Abstract
Early detection of osteoarthritis (OA) remains a critical yet unsolved multifaceted problem. To address the multifaceted nature of OA a systems model was developed to consolidate a number of observations on the biological, mechanical and structural components of OA and identify features common to the primary risk factors for OA (aging, obesity and joint trauma) that are present prior to the development of clinical OA. This analysis supports a unified view of the pathogenesis of OA such that the risk for developing OA emerges when one of the components of the disease (e.g. mechanical) becomes abnormal, and it is the interaction with the other components (e.g. biological and/or structural) that influences the ultimate convergence to cartilage breakdown and progression to clinical OA. The model, applied in a stimulus-response format, demonstrated that a mechanical stimulus at baseline can enhance the sensitivity of a biomarker to predict cartilage thinning in a five year follow-up in patients with knee OA. The systems approach provides new insight into the pathogenesis of the disease and offers the basis for developing multidisciplinary studies to address early detection and treatment at a stage in the disease where disease modification has the greatest potential for a successful outcome.
Introduction
In spite of the major impact1 of osteoarthritis (OA), the main treatments for OA address the symptoms (pain and function) rather than preventing or slowing the rate of progression of the disease process. While OA is often described as a wear and tear disease, the disease is much more complex, and the lack of treatments that address the disease is evidence of its complexity2. While the diverse appearance of the primary risk factors (aging, obesity and trauma) for developing OA suggests that there are multiple pathways to the disease, the fact that the state of the disease can be characterized by a broad scope of biological, structural and mechanical components calls for new approaches to characterizing the pathogenesis of OA. The complex nature of the disease necessitates new methods that address OA across diverse disciplines and scales.
In exploring the complexity of OA it becomes apparent that there are multiple phenotypes that can influence both the initiation and progression of the disease. For example, OA has been characterized as a disease of mechanics25, a disease with a metabolic phenotype47, a disease substantially influenced by inflammatory mediators5, 50, 53 as well as a disease that is driven by aberrant joint structure43 , morphology42 and genetics44. The complexity of OA has also been recognized in a recent workshop addressing genetics and genomic targets for OA38 where numerous candidate genes were recognized, yet, at present genetic risk prediction relative to traditional risk factors was not enhanced and it was suggested that there is a need for addressing the interaction between genetics and environmental factors. Thus the diverse nature of each of the phenotypes associated with OA has produced a large body of research that has isolated specific phenotypes without considering the interaction of the other factors associated with the disease. An interdisciplinary approach to the study of the OA as a system that consolidates the broad scope of factors that influence the disease is needed.
A broader look at the diverse phenotypic descriptions of OA in the context of the primary risk factors for the disease suggests that each of the phenotypes have abnormal characteristics in one or more of the biological, mechanical, or structural components of the disease irrespective of the initiating cause. As the disease develops, the interaction of these components at the in vivo systems levels becomes the critical factor that determines the development of clinical OA. The fact that the disease ultimately converges to pain, loss of function and breakdown of the articular cartilage provides an outcome measure to assess the interactions of these components at an in vivo systems level. Thus both early detection and treatment planning would benefit from understanding a comprehensive systems view of OA4 that incorporates the relative state and interaction of the primary components of the disease (biology, mechanics and structure) that are associated with the key risk factors.
Thus the purpose of this review was to address the question of early detection by consolidating a number of observations on the biological, mechanical and structural components associated with the pathogenesis of OA to extend a previously described framework4 and present a unified view of the diverse risk factors for knee OA. While the literature on OA is broad and diverse, this review was focused on literature that provided in vivo components (biological, mechanical and structural) of OA that could be consolidated to study the pathogenesis of OA as a unified in vivo system. As such this review is not a “systematic” review but rather presents a systems type model of the type commonly used to create a structured representation of the interaction of basic components that characterize complex systems. Specifically, a conceptual systems model was developed here to present a basis for testing the theory that there are biological, mechanical and structural components common to the early development of risk factors (aging, obesity and joint trauma) for OA, and it is the interaction of these components that determines the rate that an individual with a risk factor progresses to clinical OA. Finally, specific examples are provided to illustrate how the application of the systems model can provide new insight into the pathogenesis of knee OA.
A Systems Model for OA
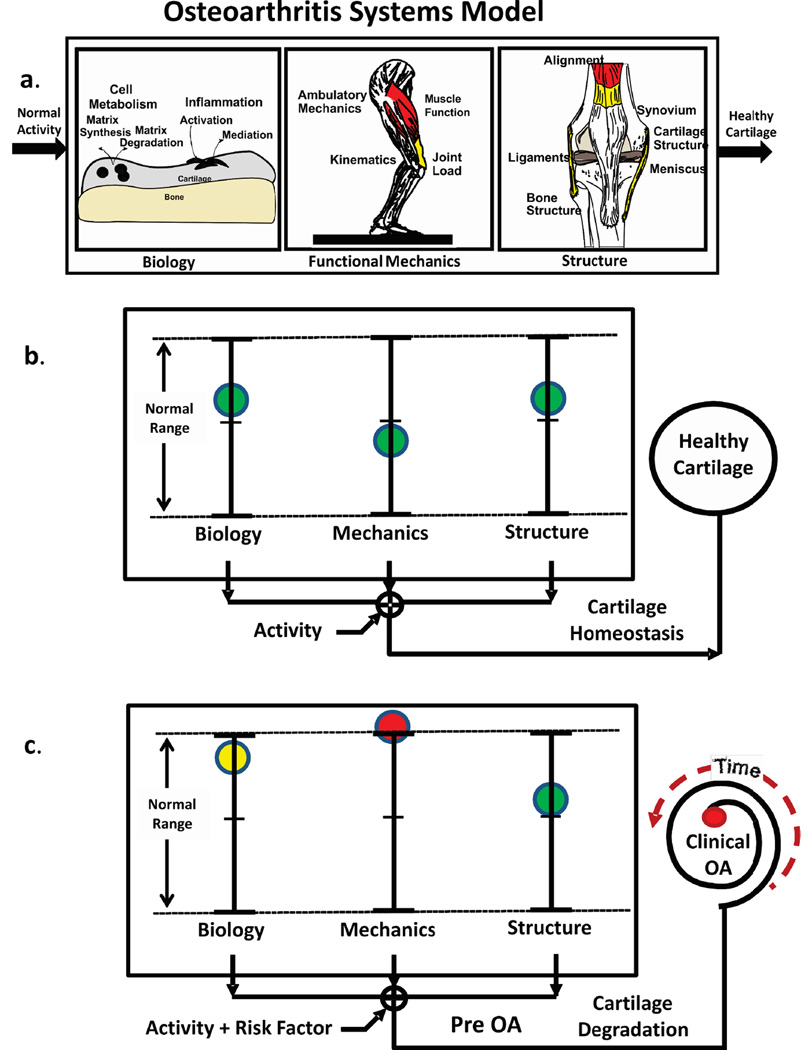
The OA systems model (Figure 1a) developed here considers cartilage health to be dependent on the integrated behavior of biological, mechanical and structural components of the disease. The Biological Component ranges from the factors that influence cell metabolism, levels of systemic inflammation and genetic etiologies. The Mechanical Component includes any signal that delivers a mechanical stimulus to the cell and spans scales from the whole body (mechanics of ambulation) to the level of the local mechanical cell environment. The Structural Component includes factors such as overall joint alignment, bony change, cartilage thickness/shape and ligament properties. Thus the model implies that healthy homeostasis is maintained when each of the components operates within normal ranges (Figure 1b).
Figure 1.
The OA systems model (a) considers cartilage health to be dependent on the integrated behavior of biological, mechanical and structural components where healthy homeostasis (b) is maintained when each of the components is within normal ranges during normal activity. Introducing a “Risk Factor” moves the system out of homeostasis (c). For example (c), if the “ Mechanics” component (red) moves out of normal range then cartilage will begin to degrade (Pre OA) and the “Time” to develop “Clinical OA” will depend the state of the other components.
A first step to developing an in vivo systems view of OA required identifying specific components of the systems model (Figure 1a) that interact to maintain healthy cartilage such that if one or more of the components moves out of the normal range for a specific individual then the risk for developing clinical OA increases (Figure 1c). The interaction between the various components of the model is critical to understanding OA at a systems level. For example, in the framework of this model previous studies7, 30 have shown that healthy cartilage responds positively to load, where specifically the ratio of medial-to-lateral cartilage thickness was associated with the adduction moment during walking for healthy young subjects, whereas cartilage thickness in patients with medial knee OA responds negatively to a higher adduction moment3. Thus at a systems level there was an association between a mechanical signal and a structural measure of cartilage health.
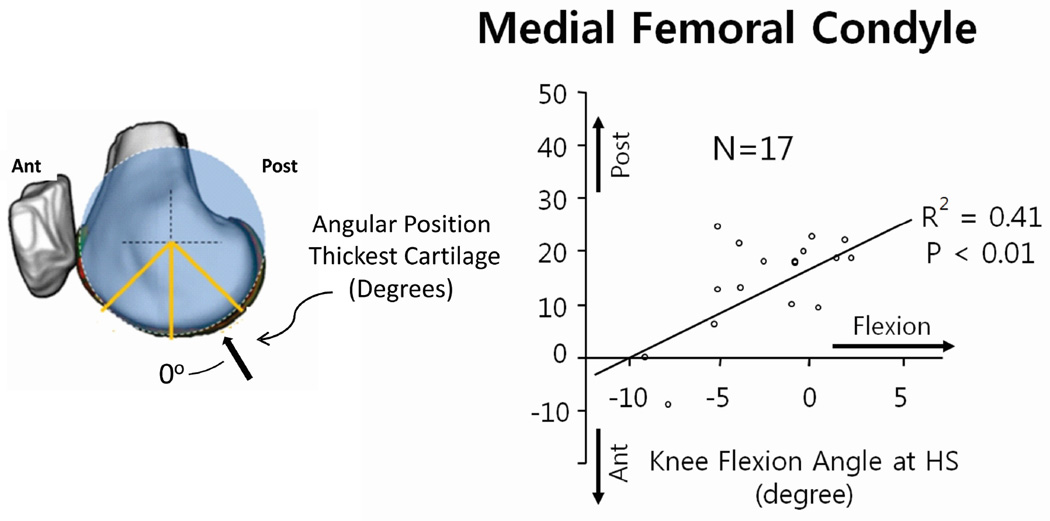
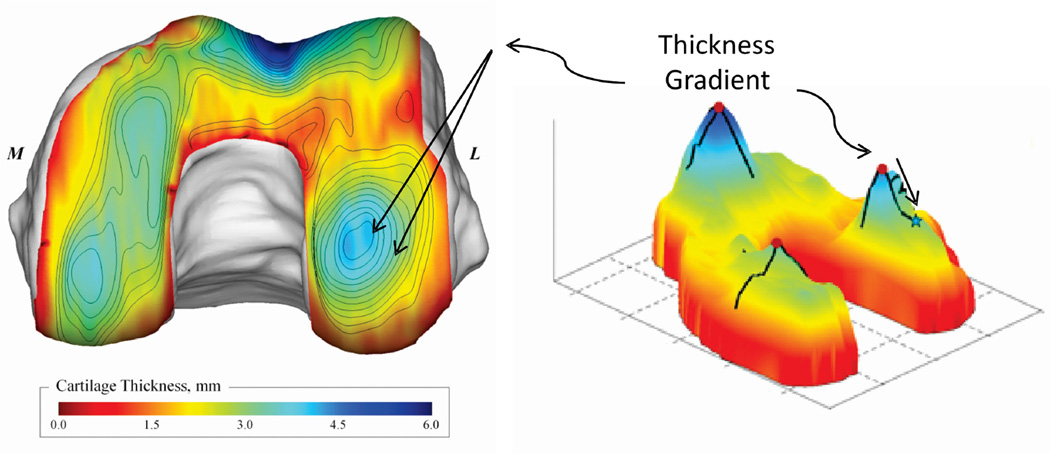
Applying the in vivo systems level model to healthy subjects brings to light the potential that cartilage adapts to the kinematic patterns of normal walking. In particular, it has been shown32, 46 that the anterior-posterior distribution of cartilage thickness was influenced by knee kinematics at heelstrike. For example (Figure 2), the location of the thickest cartilage on the medial femoral condyle was associated with the angle of knee flexion at heelstrike. That is, healthy individuals with greater knee flexion at heelstrike had a more posterior position of the thickest cartilage on the medial femoral condyle relative the contralateral knee. The findings of these studies are important as they suggest that cartilage health could be dependent on maintaining kinematic patterns in normal walking, and changes in these patterns could shift contact to regions of cartilage that cannot adapt to a change in cyclic loading. Over time the cyclic change could lead to cartilage breakdown3. The pattern of thickness distribution (Figure 3) suggests there can be substantial thickness gradients in the load bearing areas of the femoral cartilage. Thus the nature of these gradients (Figure 3) and the normal variations in healthy cartilage thickness patterns can be a factor placing some individuals at greater risk for developing OA.
Figure 2.
The interaction between gait mechanics and cartilage structure was demonstrated in a recent study32 that suggests variations in the patterns of normal cartilage thickness are influenced by knee kinematics during walking. Specifically the location of the thickest cartilage on the medial femoral condyle was associated with the angle of knee flexion at heel strike.
Figure 3.
The individual patterns of cartilage thickness distribution can influence the sensitivity of cartilage health to kinematic changes3 since relatively small kinematic changes can move contact to substantially different regions. The gradients in cartilage thickness distribution can be relatively large in the load bearing regions as illustrated by the 2d projection on the right
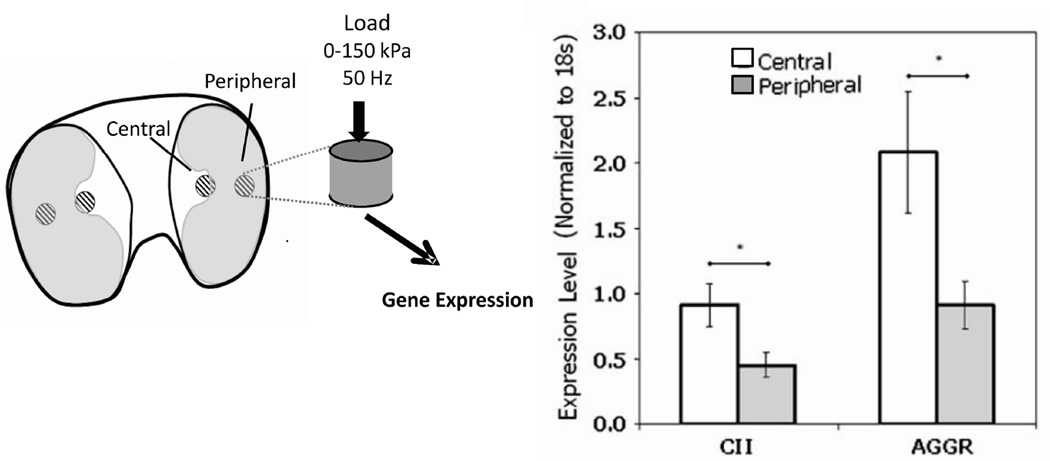
It is important to consider the biological components of the system in the context of the reports that individual regional thickness variations are associated with kinematic variations. Specifically, there is also evidence6 that regional variations in biological properties are consistent with the regional variations in the cartilage thickness (Figure 2). In particular it was shown that chondrocytes from distinct regions of the porcine tibial plateau display region-specific baseline gene expression, and respond differently to in vitro mechanical loading (Figure 4). Prior to loading mRNA levels for the structural proteins collagen II (CII) and aggrecan (AGGR) were approximately twofold greater in the central region explants compared with peripheral region explants. Further, In vitro dynamic compression strongly affected expression levels of structural proteins as well as proteases such as MMP-3. Most importantly the response differed significantly by region, with greater upregulation of CII, AGGR, and MMP-3 in central region explants. The regional variations in the biological response to mechanical load (location and magnitude) that are also associated with morphological variations in cartilage thickness provide support for incorporating biological, the mechanical and structural components into a single model of cartilage health. Thus as risk factors develop, one or more of the components in the systems model will move out of normal range required for cartilage homeostasis (Figure 1c).
Figure 4.
Regional differences6 in the biological response to mechanical load are consistent with the thickness variation shown in Figure 3 and support the interpretation that kinematic changes can load cartilage in regions that cannot respond to a new loading condition.
Consolidating the observations described above provides important insights for applying an in vivo systems model. First, the morphological and biological organization of cartilage was associated with the repetitive mechanical signals generated during walking. Second, individual variations in the normal patterns of normal walking can influence normal variations in cartilage morphology.22, 32, 45 Third, if ambulation changes in a way that places loading outside of normal ranges (increase or disuse) cartilage can respond negatively3 Thus it is possible to identify meaningful interactions at the systems level that span across scales from whole body function to the cellular level and thus provide a basis for analyzing the complexity of the factors that influence cartilage health and risk factors for developing OA.
A Systems View of OA Risk Factors
A comprehensive view of the primary risk factors for knee OA (aging, obesity and joint trauma) using a systems approach can provide new insight into the pathogenesis of the disease. While the primary risk factors seem to suggest different pathways to OA, when placed in the systems framework (Figure 1c), common features emerge among the risk factors that suggest it is the relative balance between the changes in the biological, mechanical and structural components that determines progression to clinical OA. Conditions such as substantial joint mal-alignment48 and joint laxity16 are most frequently reported after the appearance and in the advanced stages of clinical OA, and thus these conditions are beyond the scope of this paper as the focus here is on conditions that appear prior to the development of clinical OA.
A chronic change from normal in one or more of the subsystems needed to maintain cartilage health (Figure 1c) can be considered from a systems view as moving from healthy homeostasis18 to a state that puts cartilage on a pathway to OA or a pre-OA state. As previously described12, characterization of a pre-OA disease state will be critical for developing new methods for prevention and early treatment before clinical symptoms emerge. Time is an important factor (Figure 1c) in the transition from pre-OA to clinical OA because the rate that the joint develops clinical OA depends on whether the other components in the system can compensate in a manner to maintain healthy homeostasis. Theoretically a patient could remain in a state of asymptomatic pre-OA for a substantial period of time after one of the subsystems becomes abnormal if there is appropriate compensation by the other subsystems. Thus the risk for the developing clinical symptoms is dependent on the relative contribution of each of these components in mitigating or exacerbating the risk for developing OA.
Aging and OA
As the primary risk factor for developing OA, aging provides a useful basis for identifying specific systems components (biological, mechanical and structural) that are associated with the transition state of pre-OA. Age-related OA has been described as a “wear and tear” disease where the number of cycles of mechanical load builds over time causing cartilage to breakdown. The wear and tear description is supported by reports24 that the risk of developing knee OA increases substantially above the age of 45 years and the incidence of the disease increases with age past the age of 75 years. However, the fact that approximately 50% of individuals above the age range of 45 to 75 years do not develop clinical OA15, 24 suggests that others factors can mitigate the “wear and tear risk” for developing OA.
Further examining the “wear and tear” explanation, one would expect in the absence of joint trauma that healthy older, more active, moderate-weight individuals with a greater number of loading cycles would have a higher incidence of knee OA than their less active counterparts. However, it is difficult to find quantitative evidence to suggest that more active individuals have a greater incidence of knee OA. In fact, there is evidence that individuals who participate in moderately high levels of running activity do not have an increased incidence of knee OA9. It should be noted that very high levels of training or physical activity can present an increased risk of developing knee OA10. At present the available data does not support the idea that moderately increased activity and cyclic loading increases the risk of OA. Indeed, the vulnerability may arise when individuals with altered or compromised joint biology sustain injury or altered loading.12, 33 Thus a simple “wear and tear” explanation does not provide a sufficient basis to move forward with new prevention modalities or new methods for early detection of OA as a better understanding of OA at a systems level is needed.
Given that “wear and tear” alone is not sufficient to address the age-related risk for developing knee OA, it is useful to examine other elements that change with aging that have common features to the other risk factors. Specifically, there is emerging evidence that there are subtle but important changes from normal patterns of ambulation that are common to the primary risk factors (aging, obesity and joint trauma), and these ambulatory changes appear prior to developing clinical knee OA. Further these ambulatory changes have specific kinematic characteristics that taken together with reports22, 32, 45 that the structure and biology of healthy cartilage adapt to the repetitive patterns of loading, suggest that cartilage health is dependent upon maintaining kinematics within a normal envelope of function.
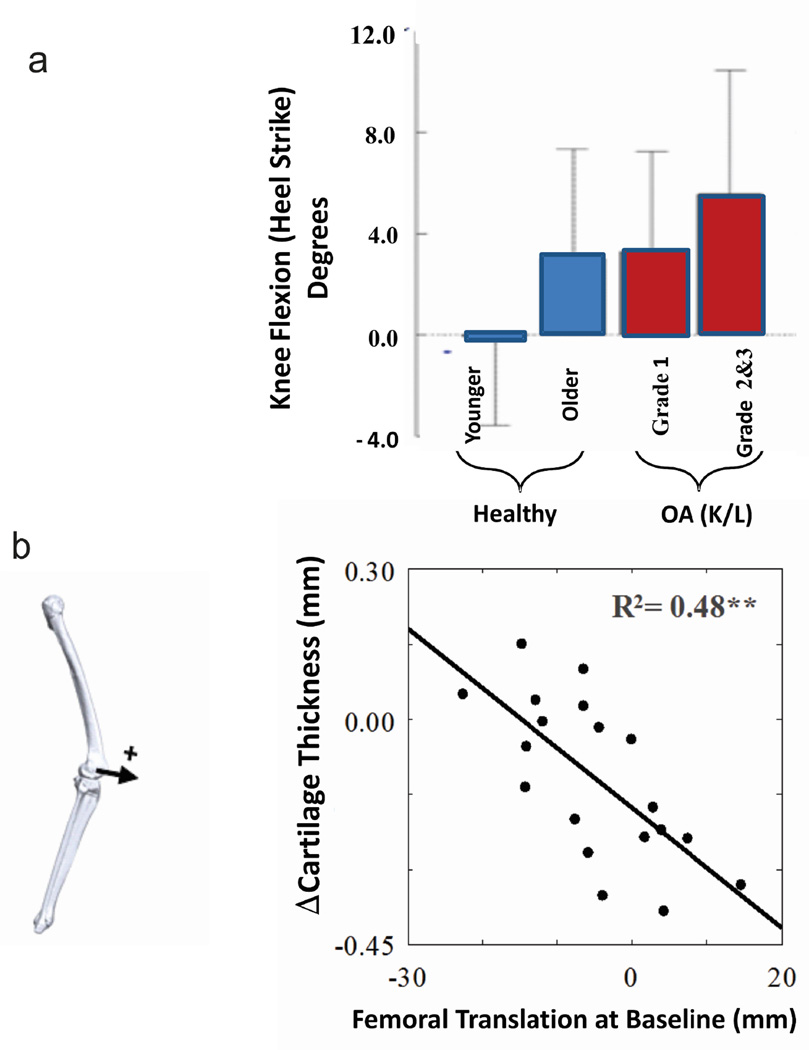
The risk of developing knee OA with aging provides an illustration of the association between early kinematic changes and the initiation of OA (Figure 1c). A recent study20 summarized several critical ambulatory changes that occur with aging and identified significant differences in sagittal plane knee kinematics between a healthy younger population (29±4 yrs) and a healthy older population (59±9 yrs). Kinematic differences were observed at heel strike with significantly less knee extension (Figure 5a), less posterior femoral displacement, and less backward shank inclination in the older healthy population as compared to the younger population. It is important to note that these differences were even more pronounced in patients with moderate and severe OA relative to the younger asymptomatic population.
Figure 5.
Age related changes in kinematics can influence the prospective cartilage changes. (a) Kinematic changes have been reported20 to progressively increase from a healthy younger population (29±4 yrs) to a healthy older population (59±9 yrs), and were even more pronounced in patients with moderate and severe OA relative to the younger asymptomatic population. The kinematic differences occurred at heel strike. (b) The AP position of the femur during walking at baseline was correlated with a reduction in cartilage thickness at a five year follow-up for patients with medial compartment knee OA. These data suggest that patients with a more anterior position at heel strike had greater loss of thickness at the 5 year follow-up21.
The nature of the kinematic differences between young and older healthy subjects20 are important when considering the finding32 that individual variations in healthy knee cartilage thickness are associated with the angle of knee flexion at heel strike (Figure 2) since this implies that healthy cartilage adapts to individual kinematic patterns during walking32. Thus any condition such as aging that causes chronic kinematic changes at the knee can move nominal contact during walking to regions of the cartilage that have different structural23 and biological6 properties.
A recent study further explored the relationship between the kinematic changes with aging and prospective changes in cartilage in a group of patients with knee OA21. Specifically, the anterior-posterior position of the femur relative to the tibia at baseline was correlated with cartilage thinning at a five year follow-up (Figure 5b). Again this example provides additional support for the interaction between the various components of the system in assessing the risk for clinical OA.
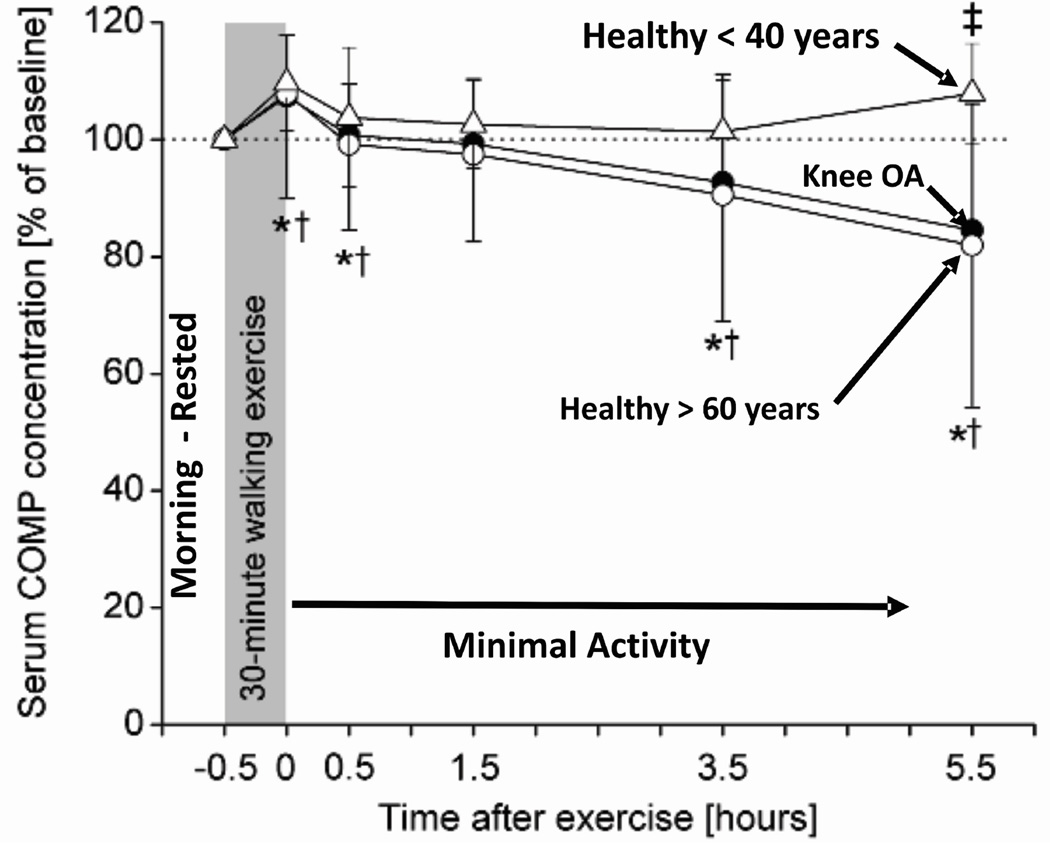
There is a biological correspondence to the kinematic similarities seen in healthy older subjects and patients with knee OA. Specifically, combining the results of two studies37, 39 shows that serum concentrations of cartilage oligomeric matrix protein (COMP) respond to a mechanical stimulus (30 minute walk) in older healthy subjects in a manner similar to that seen in patients with knee OA, whereas both groups differ from young healthy normal subjects (Figure 6). Specifically, 3.5 hours after a mechanical stimulus, the differences in serum concentration of COMP became significant between younger healthy subjects and both older healthy subjects and patients with knee OA. Given that COMP has been identified as a marker of cartilage turnover56, these observations suggest that the mechanical stimulus produced a response in the aging population suggestive of a change in cell metabolism consistent with changes observed in patients with clinical OA. While the implications of this finding require further study, the fact that the biological response was produced by a mechanical stimulus suggests the importance of treating the analysis of OA as a system where the interaction among the various components of the system provides unique insight into the pathogenesis of the disease.
Figure 6.
The importance of considering the system interaction between biology and mechanics is illustrated by combining the results of two studies39, 37 that show serum concentrations of cartilage oligomeric matrix protein (COMP) respond to a mechanical stimulus (30 minute walk) in older healthy subjects in a manner similar to that seen in patients with knee OA, whereas both groups differ from young healthy normal subjects. Specifically, 3.5 hours after a mechanical stimulus, the differences in serum concentration of COMP became significant between younger healthy subjects and both older healthy subjects and patients with knee OA.
Common Features Among the Risk Factors
The above discussion suggests that there are kinematic and biological changes that occur in an aging population that precede the development of clinical symptoms. Interestingly the other primary risk factors for knee OA (joint injury and obesity) have changes similar to those reported for aging. For example, it was reported17 that obese subjects walk with different knee kinematics than age-matched lean subjects. Similarly, differences in knee flexion at heel strike were also found45 in patients following reconstruction of the anterior cruciate ligament (ACL), where the reconstructed knee was more flexed than the healthy contralateral knee. The side-to-side differences in knee extension reported in that study45 also agree with clinical studies49 reporting an extension deficit in ACL reconstructed knees as compared to contralateral knees. Given the morphologic findings described above (Figure 2), the connection between negative subjective patient outcomes and post-operative side-to-side extension deficits49 are consistent with reports that these patients with ACL reconstruction develop significant joint space narrowing at 10–14 years after reconstruction35.
Rotational kinematic changes have also been reported3, 26, 35, 51 following joint trauma associated with ACL injury or posterior medial meniscectomy40. These changes appear to be related to a rotational offset in the position of the knee correlated with loss of constraint associated with ACL deficiency or meniscus function. As previously described3 rotational changes at the knee can produce a shift in contact location to regions of cartilage not conditioned for a regional change in loading. This shift in nominal contact is important as cartilage structure (Figure 3) and biology6 (Figure 4) can vary substantially over the weight-bearing regions of the cartilage.
The kinematic changes that are common to the primary risk factors for knee OA alone do not provide a basis for understanding the risk for developing OA. Rather, these observations beg the need for a better understanding of how normal variations in the biological and structural properties influence the risk for developing OA in association with a kinematic change. These observations suggest that an individual with cartilage having steeper thickness gradients (Figure 3) is at greatest risk for developing OA after acquiring a condition that causes kinematic changes during ambulation. Further, chondrocytes vary in their biological properties based on their location within the tissue (Figure 4)6. Thus if the chondrocytes in the new region of contact cannot adapt in a manner to maintain healthy homeostasis, then the kinematic changes can initiate a degenerative pathway. Again when taking a systems view it becomes apparent that there are biological factors that can influence the capacity of cartilage to adapt to kinematic changes. Interestingly, the primary risk factors for OA have common biological changes that include both metabolic and inflammatory changes.
Inflammatory mediators released by bone, synovioum, adipose tissue, and cartilage are important considerations in the early development of OA5. While there is an array of both metabolic and inflammatory surrogate biomarkers that have been associated with OA41, the application of these biomarkers to early detection or treatment has been limited. Further, the question remains as to why therapeutic interventions for OA that have anti-cytokine approaches have not shown significant improvement in disease modification or symptoms11, 52. It is possible that the limited success of the anti-cytokine intervention might stem from the potential interaction of the pro-inflammatory cytokines with mechanical factors. In laboratory studies6, 7, 34 it was shown that the anabolic/catabolic response of the chondrocyte to the levels of pro-inflammatory cytokines is dependent on the nature of the mechanical load. In addition, the response can be sensitive to local regional differences as illustrated in Figure 3. Thus, consideration of the overall systems behavior (Figure 1c) could be an important factor in evaluating these types of therapeutic interventions.
New Approaches to the Early Detection of OA
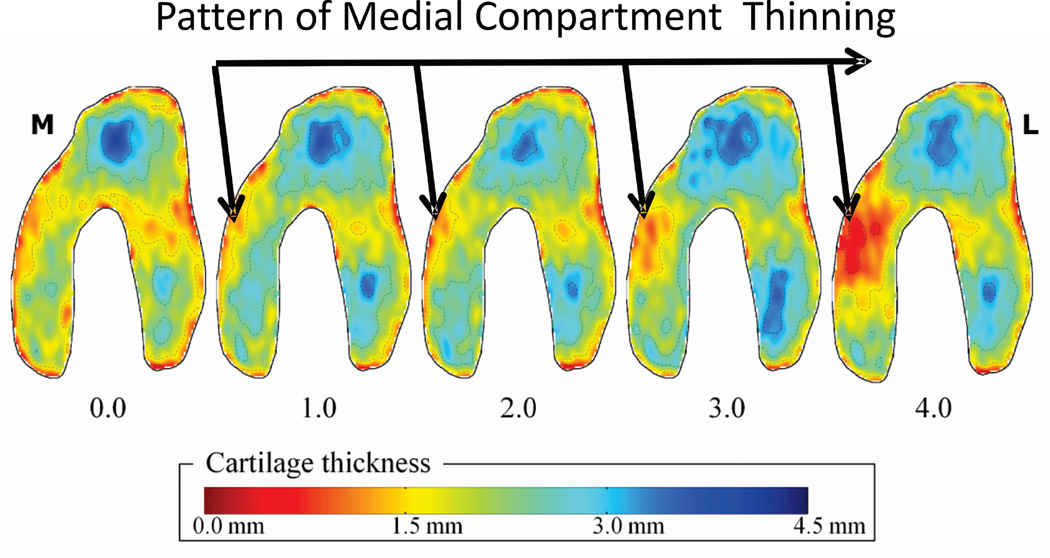
Observing OA as a system raises the opportunity for new approaches and technologies for regional assessment of cartilage structural and biological properties. For example, the reason that current imaging methods have reported limited sensitivity in detecting cartilage changes can be explained by the fact that these methods use mean thickness measurements from predefined regions,31, 55 and thus cannot account for the substantial individual variations in the regions of cartilage thickness (Figure 3). Recent work22, 23 has avoided the need for preselecting regions of interest by characterizing patterns of thickness shape23. Use of a pattern-based analysis of 140 knees from 60 asymptomatic subjects and four groups of 20 patients with mild to severe medial compartment OA identified a characteristic progression in the thickness map patterns with increasing medial knee OA (Figure 7), and the scoring method allowed for excellent differentiation between disease severities. The fact that thickness patterns could be statistically associated with increased OA severity was not trivial as cartilage thickness is known to vary among asymptomatic individuals and the response to OA is partially subject-specific. In fact the novel method introduced in that study23 may provide an important step to identify patterns of cartilage thickness that place individuals at greater risk for developing knee OA.
Figure 7.
The potential for enhancing the sensitivity to detecting cartilage changes is illustrated in a recent study where a pattern analysis23 of cartilage thickness has quantified patterns of thickness change in patients with medial compartment knee OA for conditions ranging from mild OA (1.0) to end stage disease (4.0). The disease was shown to progress from the outer anterior regions though the load bearing regions of the medial compartment.
New quantitative magnetic resonance imaging (MRI) techniques are beginning to illuminate the interplay between mechanics and structural changes to articular cartilage. Evaluation of short T2 relaxation times and ultrashort echo time enhanced (UTE) T2* mapping have been shown to assist in early detection of cartilage abnormalities27, 54. Recent work evaluating cartilage deep tissue matrix changes over time using ultrashort echo time enhanced T2* mapping suggests healing of cartilage deep tissue matrix injuries following anatomic ACL reconstruction techniques.14 It has been shown that ACL reconstruction techniques resulting in a more vertical graft orientation fails to restore rotational kinematics46 and combining these new imaging methods with kinematic studies of patients following ACL reconstruction can provide new insight into the biological changes and organizational changes following ACL injury.
A recent study13 using optical coherence tomography (OCT) offers a novel technology that can be incorporated into arthroscopes to generate cross-sectional images of articular cartilage in near real-time and at resolutions of 10–20 μm. This approach presents an opportunity to evaluate the regional variations in cartilage properties that might reflect the potential sensitivity to kinematic changes as suggested in other studies (Figures 3 and 5) and can be used clinically to identify early cartilage degeneration. Other technological improvements include the development of higher three-dimensional fast MRI methods to permit acquisition of fluid sensitive isotropic data that can be reformatted into arbitrary planes to improve delineation of cartilage contours and pathologies28.
A Stimulus-Response Model
While logical, the in vivo systems model described above introduces a level of complexity that cannot be addressed by deterministic methods, since the role and interaction of each of the components cannot be completely defined. One method to address this level of complexity can be described as a stimulus-response model where a known stimulus is applied and the response of the system is assessed based on the response of specific surrogate components. A key to reducing the conceptual model to a stimulus-response model2 is the selection of meaningful and measurable surrogates for the biological, mechanical and structural components (Figure 1). Existing literature based on an examination of individual surrogate markers that include the biological (biomarkers)41, mechanical (gait mechanics)3 and structural (imaging)29 components of the OA system can provide a basis for selecting specific surrogate components for analyzing the system behavior.
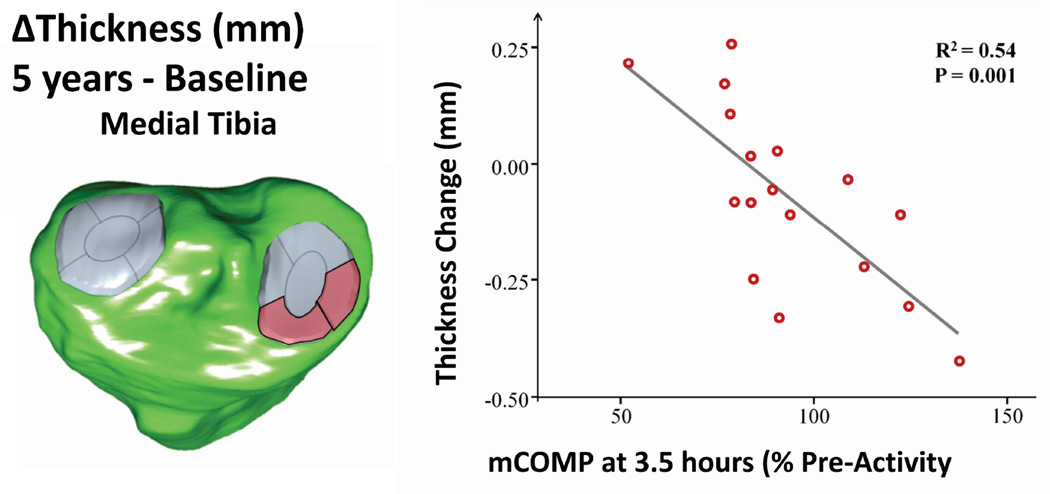
To illustrate a specific application consider the studies illustrated in Figure 4 that showed serum levels of COMP respond to a mechanical stimulus in manner that differentiates older healthy subjects and patients with knee OA from healthy younger subjects. A recent study19 used COMP as a surrogate biological component with a 30 minute walk as the mechanical component and cartilage change over 5 years as the structural component and thus reduced the conceptual systems model (Figure 1) to a practical framework with measurable components. The model was then used in a stimulus-response framework where the mechanical component (30 minute walk) was used to stimulate a biological response (change in COMP concentration from resting) with the structural component (cartilage thickness change over 5 years) investigated as the outcome. The result demonstrated that a mechanical stimulus can provoke a short-term biological response reflected by a change in serum concentrations of COMP. Most importantly the degree of cartilage thinning at 5-year follow-up was correlated with changes in COMP levels relative to resting concentrations 3.5 and 5.5 hours after the walking stimulus (Figure 8). It was important to note that resting levels of COMP were not correlated with cartilage changes. Thus using a biological response to a mechanical stimulus (mCOMP) can enhance the potential to develop methods for early detection of risk for progressing to advanced stages of OA. This example illustrates the potential for a systems approach to enhance our understanding of the pathogenesis of OA in a manner that can enable future improvements in early detection and treatment of the disease.
Figure 8.
The advantage of integrating the biological, mechanical and structural elements in a unified system is illustrated in a recent study19. The systems model was applied by combing a mechanical stimulus (30 min walk) with a biological response (change in COMP serum concentration), where the structural component was cartilage thickness. The results indicate that the change in anterior medial cartilage thickness (red) over 5 years was associated with the change in COMP in response to a mechanical stimulus (mCOMP).
Discussion and Conclusions
A unified view of the conditions common to the risk factors for knee OA emerged when a systems analysis of the risk factors for OA consolidated a number of observations from existing literature regarding the biological, mechanical and structural components associated with cartilage health. Specifically, there are kinematic changes common to each of the primary risk factors that appear prior to the development of clinical OA. Commonly the observation that kinematic changes do not occur in isolation but often occur with metabolic changes (bone and soft tissue) and changes in levels of inflammatory cytokines suggests that it is likely the interaction (Figure 1c) between these biological changes and the kinematic changes that determines the rate of progression to clinical OA. Finally the steepness of the thickness gradients in cartilage in the weight-bearing regions of the knee (Figure 3) indicates an individual’s risk for developing OA could be influenced by the nature of the gradients, where a steeper gradient might suggest greater sensitivity to kinematic changes.
The fact that there have been common biological, mechanical and structural changes associated with the primary risk factors for OA (aging, obesity and joint trauma) suggests that the diverse phenotypes of OA are not fundamentally different when viewed as a system. Rather the primary components of the disease are present to a greater or lesser extent in all manifestations of the disease. Thus the progression to clinical OA is dependent on the level of change of one or more of these components as well as the potential interaction between the components as the disease progresses (Figure 1c). The early detection of changes from normal of any of these components represents an opportunity to assess the risk for developing clinical OA as well as the potential to apply early interventions that can address the broad scope of the disease at a systems level.
The systems approach described here provides a basis for new approaches to the early detection of OA. One of the major impediments to bringing new treatments to the patient is the limited capacity to detect the risk of developing clinical OA at a stage where the course of the disease can be modified. Specifically, new approaches should consider the potential that each of the components illustrated in Figure 1 are present for all types of OA and the risk for the development of clinical OA is dependent on the relative weighting of each of these components in mitigating or exacerbating the risk for developing the disease.
Finally the systems approach provides methods to address the complexity of OA. While the complexity of each of the components cannot be completely defined at an in vivo systems level, the application of a stimulus-response model (Figure 8) provides an approach to address the complexity of OA. In summary, the systems analysis (Figure 1) approach described here can be an effective tool for analyzing the complexity of OA and opens opportunities to explore new methods for early detection, prevention and treatment. In addition, it provides a way to consolidate a number of clinical and fundamental observations from diverse disciplines on osteoarthritis in a way that provides new insights into the pathogenesis of the disease. (i.e. “...think what nobody has yet thought, about that which everybody sees…” - Schrödinger)
References
- 1.Agency for Healthcare Research and Quality. National and regional statistics in the national inpatient sample (2004–2006) Agency for Healthcare Research and Quality. 2006 [Google Scholar]
- 2.Andriacchi T, Osteoarthritis P. Probing knee OA as a system responding to a stimulus. Nat Rev Rheumatol. 2012 Apr;8(7):371–372. doi: 10.1038/nrrheum.2012.59. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009 Feb;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Muendermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004 Mar;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. Review. [DOI] [PubMed] [Google Scholar]
- 5.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013 Jan;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. Review. [DOI] [PubMed] [Google Scholar]
- 6.Bevill SL, Briant PL, Levenston ME, Andriacchi TP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage. 2009 Aug;17(8):980–987. doi: 10.1016/j.joca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Bevill SL, Boyer KA, Andriacchi TP. The regional sensitivity of chondrocyte gene expression to coactive mechanical load and exogenous TNF-α stimuli. J Biomech Eng. 2014 Sep;136(9) doi: 10.1115/1.4027937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazek K, Favre J, Asay J, Erhart-Hledik J, Andriacchi TP. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. J. Ortho Res. 2014 Mar;32(3):394–402. doi: 10.1002/jor.22530. [DOI] [PubMed] [Google Scholar]
- 9.Casper J, Berg K. Effects of Exercise on Osteoarthritis: A Review. J. Strength Conditioning Res. 1998 May;12(2):120–125. [Google Scholar]
- 10.Cheng Y, Macera CA, Davis DR, Ainsworth BE, Troped PJ, Blair SN. Physical activity and self-reported, physician-diagnosed osteoarthritis: Is physical activity a risk factor? J. Clin Epidemiol. 2000 Mar;53(3):315–322. doi: 10.1016/s0895-4356(99)00168-7. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009 Mar;61(3):344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 12.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012 Jun;14(3):212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu CR, Williams A, Tolliver D, Kwoh CK, Bruno S, 3rd, Irrgang JJ. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010 May;62(5):1412–1420. doi: 10.1002/art.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, Bruno S. Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2014 May 8; doi: 10.1177/0363546514532227. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, Dieppe PA. Risk Factors for the Incidence and Progression of Radiographic Knee Osteoarthritis. Arthritis Rheum. 2000 May;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Dayal N, Chang A, Dunlop D, Hayes K, Chang R, Cahue S, Song J, Torres L, Sharma L. The natural history of anteroposterior laxity and its role in knee osteoarthritis progression. Arthritis Rheum. 2005 Aug;52(8):2343–2349. doi: 10.1002/art.21277. [DOI] [PubMed] [Google Scholar]
- 17.DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. J. Biomech. 2003 Sep;36(9):1355–1362. doi: 10.1016/s0021-9290(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 18.Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996 Apr;325:10–18. doi: 10.1097/00003086-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Erhart-Hledik JC, Favre J, Asay JL, Smith RL, Giori NJ, Muendermann A, Andriacchi TP. A relationship between mechanically-induced changes in serum cartilage oliomeric matrix protein (COMP) changes in cartilage thickness after 5 years. Osteoarthritis Cartilage. 2012 Nov;20(11):1309–1315. doi: 10.1016/j.joca.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Favre J, Erhart-Hledik JC, Andriacchi TP. Age-related differences in sagittal-plane knee function at heel-strike of walking are increased in osteoarthritic patients. Osteoarthritis Cartilage. 2014 Mar;22(3):464–471. doi: 10.1016/j.joca.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favre J, Erhart-Hledik JC, Chehab E, Andriacchi TP. XXIV Congress of the International Society of Biomechanics. Brazil: 2013. Ambulatory kinematics correlates with future disease progression in medial osteoarthritis. [Google Scholar]
- 22.Favre J, Scanlan SF, Erhart-Hledik JC, Blazek K, Andriacchi TP. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. J. Biomech Eng. 2013 Oct;135(10):101002–101010. doi: 10.1115/1.4024629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favre J, Fasel B, Andriacchi TP. Pattern in femoral cartilage thickness map allows subtle scoring of medial compartment knee osteoarthritis severity. Osteoarthritis Cartilage. 2013 Apr;21(Supplement):S231–S232. [Google Scholar]
- 24.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30(8):914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 25.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013 Jan;21(1):10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003 Jan-Feb;31(1):75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 27.Gold GE, Thedens DR, Pauly JM, Fechner KP, Bergman G, Beaulieu CF, Macovski A. MR imaging of articular cartilage of the knee: new methods using ultrashort TEs. AJR Am J Roentgenol. 1998 May;170(5):1223–1226. doi: 10.2214/ajr.170.5.9574589. [DOI] [PubMed] [Google Scholar]
- 28.Gold GE, Hargreaves BA, Reeder SB, Block WF, Kijowski R, Vasanawala SS, Kornaat PR, Bammer R, Newbould R, Bangerter NK, Beaulieu CF. Balanced SSFP imaging of the musculoskeletal system. J Magn Reson Imaging. 2007 Feb;25(2):270–278. doi: 10.1002/jmri.20819. [DOI] [PubMed] [Google Scholar]
- 29.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, Reichmann WM, Losina E. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011 May;19(5):557–588. doi: 10.1016/j.joca.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo S, Andriacchi TP. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J. Biomech. 2007;40(13):2961–2966. doi: 10.1016/j.jbiomech.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005 Sep;13(9):782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Koo S, Rylander JH, Andriacchi TP. Knee joint kinematics during walking influences the spatial cartilage thickness distribution in the knee. J. Biomech. 2011 Apr;44(7):1405–1409. doi: 10.1016/j.jbiomech.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepus CM, Song JJ, Wang Q, Wagner CA, Lindstrom TM, Chu CR, Sokolove J, Leung LL, Robinson WH. Brief report: carboxypeptidase B serves as a protective mediator in osteoarthritis. Arthritis Rheumatol. 2014 Jan;66(1):101–106. doi: 10.1002/art.38213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Frank EH, Wang Y, Chubinskaya S, Huang HH, Grodzinsky AJ. Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-α and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthritis Cartilage. 2013 Dec;21(12):1933–1941. doi: 10.1016/j.joca.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Defrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weightbearing knee flexion. J Orthop Res. 2005 Mar;23(2):340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004 Oct;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 37.Meulenbelt I, Kraus VB, Sandell LJ, Loughlin J. Summary of the OA biomarkers workshop 2010 - genetics and genomics: new targets in OA. Osteoarthritis Cartilage. 2011 Sep;19(9):1091–1094. doi: 10.1016/j.joca.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muendermann A, King KB, Smith RL, Andriacchi TP. Change in serum COMP concentration due to ambulatory load is not related to knee OA status. J. Orthop Res. 2009 Nov;27(11):1408–1413. doi: 10.1002/jor.20908. [DOI] [PubMed] [Google Scholar]
- 39.Muendermann A, Dyrby C, Andriacchi T, King KB. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis Cartilage. 2005 Jan;13(1):34–38. doi: 10.1016/j.joca.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Netravali NA, Giori NJ, Andriacchi TP. Partial medial meniscectomy and rotational differences at the knee during walking. J. Biomech. 2010 Nov;43(15):2948–2953. doi: 10.1016/j.jbiomech.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Patra D, Sandell LJ. Recent advances in biomarkers in osteoarthritis. Curr Opin Rheumatol. 2011 Sep;23(5):465–470. doi: 10.1097/BOR.0b013e328349a32b. [DOI] [PubMed] [Google Scholar]
- 42.Quinn TM, Hauselmann HJ, Shintani N, Hunziker EB. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage. 2013 Oct;(13):S1063–S4584. 00969-2. [PubMed] [Google Scholar]
- 43.Rao C, Fitzpatrick CK, Rullkoetter PJ, Maletsky LP, Kim RH, Laz PJ. A statistical finite element model of the knee accounting for shape and alignment variability. Med Eng Phys. 2013 Oct;35(10):1450–1456. doi: 10.1016/j.medengphy.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Ryder JJ, Garrison K, Song F, Hooper L, Skinner J, Loke Y, Loughlin J, Higgins JP, MacGregor AJ. Genetic associations in peripheral joint osteoarthritis and spinal degenerative disease: a systematic review. Ann Rheum Dis. 2008;67:584–591. doi: 10.1136/ard.2007.073874. [DOI] [PubMed] [Google Scholar]
- 45.Scanlan SF, Favre J, Andriacchi TP. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J Biomech. 2013 Mar;46(5):849–854. doi: 10.1016/j.jbiomech.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 46.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J. Biomech. 2010 Jun;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellam J, Berenbaum F. Is Osteoarthritis a metabolic disease? Joint Bone Spine. 2013 Dec;80(6):568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, Torner J, Cooke TD, Hietpas J, Lynch J, Nevitt M. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010 Nov;69(11):1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shelbourne KD, Gray T. Minimum 10-year results after anterior cruciate ligament reconstruction: how the loss of normal knee motion compounds other factors related to the development of osteoarthritis after surgery. Am J Sports Med. 2009 Mar;37(3):471–480. doi: 10.1177/0363546508326709. [DOI] [PubMed] [Google Scholar]
- 50.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012 Jan;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007 Jan;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 52.Verbruggen G, Wittoek R, Vander Cruyssen B, Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann Rheum Dis. 2012 Jun;71(6):891–898. doi: 10.1136/ard.2011.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, Crish JF, Bebek G, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011 Nov;17(12):1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010 Apr;18(4):539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008 Jun;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 56.Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992 Sep;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]