Abstract
The primary cilium is essential for skin morphogenesis through regulating the Notch, Wnt, and hedgehog signaling pathways. Prior studies on the functions of primary cilia in the skin were based on the investigations of genes that are essential for cilium formation. However, none of these ciliogenic genes has been linked to ciliopathy, a group of disorders caused by abnormal formation or function of cilia. To determine whether there is a genetic and molecular link between ciliopathies and skin morphogenesis, we investigated the role of RPGRIP1L, a gene mutated in Joubert (JBTS) and Meckel (MKS) syndromes, two severe forms of ciliopathy, in the context of skin development. We found that RPGRIP1L is essential for hair follicle morphogenesis. Specifically, disrupting the Rpgril1 gene in mice resulted in reduced proliferation and differentiation of follicular keratinocytes, leading to hair follicle developmental defects. These defects were associated with significantly decreased primary cilium formation and attenuated hedgehog signaling. In contrast, we found that hair follicle induction and polarization and the development of interfollicular epidermis were unaffected. This study indicates that RPGRIP1L, a ciliopathy gene, is essential for hair follicle morphogenesis likely through regulating primary cilia formation and the hedgehog signaling pathway.
Keywords: Rpgrip1l, ciliopathy, hair follicle, cilia, hedgehog signaling
INTRODUCTION
The primary cilium is an antenna-like subcellular structure protruding from the cell surface (Goetz and Anderson, 2010; Singla and Reiter, 2006). Primary cilia are found in keratinocytes and dermal fibroblasts (Bershteyn et al., 2010; Dai et al., 2011; Ezratty et al., 2011; Lehman et al., 2009). Blocking cilium formation through disrupting ciliogenic genes can cause epidermal and hair follicle morphogenesis defects due to attenuated Notch or Hh signaling pathways (Croyle et al., 2011; Dai et al., 2013; Dai et al., 2011; Ezratty et al., 2011; Lehman et al., 2009; Woo et al., 2012). However, prior studies in the skin were based on the investigation of genes, such Ift88, Kif3a and Ift74, which have not yet been linked to ciliopathy in humans. Therefore, it remains unclear whether genes causative for ciliopathies are also essential for normal skin development.
Retinitis pigmentosa GTPase regulator interacting protein-1 like (RPGRIP1L), also known as nephronophthisis 8 (NPHP8), KIAA1005, MKS5, JBTS7, and fantom (Ftm, in mouse), encodes a 151 kDa protein, containing five coiled-coil domains in the N-terminus, two protein kinase C conserved region 2 (C2) domains in the central region, and an RPGR-interacting domain (RID) in the C-terminus (Arts et al., 2007; Delous et al., 2007; Remans et al., 2014; Vierkotten et al., 2007). Autosomal recessive mutations in RPGRIP1L can cause Joubert (JBTS) and Meckel (MKS) syndromes, two of the most severe forms of ciliopathies (Arts et al., 2007; Delous et al., 2007). JBTS is characterized by cerebellar ataxia, developmental delay, hypotonia, and the “molar tooth sign” when cerebellar vermis hypoplasia and dysplasia with accompanying brainstem defects are visualized by axial magnetic resonance imaging (Doherty, 2009; Sattar and Gleeson, 2011; Valente et al., 2013). MKS is characterized by occipital encephalocele, polydactyly, cystic kidney, and hepatic fibrosis. Interestingly, no remarkable skin manifestations were reported in JBTS and MKS patients or fetuses.
Disrupting the mouse homolog (Rpgrip1l or Ftm) of RPGRIP1L results in ciliogenesis defects in a number of cell types, such as neuroepithelial cells, embryonic fibroblasts, and cardiac cells (Besse et al., 2011; Gerhardt et al., 2013; Mahuzier et al., 2012; Vierkotten et al., 2007). In accordance, mutant mice displayed polydactyly, laterality defects, kidney cysts, liver and heart defects, craniofacial malformation, and patterning defects in the brain and in the posterior neural tube (Besse et al., 2011; Delous et al., 2007; Gerhardt et al., 2013; Vierkotten et al., 2007). Some of these phenotypes were attributed to attenuated Hh signaling pathway. Since the development of hair follicles requires primary cilia and activation of the Hh signaling pathway, it is interesting to determine whether this ciliopathy gene is also required for hair follicle morphogenesis.
Beyond controlling ciliogenesis, Rpgrip1l is required for convergent extension and the establishment of planar cell polarity (PCP) in zebra fish and in the mouse cochlea (Khanna et al., 2009; Mahuzier et al., 2012). Whether Rpgrip1l also participates in the establishment of PCP in the skin is unknown. Prior studies demonstrated that PCP is essential for the establishment of the global patterning of the hair follicles such that disrupting core PCP genes could result in hair follicle polarization defects (Chen and Chuong, 2012). Therefore, whether Rpgrip1l could influence PCP in the skin, thereafter, the polarization of the hair follicles remains to be determined.
In this study, we investigated whether Rpgrip1l is involved in the development of the skin and hair follicle. We found that Rpgrip1l is essential for primary cilia formation, Hh signaling, and follicular keratinocyte differentiation during hair follicle morphogenesis. Interestingly, disrupting Rpgrip1l did not affect hair follicle induction and polarization or epidermal differentiation. This study establishes the role of Rpgrip1l in hair follicle development at the genetic and molecular levels, and suggests that other genes involved in the development of ciliopathies may also be essential for the morphogenesis of hair follicles.
RESULTS
Expression of Rpgrip1l in the skin and hair follicle
Expression profile of Rpgrip1l was examined in developing mouse skin. Quantitative RT-PCR revealed that Rpgrip1l mRNA is expressed throughout the embryonic development of the skin (Supplemental Fig. S1). In situ hybridization showed that Rpgrip1l is expressed ubiquitously in both epidermal and dermal compartments of the skin and immunofluorescence confirms the localization of the protein at the Ciliary base in both cell types (Supplemental Fig. S1), suggesting that this gene may be involved in the function of epidermal keratinocytes and dermal fibroblasts.
Rpgrip1l is essential for the proliferation and differentiation of follicular keratinocytes but not epidermal keratinocytes
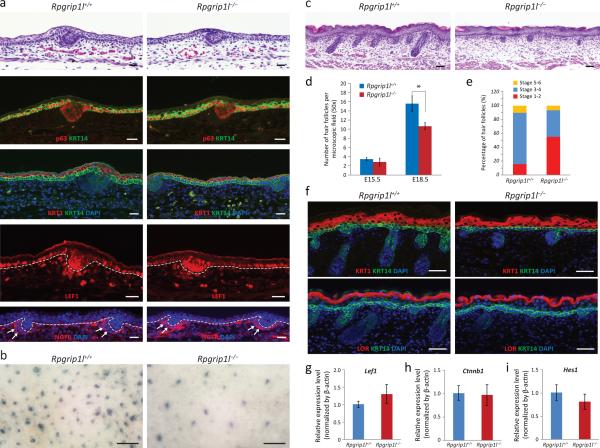
Histologically, the dorsal epidermis of E15.5 Rpgrip1l−/− fetuses did not reveal any noticeable defect (Fig. 1a). Immunofluorescence labeling of p63 and keratin 1 (KRT1) did not reveal any remarkable difference between Rpgrip1l−/− and controls at E15.5 (Fig. 1a), suggesting that Rpgrip1l is not involved in the stratification and differentiation of the epidermis. At E18.5, the proliferation of interfollicular epidermal keratinocytes, as determined by bromodeoxyuridine (BrdU) and phospho-histone H3 (Supplemental Fig. S2a and b, respectively), the expression of early and late differentiation markers (KRT1 and loricrin (LOR)), and the activation of the Notch signaling pathway were also comparable between Rpgrip1l−/− and control skins (Fig. 1f and i).
Fig. 1.
Rpgrip1l in skin morphogenesis. (a) H&E staining and immunofluorescence labeling of p63 (red), KRT1 (red), KRT14 (green), LEF1 (red) and NGFR (red) in dorsal skins of E15.5 controls (Rpgrip1l+/+) and homozygous mutants (Rpgrip1l−/−). (b−c) Alkaline phosphatase and H&E stainings of E18.5 skins. (d) Quantification of hair follicles in the dorsal skin of E15.5 and E18.5 fetuses, n ≥ 4. *, P < 0.01. (e) Distribution of developmental stages of hair follicles in dorsal skin of E18.5 fetuses. n ≥ 6. (f) Immunofluorescence labeling of KRT1 (red), LOR (red), and KRT14 (green) in E18.5 skins. Nuclei were stained with DAPI (blue). (g−i) QRT-PCR of Lef1, Ctnnb1, and Hes1 in E18.5 skins. Scale bars = 15 μm in (a); 250 μm in (b); 50 μm in (c and f).
The dorsal skins of E15.5 embryos of controls and Rpgrip1l−/− mutants contained comparable number of early hair follicles (stage 1 and stage 2) (3.44 ± 0.38 and 2.83 ± 0.86 per microscopic field, respectively), p = 0.45 (Fig. 1d), which were also positive for keratin 17 (KRT17) (Supplemental Fig. S3). The expression of canonical Wnt target genes, such as Lef1 and Ctnnb1, were also comparable between control and Rpgrip1l−/− mutants (Fig. 1a, g and h). Examination of dermal condensates of E15.5 embryos with nerve growth factor receptor (NGFR) revealed normal aggregation of hair follicle-inducing dermal mesenchymal cells (Fig. 1a). These observations suggested that the induction of hair follicle morphogenesis was unaffected in the Rpgrip1l−/− mutants.
At E18.5, a significantly reduced number of hair follicles was observed in the dorsal skin of Rpgrip1l−/− mutants. Specifically, the number of hair follicles, irrespective of developmental stages, were 15.57 ± 1.74 and 10.66 ± 0.77 per microscopic field for controls and Rpgrip1l−/− mutants, respectively, p < 0.01 (Fig. 1c and d). Furthermore, classification of the developmental stages of hair follicles (as described by Paus et al. (Paus et al., 1999)) revealed that the development of hair follicles in Rpgrip1l−/− skin was significantly delayed. Specifically, the percentage of stage 1−2, stage 3−4, and stage 5−6 hair follicles in the control skins were 15.8% ± 14.3%, 74.0% ± 10.8%, and 10.2% ± 5.9%, respectively, whereas the Rpgrip1l−/− skin contained a significantly increased proportion of stage 1−2 hair follicles (55.2% ± 17.9%, p = 0.003) and reduced proportions of stage 3−4 hair follicle (37.9% ± 15.8%, p=0.002) and stage 5−6 hair follicles (6.8% ± 2.5%, p = 0.26) (Fig. 1e). The proliferation of follicular keratinocytes contained in stage 2 and stage 3 hair follicles, as determined by BrdU and phospho-histone H3 stainings (Supplemental Fig. S2c and d, respectively), was also reduced. Interestingly, alkaline phosphatase (AP) staining of E18.5 embryonic skins demonstrated robust AP activity, albeit the number of AP-positive cell clusters was significantly reduced in Rpgrip1l−/− skin (Fig. 1b).
Disrupting Rpgrip1l in the skin causes hair follicle morphogenesis defects
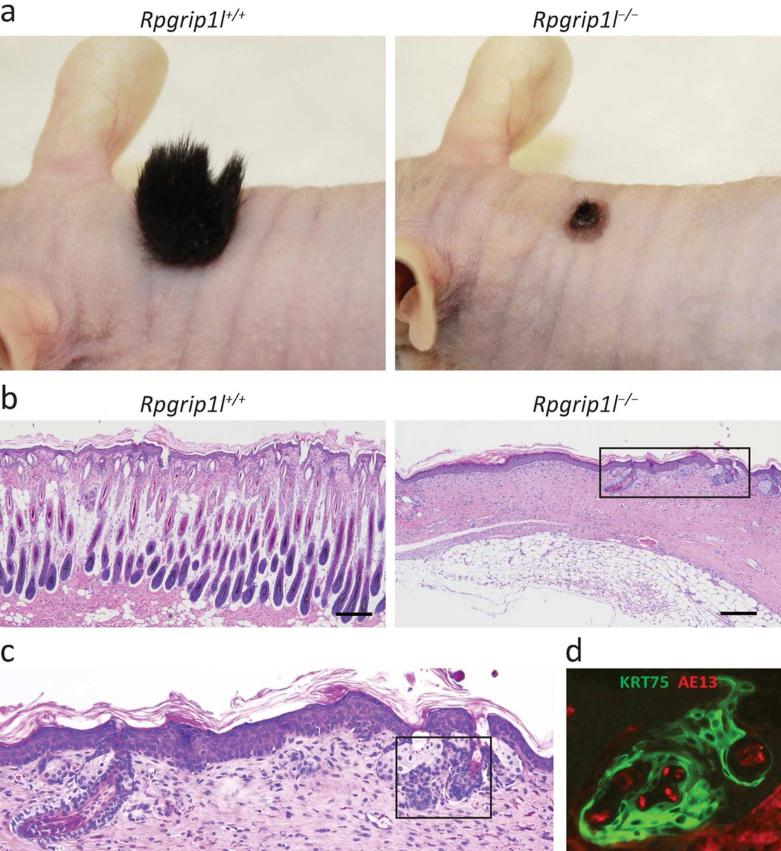
To determine the function of Rpgrip1l in postnatal skin and the fate of the developmentally delayed hair follicles, we transplanted the dorsal skins of E18.5 Rpgrip1l−/− mutants onto nude mice. Four weeks after grafting, we observed that Rpgrip1l−/− skin was able to engraft as control skin, however, the Rpgrip1l−/− skin was almost completely devoid of visible hair shafts (Fig. 2a). Histological examination confirmed that the Rpgrip1l−/− skin contained almost no hair follicles (Fig. 2b). The hair follicle-like structures in the Rpgrip1l−/− transplants were highly disorganized, despite containing keratin 75 (KRT75) and AE13 (hair cortex cytokeratin) positive cells (Fig. 2c and d). TUNEL staining showed numerous apoptotic cells along the hair canal in wild-type skin transplants, whereas there were essentially no apoptotic cells in the hair follicle-like structure in Rpgrip1l−/− transplants (Supplemental Fig. S4). This result demonstrates that Rpgrip1l is not required for the postnatal maintenance of the epidermis. However, disrupting the Rpgrip1l gene during skin development could cause permanent growth arrest and, ultimately, the clearance of developing hair follicles.
Fig. 2.
Skin transplantation of control (Rpgrip1l+/+) and mutant (Rpgrip1l−/−) littermates. (a) Representative gross images of skin transplants at 34 days post-grafting. (b) H&E staining of skin transplants in (a). (c) Magnified boxed area in (b). (d) Immunofluorescence labeling of AE13 (red) and KRT75 (green) of boxed area in (c). Note that the lack of hair in mutant skin transplants was caused by the lack of hair follicle and that the hair follicle-like structures in mutant skin contained disorganized but AE13 and KRT75 positive cells. n ≥ 4. Scale bars = 200 μm.
To gain insight into the cause of the disappearance of developing hair follicles in Rpgrip1l−/− skin, proliferation of ex vivo cultured skin explants and skin transplants was examined. We found that hair follicles in Rpgrip1l−/− skins progressively lost proliferative hair follicle matrix keratinocytes (Supplemental Fig. S5). The loss of proliferating follicular keratinocytes might have resulted in the loss of hair follicles in post-natal skin, probably in response to attenuated mitogenic signals in the hair follicles (see below).
Formation of primary cilia was disrupted in Rpgrip1l−/− skin
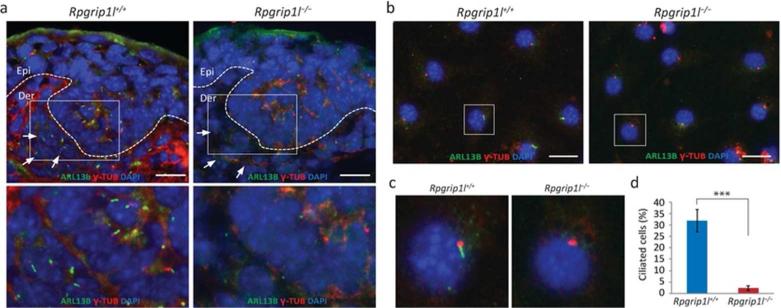
Immunofluorescence labeling of primary cilia and basal body with ARL13B and γ-tubulin, respectively, revealed significantly reduced number of ciliated keratinocytes and fibroblasts in Rpgrip1l−/− skin (Fig. 3a and Supplemental Fig. S6). Cells that were able to form cilia contained severely truncated ciliary axonemes (Supplemental Fig. S6). This observation is consistent with cilia defects described in previous reports (Besse et al., 2011; Gerhardt et al., 2013; Vierkottenet al., 2007).
Fig. 3.
Ciliogenesis was disrupted in mutant (Rpgrip1l−/−) skin. (a) Immunofluorescence labeling of primary cilia and basal bodies with ARL13B (green) and γ-tubulin (red) in dorsal skins of E15.5 control (Rpgrip1l+/+) and mutant embryos. Nuclei were stained with DAPI (blue). Dotted lines illustrate the epidermal-dermal junction; arrows point to dermal papilla cells. Epi, epidermis; Der, dermis. Lower panels are enlarged boxed area in the upper panels. n ≥ 6. (b) Labeling of cilia and basal body (as described in a) in primary dermal fibroblasts. Three independent experiments were performed with similar results. (c) Enlarged boxed area in (b). (d) Statistically analyses of the percentage of ciliated cells in (b). ***, P < 0.0001. Scale bars = 50 μm in (a) and 25 μm in (b).
To further examine the ciliogenic potential of Rpgrip1l−/− cells, we collected dermal fibroblasts from E18.5 embryos and examined their ciliogenic capability in vitro. After 48-hour serum starvation, 31.80% ± 4.90% control fibroblasts were able to form cilia; whereas only 2.32% ± 1.07% Rpgrip1l−/− cells formed cilia (p < 0.0001) (Fig. 3b − d). These data demonstrated that Rpgrip1l is indispensable for primary cilia formation in dermal fibroblasts in vitro.
Hh signaling is attenuated in Rpgrip1l−/− skin
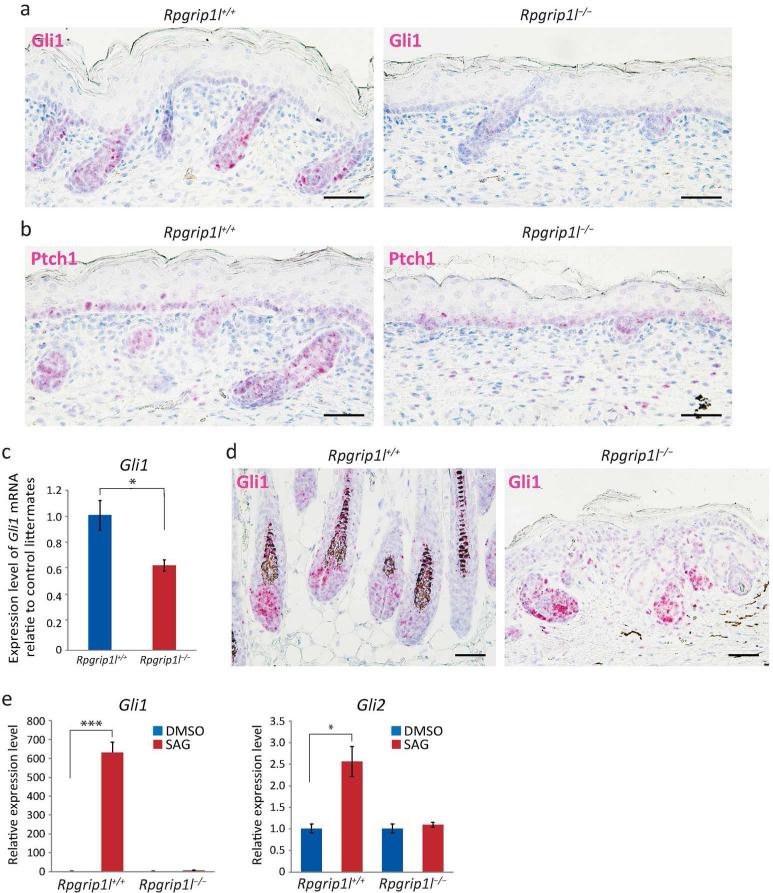
Because the primary cilium is essential for Hh signaling and the hair follicle phenotypes observed in Rpgrip1l−/− mutants were reminiscent of those in Hh mutants (Chiang et al., 1999; Gat et al., 1998; Mill et al., 2003; St-Jacques et al., 1998; Woo et al., 2012), we examined the Hh responsive genes in the Rpgrip1l−/− mutants. In situ hybridization demonstrated remarkably reduced levels of Gli1 and Ptch1 in E18.5 Rpgrip1l−/− skin (Fig. 4a and b), which was confirmed by quantitative RT-PCR (p < 0.01) (Fig. 4c). Furthermore, we found that, in the control skin graft, the hair follicle bulbs contained a large number of Gli1 positive cells; whereas in the Rpgrip1l−/− skin transplants, we were only able to observed a few Gli1-positive cells scattered along the epidermis or clustered in disorganized epidermal invaginations under a thickened epidermis (Fig. 4d and Supplemental Fig. S7). These data demonstrated that Hh signaling was severely attenuated but not completely abolished in Rpgrip1l−/− skin and that the hair follicle phenotype of the Rpgrip1l−/− mutants was associated with attenuated Hh signaling.
Fig. 4.
Hedgehog signaling is attenuated in mutant (Rpgrip1l−/−) skin. (a − b) Representative in situ hybridization of Gli1 (a) and Ptch1 (b) in dorsal skins of E18.5 controls (Rpgrip1l+/+) and Rpgrip1l mutants, n = 3. (c) Expression levels of Gli1 in skins of E18.5 fetuses by quantitative RT-PCR; n = 3; *, P < 0.01. (d) In situ hybridization of Gli1 in skin transplants. Some Gli1-positive cells scattered along the thickened epidermis and amorphic cell aggregates invaginated in the dermis. (e) Relative expression levels of Gli1 and Gli2 in SAG-treated control and mutant primary dermal fibroblasts as determined by qRT-PCR. DMSO treatment was used as control. *, p<0.05. ***, P < 0.0001. Experiments were conducted three times. Scale bars = 50 μm.
To further determine the role of Rpgrip1l in the transduction of Hh signals, we treated primary dermal fibroblasts isolated from E18.5 Rpgrip1l−/− mutants with SAG, a potent agonist of smoothened (SMO). Quantitative RT-PCR showed that SAG was able to robustly induce the expression of Hh target genes (Gli1 and Gli2) in control cells (p < 0.0001 and p < 0.001, respectively) but not in Rpgrip1l−/− cells (Fig. 4e). This experiment suggested that Rpgrip1l is essential for primary dermal fibroblasts to respond to Hh signals.
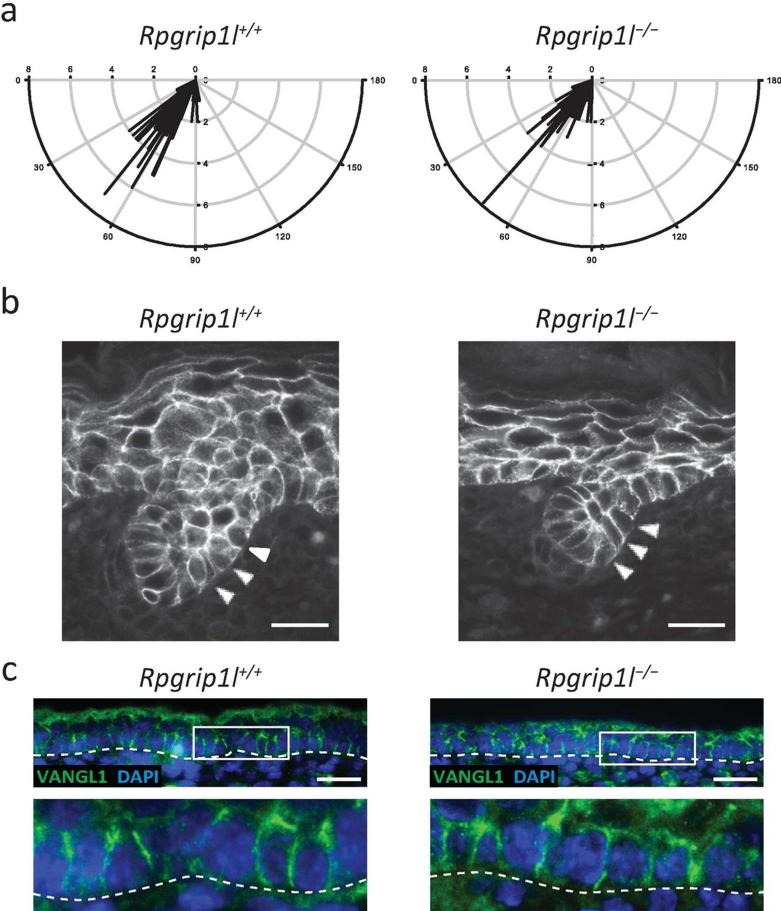
Polarization of hair follicles was unaffected in Rpgrip1l−/− skin
Since Rpgrip1l was implicated in the establishment of PCP, such as the polarization of cochlear hair cells in mice, (Mahuzier et al., 2012) and because PCP is essential for establishing the global polarization of hair follicles (Devenport and Fuchs, 2008; Guo et al., 2004; Tissir et al., 2010; Wang et al., 2010), it is of interest to determine whether Rpgrip1l is also involved in polarizing the hair follicles along major body axes of Rpgrip1l−/− mice. First, we observed that hair germs in E15.5 embryos were consistently polarized, which is especially evidenced by the asymmetric localization of dermal condensate cells (Fig. 1a). Then, we determined the acute angles formed between the hair follicle projection and the planar plane (surface) along the midline of the dorsal skin of E18.5 embryos (Fig. 5 and Supplemental Fig. S8). The angles formed in wild-type and Rpgrip1l−/− skins were essentially indistinguishable at 60.74° ± 1.42° (n = 137) and 52.10° ± 1.88° (n = 79), respectively (Fig. 5a), suggesting a normal polarization of the hair follicles in Rpgrip1l−/− mutants. To further determine whether mutant hair follicles were also molecularly compartmentalized, the distribution of a polarity marker, E-cadherin, in stage 2 hair follicles was examined in control and Rpgrip1l−/− hair follicles. We found that E-cadherin was asymmetrically distributed to cells that were localized to the posterior side of control and Rpgrip1l−/− hair follicles (Fig. 5b). Finally, immunofluorescence labeling of VANGL1 revealed normal distribution of this core PCP protein at the lateral membrane of basal keratinocytes in Rpgrip1l−/− skin (Fig. 5c).
Fig. 5.
Orientation of hair follicles in sagittal sections of E18.5 dorsal skins of control (Rpgrip1l+/+) and Rpgrip1l mutant (Rpgrip1l−/−) skins. (a) Quantification of the acute angle between hair follicle and the surface of the epidermis. (b) Polarized distribution of E-cadherin in follicular keratinocytes. Triangles point to E-cadherin positive cells on the posterior side of the hair germs. (c) Immunofluorescence labeling of VANGL1 (green). Lower panels represent magnified boxed areas in upper panels. Scale bar = 25 μm.
DISCUSSION
Here, we show that Rpgrip1l is essential for primary cilia formation, Hh signaling, and hair follicle keratinocyte differentiation during hair follicle morphogenesis, but is dispensable for hair follicle induction and polarization and for epidermal differentiation. This study suggests that genes involved in the development of ciliopathies may also be essential for the morphogenesis of hair follicles.
Ciliopathies represent a number of clinically and genetically heterogeneous syndromes caused by dysfunctions of primary or motile cilia (Badano et al., 2006; Hildebrandt et al., 2011; Tobin and Beales, 2009). Clinical manifestations of ciliopathies are heterogeneous due to the diverse morphogenetic functions of cilia during the development of multiple tissues (Eggenschwiler and Anderson, 2007). Dermatological manifestations are generally unremarkable and only described in a subset of ciliopathies, notably skin laxity and sparse hair in cranioectodermal dysplasia (CED, also known as Sensenbrenner syndrome) (Arts and Knoers, 1993; Lin et al., 2013; Walczak-Sztulpa et al., 2010) and Ellis–van Creveld (EvC) syndrome (also known as chondroectodermal dysplasia) (Atasu and Biren, 2000; Baujat and Le Merrer, 2007). The lack of documentation of skin phenotypes in most ciliopathy patients may be due to the fact that there is no remarkable dermatological manifestation associated with ciliopathies. It is also possible that skin phenotypes are dwarfed by more severe clinical conditions, such as cystic kidney, cardiac anomalies, and developmental delay, or that skin phenotypes are frequently associated with lethal developmental defects. Since the current study provides evidence that a ciliopathy gene, Rpgrip1l, is essential for the normal development of hair follicles, together with the recently established role of primary cilia in skin and hair follicle development, this suggests that skin phenotypes may be more prevalent in ciliopathy patients than currently reported. However, because hair follicle morphogenesis requires complex neuroectodermal/mesodermal interactions, which is also under the influence of many temporally and spatially regulated genetic and environmental factors, it is also possible that genetic make-ups and species differences are confounding factors underlying variations in phenotypic outcome or penetrance. Genetic backgrounds of the Rpgrip1l−/− mice might affect the phenotypic presentations of this mutant allele, as previously shown for TMEM67 mutant ((Abdelhamed et al., 2013) and CL personal communication). Also, as human hair follicle morphogenesis is completed already in utero, subtle phenotypes in the skin of ciliopathy patients may get lost during the substantial post-natal remodeling that human skin undergoes.
Ciliopathies are caused by mutations in genes encoding the cilium or basal body/centrosome-related proteins. The mechanisms of genotype-phenotype correlation of ciliopathy remains perplexing, which are likely under the influence of heterogeneity of the genetic locus, multiple allelism (mutational load), modifier genes (effect), and true oligogenicity (oligo-genetic inheritance) (Hildebrandt et al., 2011). Ciliopathies caused by RPGRIP1L mutations are often associated with more severe or even lethal cases of ciliopathy, such as for JBTS and MKS, in which ciliary functions are more severely impaired. Because strong cilia and hair follicle phenotypes were observed in the Rpgrip1l−/− mice, we speculate that hair follicle or skin abnormalities may be more prominent in severe or lethal cases of ciliopathies. However, it is worth noting that phenotypes seen in JBTS and MKS patients are caused by point mutations in the RPGRIP1L gene; whereas the phenotypes in Rpgrip1l−/− mice resulted from targeted gene disruption. Although some patient mutations could theoretically result in truncation of most of the functional domains of the RPGRIP1L protein (Arts et al., 2007; Delous et al., 2007), variations in phenotypic severity warrant further investigation of the impact of a particular mutation on the functional domains of RPGRIP1L. A number of recent studies have shed light on protein-protein interactions mediated by specific domains of RPGRIP1L (Arts et al., 2007; Delous et al., 2007; Remans et al., 2014; Sang et al., 2011).
CED and EvC are the only two ciliopathies that demonstrate hair phenotypes, namely, fine, sparse and slow-growing hair. Mutant mouse models of the CED genes (Ift121, Ift122 and Ift144) were unable to develop beyond midgestation for further analysis (Ashe et al., 2012; Cortellino et al., 2009; Mill et al., 2011); mutant mouse models of the EvC genes (Evc1 and Evc2) display perinatal lethality (Caparros-Martin et al., 2013; Ruiz-Perez et al., 2007), however, the skin remained uncharacterized. Overlapping phenotypes observed in the Rpgrip1l mutants and CED or EvC mutants, such as bone and cardiovascular defects, and disrupted Hh signaling suggest that CED and EvC genes may control hair follicle formation in a similar fashion to Rpgrip1l. Whether the hair phenotypes in CED and EvC patients are caused by impaired cilia formation or function and, consequently, attenuated Hh signaling, remains to be determined.
Birt–Hogg–Dube (BHD) syndrome is a recently characterized ciliopathy, caused by autosomal dominant mutations in the folliculin (FLCN) gene (Luijten et al., 2013). BDH patients are frequently affected by fibrofolliculomas, benign tumors of hair follicles (Menko et al., 2009). The formation of fibrofolliculoma in BHD patients are associated with increased activation of the canonical Wnt signaling pathway (Luijten et al., 2013), whereby represents another type of aberrant signaling due to ciliary dysfunction. It will be interesting to determine whether the Hh signaling pathway is also involved in the development of the hair follicle phenotypes in BHD patients.
PCP is essential for global polarization of hair follicles. Interruption of PCP in the skin through the disrupting core PCP genes, such as Fzd6, Vangl2, and Celr1, could result in disrupted polarization of the hair follicles and altered asymmetric localization of molecules within the hair follicles. In the current study, we found no morphogenetic evidence of disrupted hair follicle polarization. This observation is reminiscent of findings in the Ift88 mutant, in which apparent PCP defects was observed in the cochlea (Jones et al., 2008), however without apparent hair follicle polarization defects (Croyle et al., 2011; Ezratty et al., 2011; Lehman et al., 2009). These findings suggest that these ciliary gene may function downstream of core PCP genes or that disrupting them in the skin is not sufficient to cause hair follicle polarization defects.
In summary, the current study establishes the genetic and molecular basis of hair phenotypes in association with a ciliopathy gene, RPGRIP1L. Further characterization of the ever growing list of ciliopathy genes, which are essential for the development and function of many tissues and organs, will advance our understanding of their functions in skin development and homeostasis.
MATERIALS AND METHODS
Rpgrip1l mouse model
Genetic modification and PCR genotyping of the Rpgrip1l locus was described previously in the Ftm mouse (Besse et al., 2011; Gerhardt et al., 2013; Vierkotten et al., 2007). Rpgrip1l−/− homozygous mutants were obtained by crossing Rpgrip1l+/− heterozygous. E15.5 and E18.5 fetuses were obtained by timed-mating. All procedures related to mice were conducted according to relevant European rules and approved by a French ethic committee and the Institutional Animal Care and Use Committee of Stony Brook University.
BrdU labeling and tissue processing
BrdU labeling was performed by injecting BrdU labeling reagent at 10 μg per gram body weight intraperitoneally two hours prior to euthanization. Skin specimens were obtained by removing the full thickness dorsal skin along the midline and processed for routine histology analysis. For all analyses, a minimum of three embryos obtained from at least two different litters were examined.
Alkaline phosphatase staining
Alkaline phosphatase (AP) staining was performed as previously described (Tsai et al., 2010). Specifically, fresh skin was fixed in 4% paraformaldehyde for 10 minutes, soaked in B3 buffer (0.1 M Tris, pH 9.5, 0.1 M NaCl, and 0.05 M MgCl2) for 10 minutes, and NBT/BCIP solution (1:200 of Nitro Blue tetrazolium and 1:267 of 5-bromo-4-chloro-3-indolyl phosphate, Roche, Indianapolis, IN) for 20 minutes. Stained skins were imaged on a Zeiss Stemi 2000-C dissecting microscope fitted with an Infinity 2 camera.
Cell culture and in vitro assays
The isolation of primary skin keratinocyte and dermal fibroblast was conducted as described elsewhere (Dai et al., 2011). Briefly, skins of E18.5 fetuses were digested by dispase II (Roche) to separate epidermis and dermis. Dermis was then digested with collagenase to dissociate dermal fibroblasts. Fibroblasts were cultured in Dulbecco's modified Eagle's medium (4.5 g/l glucose) supplemented with 10% fetal bovine serum and antibiotics. To examine ciliogenesis, cells were grown to confluency and serum-starved for 48 hours before being fixed in cold methanol. To examine Hh responsiveness, fibroblasts were grown as described above, serum-starved for 24 hours, treated with 100 nM SAG (Calbiochem, Danvers, MA) for 24 hours, before lysed in RLT buffer (Qiagen, Valencia, CA) for RNA extraction.
Quantitative RT-PCR
RNA was isolated with RNeasy kit (Qiagen) and quantitative RT-PCR analyses were performed as described previously (Dai et al., 2013). The following probes were used for TaqMan analysis: Lef1, Mm00550265_m1; Ctnnb1, Mm00483039_m1; Hes1, Mm01342805_m1; Gli1, Mm00494645_m1; Gli2, Mm01293111_m1; and β-actin, Mm00607939_m1 (Life Technologies, Grand Island, NY). Results were analyzed using ΔΔCt method. Relative expression levels of target genes were determined by comparing with wild type or treatment controls after normalizing with β-actin.
Immunofluorescence labeling and microscopy
Immunofluorescence and TUNEL staining were performed as described previously (Dai et al., 2013). The following primary antibodies were used: RPGRIP1L, 1:100 (Origene, Rockville, MD); p63, 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA); KRT14, 1:1,000 (Covance, Princeton, NJ); KRT1, 1:500 (Roop et al., 1987); KRT17, 1:1,000 (Abcam, Cambridge, MA); LOR, 1:100 (Covance); NGFR, 1:100 (Promega); LEF1, 1:100 (Cell Signaling, Danvers, MA); BrdU, 1:50 (Life Technologies); acetylated α-tubulin, 1:1,000 (Sigma, St. Louis, MO); γ-tubulin, 1:1,000 (Abcam); ARL13B, 1:1,000 (#73-287, NeuroMab, Davis, CA); phospho-histone H3, 1:100 (Cell Signaling). AlexaFluor-conjugated secondary antibodies (1:250) were from Life Technologies. Sections were sealed in mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired by Nikon 80i fitted with Nikon DS-Qi1Mc camera and processed with Photoshop 5.5 CS.
In situ hybridization
In situ hybridization was carried out on formalin-fixed paraffin embedded tissue sections using the RNAScope system (Advanced Cell Diagnostic, Hayward, CA) per manufacturer's instructions and as previously described (Wang et al., 2012).
Skin transplantation and explant culture
Full-thickness skin transplantation was performed as previously described (Dai et al., 2011). Briefly, dorsal skins of E18.5 littermates were dissected and seeded in silicon grafting chambers placed on the back of 8 – 12 week old nude mice (Foxn1−/−). Graft chambers were removed 10 days later. Skin grafts were harvested two weeks thereafter. All experiments were performed at least three times. Skin explants were cultured as previously described (Zhang et al., 2009). Specifically, dorsal skins were harvested at E18.5 embryos and cultured at the air-liquid interface in Corning Transwell plates containing DMEM and 10% FBS. These ex vivo cultured skin explants were then fixed in formalin and processed for routine histology analysis.
Statistical analyses
All quantifications are presented as means ± S.D. Student t-test was used for statistical analysis. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Mallory Korman of the Stony Brook Research Histology Core Lab for assistance in histology. This study was supported by start-up funds provided by the Department of Pathology and the Cancer Center of Stony Brook University, and a research grant from NIH/NIAMS (AR061485) to JC. Work in the SSM lab was funded by the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (Inserm), Université Pierre et Marie Curie (UPMC Paris 06), the Agence Nationale de la Recherche (SSM grant “Ciliainthebrain”), the Fondation ARC pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale (‘Equipe FRM’ grant DEQ20140329544 to SSM).
Footnotes
CONFLICT OF INTEREST
None to declare.
REFERENCES
- Abdelhamed ZA, Wheway G, Szymanska K, et al. Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects. Hum Mol Genet. 2013;22:1358–72. doi: 10.1093/hmg/dds546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts H, Knoers N. Cranioectodermal Dysplasia. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Ashe A, Butterfield NC, Town L, et al. Mutations in mouse Ift144 model the craniofacial, limb and rib defects in skeletal ciliopathies. Hum Mol Genet. 2012;21:1808–23. doi: 10.1093/hmg/ddr613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasu M, Biren S. Ellis-van Creveld syndrome: dental, clinical, genetic and dermatoglyphic findings of a case. J Clin Pediatr Dent. 2000;24:141–5. [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2:27. doi: 10.1186/1750-1172-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, et al. MIM and cortactin antagonism regulates ciliogenesis and Hedgehog signaling. Dev Cell. 2010;19:270–83. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse L, Neti M, Anselme I, et al. Primary cilia control telencephalic patterning and morphogenesis via Gli3 proteolytic processing. Development. 2011;138:2079–88. doi: 10.1242/dev.059808. [DOI] [PubMed] [Google Scholar]
- Caparros-Martin JA, Valencia M, Reytor E, et al. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet. 2013;22:124–39. doi: 10.1093/hmg/dds409. [DOI] [PubMed] [Google Scholar]
- Chen J, Chuong CM. Patterning skin by planar cell polarity: the multi-talented hair designer. Exp Dermatol. 2012;21:81–5. doi: 10.1111/j.1600-0625.2011.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- Cortellino S, Wang C, Wang B, et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev Biol. 2009;325:225–37. doi: 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MJ, Lehman JM, O-Connor AK, et al. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development. 2011;138:1675–85. doi: 10.1242/dev.060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Li L, Huebner A, et al. Planar cell polarity effector gene Intu regulates cell fate-specific differentiation of keratinocytes through the primary cilia. Cell Death Differ. 2013;20:130–8. doi: 10.1038/cdd.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Zhu H, Wlodarczyk B, et al. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–10. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16:143–54. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–73. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–41. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, et al. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt C, Lier JM, Kuschel S, et al. The ciliary protein Ftm is required for ventricular wall and septal development. PLoS One. 2013;8:e57545. doi: 10.1371/journal.pone.0057545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A. 2004;101:9277–81. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–45. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Laag E, Michaud EJ, et al. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol. 2009;129:438–48. doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Traum AZ, Sahai I, et al. Sensenbrenner syndrome (Cranioectodermal dysplasia): clinical and molecular analyses of 39 patients including two new patients. Am J Med Genet A. 2013;161A:2762–76. doi: 10.1002/ajmg.a.36265. [DOI] [PubMed] [Google Scholar]
- Luijten MN, Basten SG, Claessens T, et al. Birt-Hogg-Dube syndrome is a novel ciliopathy. Hum Mol Genet. 2013;22:4383–97. doi: 10.1093/hmg/ddt288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuzier A, Gaude HM, Grampa V, et al. Dishevelled stabilization by the ciliopathy protein Rpgrip1l is essential for planar cell polarity. J Cell Biol. 2012;198:927–40. doi: 10.1083/jcb.201111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- Mill P, Lockhart PJ, Fitzpatrick E, et al. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet. 2011;88:508–15. doi: 10.1016/j.ajhg.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–94. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Remans K, Burger M, Vetter IR, et al. C2 domains as protein-protein interaction modules in the ciliary transition zone. Cell Rep. 2014;8:1–9. doi: 10.1016/j.celrep.2014.05.049. [DOI] [PubMed] [Google Scholar]
- Roop DR, Huitfeldt H, Kilkenny A, et al. Regulated expression of differentiation-associated keratins in cultured epidermal cells detected by monospecific antibodies to unique peptides of mouse epidermal keratins. Differentiation. 1987;35:143–50. doi: 10.1111/j.1432-0436.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–12. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–28. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S, Gleeson JG. The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev Med Child Neurol. 2011;53:793–8. doi: 10.1111/j.1469-8749.2011.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell-s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–7. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Clavel C, Kim S, et al. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28:221–8. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- Valente EM, Dallapiccola B, Bertini E. Joubert syndrome and related disorders. Handb Clin Neurol. 2013;113:1879–88. doi: 10.1016/B978-0-444-59565-2.00058-7. [DOI] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, et al. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, et al. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–56. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang H, Nathans J. When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development. 2010;137:4091–9. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–46. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.