS model (Table 2a-1), age, INSS, and DNA index were retained; patients with L-MKI tumor diagnosed at over 60 months of age were 2.5 times more likely to experience an event than patients with L-MKI tumor diagnosed at less than 60 months of age. In the final backward-selected OS model (Table 2a-2), age, INSS, MYCN status, and DNA index were retained; patients with L-MKI tumor diagnosed at over 60 months of age were 3.2 times more likely to die than patients with L-MKI tumor diagnosed at less than 60 months of age.
Table 2.
Final backward-selected Cox model tests: age at diagnosis and covariates INSS, MYCN status, DNA index, and grade of neuroblastic differentiation
| Table 2a-1. EFS for Patients with Low MKI
| ||||
|---|---|---|---|---|
| Variable (n = 2273)a | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| Age (<60 months vs ≥60 months) | 1 | <0.0001 | 2.458 | (1.926, 3.137) |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 2.772 | (2.249, 3.416) |
| DNA index (hyperdiploid vs diploid) | 1 | <0.0001 | 1.555 | (1.265, 1.911) |
| Table 2a-2. OS for patients with low MKI
| ||||
|---|---|---|---|---|
| Variable (n = 2273)b | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| Age (<60 months vs ≥60 months) | 1 | <0.0001 | 3.189 | (2.360, 4.310) |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 7.566 | (5.313, 10.774) |
| MYCN status (not amplified vs amplified) | 1 | 0.0118 | 1.766 | (1.135, 2.749) |
| DNA index (hyperdiploid vs diploid) | 1 | <0.0001 | 1.846 | (1.392, 2.450) |
| Table 2b-1. EFS for patients with intermediate MKI
| ||||
|---|---|---|---|---|
| Variable (n = 1009)a | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| Age (<18 months vs ≥18 months) | 1 | <0.0001 | 2.218 | (1.647, 2.986) |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 1.982 | (1.484, 2.647) |
| MYCN status (not amplified vs amplified) | 1 | 0.0349 | 1.351 | (1.022, 1.787) |
| DNA index (hyperdiploid vs diploid) | 1 | 0.0146 | 1.356 | (1.062, 1.732) |
| Table 2b-2. OS for patients with intermediate MKI
| ||||
|---|---|---|---|---|
| Variable (n = 1009)b | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| Age (<18 months vs ≥18 months) | 1 | <0.0001 | 3.188 | (2.083, 4.877) |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 3.196 | (2.108, 4.846) |
| MYCN status (not amplified vs amplified) | 1 | <0.0001 | 2.002 | (1.466, 2.733) |
| DNA index (hyperdiploid vs diploid) | 1 | 0.0260 | 1.396 | (1.041, 1.873) |
| Table 2c-1. EFS for patients with high MKI
| ||||
|---|---|---|---|---|
| Variable (n = 809)a | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 2.281 | (1.699, 3.061) |
| DNA index (hyperdiploid vs diploid) | 1 | 0.0015 | 1.411 | (1.142, 1.745) |
| Table 2c-2. OS for patients with high MKI
| ||||
|---|---|---|---|---|
| Variable (n = 809)b | DF | P value | Hazard ratio | 95% CI on hazard ratio |
| INSS stage (not stage 4 vs stage 4) | 1 | <0.0001 | 2.210 | (1.578, 3.095) |
| MYCN status (not amplified vs amplified) | 1 | 0.0011 | 1.605 | (1.207, 2.135) |
| DNA index (hyperdiploid vs diploid) | 1 | 0.0053 | 1.421 | (1.110, 1.819) |
Variables tested and not statistically significant for EFS: MYCN status and grade; for OS: grade.
INSS indicates International Neuroblastoma Staging System; EFS, event-free survival; OS, overall survival; DF, degrees of freedom; CI, confidence interval.
Variables tested and not statistically significant: grade.
INSS indicates International Neuroblastoma Staging System; EFS, event-free survival; OS, overall survival; DF, degrees of freedom; CI, confidence interval.
Variables tested and not statistically significant for EFS: grade, MYCN status, and age; for OS: age and grade.
INSS indicates International Neuroblastoma Staging System; EFS, event-free survival; OS, overall survival; DF, degrees of freedom; CI, confidence interval.
Patients with I-MKI tumors
As shown in Figure 3B, any age between 12 and 18 months, including the standard INPC age of 18 months, could be considered as a cut-off point, since these all effectively differentiated between 2 groups of patients with I-MKI tumors in terms of an increased risk of event for older patients. For further analysis, an age cut-off of 18 months was used (RR 3.6): There were 568 I-MKI tumors diagnosed at less than 18 months of age (83.8% ± 2.0% EFS, 93.7% ± 1.3% OS) and 500 I-MKI tumors diagnosed at over 18 months of age (51.4% ± 2.9% EFS, 66.7% ± 2.7% OS) (P < 0.0001 for EFS; P < 0.0001 for OS). As shown in Table 1b, a significant association was found between age group and other prognostic factors for patients with I-MKI tumors. Patients <18 months of age were more likely to have non–stage 4 disease, nonamplified MYCN, and hyperdiploid tumors. In contrast, patients ≥18 months of age were more likely to have stage 4 disease, amplified MYCN, and diploid tumors. In the final backward-selected EFS model (Table 2b-1), age, INSS, MYCN status, and DNA index were retained; patients with I-MKI tumors diagnosed at over 18 months of age were 2.2 times more likely to experience an event than patients with I-MKI tumors diagnosed at less than 18 months of age. In the final backward-selected OS model (Table 2b-2), age, INSS, MYCN status, and DNA index were retained; patients with I-MKI tumors diagnosed at over 18 months of age were 3.2 times more likely to die than patients with I-MKI tumors diagnosed at less than 18 months of age.
Table 1b.
Cross-tabulation of age group with prognostic factors for patients with intermediate MKI
| Prognostic factor | Age group
|
Chi-square test P value | |
|---|---|---|---|
| <18 months | ≥18 months | ||
| INSS stagea | < 0.0001 | ||
| Not stage 4 | 406 | 124 | |
| Stage 4 | 157 | 374 | |
| MYCN status | < 0.0001 | ||
| Not amplified | 547 | 360 | |
| Amplified | 21 | 140 | |
| DNA indexa | < 0.0001 | ||
| Hyperdiploid | 451 | 266 | |
| Diploid | 94 | 205 | |
Seven patients were missing INSS stage and 52 were missing DNA index data.
Patients with H-MKI tumors
As mentioned earlier, the EFS and OS rates for patients with H-MKI tumors were generally low. As shown in Figure 3C, however, an age of either 3 or 4 months could be considered as a cut-off point, since this effectively differentiated between 2 groups of patients with H-MKI tumors in terms of an increased risk of events for older patients. For further analysis, an age cut-off of 4 months was used (RR 3.1). There were 38 H-MKI tumors diagnosed at less than 4 months of age (82.3% ± 8.4% EFS, 81.8% ± 8.5% OS) and 811 H-MKI tumors diagnosed at over 4 months of age (49.6% ± 2.2% EFS, 63.7% ± 2.1% OS) (P = 0.0034 for EFS, P = 0.0437 for OS). As shown in Table 1c, a significant association was found between age group and other prognostic factors for patients with H-MKI tumors. Patients <4 months of age were more likely to have non–stage 4 disease and nonamplified MYCN. In contrast, patients ≥4 months of age were more likely to have stage 4 disease and amplified MYCN. As shown in Table 2c-1, the final backward-selected model for EFS included INSS and DNA index, while grade, MYCN status, and age were dropped. The final model for OS included INSS, MYCN status, and DNA index, while age and grade were dropped (Table 2c-2).
Table 1c.
Cross-tabulation of age group with prognostic factors for patients with high MKI
| Prognostic factor | Age group
|
Chi-square test P value | |
|---|---|---|---|
| <4 months | ≥4 months | ||
| INSS stagea | < 0.0001 | ||
| Not stage 4 | 26 | 190 | |
| Stage 4 | 12 | 617 | |
| MYCN Status | 0.0004 | ||
| Not amplified | 22 | 247 | |
| Amplified | 16 | 564 | |
| DNA indexa | 0.8906 | ||
| Hyperdiploid | 18 | 386 | |
| Diploid | 19 | 389 | |
Three patients were missing INSS stage, 36 were missing DNA index data, and 1 was missing both.
Summary of prognostic impact
In summary (Fig. 4), among the patients with L-MKI and I-MKI tumors, when age cut-offs of 60 months and 18 months, respectively, were used, as incorporated in the standard INPC system, age was found to be highly independently prognostic of survival, in addition to the other prognostic factors (INSS, MYCN status, and DNA index). In the cohort of patients with H-MKI tumors, although age was found to be highly prognostic of outcome in univariate analyses using a cut-off of 4 months, this failed to reach statistical significance in the presence of the more prognostic variables of INSS and DNA index.
Figure 4.
Event-free survival for patients with different MKI tumors based on the age cut-offs incorporated in the standard International Neuroblastoma Pathology Classification system. Patients with L-MKI tumors diagnosed at < 60 months and with I-MKI tumors diagnosed at <18 months had a significantly better prognosis than patients with L-MKI tumor diagnosed at ≥60 months, with I-MKI tumor diagnosed at ≥18 months, and with H-MKI tumor diagnosed at any age (P < 0.0001). L-MKI indicates low-MKI tumors; I-MKI, intermediate-MKI tumors; H-MKI, high-MKI tumors; MKI, mitosis-karyorrhexis index.
DISCUSSION
According to the INPC, mitotic and karyorrhectic activities of neuroblastoma cells demonstrating cellular proliferation (mitosis) and cellular death due to severe genomic instability (with karyorrhexis presenting as nuclear fragmentation) have been evaluated, and 3 MKI classes (L-MKI, I-MKI, and H-MKI) [8,9] have been defined. Four key points are important for routine practice in MKI class determination, as noted through our central review at the COG Neuroblastoma Pathology Reference Laboratory. (1) Clear distinction between mitotic nuclei and karyorrhectic nuclei is sometimes difficult. (2) The karyorrhectic cells, especially in the I-MKI and H-MKI cases, almost always outnumber the mitotic cells in the same tumor tissues. This is not surprising, as mitoses are short lived (on the order of minutes) based on our tissue culture experience, while karyorrhex would stay a considerably longer period of time (days or weeks before being taken away by scavenger cells). (3) These activities are distributed rather uniformly in most of the L-MKI (diffusely low) and H-MKI (diffusely high) tumors, while the activities often vary markedly from area to area in I-MKI tumors. (4) There are rare tumors composed of multiple and clearly distinct neuroblastoma components (clones), tentatively named composite neuroblastoma, in which MKI determination should be made separately in each component [17,23].
Based on the Cox PH model, the effective range of age cut-off for prognostic distinction of the patients with L-MKI tumors was relatively broad (any point between 28 and 60 months), and we selected 60 months for prognostic distinction for the following reasons: (1) the INPC system using a 60-month cut-off for L-MKI could successfully identify a group of favorable histology patients who had an excellent prognosis without intensive treatment, (2) relative risk was higher for the 60-month cut-off than for the 28-month cut-off, and (3) the current INPC system has been using the same 60-month cut-off for prognostic distinction by grade. The effective range of age cut-off for I-MKI tumors was narrow, between 12 and 18 months, and we selected 18 months since the same age cut-off has been used for prognostic distinction of grade in the current INPC system. As for the H-MKI tumors, whose prognosis is defined as poor in any age group by the INPC, univariate analysis of the Cox PH model suggested either 3 or 4 months of age as a new cut-off for distinguishing patients into different prognostic groups. However, in the presence of other prognostic factors, age with a 4-month cut-off within H-MKI class did not reach statistical significance.
This study confirmed a significant relationship between MYCN amplification and increased MKI class, as shown in previous reports [12,20]. In the embryo, the MYCN oncogene is expressed without amplification and plays a critical role in the early stage of organogenesis through the MYC-MAX (MYC-associated factor X) transcription factor network [24–29]. It is known that MYCN protein expression is eventually terminated during organogenesis [25,26]. In neuroblastoma, MYCN amplification, subsequent MYCN protein expression in excess amounts, and MYC-MAX protein heterodimer formation can activate various downstream genes related to cellular proliferation, inhibition of cellular differentiation, genomic instability, and apoptosis, leading to increasing mitotic and karyorrhectic activities of H-MKI tumors [12,20,30,31]. In this study, it was demonstrated that older children were more likely to have MYCN amplification than younger children in each MKI class. Although this finding might explain a part of the reasons for age-dependent prognostic effects by MKI, this study also proved the independent prognostic effect of 18-month and 60-month cut-offs for the patients with L-MKI and I-MKI tumors, respectively.
It was noted that 3% of L-MKI tumors had amplified MYCN. It is speculated that those rare tumors did not seem to have MYCN protein expression, despite the gene amplification [32]. In contrast, 32% of H-MKI tumors did not have amplified MYCN. Either MYCN overexpression without gene amplification or other molecular mechanism(s) should be responsible for the increased mitotic and karyorrhectic activities of those tumors. In this series of cases, 85% of I-MKI tumors did not have amplified MYCN. Further and detailed investigation should be conducted for the level (high or low) and distribution (diffuse or heterogeneous) as well as pattern (continuously expressed or to be terminated as observed in normal organogenesis) of MYCN protein expression, so that we may understand more about I-MKI tumors, especially concerning the marked variability of mitotic and karyorrhectic activities often encountered in the same tumor tissues. Other molecular mechanism(s), including MYC (C-myc) expression, should be investigated in those I-MKI tumors as well [33].
CONCLUSIONS
Results of this study support the current INPC system, and the same age cut-offs for defining the prognostic effects of age within subgroups by grade of neuroblastic differentiation (no cut-off for undifferentiated, 18 months for poorly differentiated, and 60 months for differentiated) can be applied to subgroups of H-MKI (no cut-off), I-MKI (18 months), and L-MKI (60 months) classes, respectively.
Acknowledgments
This work was partly supported by grants from the National Institute of Health (U10 CA98413 and U10 CA98543).
References
- 1.Gurney JG, Ross JA, Wall DA, Bleyer WA, Severson RK, Robison LL. Infant cancer in the US: histology-specific incidence and trends, 1973 to 1992. J Pediatr Hematol Oncol. 1997;19:428–432. doi: 10.1097/00043426-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans AE, D’Angio GJ, Randolph J. A proposed staging for children with neuroblastoma: Children’s Cancer Study Group A. Cancer. 1971;27:374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Evans AE, D’Angio GJ, Propert K, Anderson J, Hann HW. Prognostic factors in neuroblastoma. Cancer. 1987;59:1853–1859. doi: 10.1002/1097-0142(19870601)59:11<1853::aid-cncr2820591102>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 6.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for Neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 7.Shimada H, Chatten J, Newton WA, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 8.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: Recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. [PubMed] [Google Scholar]
- 9.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada System) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 10.Shimada H, Umehara S, Monobe Y, et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors. Cancer. 2001;92:2451–2461. doi: 10.1002/1097-0142(20011101)92:9<2451::aid-cncr1595>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 12.Shimada H, Stram DO, Chatten J, et al. Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J Natl Cancer Inst. 1995;87:1470–1476. doi: 10.1093/jnci/87.19.1470. [DOI] [PubMed] [Google Scholar]
- 13.Oppedal BR, Storm-Mathisen I, Lie SO, Brandtzaeg P. Prognostic factors in neuroblastoma. Clinical, histopathologic, and immunohistochemical features and DNA ploidy in relation to prognosis. Cancer. 1988;62:772–780. doi: 10.1002/1097-0142(19880815)62:4<772::aid-cncr2820620422>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Look AT, Hayes FA, Nitschke R, McWilliams NB, Green AA. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984;311:231–235. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- 15.Fong CT, White PS, Peterson K, et al. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992;52:1780–1785. [PubMed] [Google Scholar]
- 16.Attiyeh EF, London WB, Mossé YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 17.Peuchmaur M, d’Amore ES, Joshi VV, et al. Revision of the International Neuroblastoma Pathology Classification: confirmation of favorable and unfavorable prognostic subsets in ganglioneuroblastoma, nodular. Cancer. 2003;98:2274–2281. doi: 10.1002/cncr.11773. [DOI] [PubMed] [Google Scholar]
- 18.Shimada H, Nakagawa A, Peters J, et al. TrkA expression in peripheral neuroblastic tumors: prognostic significance and biological relevance. Cancer. 2004;101:1873–1881. doi: 10.1002/cncr.20557. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Bogenmann E, Shimada H, Stram D, Seeger RC. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst. 1993;85:377–384. doi: 10.1093/jnci/85.5.377. [DOI] [PubMed] [Google Scholar]
- 20.Goto S, Umehara S, Gerbing RB, et al. Histopathology (International Neuroblastoma Pathology Classification) and MYCN status in patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer. 2001;92:2699–2708. doi: 10.1002/1097-0142(20011115)92:10<2699::aid-cncr1624>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II: analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umehara S, Nakagawa A, Matthay KK, et al. Histopathology defines prognostic subsets of ganglioneuroblastoma, nodular. Cancer. 2000;89:1150–1161. [PubMed] [Google Scholar]
- 24.Baudino TA, Cleveland JL. The Max network gone mad. Mol Cell Biol. 2001;21:691–702. doi: 10.1128/MCB.21.3.691-702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6:2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- 26.Mugrauer G, Alt FW, Ekblom P. N-myc proto-oncogene expression during organogenesis in the developing mouse as revealed by in situ hybridization. J Cell Biol. 1988;107:1325–1335. doi: 10.1083/jcb.107.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charron J, Malynn BA, Fisher P, et al. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- 28.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 29.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer C, Lee M, Westerhof M, et al. Myc-dependent genome instability and lifespan in Drosophila. PloS one. 2013;8:e74641. doi: 10.1371/journal.pone.0074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwab M, Corvi R, Amler LC. N-MYC oncogene amplification: a consequence of genomic instability in human neuroblastoma. Neuroscientist. 1995;1:277–285. [Google Scholar]
- 32.Suganuma R, Wang LL, Sano H, et al. Peripheral neuroblastic tumors with genotype-phenotype discordance: a report from the Children’s Oncology Group and the International Neuroblastoma Pathology Committee. Pediatr Blood Cancer. 2013;60:363–370. doi: 10.1002/pbc.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LL, Suganuma R, Ikegaki N, et al. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: a report from the Children’s Oncology Group. Cancer. 2013;119:3718–3726. doi: 10.1002/cncr.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]