Abstract
Purpose
Evaluate combination of heat and elevated pressure to enhance protein extraction and quality of formaldehyde-fixed (FF), and FF paraffin-embedded (FFPE) aorta for proteomics.
Experiment design
Proteins were extracted from fresh frozen aorta at RT. FF and FFPE aortas (3 months and 15 years) were extracted at RT, heat alone, or a combination of heat and high pressure. Protein yields were compared, and digested peptides from the extracts were analyzed with mass spectrometry.
Results
Combined heat and elevated pressure increased protein yield from human FF or FFPE aorta compared to matched tissues with heat alone (1.5 fold) or at RT (8.3 fold), resulting in more proteins identified and with more sequence coverage. The length of storage did adversely affect the quality of proteins from FF tissue. For long term storage, aorta was preserved better with FFPE than FF alone. Periostin and MGF-E8 were demonstrated suitable for MRM assays from FFPE aorta.
Conclusions and clinical relevance
Combination of heat and high pressure is an effective method to extract proteins from FFPE aorta for downstream proteomics. This method opens the possibility for use of archival and often rare FFPE aortas and possibly other tissues available to proteomics for biomarker discovery and quantification.
Keywords: aorta, FFPE, formalin-fixed paraffin-embedded, heat and high-pressure protein extraction, mass spectrometry
1 Introduction
Recent proteomic studies have demonstrated that archived formaldehyde-fixed (FF), paraffin-embedded (FFPE) tissues could be used for both qualitative and quantitative mass spectrometry (MS)-based analysis [1, 2].The major advantage of using FF or FFPE tissues is the availability of archived samples from longitudinal investigations in rare populations where the clinical course of disease and response to therapy have been established. However, protein modifications by formaldehyde treatment and histological processing [1, 2] have hampered protein extraction and downstream MS analysis resulting in decreased protein identification and protein sequence coverage limiting their value. Recently, researchers have demonstrated that high temperature, augmented by elevated hydrostatic pressure improves the extractionof proteins and DNA from FFPE liver or colon tissues, respectively [2, 3]. In this study, we expand this work and compare the efficiency of protein extraction from unfixed, FF as well as FFPE human aorta, a challenging tissue to extract at baseline because of significant connective tissue content.
In this report, we test a new combined method of elevated hydrostatic pressure and heat to improve protein extraction efficiency from archival FFPE and FF (15 years old) aortas. The overall quality of proteins extracted compared to tissue heat alone, is demonstrated with higher protein recovery, more protein identification, and with greater amino acid sequence coverage.
2 Materials and Methods
2.1 Protein extraction from unfixed, FF and FFPEhuman aortas
As a comparison to archival aortic tissue, unfixed aortic tissue samples from three individuals were obtained from the Johns Hopkins Hospital Pathology Department and were in compliance with the Johns Hopkins HIPAA/IRB. Unfixed aortas were: A) snap frozen and stored at-80°C forthree months or 2 years or B)10% formalin fixed at 4°C for three months before being processed. Those samples were obtained from subjects with no pathologic evidence of vascular disease based on gross pathology and on examination by hematoxylin and eosin stain as part of the routine anatomic autopsy procedure. Human FF and FFPE aorta samples that had been stored for 15 years were obtained from the NHLBI Pathobiological Determinants of Atherosclerosis (PDAY)repository [4].The PDAY aortic specimens were collected and subdivided according to the PDAY study protocol[4]with storage until use in this study for 15 years. Briefly, the FF aortas were stored in a solution (10% buffered formalin, pH 7.5) at 4°C. Alternatively, the aortas were processed for FFPE. Briefly, aortas were fixed 10% buffered formalin solution for 24 hours, then washed for 30 min with water, dehydrated through a graded series of alcohols (70, 80, and 95% by volume) followed by two changes of xylene, with each step being 30 mins. The tissue was embedded in paraffin following established histology protocols [4]. For sample storage time and origin, please see Supplementary Table 1.
To extract protein from the FFPE PDAY aortas, they were first deparaffinized by incubation through two changes of xylene, and rehydrated through a series of graded alcohols and water washes for 5 min[5, 6 7]. Approximately 20-60 mg (wet weight) of unfixed, FF, and FFPE aorta tissues were minced with scissors, dounce homogenized in 0.78 ml of a homogenization buffer (100 mMTris-HCl, pH 8.0, 100 mM DTT) at room temperature with subsequent addition of a 10% SDS to make a final concentration of 4% SDS. To test the efficiency of heat and pressure to extract aortic proteins the homogenized FFPE aortas were incubated at 24°Cfor one hour at 14.7 psi, 95 °C for one hour at 14.7 psi, or at 95 °C for one hour at 40,000 psi using a NEP 2320 Barocycler (Pressure Biosciences, South Easton, MA) modified by the manufacturer to hold isobaric pressureas previously reported by Fowler et al. [2]. Samples were centrifuged at 16,000xg for 30 mins at room temperature and the supernatants stored at -80°C.The protein concentrations were determined using the CB-X™ Protein Assay kit (G-Biosciences, St. Louis) in duplicate. Each individual biologic sample was replicated three times for protein extraction except that FFPE samples which were combined from three separate blocks (from each individual) due to limited sample availability.
2.2 MS and data analysis
Approximately 100 μg of the protein extracts were desalted with a 2-D clean-up kit (GE Healthcare) and reconstituted in 100mMTris-HCl, 6M urea, pH 7.8.The proteins were reduced with DTT and alkylated with iodoacetimide, digested with trypsin(1:20) for 16 hours at 37°C and the digested peptides were desalted with Oasis HLB plates. LC-MS/MS of the resulting peptideswas performed on an Agilent 1200 nanoflow LC system coupled on-line to a LTQ OrbiTrap mass spectrometer (Thermo Scientific,). The peptides were separated by a BioBasic C18 reverse-phase PicoFrit column (300 A, 5 μm, 75 μm ×10 cm, 15 μm tip, New Objective). Peptides were eluted with a142-min linear gradient from 5 to 45% B (mobile phase A: 2% v/v ACN containing 0.1% v/v formic acid; mobile phase B: 90% v/v ACN containing 0.1% v/v formic acid) at 200nl/min flow rate. The OrbiTrap was operated with an applied electrospray potential of 1.71 kV and capillary transfer tube temperature of 185 °C in a data-dependent mode where each full MS scan was followed by ten MS/MS scans in which the ten most abundant peptide molecular ions detected from the MS scan were dynamically selected for MS/MS analysis using a normalized CID energy of 35%. A dynamic exclusion of 60-s was applied to reduce redundant selection of peptides. The MS/MS spectra were analyzed using SEQUEST (ThermoElectron) and Mascot (Matrix Science) search engines. The data for the aorta extracts was analyzed against a UniProt human proteome database (July, 2012) containing 14770 protein sequences. Only peptides with delta-correlation scores (ΔCn) >0.08 and charge state-dependent cross-correlation scores (Xcorr) with the following criteriawere considered as legitimate identifications:>1.9 for +1 charged peptides, >2.2 for +2 charged peptides, and >3.1 for +3 charged peptides. Reverse-database searches, performed using the respective databases, resulted in calculated false-positive rates of 0.1%for proteins and 0.3% for peptides. Protein isoforms were included only if the peptide sequence was unique to the particular isoform was observed. In Mascot searches, the same UniProt database was searched and the following parameters were used: taxonomy, Homo sapiens; enzyme, semi-trypsin; and allowance of one missed cleavage. Carbamidomethylation was selected as afixed modification and the oxidation of methionine was allowed to vary. Thepeptide and fragment mass tolerance was set at 50ppm and 0.5 Da, respectively. Proteins with one peptide hit were manually validated, and had to be identified with >99% confidence.
2.3 Multiple Reaction Monitoring (MRM) assay
Quantitative MRM assays were developed for periostin and MFG-E8. Two or more peptides unique for each protein were selected for MRM analysis. Three MRM transitions per peptide were designed based on MS/MS identification acquired data. Each MRM assay was optimized manually on a 4000 QTRAP hybrid triple quadrupole/linear IT mass spectrometer (AB SCIEX, Framingham, MA) operating with Analyst 1.4.2 software. The instrument was operatedin positive ion mode. Peptides were separated by an Eksigent Temponano-LC system (Eksigent Technology) onto a BioBasic C18 reverse-phase PicoFrit column (300 Å, 5 μm, 75 μm ×10 cm, 15 μm tip, New Objective). Peptides were eluted with a 36min linearA/B gradient from 5 to 40% B (where mobile phase A: 2% v/v ACN containing 0.1% v/v formic acid; mobile phase B: 98% v/v ACN containing 0.1% v/v formic acid) at a flow rate of 500 nl/min. Samples were analyzed using the following settings curtain gas (CUR):15; collision gas (CAD): high; ion spray voltage (IS): 2.5 kV; ion source gas1 (GS1): 25; ion source gas 2 (GS2): 0; resolution Q1 and Q3: unit; heater interface temperature: 150 °C.
MRM assay data analysis was performed using MultiQuant 2.1 (ABSciex, Framingham, MA). The lower limit of detection (LLOD) was determined from the concentration of a stable isotope labeled synthetic peptide with a signal-to-noise ratio (S/N) above 3. The lower limit of quantification (LLOQ)was determined as the lowest concentration with an S/N above 10 and a recovery between 80-120%. The S/N ratio was determined using MultiQuant 2.1 by injecting known amounts of synthetic peptides. Each sample was analyzed in triplicate.
3 Results
3.1 Protein extraction yield from fresh, FF, FFPE aortas
The different aorta samples and their characteristics are listed in Supplementary Table 1. Figure 1 compares the quantity of proteins extracted after each of the different fixation and storage methods. The protein yield obtained from unfixed, fresh frozen tissue was used as a standard for comparison with the other samples (100%). For FF aortas which had been preserved for 3 months, heat and pressure was superior to heat alone, or room temperature in protein recovery (73.8 ± 23.5%, 46.2 ± 20.1% and 16.1 ± 2.0%, respectively compared to the unfixed samples as 100%). In the FF aortas which had been stored for 15 years, protein recovery was superior with pressure and heat was superior to heat alone, or room temperature (69.3 ± 10.9%, 50.0 ± 12.8% and 4.7 ± 1.7%, respectively). For FF aortas, the storage time of the samples did not adversely affect the efficiency of extraction whether heat or heat and pressure were used; however, the addition of elevated hydrostatic pressure with heat did significantly improve the protein extraction yield compared to heat alone or room temperature. This improved extraction efficiency was also observed with FFPE aorta that had been preserved for 15 years regardless if the samples were extracted using pressure and heat (88.7 ± 3.2%) or heat alone(63.7 ± 5.5%).
Figure 1.
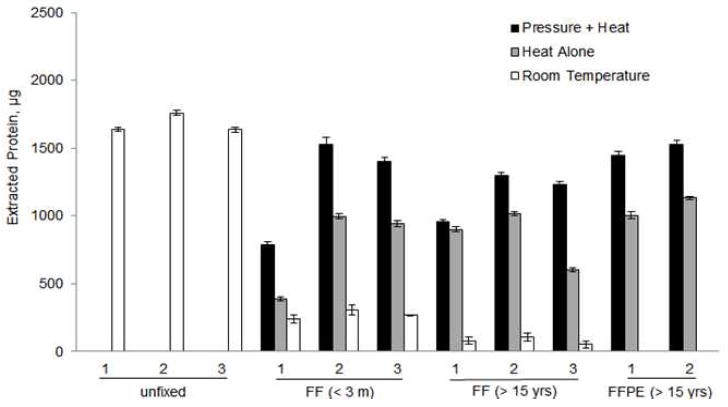
Efficiency of protein extraction from 60 mg unfixed, FF and FFPE aortas at room temperature, with heat or heat plus pressure.*All proteins were extracted from 60 mg of wet tissues except the FFPE aortas were from 20, and 31mg and normalized to 60 mg for comparison.
3.2 MS analysis oftrypsin digestedaorta peptides
Table 1 summarizes the number of non-redundant proteins identified using either SEQUEST or Mascot search engines for the various aortic samples extracted with heat and pressure, heat alone or at room temperature. Most proteins were identified with 2 or more peptides, with a small percentage of single peptide hits (<5%) that met the 99% or greater confidence threshold and passed manual validation. Regardless of the search engine used, the largest number of non-redundant proteins which were identified from samples extracted from fresh frozen, unfixed samples. Mascot identified more proteins than SEQUEST even when using similar search criteria. SEQUEST and Mascot are complementary in that both showed the same trend with respect to comparing the samples preserved under different conditions. However, the differences in the number of identified proteins by SEQUEST was most likely due to its difference in algorithms from Mascot [8, 9].The numbers of total MS spectra were similar for unfixed, FFPE and FF ( 3 months) extracted with heat and pressure or heat alone. FF (3 months) had 50% or less spectra observed extracted at room temperature. In FF (15 years) samples, heat only did not yield any spectra while heat and pressure produced only a third of spectra compared to unfixed samples. The method of fixation and protein extraction had an effect on the number of assigned spectra which influenced both the number of proteins identified and the percent sequence coverage. As shown in Table 1, the number of assigned spectra decreased in the FF and FFPE samples compared to the unfixed frozen samples. For fresh frozen unfixed aorta, there was an average 42% of the total MS spectra were assigned using SEQUEST search engine. In contrast, for FF (3 months), processed at room temperature, or with heat only or with heat and pressure had an average 13.3%, 25%, 30% of the MS spectra identified, respectively. Interestingly, for FF (15 yrs) extracted with heat and pressure, although a third of total MS spectra were observed compared to frozen and FFPE samples, a limited number of proteins were identified, even though the amount of total protein extracted was similar to that of the new FF samples (3 months) (Figure 1) measured with protein assay. Comparing matched samples (15 years) preserved by FFPE and FF, a larger number of proteins were identified in the FFPE samples. There were average 283 proteins identified in the three unfrozen samples using SEQUEST search. FFPE and FF (15 years) samples had average 213 and 20 proteins identified, resulting in a recovery rate of 78% and 7% compared to unfixed tissue. In fact, the number of proteins identified for FFPE (15 yrs) was on par to that observed for the new FF samples (3 months). Comparing proteins extracted with heat and pressure, an average of 213 and 527 proteins were identified for FFPE compared to 224 and 564 proteins for FF (3 months) using SEQUEST or Mascot search engines, respectively (Table 1). In FFPE samples, in addition to more identified proteins with pressure and heat extraction, a significant number of these proteins identified in both conditions were also found with greater sequence coverage than in identical tissue extracted with heat alone (Figure 3). In FFPE sample 1, 90 of 125 proteins identified in both conditions had more spectra (same peptide was detected more times) in heat and pressure compared to heat alone. In FFPE sample 2, 88 of 162 proteins identified in both conditions had more spectra in heat and pressure vs heat only. For example, detailed mass spectra data for fibronectin and filament-A are listed on Table 2. Both proteins were identified with more peptides and larger sequence coverage in FFPE samples in heat and pressure vs. heat only.
Table 1. Number of proteins identified from 0.5ug of trypsin digest of protein.
| Sample Type | Sample and extraction condition | Identified protein (Mascot) | Identified Protein (SEQUEST) | ||||
|---|---|---|---|---|---|---|---|
| Number | Identified Spectra | Total Spectra | %Identified Spectra | ||||
| unfixed | 1 | Room Temperature | 706 | 312 | 8465 | 19497 | 43 |
| 2 | Room Temperature | 629 | 234 | 7597 | 18482 | 41 | |
| 3 | Room Temperature | 679 | 302 | 7948 | 18720 | 42 | |
| FF (3 months) | 1 | Room Temperature | 112 | 36 | 533 | 6843 | 8 |
| Heat only | 469 | 167 | 5257 | 18488 | 28 | ||
| Heat + Pressure | 585 | 202 | 6666 | 18905 | 35 | ||
| 2 | Room Temperature | 180 | 74 | 1230 | 9616 | 13 | |
| Heat only | 561 | 217 | 6273 | 21752 | 29 | ||
| Heat + Pressure | 581 | 259 | 7416 | 21732 | 34 | ||
| 3 | Room Temperature | 106 | 65 | 1440 | 7777 | 19 | |
| Heat only | 359 | 162 | 2693 | 14008 | 19 | ||
| Heat +pressure | 527 | 213 | 3688 | 16919 | 22 | ||
| FF (15 yrs) | 4 | Heat only | 0 | 0 | 0 | 0 | 0 |
| Heart + Pressure | 22 | 12 | 73 | 6242 | 1.2 | ||
| 5 | Heat only | 0 | 0 | 0 | 0 | 0 | |
| Heart+ Pressure | 45 | 21 | 118 | 6775 | 1.8 | ||
| 6 | Heat only | 0 | 0 | 0 | 0 | 0 | |
| Heart+ Pressure | 79 | 28 | 28 | 6242 | 2.0 | ||
| FFPE (15 yrs) | 1 | Heat only | 471 | 149 | 2752 | 21137 | 13 |
| Heat+ Pressure | 565 | 206 | 6030 | 22662 | 27 | ||
| 2 | Heat only | 521 | 198 | 5456 | 22602 | 24 | |
| Heat + Pressure | 589 | 219 | 7479 | 22504 | 33 | ||
Figure 3.
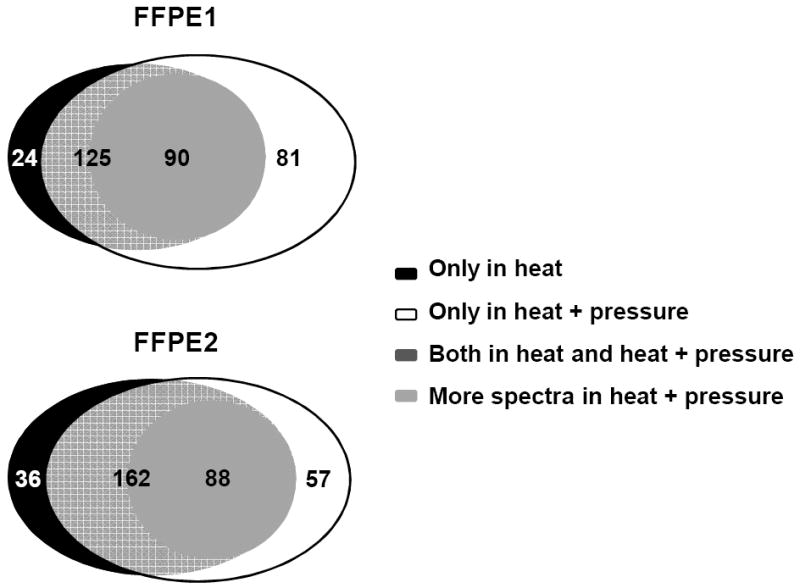
Comparison of identified proteins in FFPE aortas. More spectra in heat and pressure means that the peptide has been detected and scanned more times in mass spectrometer in the same settings.
Table 2. Comparison of peptide and sequence coverage of fibronectin and filament Ain FFPE samples on different extraction condition.
| Protein | FFPE Sample and extraction condition | Unique peptide | Unique spectra | Total Spectra | %Sequence coverage | |
|---|---|---|---|---|---|---|
| Fibronectin | 1 | Heat only | 37 | 39 | 90 | 18 |
| Heat+Pressure | 51 | 58 | 215 | 25 | ||
| 2 | Heat only | 44 | 50 | 244 | 25 | |
| Heat + Pressure | 55 | 65 | 345 | 32 | ||
| Filament A | 1 | Heat only | 38 | 38 | 87 | 19 |
| Heat+ Pressure | 63 | 69 | 299 | 32 | ||
| 2 | Heat only | 60 | 71 | 355 | 33 | |
| Heat + Pressure | 65 | 79 | 419 | 35 | ||
There were 263 different proteins identified in the two FFPE samples extracted with heat and pressure, 190 of which were identified inthe three unfixed samplesusing SEQUEST search[Table 1, Supplementary Table 2].Usingthe proteins identified by SEQUEST for the unfixed and FFPE extracted with heat and pressure, the gene ontology program GoMiner software (http://discover.nci.nih.gov/gominer/index.jsp) was used to categorize the proteins based on annotated biological process, cellular componentor molecular function. The results indicated that the proteins spanned a wide range of cellular component, and were involved inimportant biologicalprocesses and molecular functions. Similar percentages of proteins were involved in cell adhesion, proliferation, metabolic and signaling process from unfixed and FFPE samples (Supplementary Figure 1).
3.3 MS Based quantification of aortic proteins from FFPE samples
MRM assays were developed and used to test the quality of the extracted aortic proteins for targeted quantitative measures. For example, in Figure 2, the MRM assay for periostin, a smooth muscle cell protein previously identified in aorta [10], was able to quantify this protein in FFPE samples regardless of storage time. The various prototypic peptides for periostin were detected and co-eluted with the N15 labeled internal peptide standards in the two PDAY FFPE samples studied. Of importance, usingan MRM assay, periostin was detected in unfixed, the FF (3 months) and FFPE (15 years) tissue. Another protein, MFG-E8, a low abundant aortic protein [10], was also detected in, unfixed, FF (3 months) and FFPE aorta with MRM (Supplementary Figure 2), indicating the feasibility to also quantify proteins in old archival samples such as the PDAY cohort (Figure 2).
Figure 2.
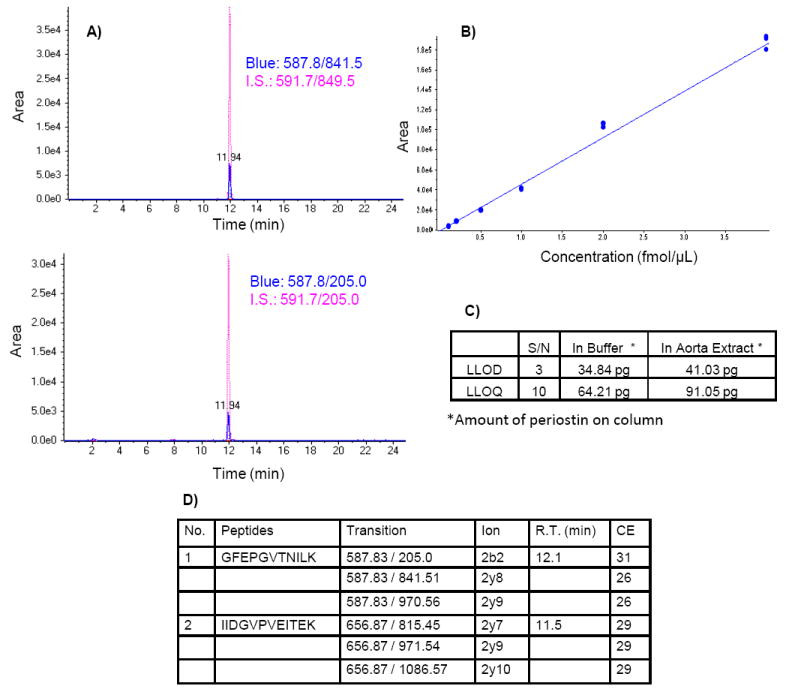
MRM assay of periostin in FFPE aortas. A) overlapped peptides with standard; B) standard curve based on isotope labeled standard peptides; C) lower limit of detection (LLOD) and quantification (LLOQ) of periostin; D) chosen peptides and transitions
4 Discussion
Previously, it has been shown thatthe addition of sodium dodecyl sulfate (SDS) in heat-induced antigen retrieval (HIAR) protocols for the reversal of formalin-induced cross-links iseffective for subsequent recognition of proteins by antibodies in immunohistochemistry [11]. Recently, Fowler and colleagues demonstrated a dramatic increase in protein extraction efficiency usingheat augmented with high hydrostatic pressure to extract proteins from FFPE rat liver samples [2]. However, it is not known whether heat and or pressure will be useful in extraction of FF as well as FFPE samples stored for long term and whether it would be useful for challenging tissuesthat have considerable connective tissue. FFPE aorta, is particularly difficult to homogenize because of its significant connective tissue content making it a challenge to prepare extracts suitable for proteomic analysis [4, 10].
In this study, we reconfirmed that the combination of elevated pressureand heating significantly improved protein extraction, protein identifications and proteome coveragein tissues not previously tested, the FFPE aorta. An increase in pressure to 40,000 psiin combination with heat treatment, improved protein extraction efficiency by approximately 1.5-foldfrom the FFPE aorta, compared to heat alone. Compared to samples extracted at room temperature without pressure, heat and pressure improved extraction efficiency by 8.3 fold. This was complimented in FFPE aorta samples where more proteins were identified with more peptide sequence coverage when extracted with heat and pressure. This demonstrated that heating improved protein extraction and that combination of pressure and heat further improved the yield. An important finding is that the old FF aorta (15 yrs) yielded a similar amount oftotal protein extract. However, the quality of the protein for proteomic analysis was poor, yieldingextremely limited numbers of positive protein identifications, suggesting that the proteins were degradedor cross-linked even though the extracts still reacted with protein assay chemicals. Actually, with FF there is the real possibility of cross-linked proteins generating cross-linked and modified peptides which will not be identified using traditional search parameters that are applied with MS analysis [12]. Yet, it would contribute towards the total protein yield determined using a proteinconcentration assay. Additional methods are necessary to improve the yield of proteins from old FF aortasfor downstream MS. Yet, it is remarkable that the archival FFPE samples stored over 15 years from the PDAY study still yielded a remarkable number of peptide and protein identifications. These results suggest that FFPE preservation of tissue is better than FF alone for long term preservation of the aorta. Furthermore, we have demonstrated that the extracted proteins are of high quality and suitable for analytic studies using MRM assays. The two proteins, periostin and MFG-E8, found to be quantifiable in these archival samples have been implicated in cardiovascular disease [10, 13].
This is major concern that range of proteins that can be recovered from archival tissue will be limited and therefore of little value for disease pathway analysis. In this report we found that the major proteins identified from the heat and pressure extraction of FFPE samples were involved in important functions (Supplementary Figure 1), and also represented those proteins previously identified from fresh rat aorta by Fu et al [10].While most published proteomic studies of FFPE tissue analyze only a few thousand cells from micro-dissected tissue [14, 15], the high-pressure method has the advantage of improving protein extraction from whole tissue fragments. The ability to extract proteins from whole tissue is particularly useful for samples that contain large amounts of connective tissue, like aorta, in instances where tissue micro-dissection is not practical, or when a more global proteomic analysis is desired [2,4].
It has been suggested by Balnyand colleagues that pressure promotes water penetration into the protein core causing denaturation, whereas heat alone causes protein unfolding followed by aggregation [2,16]. Consequently, the combined effect of heat, augmented by elevated pressure, would facilitate the re-hydration of highly aggregated protein in FFPE tissue, leading to protein solubilization and the reversal of protein formaldehyde adducts and cross-links. This method improves protein extraction efficiency, recoversmore proteins, and yields tryptic digests that closely resemble matched unfixed frozen tissue.
In conclusion, the combination of both pressure and heat make it possible to take advantage of old archival FFPE aorta tissue for MS proteomic analysis and quantification with high protein identification confidence and sequence coverage that rivals fresh frozen tissue. Thus, FFPE appears to be the optimal archival media for tissue preservation for proteomics.
Supplementary Material
Clinical Relevance.
Formaldehyde-fixed (FF), paraffin-embedded (FFPE) arterial repositories are a valuable resource for studying vascular disease; however, such repositories presently offer little opportunities for proteomics. This is due, in part, to the fact that arterial tissues, including aorta, have significant connective tissue component which limits yield of protein extraction. This problem is exacerbated by the covalent cross-linking and protein degradation that occurs with formalin fixation and long time storage. Here an improved protein extraction method for FF and FFPE aortas is described, which uses high temperature (95°C) and high hydrostatic pressure (40,000 psi) with a significant improvement in protein yields. More proteins were identified with more amino acid sequence coverage from the same amount of proteins. The length of storage had a detrimental effect on the quality of protein from FF samples based on mass spectrometry analysis. However, for aorta (and possibly other tissues that may be stored for long periods of time), the quality and quantity of protein extraction was significantly better with FFPE than FF alone. In addition, the extracted proteins from archived FFPE aorta (15 years old) were of high quality and could be quantified using MRM assays for two medium to low abundance proteins. In conclusion, a combination of high temperature and pressure make it feasible to utilize archives of FFPE arterial samples, including samples representing rare conditions, and possibly other tissue types for detailed proteomic analysis.
Acknowledgments
This project has been funded in whole or in part with federal funds from grant 5RC1HL100021-02 (JVE) and grant NHLBI-HV-10-05 (JVE).
References
- 1.Ostasiewicz P, Zielinska DF, Mann M, Wisniewski JR. Proteome, phosphoproteome, and N-glycoproteome are quantitatively preserved in formalin-fixed paraffin-embedded tissue and analyzable by high-resolution mass spectrometry. J Proteome Res. 2010;9:3688–3700. doi: 10.1021/pr100234w. [DOI] [PubMed] [Google Scholar]
- 2.Fowler CB, Waybright TJ, Veenstra TD, O’Leary TJ, Mason JT. Pressure-assisted protein extraction: a novel method for recovering proteins from archival tissue for proteomic analysis. J Proteome Res. 2012;11:2602–2608. doi: 10.1021/pr201005t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung JY, Yi JM, Xie R, Brown V, Lee O, Ahuja N, Braunschweig T, Hewitt SM. A pressure cooking-based DNA extraction from archival formalin-fixed, paraffin-embedded tissue. Anal Biochem. 2012;425:128–134. doi: 10.1016/j.ab.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, 3rd, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the pathobiological determinants of atherosclerosis in youth study. JAMA. 1999;281:727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 5.Fowler CB, et al. Elevated hydrostatic pressure promotes protein recovery from formalin-fixed, paraffin-embedded tissue surrogates. Lab Invest. 2008;88:185–195. doi: 10.1038/labinvest.3700708. [DOI] [PubMed] [Google Scholar]
- 6.Nirmalan NJ, Harnden P, Selby PJ, Banks RE. Development and validation of a novel protein extraction methodology for quantitation of protein expression in formalin-fixed paraffin-embedded tissues using western blotting. JPathol. 2009;217:497–506. doi: 10.1002/path.2504. [DOI] [PubMed] [Google Scholar]
- 7.Nirmalan NJ, Hughes C, Peng J, McKenna T, Langridge J, Cairns DA, Harnden P, Selby PJ, Banks RE. Initial development and validation of a novel extraction method for quantitative mining of the formalin-fixed, paraffin-embedded tissue proteome for biomarker investigations. J Proteome Res. 2011;10:896–906. doi: 10.1021/pr100812d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves G, Wu WW, Wang G, Shen RF, Yu YK. Enhancing peptide identification confidence by combining search methods. J Proteome Res. 2008;7:3102–3113. doi: 10.1021/pr700798h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 10.Fu Z, Wang M, Gucek M, Zhang J, Wu J, Jiang L, Monticone RE, Khazan B, Telljohann R, Mattison J, Sheng S, Cole RN, Spinetti G, Pintus G, Liu L, Kolodgie FD, Virmani R, Spurgeon H, Ingram DK, Everett AD, Lakatta EG, Van Eyk JE. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104:1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 12.Sprung RW, Jr, Brock JW, Tanksley JP, Li M, Washington MK, Slebos RJ, Liebler DC. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol Cell Proteomics. 2009;8:1988–1998. doi: 10.1074/mcp.M800518-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hixson JE, Shimmin LC, Montasser ME, Kim DK, Zhong Y, Ibarguen H, Follis J, Malcom G, Strong J, Howard T, Langefeld C, Liu Y, Rotter JI, Johnson C, Herrington D. Common variants in the periostin gene influence development of atherosclerosis in young persons. ArteriosclerThrombVasc Biol. 2011;31:1661–1667. doi: 10.1161/ATVBAHA.111.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didangelos A, et al. Proteomics Characterization of Extracellular Space Components in the Human Aorta. Molecular & Cellular Proteomics. 2010;9:2048–2062. doi: 10.1074/mcp.M110.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo T, Wang W, Rudnick PA, Song T, Li J, Zhuang Z, Weil RJ, DeVoe DL, Lee CS, Balgley BM. Proteome analysis of microdissected formalin-fixed and paraffin-embedded tissue specimens. J HistochemCytochem. 2007;55:763–772. doi: 10.1369/jhc.7A7177.2007. [DOI] [PubMed] [Google Scholar]
- 16.Balny C, Masson P, Heremans K. High pressure effects on biologicalmacromolecules: from structural changes to alteration of cellular processes. Biochim Biophys Acta. 2002;1595:3–10. doi: 10.1016/s0167-4838(01)00331-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


