Abstract
Egg chambers from starved Drosophila females contain large aggregates of processing (P) bodies and cortically enriched microtubules. As this response to starvation is rapidly reversed upon re-feeding females or culturing egg chambers with exogenous bovine insulin, we examined the role of endogenous insulin signaling in mediating the starvation response. We found that systemic Drosophila insulin-like peptides (dILPs) activate the insulin pathway in follicle cells, which then regulate both microtubule and P body organization in the underlying germline cells. This organization is modulated by the motor proteins Dynein and Kinesin. Dynein activity is required for microtubule and P body organization during starvation, while Kinesin activity is required during nutrient-rich conditions. Blocking the ability of egg chambers to form P body aggregates in response to starvation correlated with reduced progeny survival. These data suggest a potential mechanism to maximize fecundity even during periods of poor nutrient availability, by mounting a protective response in immature egg chambers.
Keywords: P body, Oogenesis, Insulin, Dynein, Kinesin, Microtubule
Introduction
Drosophila oogenesis is a tractable system to study how insulin/TOR signaling relays cause cellular changes in response to starvation. Females devote significant resources to reproduction, producing up to 60 eggs per female per day on a nutrient-rich diet (King, 1970). Mechanisms used by fruit flies to maximize reproductive capacity also provide valuable insights into how animals protect their germline under varying environmental conditions. We investigated the effects of disrupting components of the insulin/TOR pathway in specific cell types in Drosophila ovaries to control their cellular response to starvation, and the consequences of interfering with the ability of egg chambers to launch a starvation response.
The production of Drosophila eggs is accomplished in “assembly lines” called ovarioles that contain developing egg chambers. Oogenesis initiates in the germarium located at the anterior tip of each ovariole when a germline stem cell daughter undergoes four rounds of mitosis to produce a cluster of 16 cells (Roth and Lynch, 2009). The mitotic divisions are characterized by incomplete cytokinesis and stabilization of arrested cleavage furrows into intercellular bridges called ring canals. Somatic follicle cells form an epithelium around the germline cells to complete egg chamber formation. One of the 16 germline cells becomes the oocyte, while the other 15 cells, called nurse cells, provide the oocyte with maternal protein, mRNA and organelles by intercellular transport through ring canals.
Many maternal mRNAs become oocyte-enriched during oogenesis (Lasko, 2012). They reside within ribonucleoprotein complexes (mRNPs) containing proteins that repress mRNA translation until they reach their destination in the oocyte. Enrichment of mRNPs in the oocyte depends on the microtubule (MT) network extending from the oocyte into nurse cells and dynein-mediated transport to the oocyte, combined with diffusion and localized retention in the oocyte (Koch and Spitzer, 1983; Shimada et al., 2011; Theurkauf et al., 1993). The oskar mRNP is one such complex, and includes the initiation factor eIF4E, the Y-box protein Yps, and the RNA helicase Me31B (Nakamura et al., 2001; Nakamura et al., 2004; Wilhelm et al., 2000). oskar mRNA is silenced in this complex until it reaches the oocyte posterior, where its translational de-repression is mediated by Orb (Castagnetti and Ephrussi, 2003; Mansfield et al., 2002).
The distribution of germline mRNPs and MTs, as well as the general development of egg chambers, is highly sensitive to nutrition. In protein-poor (starved) conditions, egg chambers do not complete development and instead undergo apoptosis during stage 8 just before the onset of yolk uptake, or vitellogenesis (Drummond-Barbosa and Spradling, 2001; Mazzalupo and Cooley, 2006; Pritchett et al., 2009). This spares the female from making a large metabolic investment of yolk and eggshell production when nutrients are limited and progeny survival is uncertain. Starvation conditions also result in a fourfold decrease in follicle stem cell and germline stem cell proliferation, thus slowing the overall rate of egg chamber production (Drummond-Barbosa and Spradling, 2001). We previously described a third response to starvation in egg chambers by demonstrating that germline cells in pre-vitellogenic egg chambers (stages 2–7) from starved females have aggregates of mRNP components that resemble Processing bodies (P bodies), as well as cortically condensed MTs (Shimada et al., 2011). Nutrient sensitive accumulation of P body components has also been shown to occur in reticular sponge bodies, which appear to be enlarged P body aggregates (Snee and Macdonald, 2009). Importantly, providing starved females with rich food (yeast) reverses the effects on P body organization caused by starvation within two hours, suggesting this starvation response is tightly regulated. We also observed that culturing egg chambers with exogenous insulin for one hour reversed the germline starvation response, suggesting the involvement of endogenous insulin signaling in controlling the egg chamber’s response to nutrition (Shimada et al., 2011).
In Drosophila, eight insulin-like peptides (dILPs) are secreted from various tissues in the fly to regulate metabolism, longevity and the cell cycle (Colombani et al., 2012; Grönke et al., 2010). dILP3 and dILP5 expression from the insulin-producing cells (IPCs) of the central nervous system decreases in flies reared in starvation conditions (Ikeya et al., 2002). Ablating the IPCs, and thereby depleting systemic dILPs, results in egg chamber arrest and impaired ability of follicle cells to increase proliferation in response to rich nutrient conditions (Ikeya et al., 2002; King, 1970; LaFever and Drummond-Barbosa, 2005), suggesting systemic dILPs control at least some of the responses of oogenesis to nutrient availability. Furthermore, removal of the insulin/IGF signaling (IIS) pathway and TOR signaling components from ovarian cells results in severe growth retardation (Hsu et al., 2011; King, 1970; LaFever and Drummond-Barbosa, 2005; LaFever et al., 2010), indicating that insulin pathway function contributes to regulation of overall egg chamber growth.
Here, we show that systemic dILPs secreted from the brain also control the dramatic reorganization of P bodies and MTs in nurse cells of previtellogenic egg chambers during starvation. Furthermore, using cell type-specific manipulations, we show that somatic follicle cells mediate this dILP-dependent germline starvation response. We provide evidence that aggregation of P bodies in response to starvation happens as a consequence of the depletion of cytoplasmic MTs. Importantly, females that cannot respond to starvation because of constitutive systemic dILP expression produce embryos with a reduced hatching rate. These data show for the first time that the nutrient stress response in immature egg chambers may provide a protective mechanism important for maximizing embryo survival.
Materials and Methods
Fly Stocks
Two Flytrap (http://medicine.yale.edu/lab/cooley/flytrap/index.aspx#page1) lines were used: ZCL3710 (GFP-RpS2) and YB0151 (GFP-RpL30) (Kelso et al., 2002; Roth and Lynch, 2009). For germline-specific expression, Maternal-α-tubulin-GAL4 (Mat-GAL4, Bloomington Drosophila Stock Center [BDSC] #7063) was used. We additionally used UASp-RFP::YpsN (Lasko, 2012; Shimada et al., 2011); pUASp-GFP::tubulin (Grieder et al., 2000; Koch and Spitzer, 1983; Shimada et al., 2011; Theurkauf et al., 1993); UAS-2x-GFP (BDSC #6874) osk-MS2/MS2::GFP/TM3 (Nakamura et al., 2001; Nakamura et al., 2004; Wilhelm et al., 2000; Zimyanin et al., 2008); bcd-MS2, MCP::GFP (Castagnetti and Ephrussi, 2003; Mansfield et al., 2002; Weil et al., 2006); grk-MS2, MCP::GFP (Drummond-Barbosa and Spradling, 2001; Jaramillo et al., 2008; Mazzalupo and Cooley, 2006; Pritchett et al., 2009); UAS-dTrpA1 (Drummond-Barbosa and Spradling, 2001; Hamada et al., 2008); UAS-Kir2.1 (Baines et al., 2001; Shimada et al., 2011), dILP2-GAL4 (Ikeya et al., 2002; Snee and Macdonald, 2009); UAS-TSC1 (Potter et al., 2001); UAS-InR (Huang et al., 1999); traffic jam-GAL4 (Hayashi et al., 2002; Shimada et al., 2011); and T80-GAL4 (Brand and Perrimon, 1993; Colombani et al., 2012; Grönke et al., 2010). shRNA lines directed to Khc (BDSC# 35409), Dhc64c (BDSC #36583), TOR (BDSC #35578) and InR (BDSC #35251) were from the Transgenic RNAi Project (TRiP) at Harvard University (http://www.flyRNAiRNA.org/TRiP-HOME.html) (Ikeya et al., 2002; Ni et al., 2008).
Live Imaging
Ovaries were removed from flies and ovarioles were carefully dissected in 200 μL Schneider’s Drosophila Medium (SDM) in a glass-bottom culture dish (P35G-1.0-14-C, MatTek). In Rapamycin culturing experiments, 10 mM Rapamycin was added immediately after dissection before imaging. An 18 mm cover glass was mounted to avoid desiccation of culture medium. Images were taken on a Zeiss LSM-510 META laser-scanning confocal microscope or on a Zeiss Axiovert 200 equipped with a CARV II confocal imager (BD Bioscience) and CoolSnap HQ2 camera (Roper Scientific). Images were processed using ImageJ and P body voxels were quantified using Imaris.
For analysis of microtubules using GFP::tubulin, newly-eclosed adult females were fed on regular fly food with yeast paste for 2 days. Then they were transferred to a vial containing grape juice agar with or without yeast paste for 2 days. Dissected ovaries were placed on a glass-bottom culture dishes filled with SDM containing 10 μg/ml FM4-64. Images were taken within 30 minutes after dissection.
For data analysis, we use “Interactive 3D Surface Plot” and “Plot Profile” plugin in ImageJ (NIH) to examine the fluorescence intensity distribution of GFP::tubulin. avoid cytoplasmic fluorescence from contributing to the apparent cortical signal, we analyzed single optical sections near the center of the cell so that cortical signal would not be superimposed on cytoplasmic signal, as can happen with projections of Z stacks.
Immunohistochemistry
Ovaries were dissected in IMADS (100 mM sodium glutamate, 25 mM KCl, 15 mM MgCl2, 5 mM CaSO4, and 2 mM sodium phosphate buffer, pH 6.9) and fixed in 100 μL Devit Buffer (66 μL ddH2O, 17μL 36% paraformaldehyde, 17μL Buffer B [100mM K phosphate buffer, pH6.8, 450mM KCl, 150 mM NaCl, 20mM MgCl2•6H2O]), with 600 μL Heptane for 20 min, rinsed three times for 10 minutes with PBS + 0.3% Triton (PBST) and blocked for one hour with 4% BSA in PBST. Ovaries were then incubated with primary antibodies for 2 hours at room temperature or overnight at 4°C. Ovaries were then washed four times for 15 minutes each with PBST, and incubated with secondary antibodies for 2 hours at room temperature or overnight at 4°C. Ovaries were washed four times for 15 minutes with PBST before being incubated with Aqua Poly/Mount (Polysciences, Inc.) overnight at 4°C. To visualize DNA, 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes) was added, if needed, to the second of the four final washes at a concentration of 1000 ng/ml. Primary antibodies used were rat Me31B antiserum at 1:500 (gift of A. Nakamura) and eIF4E antibody at 1:500 (Nakamura et al., 2004). Secondary antibodies were Alexa Fluor 488 and 568 (Invitrogen) at 1:500.
For the colchicine treatment experiment, dissected ovaries were incubated in SDM containing 100 μg/ml colchicine or EtOH (vehicle) as a control. After 2 hour-treatment, ovaries were fixed with 3.7% paraformaldehyde in PBS for 20 minutes, and then immunostained with anti-eIF4E and anti-α-tubulin. F-actin was labeled with phalloidin. Images were taken on a Zeiss LSM700 microscope.
To measure levels of dILP2 in IPCs, brains were dissected from adult flies, fixed in 1% paraformaldehyde overnight at 4°C, and processed for immunostaining as described in (Pfeiffer et al., 2010) except incubation in secondary antibody was for 4–5 days at 4°C. Rabbit anti-dILP2 (Géminard et al., 2009) was used at 1:200 and Alexa Fluor 568-labeled secondary at 1:500. dILP2-expressing IPCs were imaged using the CARVII spinning disc system described above, using identical exposure times for all samples. We then determined the mean pixel intensity for the IPCs in each brain after identifying the dILP2-expressing IPCs using Imaris software (Bitplane).
Starvation Protocol
Females less than 48 hours post eclosion were cultured with standard fly food supplemented with yeast paste for 2 days. These females were then transferred to a vial containing grape juice agar with or without yeast paste (the protein-rich/poor conditions) for two days before dissection. Females expressing dTrpA1 in IPCs were shifted from ambient temperature to 28°C or 18°C for the two days before dissection. Ovaries from 5–10 females were dissected per experiment, and images were collected from 2–4 sets of experiments. To determine if there was a change in the germline starvation response as a result of our genetic manipulations, we assigned Aggregation Scores to imaged stage 6–7 egg chambers by a four-degree severity scale of P body aggregation in the germline, with 1 having no visible aggregates and 4 having the germline largely filled with aggregates (Fig S2). Egg chambers from control well-fed flies had an average score of 1.2, and egg chambers from starved flies had an average score of 3.2.
Q RT PCR
Previtellogenic chambers (stage 7 and younger) were dissected in IMADS from the ovaries of females exposed to rich or poor food conditions (Shimada et al., 2011; Singleton and Woodruff, 1994). These chambers were homogenized by passing through a 23-gauge needle 20 times, and RNA was extracted with a Qiagen RNEasy kit (Qiagen Sciences). RNA was then diluted to a concentration of 50 ng/uL. Q RT PCR was performed using Applied Biosystems Power SYBR Green RNA-to-CT 1-Step Kit with an Applied Biosystems OneStep Machine. Three full runs were performed, and each run contained triplicates from which CT was averaged. Significance of the ratio of starved mRNA amount to fed mRNA amount was calculated using an unpaired T-test for average triplicate comparative CT from each of the three runs. RNA was normalized to actin5c. Primers used were as followed: act5CF: CACCGGTATCGTTCTGGACT, act5CR: GGACTCGTCGTACTCCTGCT, oskF2:ACGCCCAGAATGGAATCAC, oskR2: AGGAAATCCGTCACGTTGTC, bcdF: GACCTTGCGCCATCGCCGTT, bcdR: ACCCTTCAAAGGCTCCAAGAT, grkF:AAGCAGCAGCAACTACACC, grkR: AGCAAATACTAACTTGTGCGTC.
Egg Laying and Hatching Assays
Egg hatching assays were performed on grape juice agar plates. 5–10 females and two male flies were collected within 48 hours of eclosion and placed in a vial with standard food and yeast paste for two days. These groups of flies were evenly distributed into two chambers with a 35mm plate of grape juice agar, one with and one without supplemental yeast paste on the agar. Plates were collected and exchanged every 12 hours for 3 days. Embryo survival was evaluated by counting hatched versus unhatched eggs observed 24 hours after plate collection. Results from collections on day 2 and 3 were used to calculate both egg laying and hatching rates to ensure eggs being assayed developed under starvation conditions. Significance was calculated using an unpaired T-test. Three replicates of Kir2.1 experiments were performed, and seven of TrpA1.
Results
Insulin/TOR Signaling Controls the Germline Starvation Response
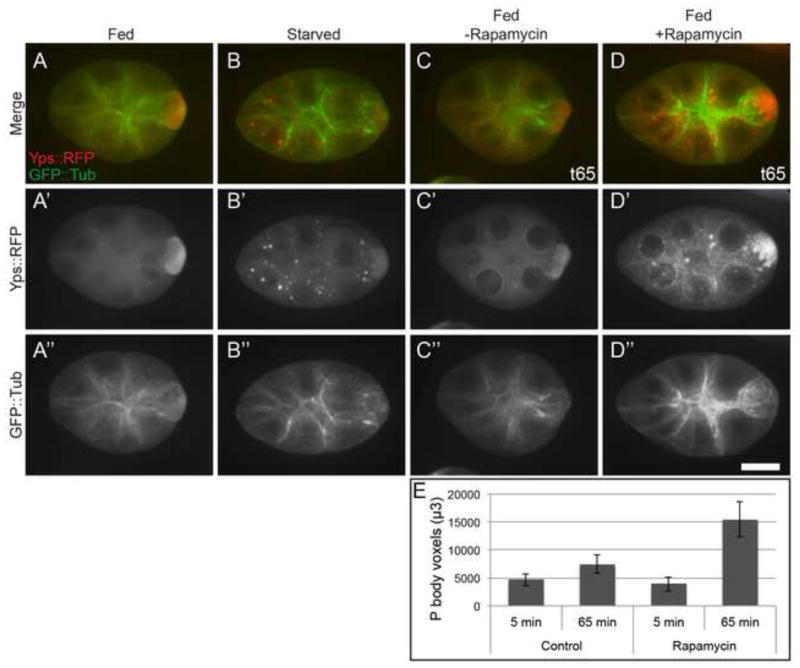
Previtellogenic egg chambers from starved females have cortically condensed MTs and large P body aggregates in nurse cell cytoplasm, which we refer to as the “germline starvation response” throughout this paper (Fig. 1A–B) (Shimada et al., 2011). Culturing egg chambers from starved flies with exogenous insulin reversed the starvation response (Shimada et al., 2011), and suggested that the insulin/TOR pathway is involved in communicating nutrient availability to the germline. To complement this experiment, we reduced TOR signaling by culturing stage 6 egg chambers dissected from well-fed females in the absence or presence of Rapamycin. Egg chambers cultured with Rapamycin for one hour displayed a dramatic increase in P body aggregates and cortical MT condensation (Fig. 1 C–D, E), very similar to the germline starvation response. These data imply that decreased insulin/TOR signaling causes the germline starvation phenotype.
Figure 1. Insulin/TOR signaling controls the germline starvation response.
(A–B) Stage 6–7 egg chambers dissected from females starved for 48 hours contain numerous P body aggregates (Yps::RFP, red) in nurse cells, and MTs (GFP::tub, green) become cortically condensed. (C–D) Egg chambers dissected from well-nourished females and cultured with 10mM Rapamycin (+Rapamycin) appear “starved” with abundant P body aggregates and MT cortical condensation. (E) Imaris quantification of P body aggregates formed in fed egg chambers cultured with or without Rapamycin for one hour. The separate channels are indicated with a prime and double prime. Images were taken 5 min (t5) or 65 min (t65) after adding Rapamycin to the culture medium. Control n=15, +Rapamycin n=12. Scale bar is 20 μm.
Systemic dILPs Communicate Nutrient Availability to the Ovary
We tested whether the starvation response is mediated by systemic Drosophila insulin-like peptides (dILPs) secreted from neurosecretory insulin producing cells (IPCs) in the central nervous system. As total ablation of IPCs leads to severe growth defects within the ovary (Ikeya et al., 2002; LaFever and Drummond-Barbosa, 2005), we instead sought to manipulate the activity of these neurons with temporal precision by using engineered ion channels to modulate dILP secretion. We hypothesized that egg chambers from flies with depleted dILP signaling should behave starved (have P body aggregates) even when well nourished, and egg chambers from flies with increased dILP signaling should appear well nourished (have few P body aggregates) even when starved.
To increase dILP secretion, we used the dILP2-GAL4 driver (Ikeya et al., 2002) to express in IPCs the temperature sensitive TrpA1 channel (dILP2>TrpA1), which increases neuronal activity when flies are incubated above 26°C (Hamada et al., 2008). To determine the effect of TrpA1 expression on dILP, we used dILP2 antibody (Géminard et al., 2009) to stain brains dissected from control females kept at 18°C and females shifted to 28°C for two days. In controls, brains from starved females had elevated dILP2; however, 28°C brains from fed or starved animals had lower dILP2 levels similar to fed controls (supplementary material Fig. S1). These data suggest secretion of dILP2 is inhibited by starvation, leading to accumulation of the hormone in neurosecretory cells as seen in Géminard et al. (Géminard et al., 2009).
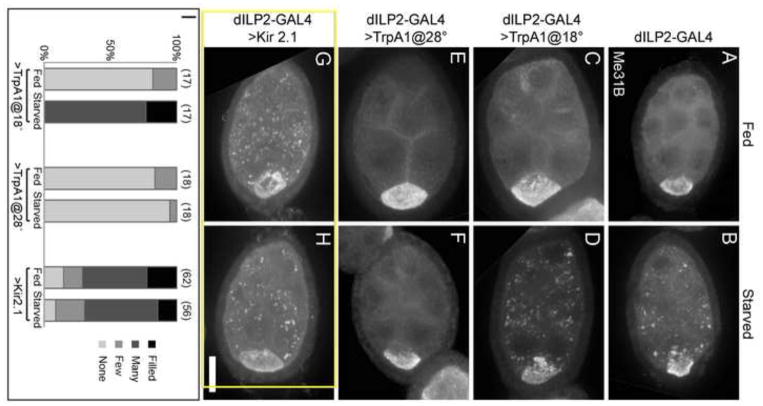
To determine whether systemic insulin had an effect on P body organization, we imaged stage 6–7 egg chambers and rated the severity of P body aggregation using a qualitative scoring system (supplementary material Fig. S2). Control flies and dILP2>TrpA1 flies at 18°C responded normally to starvation, with starved egg chambers displaying enlarged P body aggregates (Fig. 2 A–D, I). Importantly, egg chambers from dILP2>TrpA1 females incubated at 28°C formed very few enlarged P body aggregates in response to starvation (Fig. 2 E, F, I). We also used dILP2-GAL4 to express UAS-Kir2.1 in IPCs. When overexpressed, this potassium ion channel decreases neuronal excitability, leading to decreased dILP release (Baines et al., 2001). The egg chambers of these females contained enlarged P body aggregates even in the presence of food (Fig. 2 G, H, I). These results indicate that systemic dILPs in the hemolymph secreted by the IPCs are responsible for communicating external nutrient conditions to the germline cells.
Figure 2. IPC activity communicates nutrient availability to the ovary.
(A–B) Control egg chambers with dILP2-GAL4 alone form P body aggregates in response to starvation. (C–D) Control egg chambers from flies expressing TrpA1 in IPCs and cultured at 18°C respond to starvation normally with enlarged P body aggregates. (E–F) Egg chambers with increased systemic insulin due to expression of TrpA1 in flies cultured at 28°C do not form P body aggregates in response to starvation. (G–H) Egg chambers with reduced systemic dILP secretion due to expression of Kir2.1 in the IPCs form enlarged P body aggregates even in the presence of abundant food. (I) P body aggregation is prevented in starved egg chambers from flies expressing active TrpA1 (at 28°C) in IPCs, and induced in fed egg chambers from flies expressing Kir2.1 in IPCs. Aggregation scores are listed above the columns, and sample sizes are indicated in parentheses. Egg chambers were stained with Me31B antibodies. Images are Z projections of 20 1-μm slices. Scale bar is 20 μm.
Recently it has been discussed whether indirect measures to infer insulin secretion levels may be misleading. Using dILP antibody fluorescence data, trehalose levels in the hemolymph, or dILP protein and RNA levels does not necessarily provide an accurate reflection of the insulin levels being secreted into the hemolymph (Park et al., 2014). Despite that caveat, it has been demonstrated that genetically manipulating the ion channels in IPCs to influence insulin secretion is an experimental strategy that results in measurable differences in the amounts of tagged dILP2 found in hemolymph (Park et al., 2014), supporting our use of TrpA1 and Kir2.1 expression in IPCs to effect systemic insulin levels.
Manipulating dILP Secretion Does Not Override Starvation-induced Egg Chamber Apoptosis
We first examined the effect of blocking or inducing the germline starvation response on the overall ovarian response to starvation by measuring egg chamber progression. Using TrpA1 expression in IPCs, we tested whether increased systemic dILPs were sufficient to block the onset of stage 8 egg chamber apoptosis in response to starvation. Interestingly, we observed the usual increase in stage 8 apoptosis (Fig. 3 A–D, E), showing that despite the constitutive presence of circulating dILPs, ovaries still responded to starvation by dramatically slowing egg production. Thus, constitutive dILPs blocked P body aggregation in very young pre-vitellogenic egg chambers, but not the resorption of stage 8 egg chambers or reduced egg production.
Figure 3. IPC activity does not influence apoptosis in response to starvation.
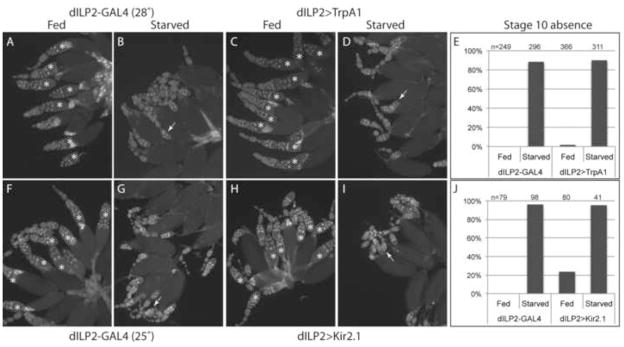
Ovaries from fed and starved control (A, B, F, G) dILP2>TrpA1 (C, D) and dILP2>Kir2.1 (H, I) females were dissected and stained with DAPI to detect apoptotic egg chambers. Ovarioles were scored for the presence (*) or absence of a stage 10 egg chamber as a proxy for apoptotic behavior. Examples of apoptotic egg chambers are indicated with arrows. (E, J) Ovarioles lacking a stage 10 egg chamber were counted and plotted as a percentage of the total number scored in each condition.
We also tested the complementary effect on stage 8 egg chambers of reduced dILP secretion in both fed and starved females. We predicted that expression of Kir2.1 in IPCs could mimic starvation in well-fed females, causing an increase in apoptosis. We did observe a mild increase in apoptosis, though not as severe as in starved females (Fig. 3 F–I, J). These data indicate that systemic dILPs control a subset of the ovarian responses to starvation, including P body aggregation in pre-vitellogenic egg chambers, but do not play a major role in modulating egg chamber progression to vitellogenic stages.
The Germline Starvation Response Correlates with Enhanced Embryo Survival
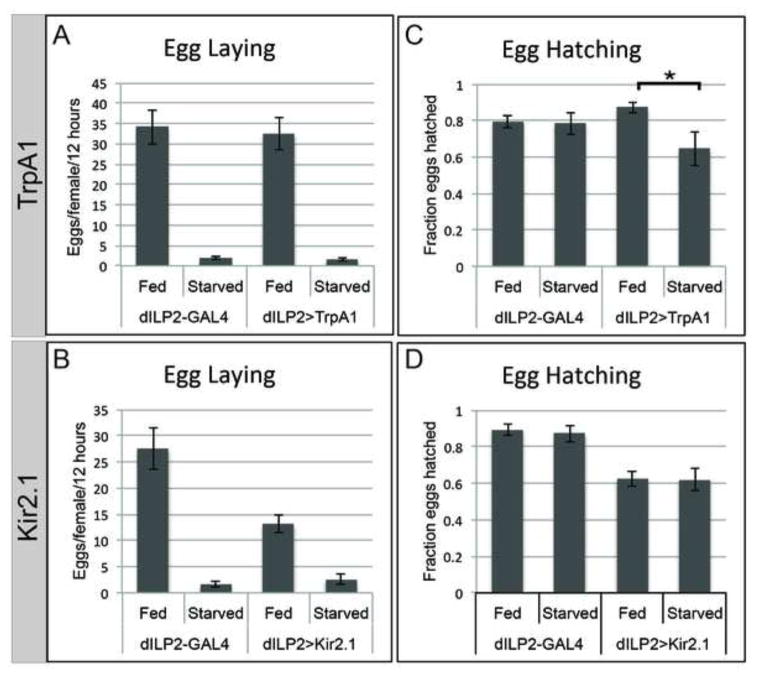
The ability to induce or block germline P body aggregation by changing systemic dILP levels provided a means to investigate the physiological significance of the germline starvation response. Similar to controls, females expressing TrpA1 or Kir2.1 in the IPCs laid few eggs in reponse to starvation (Fig. 4 A, B). Females expressing Kir2.1 had reduced egg laying even when well fed, though not as severe as when starved (Fig. 4B). The egg laying data were consistent with increased egg chamber apoptosis (Fig. 3).
Figure 4. The germline starvation response enhances embryo survival.
(A) Increasing systemic dILP secretion by expression of TrpA1 (28°C) in the IPCs does not prevent the starvation-induced reduction in egg laying rate. (B) Constitutively decreasing systemic dILP secretion by expression of Kir2.1 in IPCs reduces egg laying, but not to the same levels as starvation. (C) Embryo survival from eggs laid by starved mothers expressing TrpA1 in IPCs was significantly reduced. (D) Decreasing systemic dILP secretion by expressing Kir2.1 in IPCs did not affect hatching of eggs produced by starved compared to fed flies. Error bars represent s.e.m. * = p<0.025.
We next tested whether embryo viability was affected by manipulating dILP secretion. The few eggs produced by control starved females developed from egg chambers fully capable of responding to starvation by reorganizing mRNP-containing P bodies into large aggregates. These starved control eggs hatched at the same rate as eggs from well-fed females (Fig. 4 C, dILP2-GAL4 Fed and Starved). However, eggs laid by TrpA1-expressing females reared under starvation conditions developed from egg chambers that could not respond normally to starvation (“unstarved” eggs). Interestingly, unstarved eggs hatched at a significantly lower frequency (70.5% hatching, n=28 embryo collections, 357 embryos) than their starved control counterparts (86.5% hatching, n=28 embryo collections, 657 embryos; P value of 0.025; Fig. 4 C, dILP2>TrpA1), suggesting that the germline starvation response is needed for normal survival of embryos. Embryos from well-fed females expressing TrpA1 hatched at a normal rate, indicating that increased dILP had no additive effect on the quality of eggs (Fig. 4 C).
We also tested the effect on embryo hatching of inducing rather than blocking the germline starvation response by expressing Kir2.1 in IPCs. In this experiment, expression of Kir2.1 was not temporally controlled, and flies had reduced viability, an indication that compromising the insulin/TOR pathway affects overall fly health. Embryos produced by fed or starved females had similar reductions in hatching rates: 87.5% to 61.5% in fed, and 87.1% to 60.5% in starved (n= 18 embryo collections, 1591 fed embryos and 313 starved embryos) (Fig. 4 D, dILP2>Kir2.1). Therefore, reduction of dILP secretion was deleterious to overall egg quality regardless of nutrient conditions, emphasizing the importance of insulin/TOR signaling during oogenesis and subsequent development.
These data provide the first evidence that the germline starvation response in previtellogenic egg chambers is correlated with increased health of progeny. Constitutive dILP secretion in dILP2>TrpA1 flies prevented the germline starvation response (Fig. 2 E, F, I) and reduced the viability of the few eggs produced under nutrient-stressed conditions (Fig. 4 C). The 16% reduction in hatching from “unstarved” compared to starved eggs corresponds to a selection coefficient of 0.18 (calculated as 16%/86.5%), indicating a reduction in reproductive fitness. Thus, the reduction of dILPs that normally occurs in response to starvation not only slows the progression of oogenesis as previously documented (Ikeya et al., 2002), but is also correlated with a significant improvement in egg quality following starvation.
P body Aggregates Include Oocyte-enriched mRNA but Not Ribosomes, and Do Not Increase RNA Degradation
We sought a better understanding of the function of germline P bodies. Depending on the cellular context, P bodies have been shown to be important for both mRNA decay and mRNA storage, (Anderson and Kedersha, 2009; Rajgor and Shanahan, 2014). Our hypothesis was that germline P body aggregates served to store protect maternal mRNAs from degradation. If so, measurements of mRNA levels in fed and starved flies should detect static or elevated levels of mRNA in starved egg chambers; this would argue against a mechanism in which P body aggretgates function to promote mRNA degradation.
We first confirmed that key maternal mRNAs were present in P body aggregates using the MS2::GFP reporter system (Lin et al., 2008; Weil et al., 2006). bcd, grk and osk mRNAs became enriched in germline P body aggregates in starved egg chambers (supplementary material Fig. S3 A–C), similar to results for oskar reported in Lin et al. (2008). The P body aggregates also excluded both small and large subunits of ribosomes (supplementary material Fig. S3 D, E), consistent with the aggregates being translationally inactive, as was recently reported (Weil et al., 2012).
To determine whether germline P body aggregrates were mediating mRNA degradation or storage, we examined the effect of P body aggregation on the levels of mRNP-enriched mRNA transcripts in previtellogenic egg chambers using quantitative reverse transcription polymerase chain reaction (QRT PCR). We found that osk, bcd, and grk showed no significant change in levels in starved egg chambers compared to matched controls from well-nourished flies (supplementary material Fig. S3 F, Control). Additionally, no significant nutrient condition-induced change in mRNA quantity was observed in previtellogenic egg chambers dissected from flies expressing Kir2.1 or TrpA1 in IPCs, which either have or do not have large P body aggregates regardless of nutrient availability, respectively (supplementary material Fig. S3 F, Kir2.1 and TrpA1). Therefore, as in C. elegans, enlarged P body aggregates do not appear to promote mRNA degeneration, and may instead play a role in mRNA storage.
Follicle Cells Relay Nutrient Availability to the Germline
Egg chamber responses to levels of circulating dILPs could be mediated by activation of the insulin pathway in follicle cells, germline cells or both, since mosaic analyses in both cell types using mutations in the insulin response pathway have shown severe effects on the growth of germline cells (LaFever and Drummond-Barbosa, 2005; LaFever et al., 2010). Therefore, we used tissue-specific expression to investigate whether systemic dILPs are detected by follicle cells which then control P body organization in the germline, or directly by the germline. We used follicle cell specific Gal4 drivers (supplementary material Fig. S4) to express transgenes that modulate the insulin/TOR pathway and germline Gal4 drivers to express transgenic short hairpin RNAi (shRNA) to reduce expression in the germline. We opted for tissue-specific expression rather than clonal analysis of mutations to avoid the additional stress of heat shock, which is known to induce germline aggregation of P body components (Jud et al., 2008), and also to avoid severely impairing germline growth (LaFever and Drummond-Barbosa, 2005; LaFever et al., 2010), which could obfuscate the germline starvation response in these cells.
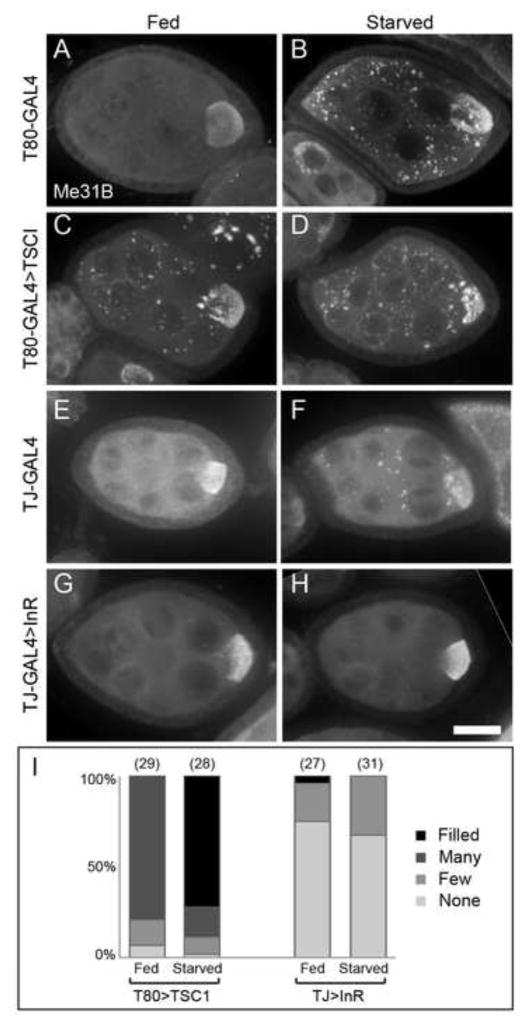
Depleting insulin/TOR signaling in the follicle cells by overexpression of the TOR antagonist Tuberous Sclerosis 1 (TSC1) induced P body aggregation in the germline of well-nourished egg chambers (Fig. 5 A–D, I). Conversely, stimulating the insulin pathway by overexpression of the insulin receptor InR in follicle cells prevented the aggregation of P bodies in starved egg chambers (Fig. 5 E–I). These manipulations did not block the growth of egg chambers since ovarioles contained progressively larger egg chambers. These results provide strong evidence that follicle cells monitor nutrient status and transmit information about dILP signaling activity that affects P body organization in the germline.
Figure 5. Insulin/TOR signaling in somatic follicle cells regulates P body aggregation in response to nutrient availability.
(A–B) Control egg chambers with follicle cell-specific T80-GAL4 alone form P body aggregates in response to starvation. (C–D) Egg chambers with reduced follicle cell TOR signaling caused by overexpression of TSCI form enlarged P body aggregates in egg chambers from both fed and starved females. (E–F) Control egg chambers with TJ-GAL4 form enlarged P body aggregates in response to starvation. (G–H) Egg chambers with increased follicle cell insulin signaling caused by overexpression of InR do not form enlarged P body aggregates in response to starvation. (I) P body aggregation is induced in fed egg chambers with follicle cell TSCI overexpression and prevented in egg chambers with follicle cell InR overexpression. The aggregation scores are listed above the columns, and the sample sizes are in parentheses. Egg chambers were immunostained with Me31B antibodies. Scale bar is 20 μm.
We also investigated whether insulin pathway activity in the germline is involved in the P body and MT responses to starvation. Reducing germline insulin/TOR signaling by expressing shRNA against InR or TOR in the germline caused fully penetrant egg chamber growth inhibition (Fig. 6 A, D, G) similar to the effect of germline clones of InR and TOR mutations (LaFever and Drummond-Barbosa, 2005; LaFever et al., 2010). Interestingly, previtellogenic egg chambers in well-fed females did not contain P body aggregates (Fig. 6 B, E, H), suggesting reduced insulin/TOR activity did not mimic starvation, or the knockdown is too mild to trigger P body aggregation. However, starved egg chambers expressing germline InR or TOR shRNA also did not display a typical germline starvation response; aggregates of P bodies were present only in egg chambers in the process of degenerating, and not in younger egg chambers (Fig. 6 C, F, I). These data suggest that reduced insulin/TOR pathway activity in the germline impairs the ability to aggregate P bodies in egg chambers in response to starvation. Alternatively, growth arrest may render egg chambers unable to respond to nutrient conditions. However, the growth arrest of TOR-knockdown egg chambers was relatively mild (Fig. 6 G), arguing in favor of a role for the pathway in the germline to mount a response to starvation.
Figure 6. Disrupting insulin/TOR signaling in the germline causes reduced growth, but does not induce P body aggregation.
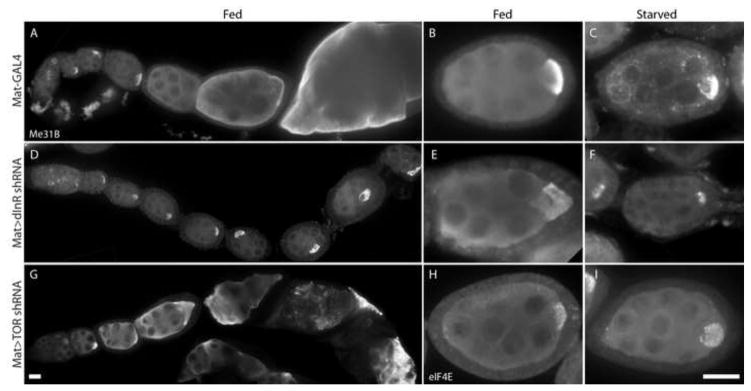
(A–C) Control egg chambers with germline-specific Mat-GAL4 alone develop normally and exhibit large P body aggregates when females are starved. (D–F) The growth of egg chambers in females expressing InR shRNA in the germline is severely inhibited resulting in chains of similarly-sized egg chambers that finally degenerate. Enlarged P body aggregates do not form in fed or starved conditions. (G–I) Egg chambers expressing TOR shRNA in the germline have inhibited growth and do not form many enlarged P body aggregates in either fed or starved conditions. Egg chambers were stained with Me31B antibodies (A–G) or eIF4E antibodies (H, I). Scale bars are 20 μm.
MT Distribution Controls P body Organization
MTs appear to be important for P body distribution since dissociation of MTs altogether with colchicine causes P body aggregation (Shimada et al., 2011). Furthermore, a prominent aspect of the germline starvation response is accumulation of MTs in the cortical region of nurse cells (Shimada et al., 2011). We re-examined MTs in fed versus starved egg chambers to determine if starvation also causes a reduction in cytoplasmic MTs. Previous analysis used projections of confocal Z-stacks in which some of the cytoplasmic signal could have been from cortical MTs. Therefore, we carefully quantified GFP::tubulin in single Z-sections through the center of cells. This new analysis again showed increased cortical tubulin in starved egg chambers, and also revealed a decrease in the cytoplasmic pool (Fig. 7 A–E). This provides evidence that starved egg chambers have fewer MT’s dispersed in the cytoplasm, which correlates with P body aggregation.
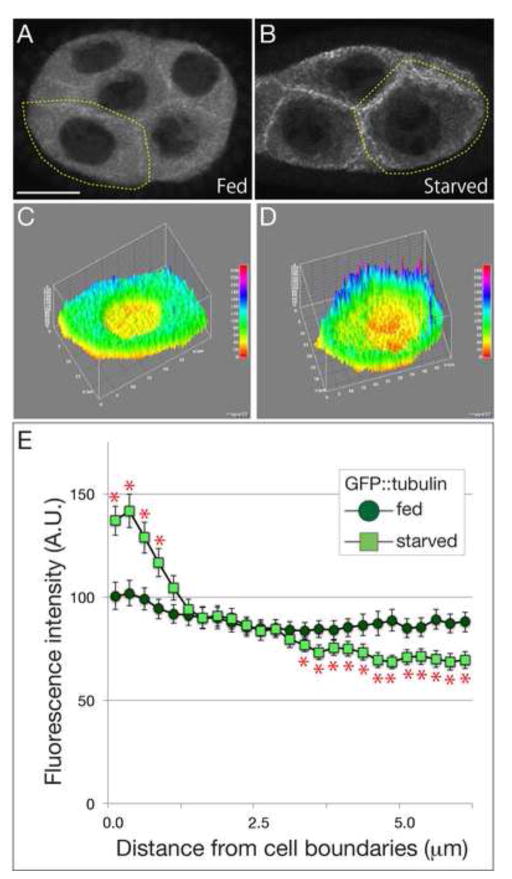
Figure 7. MT distribution is affected by nutrient availability.
(A–D) Stage 6 egg chambers expressing GFP::tubulin dissected from females fed (A and C) or starved for 48 hours (B and D). While GFP::tubulin was distributed throughout nurse cell cytoplasm in fed egg chambers, the cytoplasmic MTs decreased and cortical MTs increased in response to starvation. (C and D) The 3-D graph of flourescence intensities of GFP::tubulin in representative cells indicated by yellow dotted lines in A and B, respectively. The level of flourescence intensity corresponds to the color spectrum. (E) A quantitative analysis of MT distribution in single focal planes from the membrane to cytoplasm in nurse cells. The position of membrane was normalized with FM4-64 labeling. Approximately 20 boundaries from 12 egg chambers were analyzed in each condition. Asterisks show statistical significance (Student’s t-test, p<0.05). Scale bar is 20 μm.
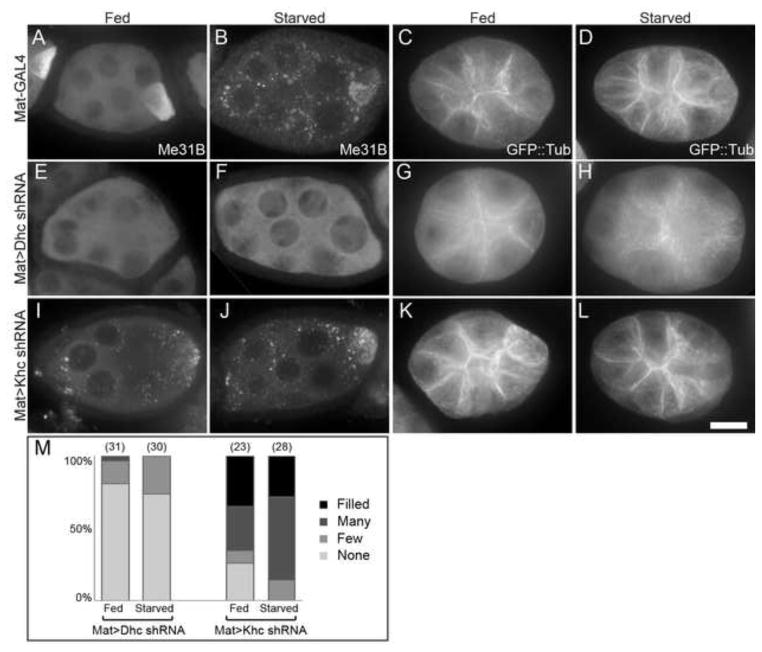
mRNP aggregates caused by oxidative stress in mammalian cells require Dynein to form and Kinesin to disperse (Loschi et al., 2009), though how MT organization might be affected was not reported. To investigate the connection between MT organization and P bodies, we tested the effects of inhibiting the Kinesin and Dynein MT motors using germline-specific shRNA knockdown. In egg chambers from well-fed females, Dynein heavy chain 64c (Dhc) knockdown in germline cells blocked oocyte enrichment of Me31B (Fig. 8 A, E), indicating that the shRNA line was effective at reducing Dynein activity needed for enriching maternal proteins in the oocyte, as previously shown with Dhc mutants (McGrail and Hays, 1997). Interestingly, MTs were more uniformly distributed in Dhc knockdown egg chambers (Fig. 8 C, G), suggesting that some Dynein activity is required for MT distribution in fed conditions. Furthermore, germline knockdown of Dhc prevented egg chambers from undergoing MT reorganization during starvation. Instead, MTs remained dispersed in the cytoplasm without cortical enrichment (Fig. 8 D, H). These results suggest Dynein is involved in localizing MTs to the cortex, which is especially important during starvation. Importantly, blocking MT cortical condensation with Dhc shRNA also prevented the aggregation of P bodies in response to starvation in egg chambers (Fig. 8 B, F, M), supporting the conclusion that P body aggregation depends on shifting the distribution of MTs from the cytoplasm to the cortex.
Figure 8. MT motors regulate MT distribution and P body formation in response to starvation.
(A–D) Control egg chambers from flies with Mat-GAL4 respond to starvation with enlarged P body aggregates (A, B) and cortical enrichment of MTs (C, D). (E–H) Egg chambers expressing germline Dhc shRNA do not accumulate Me31B in oocytes, do not accumulate P body aggregates in response to starvation (E, F), and contain dispersed cytoplasmic MTs in both fed and starved conditions (G, H). (I–L) Germline Khc shRNA causes P body aggregation and cortical accumulation of MTs in egg chambers from well-fed flies, phenocopying the germline starvation response. (M) P body aggregation is prevented in starved Dhc shRNA expressing egg chambers, and induced in fed Khc shRNA expressing egg chambers. Sample sizes are indicated in parentheses. Egg chambers expressing GFP::tubulin were stained with Me31B antibodies to view P bodies. Scale bar is 20 μm.
Kinesin heavy chain (Khc) has been shown to contain domains necessary for ooplasm streaming and cargo transport (Williams et al., 2013). Reducing Khc with germline-specific shRNA had the opposite effect from Dhc shRNA. Egg chambers had a constitutive starvation phenotype with cortically enriched MTs in egg chambers from well fed females (Fig. 8 K, L) and aggregates of P bodies under both fed and starved conditions (Fig. 8 I, J, M). These results are consistent with the idea that MT distribution in response to nutrition controls P body organization. Thus, Khc knockdown in egg chambers from well-fed females phenocopies the normal germline starvation response.
To determine if MT motor activity controls P body organization directly, perhaps by carrying RNP cargo, or indirectly by controlling MT organization, we tested the effect of colchicine treatment on egg chambers with reduced motor activity. In Dhc knockdown egg chambers, P body aggregates were present in both fed and starved egg chambers when MTs were depolymerized with colchicine (supplementary material Fig. S5 C, G). Similarly, P body aggregates were still present in Khc knockdown egg chambers in the absence of microtubules (supplementary material Fig. S5 K, O). Thus, the motors appear to be indirectly affecting P body organization by controlling MT organization.
Taken together, these data suggest that nutrient conditions may control the balance of Kinesin and Dynein activities. In this model, Kinesin activity is important for dispersal of MTs and P bodies in the egg chambers of fed females, and Kinesin motor function is reduced or altered in response to low dILP levels in starved flies. In contrast, Dynein’s role in condensing MTs at the cortex is activated or enhanced in starved females, and results in P body accumulation in the cytoplasm.
Discussion
Abundant evidence has been gathered for the involvement of the insulin pathway in regulating cell growth during oogenesis (Drummond-Barbosa and Spradling, 2001; Ikeya et al., 2002; LaFever and Drummond-Barbosa, 2005). Here we have demonstrated that dILPs secreted from the IPCs in the brain relay information about nutrient conditions to the ovary to control a physiologically important response in immature egg chambers to transient nutrient shortage. We propose a model in which the signal is relayed to the germline cells by the somatic follicle cells and influences the organization of MTs and P bodies (Fig. 9). Our data suggest that MT organization might be controlled by shifting the balance of MT motor activity. MT dispersion in well-fed, high dILP conditions is dependent on Kinesin motor activity, while cortical condensation and cytoplasmic depletion of MTs in response to starvation is dependent on Dynein motor activity.
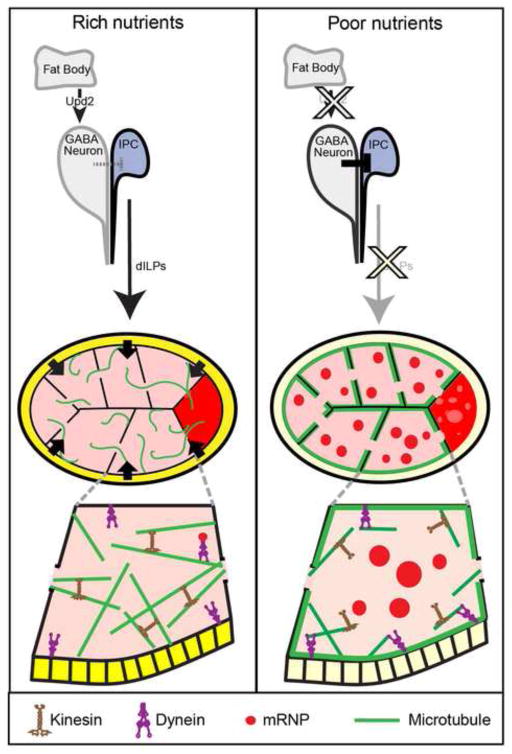
Figure 9. A model for the control of the germline starvation response.
In rich nutrient conditions, dILP secretion by the IPCs is likely downstream of Upd2 secretion from the fat body (Rajan and Perrimon, 2012). The secreted dILPs then signal to the follicle cells (shaded dark yellow), which communicate to the germline, allowing Kinesin to keep MTs diffuse and support Dynein-dependent mRNP transport to the oocyte. In poor nutrient conditions, fewer dILPs are secreted from IPCs, leading to decreased insulin/TOR pathway activity in follicle cells (shaded light yellow). This decreased signaling alters Dynein activity in the germline to promote cortical condensation of MTs (green) and consequently the accumulation of P bodies (red).
The germline starvation response we are studying may be protective for the germline, as impairing the ability of egg chambers to respond to nutrient stress was correlated with decreased progeny survival. However, the method we used to block the response of MTs and P bodies to starvation was indirect – constitutive dILP secretion from the brain. Thus, it is possible that the inability to reorganize MTs and P bodies was only partly responsible for the significant reduction in embryo viability we measured. We cannot rule out the possibility that forced dILP secretion during nutrient stress has other deleterious effects on embryo quality independent of the P body starvation response. Although the ovaries in TrpA1-expressing, starved females were similar in overall appearance to controls, and the experiments were tightly controlled for temperature, crowding and nutrition, the MT and P body phenotypes we observe in the germline cells may be correlated with egg quality without having a direct causal relationship.
Our data indicate that nutrient availability is signaled to follicle cells in previtellogenic egg chambers by systemic dILP secretion from the IPCs of the brain. dILP secretion is very likely controlled by increased upd2 expression in the fat body in response to food, which indirectly stimulates dILP secretion by activating JAK/STAT signaling in GABAergic neurons adjacent to IPCs (Rajan and Perrimon, 2012) (Fig. 9). dILP secretion also controls follicle cell division rate and follicle growth in response to food (LaFever and Drummond-Barbosa, 2005). Thus, dILPs control physiological responses aimed at modulating oogenesis in response to food availability with the goal of preserving egg chambers at least until they reach the end of previtellogenic stages.
One function of P body aggregation appears to be protecting mRNAs from degradation. We saw no significant decrease in the population of P body-enriched mRNAs in the previtellogenic ovary of starved females, suggesting that these P bodies are similar to germline P bodies of C. elegans, which serve as storage sites for CGH-1 (Me31B) associated mRNA, such as nos-2 (Boag et al., 2008), or P bodies in mammalian cells that appear to store mRNAs during amino acid deprivation (Aizer et al., 2014).
Interestingly, sperm deprivation in C. elegans causes changes in immature oocytes similar to what we have described in Drosophila starved egg chambers, including the formation of large P body-like aggregates and cortically condensed MTs (Boag et al., 2008; Harris et al., 2006; Noble et al., 2008). Similarly, the egg chambers of sperm-deprived Drosophila virgins contain reticulated bodies (Snee and Macdonald, 2009). We do not observe the two fold increase in mRNP-associated mRNA levels that are present in the sperm-deprived C. elegans oocytes (Boag et al., 2008). Nonetheless, the starvation-induced phenotype in Drosophila and the sperm-deprivation induced phenotype in C. elegans are similar, suggesting an evolutionarily conserved mechanism used by the germline to respond to stress (Boag et al., 2008; Harris et al., 2006; Noble et al., 2008; Schisa, 2012). P bodies in yeast also serve to store mRNA, as transcripts temporarily triaged in P bodies can proceed through translation once they leave these storage bodies (Brengues et al., 2005). We propose that in Drosophila, P body enriched mRNA in aggregates with their associated mRNPs are available for rapid resumption of polarized transport to the oocyte via the MT network when food becomes available.
An important question that remains to be addressed is how the follicle cells communicate their altered insulin/TOR signaling activity to the germline. Insulin/TOR - signaling from the follicle cells to the Drosophila germline was previously shown to control egg chamber growth, as germline cysts surrounded by large TOR mutant follicle cell clones display slowed growth (LaFever et al., 2010). Follicle cells also signal a germline MT rearrangement later in oogenesis. Activation of the EGF receptor in follicle cells by the Gurken ligand produced in the germline plays a central role in establishing both the anterior/posterior and dorsal/ventral axes (González-Reyes and St Johnston, 1994). The signal resulting from activated EGFR that establishes the posterior pole of oocytes involves a dramatic rearrangement of the germline MT cytoskeleton. Thus, in both posterior patterning and the germline response to starvation, activation of a receptor tyrosine kinase pathway in the follicle cells results in global reorganization of MTs in the underlying germline (González-Reyes and St Johnston, 1994; Newmark et al., 1997; Poulton and Deng, 2007).
However, it remains unclear how signaling from the follicle cells to the germline is carried out in either case. In C. elegans oocytes, cortical MT organization and P body aggregation occur in response to decreased Major Sperm Protein, and this response relies on functional gap junctions between the oocyte and the surrounding sheath cells (Harris et al., 2006). As gap junctions connect Drosophila follicle cells to the germline (Mahowald, 1972), it is possible that signaling molecules diffuse through these channels to control the Drosophila germline starvation response. Alternatively, follicle cells may produce a secreted signaling molecule that is received by the germline to initiate changes to the MT cytoskeleton. Communication in the opposite direction, from the germline to the follicle cells, has been shown to employ the apical actomyosin cytoskeleton in follicle cells to coordinate the growth of the cyst by coupling somatic epithelium cell proliferation with the expansion of the underlying germline cells. This signaling is suspected to rely on the mechanical tension produced by the growing germline, as detected by the cyskeleton of the follicle cells (Wang and Riechmann, 2007). Perhaps under starvation conditions epithelial tension caused by slowed follicle cell growth signals to the germline cells, which results in the reorganization of MTs and P bodies.
We propose that follicle cell signaling in response to nutrient availability shifts the balance between the activities of Kinesin and Dynein in the germline to control MT distribution (Fig. 9). In this model, Kinesin activity predominates in fed egg chambers to maintain a dispersed cytoplasmic MT network. In starved flies, increased Dynein activity and/or decreased Kinesin activity results in cortical MT accumulation and depletion of cytoplasmic MTs, thereby allowing P body aggregate formation. Khc is required in multiple cell types for the proper organization of cellular MTs (Jolly et al., 2010). In S2 cells, disruption of Khc results in large parallel arrays of MTs, which is similar to the reorganization we observe in starved and Khc knockdown egg chambers. Dynein and Kinesin motors have also been shown to mediate MT function in mRNP trafficking and maintenance in the germ plasm (Sinsimer et al., 2013). Furthermore, Kinesin and Dynein are known to act antagonistically in the control of ooplasmic streaming in stage 10A–10B egg chambers, as Dynein inhibition allows premature Kinesin-driven fast streaming (Serbus et al., 2005). In another case, oxidative stress-induced Stress Granules and P bodies in mammalian cells are dependent on Dynein for their formation and Kinesin for their dissolution (Loschi et al., 2009). Thus, modulating the relative activities of Kinesin and Dynein could provide a highly responsive mechanism for controlling the arrangement of MTs in previtellogenic egg chambers.
MT depletion in the cytoplasm appears to be necessary for P body aggregation during the starvation response. Depolymerizing MTs with colchicine induces the formation of germline P body aggregates (Shimada et al., 2011), and cytoplasmic MTs are depleted in starved egg chambers containing numerous P body aggregates (Fig. 7). Similarly, disruption of MTs in yeast by treatment with benomyl or a lesion in the Tub-1 gene can induce P bodies (Sweet et al., 2007). We propose that when MTs accumulate at the cell periphery and diminish in the cytoplasm of starved egg chambers, mRNPs have decreased access to MTs and thus aggregate into large P body foci in the cytoplasm. When food is plentiful and MTs are dispersed in the cytoplasm, active transport of mRNPs along MTs prevents P body aggregation and mediates oocyte enrichment of maternal components. Importantly, our data suggest that the ability of egg chambers to respond to changes in nutrient availability by altering cytoskeletal organization, and thus mRNP organization, contributes to protecting and preserving immature previtellogenic egg chambers and enhancing egg viability.
Conclusions
The role of the cytoskeleton in controlling P body organization is an active area of research (Rajgor and Shanahan, 2014). Kinesin (KIF5) is involved with transporting P bodies to the dendrites in cultured hippocampal neurons (Oh et al., 2013) and Nesprin-1 has recently been shown to recruit P bodies to MTs in human cell lines (Rajgor et al., 2014). Here we have shown that Drosophila egg chambers provide an excellent system for studying P body dynamics in response to nutrient availability. In characterizing the germline starvation response, we have shown that systemic insulin signals are relayed to the germline by somatic follicle cells, and that the germline starvation response may contribute to maintaining the quality of eggs during periods of nutrient deprivation. P body aggregates that formed in response to starvation depend on the MT organization, and that MT organization is regulated by the Dynein and Kinesin motors. Future work will reveal how signaling from follicle cells causes changes in MT organization in the germline.
Supplementary Material
Highlights.
P bodies aggregate in the Drosophila germline in response to starvation.
Systemic insulin signals are relayed to the germline by somatic follicle cells.
Blocked starvation response correlates with reduced egg quality.
P body distribution is controlled by microtubule organization.
Kinesin and Dynein motors are required for microtubule response to nutrients.
Acknowledgments
We are grateful to the TRiP Project at Harvard University, James Wilhelm, Akira Nakamura, Tze-Bin Chou, William Theurkauf, Daniel St. Johnston, Trudi Schüpbach, Elizabeth Gavis, Pierre Léopold and Tobias Meyer for fly stocks and/or valuable reagents. We also thank members of the Cooley lab for review of this manuscript. This work was supported by NIH grant GM043301 to LC. KMB received support from the National Institutes of Health Developmental Biology Training Grant (T32 HD007180). YS is a recipient of fellowship from the Japan Society for the Promotion of Science.
Footnotes
Author Contributions
K.M.B., Y.S., K.A. and L.C. developed the project and experimental approaches. K.M.B., Y.S. and K.A. performed the analysis of egg chambers including genetics, egg chamber staining and imaging. F.L. carried out the dILP staining of brains. Y.S. performed experiments on microtubule distribution and the effects of colchicine on P body organization. A.M.H and L.C. did microscopy and carried out image analysis of egg chamber and brain data. K.M.B, K.A. and L.C. wrote the paper and all the authors edited the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizer A, Kalo A, Kafri P, Shraga A, Ben-Yishay R, Jacob A, Kinor N, Shav-Tal Y. Quantifying mRNA targeting to P bodies in living human cells reveals a dual role in mRNA decay and storage. J Cell Sci. 2014;127:4443–4456. doi: 10.1242/jcs.152975. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Atalay A, Robida S, Reinke V, Blackwell TK. Protection of specific maternal messenger RNAs by the P body protein CGH-1 (Dhh1/RCK) during Caenorhabditis elegans oogenesis. J Cell Biol. 2008;182:543–557. doi: 10.1083/jcb.200801183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnetti S, Ephrussi A. Orb and a long poly(A) tail are required for efficient oskar translation at the posterior pole of the Drosophila oocyte. Development. 2003;130:835–843. doi: 10.1242/dev.00309. [DOI] [PubMed] [Google Scholar]
- Colombani J, Andersen DS, Léopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Géminard C, Rulifson EJ, Léopold P. Remote Control of Insulin Secretion by Fat Cells in Drosophila. Cell Metabolism. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, St Johnston D. Role of oocyte position in establishment of anterior-posterior polarity in Drosophila. Science. 1994;266:639–642. doi: 10.1126/science.7939717. [DOI] [PubMed] [Google Scholar]
- Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Govindan JA, Yamamoto I, Schwartz J, Kaverina I, Greenstein D. Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Dev Biol. 2006;299:105–121. doi: 10.1016/j.ydbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Ito K, Sado Y, Taniguchi M, Akimoto A, Takeuchi H, Aigaki T, Matsuzaki F, Nakagoshi H, Tanimura T, et al. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis. 2002;34:58–61. doi: 10.1002/gene.10137. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, Hafen E, Sun H, Xu T. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Jaramillo AM, Weil TT, Goodhouse J, Gavis ER, Schüpbach T. The dynamics of fluorescently labeled endogenous gurken mRNA in Drosophila. J Cell Sci. 2008;121:887–894. doi: 10.1242/jcs.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proceedings of the National Academy of Sciences. 2010;107:12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud MC, Czerwinski MJ, Wood MP, Young RA, Gallo CM, Bickel JS, Petty EL, Mason JM, Little BA, Padilla PA, et al. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol. 2008;318:38–51. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso RJ, Hudson AM, Cooley L. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J Cell Biol. 2002;156:703–713. doi: 10.1083/jcb.200110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RC. Ovarian development in Drosophila melanogaster. Academic Press; New York City and London: 1970. [Google Scholar]
- Koch EA, Spitzer RH. Multiple effects of colchicine on oogenesis in Drosophila: induced sterility and switch of potential oocyte to nurse-cell developmental pathway. Cell Tissue Res. 1983;228:21–32. doi: 10.1007/BF00206261. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137:2117–2126. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P. mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2012;4:a012294. doi: 10.1101/cshperspect.a012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M-D, Jiao X, Grima D, Newbury SF, Kiledjian M, Chou T-B. Drosophila processing bodies in oogenesis. Dev Biol. 2008;322:276–288. doi: 10.1016/j.ydbio.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Loschi M, Leishman CC, Berardone N, Boccaccio GL. Dynein and kinesin regulate stress-granule and P-body dynamics. J Cell Sci. 2009;122:3973–3982. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP. Ultrastructural observations on oogenesis in Drosophila. J Morphol. 1972;137:29–48. doi: 10.1002/jmor.1051370103. [DOI] [PubMed] [Google Scholar]
- Mansfield JH, Wilhelm JE, Hazelrigg T. Ypsilon Schachtel, a Drosophila Y-box protein, acts antagonistically to Orb in the oskar mRNA localization and translation pathway. Development. 2002;129:197–209. doi: 10.1242/dev.129.1.197. [DOI] [PubMed] [Google Scholar]
- Mazzalupo S, Cooley L. Illuminating the role of caspases during Drosophila oogenesis. Cell Death Differ. 2006;13:1950–1959. doi: 10.1038/sj.cdd.4401892. [DOI] [PubMed] [Google Scholar]
- McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Amikura R, Hanyu K, Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Developmental Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Mohr SE, Gong L, Boswell RE. mago nashi mediates the posterior follicle cell-to-oocyte signal to organize axis formation in Drosophila. Development. 1997;124:3197–3207. doi: 10.1242/dev.124.16.3197. [DOI] [PubMed] [Google Scholar]
- Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SL, Allen BL, Goh LK, Nordick K, Evans TC. Maternal mRNAs are regulated by diverse P body-related mRNP granules during early Caenorhabditis elegans development. J Cell Biol. 2008;182:559–572. doi: 10.1083/jcb.200802128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J-Y, Kwon A, Jo A, Kim H, Goo Y-S, Lee J-A, Kim HK. Activity-dependent synaptic localization of processing bodies and their role in dendritic structural plasticity. J Cell Sci. 2013;126:2114–2123. doi: 10.1242/jcs.125690. [DOI] [PubMed] [Google Scholar]
- Park S, Alfa RW, Topper SM, Kim GES, Kockel L, Kim SK. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 2014;10:e1004555. doi: 10.1371/journal.pgen.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TTB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Deng W-M. Cell-cell communication and axis specification in the Drosophila oocyte. Dev Biol. 2007;311:1–10. doi: 10.1016/j.ydbio.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett TL, Tanner EA, McCall K. Cracking open cell death in the Drosophila ovary. Apoptosis. 2009;14:969–979. doi: 10.1007/s10495-009-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–137. doi: 10.1016/j.cell.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgor D, Shanahan CM. RNA granules and cytoskeletal links. Biochem Soc Trans. 2014;42:1206–1210. doi: 10.1042/BST20140067. [DOI] [PubMed] [Google Scholar]
- Rajgor D, Mellad JA, Soong D, Rattner JB, Fritzler MJ, Shanahan CM. Mammalian microtubule P-body dynamics are mediated by nesprin-1. J Cell Biol. 2014;205:457–475. doi: 10.1083/jcb.201306076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Lynch JA. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2009;1:a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa JA. New insights into the regulation of RNP granule assembly in oocytes. Int Rev Cell Mol Biol. 2012;295:233–289. doi: 10.1016/B978-0-12-394306-4.00013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus LR, Cha BJ, Theurkauf WE, Saxton WM. Dynein and the actin cytoskeleton control kinesin-driven cytoplasmic streaming in Drosophila oocytes. Development. 2005;132:3743–3752. doi: 10.1242/dev.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Burn KM, Niwa R, Cooley L. Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev Biol. 2011;355:250–262. doi: 10.1016/j.ydbio.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton K, Woodruff RI. The osmolarity of adult Drosophila hemolymph and its effect on oocyte-nurse cell electrical polarity. Dev Biol. 1994;161:154–167. doi: 10.1006/dbio.1994.1017. [DOI] [PubMed] [Google Scholar]
- Sinsimer KS, Lee JJ, Thiberge SY, Gavis ER. Germ Plasm Anchoring Is a Dynamic State that Requires Persistent Trafficking. Cell Rep. 2013;5:1169–1177. doi: 10.1016/j.celrep.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snee MJ, Macdonald PM. Dynamic organization and plasticity of sponge bodies. Dev Dyn. 2009;238:918–930. doi: 10.1002/dvdy.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet TJ, Boyer B, Hu W, Baker KE, Coller J. Microtubule disruption stimulates P-body formation. RNA. 2007;13:493–502. doi: 10.1261/rna.355807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Alberts BM, Jan YN, Jongens TA. A central role for microtubules in the differentiation of Drosophila oocytes. Development. 1993;118:1169–1180. doi: 10.1242/dev.118.4.1169. [DOI] [PubMed] [Google Scholar]
- Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–1355. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Developmental Cell. 2006;11:251–262. doi: 10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Weil TT, Parton RM, Herpers B, Soetaert J, Veenendaal T, Xanthakis D, Dobbie IM, Halstead JM, Hayashi R, Rabouille C, et al. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat Cell Biol. 2012;14:1305–1313. doi: 10.1038/ncb2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Mansfield J, Hom-Booher N, Wang S, Turck CW, Hazelrigg T, Vale RD. Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J Cell Biol. 2000;148:427–440. doi: 10.1083/jcb.148.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LS, Ganguly S, Loiseau P, Ng BF, Palacios IM. The auto-inhibitory domain and ATP-independent microtubule-binding region of Kinesin heavy chain are major functional domains for transport in the Drosophila germline. Development. 2013;141:176–186. doi: 10.1242/dev.097592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimyanin VL, Belaya K, Pecreaux J, Gilchrist MJ, Clark A, Davis I, St Johnston D. In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell. 2008;134:843–853. doi: 10.1016/j.cell.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.