Abstract
Understanding the cellular populations and mechanisms responsible for overcoming immune compartmentalization is valuable for designing vaccination strategies targeting distal mucosae. In this study we show that the human pathogen, Chlamydia trachomatis, infects the murine respiratory and genital mucosa and that T cells, but not antibodies, elicited through intranasal immunization can protect against a subsequent transcervical challenge. Unlike the genital infection where CD8+ T cells are primed, yet fail to confer protection, we found that intranasal priming engages both CD4+ and CD8+ T cells, allowing for protection against genital infection with C. trachomatis. The protection is largely dependent on IFNγ secretion by T cells. Moreover, different chemokine receptors are critical for C. trachomatis-specific CD4+ T cells to home to the lung, rather than the CXCR3 and CCR5-dependent migration observed during genital infection. Overall, this study demonstrates that the cross-mucosa protective immunity against genital C. trachomatis infection following intranasal immunization is not dependent on antibody response but is mediated by not only CD4+ T cells but also CD8+ T cells. This study provides insights for the development of vaccines against mucosal pathogens that threaten reproductive health worldwide.
INTRODUCTION
Mucosal surfaces serve as both entry and transmission routes for most pathogens. Therefore, an important aspect in designing effective vaccines against these pathogens is the stimulation of immunity at the relevant mucosal sites. Although there are studies suggesting that systemic immunization can provide mucosal protection (1), others have suggested that mucosal immunization is required for effective T cell-dependent mucosal immunity (2).
There are important distinctions between different mucosal tissues. For example, the lower respiratory and upper genital tracts are relatively sterile and intolerant of flora compared to the gastrointestinal tract. Another example is the distinctive lympho-epithelial structure of the intestinal Peyer’s patches, in contrast to the genital mucosa that lacks organized lymphoid elements. T cell migration among mucosal surfaces is also tightly regulated by the interaction of adhesion molecules and chemokine receptors that are differentially expressed on T cells and their target tissues (3, 4). For instance, skin-homing T cells express ligands for E- and P-selectins, as well as the chemokine receptors, CCR4 and CCR10 (5–7), while gut-homing effector and memory cells express the α4β7 integrin and CCR9 chemokine receptor (8, 9).
Despite these differences, the presence of shared immune elements between mucosal sites is also well recognized. For instance, other than well-described skin-homing properties, the E- and P-selectins are also involved in the migration of activated T cells to the peritoneal cavity during inflammation (6). Furthermore, the ability to use remote-site immunization to generate protective immunity at a distinct tissue also suggests that there are aspects of the immune system shared by various mucosal surfaces (10–12). Intranasal immunization with Neisseria gonorrhoeae, C. trachomatis or HIV antigens has been shown to confer some protection in the genital tract and the protection is correlated with mucosal antibody responses and sometimes heightened cell-mediated responses (10, 12, 13). However, it is not clear which of these elevated responses is responsible or sufficient for cross-mucosal protection.
Given its ability to infect several mucosal sites, Chlamydia trachomatis provides a unique opportunity to explore how tissue-specific immunity might be overcome. C. trachomatis is responsible for significant morbidity worldwide. Infection of the ocular epithelium causes blinding trachoma and infection of the genital mucosa can result in ectopic pregnancy and infertility (14–18). Moreover, if infection of pregnant women is not detected, perinatal transmission of C. trachomatis to the lungs of the newborn can ultimately result in pneumonia (19).
Using murine infection models, researchers have shown that although antibodies can provide limited protection against Chlamydia species (20, 21), the host response to C. trachomatis infection is primarily dependent on IFNγ (22–26). Both CD4+ and CD8+ T cells are stimulated during infection and secrete IFNγ. However, elimination of CD8+ T cell response does not appear to compromise protection against Chlamydia genital infection (20, 27, 28). In contrast, CD4+ T cells are both necessary and sufficient to confer protection against subsequent infection (22, 29). The signals that govern CD4+ T cell trafficking to the genital mucosa have not been completely elucidated but it is known that efficient migration of C. trachomatis-specific CD4+ T cells to the genital mucosa requires the expression of both CXCR3 and CCR5 (30). Here we explore the signals mediating homing of T cells to another mucosal site, the respiratory tract, and show that cross-mucosal protection can be induced in the genital tract through IFNγ secreted by not only CD4+ T cells but also CD8+ T cells.
MATERIAL AND METHODS
Mice
C57BL/6J, B6.PL-Thy1a/CyJ (CD90.1), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1), B6.129S2-Ighmtm1Cgn/J (µMT), B6.129S7-Ifngtm1Ts/J (IFNγ−/−), B6.129S7-Ifngr1tm1Agt/J (IFNγR−/−), B6.129P2-Ccr5tm1kuz/J (CCR5−/−), and B6.129P2-Cxcr3tm1Dgen/J (CXCR3−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME). NR1 transgenic mice expressing a TCR transgene specific for the C. trachomatis antigen Cta1133–152 have been described previously (25). CXCR3−/−CCR5−/− mice were generated by crossing CXCR3−/− and CCR5−/− mice. Mice were maintained within the Harvard Medical School Center for Animal Resources and Comparative Medicine. All experiments in this report were approved by Harvard’s Institutional Animal Care and Use Committee.
Growth, isolation, and detection of bacteria
C. trachomatis serovar L2 (434/Bu) was propagated within McCoy cell monolayers as previously described (30, 31). Aliquots of purified elementary bodies were stored at −80° C in medium containing 250 mM sucrose, 10 mM sodium phosphate, and 5 mM L-glutamic acid (SPG).
Infection of mice and preparation of tissue
For intranasal inoculation, mice were sedated with 5% isoflurane (Vedco Inc, St. Joseph, MO) in oxygen and inoculated with 40 µL SPG containing 105 IFU of C. trachomatis, unless otherwise stated, onto the external nares. For leukocyte quantification, lungs were perfused with 3 ml ice-cold PBS before harvest. Lungs were meshed in between microscope slides, enzymatically dissociated in HBSS/Ca2+/Mg2+ containing 1 mg/ml type XI collagenase and 50 Kunitz/ml DNase (Sigma) for 45 minutes at 37° C, and washed in PBS containing 5 mM EDTA.
To infect the genital tract, mice were treated subcutaneously with 2.5 mg medroxyprogesterone acetate (Pfizer Inc, New York, NY) and infected transcervically one week later as previously described (29). Briefly, 10 µl of SPG containing 5×106 IFU of C. trachomatis was deposited using the NSET pipet tip (ParaTechs, Lexington, KY). Uteri were minced with scalpels and enzymatically dissociated in HBSS/Ca2+/Mg2+ containing 1 mg/ml type XI collagenase and 50 Kunitz/ml DNase for 30 minutes at 37° C, washed in PBS containing 5 mM EDTA, and ground between microscope slides before filtration through a 70-µm mesh (32).
To determine C. trachomatis levels in systemic organs, peripheral blood was collected in 10% sodium citrate, lysed with 100 U mutamolysin, and processed with QIAamp DNA mini kit (Qiagen). Spleen, stomach, liver, uterus, and lymph nodes were homogenized by mechanical disruption before DNA extraction with the QIAamp DNA min kit (Qiagen).
Flow cytometry
After isolation, cells were immediately stained for surface and activation markers. For intracellular cytokine staining, cells were stimulated for 4–5 hours with 50 ng/ml of phorbol myristate acetate (PMA) (Enzo Life Sciences, Farmingdale, NY) and 500 ng/ml of ionomycin (EMD Millipore, Darmstadt, Germany) in the presence of brefeldin A (Biolegend, San Diego, CA). Antibodies were purchased from Biolegend except for CD16/CD32 (Bio X-Cell, West Lebanon, NH), anti-CD4 Qdot 605 (Invitrogen), and anti-IFNγ APC-Cy7 (BD Biosciences, San Jose, CA). Cells were pre-incubated with CD16/CD32 (2.4G2) before staining with fluorochrome-conjugated antibodies against CD4 (RM4-5), CD8 (53–6.7), CD90.1 (OX-7), CD90.2 (53–2.1), CD11b (M1/70), Gr1 (RB6-8C5), CD11c (N418), F4/80 (BM8), NK1.1 (PK136), TCRγδ (GL3), MHC I-Ab (AF6-120.1), CD127 (A7R34), CD62L (Mel-14), or Db/ASFVNPIYL (CrpA63–71) MHCI tetramer. A LIVE/DEAD fixable aqua dead cell stain kit (Invitrogen) was used to exclude dead cells. For intracellular staining, cells were permeabilized with a Cytofix/Cytoperm Plus Kit according to the manufacturer’s instructions (BD Biosciences), and stained with anti-IFNγ (XMG 1.2). The absolute cell number was determined using AccuCheck Counting Beads (Invitrogen). Data were collected on a LSRII (BD Bioscience) and analyzed using FlowJo (Tree Star Industries, Ashland, OR).
T cell or IFNγ depletion
All antibodies were from Bio X Cell. For T cell depletion immune mice were injected i.p. with 200 µg anti-CD4 (GK1.5), anti-CD8 (2.43), or isotype control (LTF-2) in 200 µl PBS, starting 5 days before secondary challenge and every other day after the secondary challenge. One, 3, and 5 days prior to secondary challenge, each mouse also received 10 µg of depleting or control antibodies transcervically in 10 µl of PBS. T cell depletion was confirmed in the SLOs and uterus by flow cytometry.
For IFNγ depletion, intranasally-immunized mice were injected i.p. with 250 µg of anti-IFNγ (XMG1.2), or isotype control (HRPN) in 200 µl PBS every other day starting 3 days before secondary challenge. XMG1.2 or HRPN antibodies (12.5 µg in 10 µl PBS) were also delivered transcervically on days 1 and 3 before secondary challenge. IFNγ depletion was confirmed in the sera by ELISA as previously described (33).
T cell or serum transfer
For transgenic T cell transfer, C. trachomatis-specific CD4+ T cells were isolated from the SLOs of NR1 mice. Recipient mice were injected intravenously (i.v.) with 105 cells one day before infection. For polyclonal T cell transfer, SLOs were isolated from naïve or immune mice, and homogenized into single cell suspensions. CD4+ or CD8+ T cells were isolated using mouse CD4 or CD8 negative isolation kits (Invitrogen). Isolated cells were labeled with 10 µM of carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) as previously described (22). Ptx treatment was performed as previously described (34). Unless otherwise stated, 5×106 CD4+ or 3×106 CD8+ T cells were injected i.v. into naïve mice 4 hours before transcervical infection. For serum transfer, sera were extracted from peripheral blood of naïve animals or intranasally-immunized animals. Two hundred µl of pooled serum was injected i.v. into naïve mice 4 hours before challenge.
Real-time PCR
Uterine and lung CD45.2+ cells were sorted from tissue single cell suspensions by magnetic columns (Miltenyi Biotec) and resuspended in TRIzol (Invitrogen). RNA was isolated using an RNAeasy mini kit (Qiagen, Valencia, CA) and then diluted to 4 ng/µl. Primers for chemokine receptors were CXCR3 forward-5′-GCCCTCACCTGCATAGTTGT-3′, reverse-5′-ATTGAGGCGCTGATCGTAGT-3′; CCR5 forward-5′-CGAAAACACATGGTCAAACG-3′, reverse-5′-GTTCTCCTGTGGATCGGGTA-3′; and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) forward-5′-GGTGCTGAGTATGTCGTGGA-3′, reverse-5′-CGGAGATGATGACCCTTTTG-3′. Real-time PCR was conducted using the Quantitect SYBR Green reverse transcriptase-PCR kit (Qiagen) on an ABI prism 7000 sequence detection system (Applied Biosystems, Carlsbad, CA). The data were analyzed using the ΔΔCt method, and expression of all genes was normalized against GAPDH. Fold induction was calculated based on expression of uninfected tissue.
Quantitative PCR
The levels of C. trachomatis in the tissue were quantified using a previously described quantitative PCR assay (35). Briefly, total nucleic acid from tissue homogenates was prepared using the QIAamp DNA mini kit (Qiagen). Chlamydia 16S DNA and mouse GAPDH DNA content was quantified using primer pairs and dual-labeled probes (IDT, San Jose, CA, or Applied Biosystems).
Statistical analysis
A two-tailed Mann-Whitney U test was applied to determine the statistical significance for bacterial burden among groups. All other data were evaluated with an unpaired two-tailed t test. P values < 0.05 were considered statistically significant. Representative results from at least two experiments were shown as mean ± standard error.
RESULTS
C. trachomatis infects the lungs of mice and stimulates a potent immune response
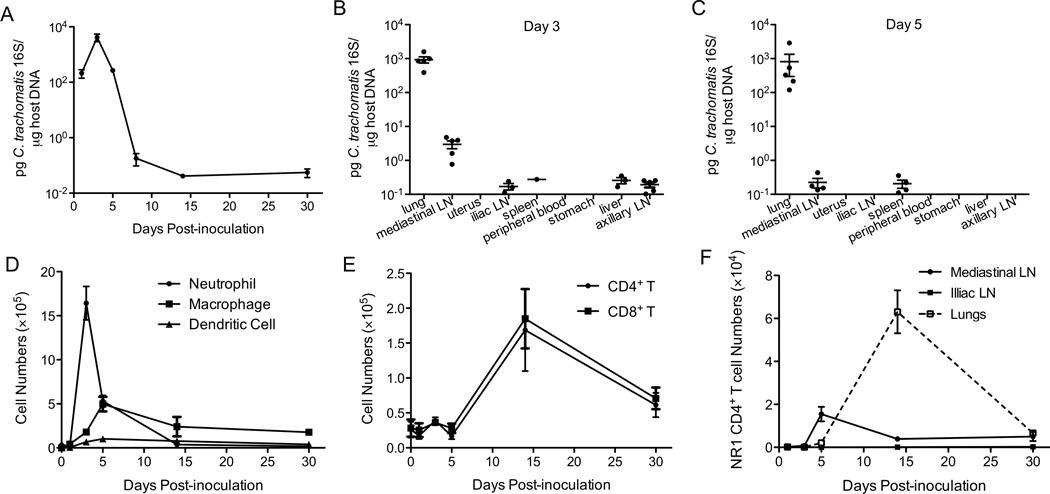
To characterize C. trachomatis replication in the lungs of C57BL/6 (wt) mice following intranasal inoculation, we first inoculated mice with 106 IFU of C. trachomatis, a dose that efficiently infects uterine tissues (29). Three days post-inoculation (p.i.), some mice showed symptoms such as ruffled fur, and diminished responsiveness. By day 11 p.i., 70% of inoculated mice succumbed to C. trachomatis infection (data not shown). When we inoculated mice intranasally with 105 IFU of C. trachomatis, none of the mice showed signs of disease. In these mice, C. trachomatis replicated in the lungs during the first three days p.i, but bacterial burden then declined to below the limit of detection by day 14 (Figure 1A). We then determined whether intranasal infection disseminated to the lymphatic tissues or other organs by measuring bacterial burden in the blood, spleen, liver, stomach, uterus, and lymph nodes of infected mice. We detected almost no C. trachomatis 16S DNA in these organs, except for some minimal levels in the mediastinal lymph node on day 3 but not day 5 (Figure 1B and 1C). This might be due to active antigen presentation in the lymph nodes draining the lung. Taken together, these data indicate that intranasal infection of C. trachomatis is mostly restricted to the respiratory tract and does not seem to replicate or spread to other organs.
Figure 1.
C. trachomatis replicates in the lungs and induces an effective immune response. Groups of 5 C57BL/6 mice were intranasally infected with C. trachomatis. (A) On the indicated days p.i., bacterial burden in the lungs was determined by quantitative PCR. (B) On day 3 and (C) day 5 p.i., bacterial burden was determined in the indicated organs by quantitative PCR. (D) Absolute numbers of neutrophils (Gr1hiCD11bhi), macrophages (CD11b+F4/80+), dendritic cells (CD11c+MHCII+), (E) CD4+ T cells (CD4+CD3+NK1.1), and CD8+ T cells (CD8+CD3+NK1.1−) in the lungs were determined by flow cytometry. (F) Chlamydia-specific CD90.1+ NR1 CD4+ T cells were transferred into CD90.2+ mice one day prior to intranasal infection. On the indicated days p.i., the numbers of NR1 CD4+ T cells in the lungs, mediastinal lymph nodes, and iliac lymph nodes were determined by flow cytometry.
To further characterize the immune response after intranasal inoculation, we examined the recruitment of inflammatory cells to the lungs. Recruitment of neutrophils to the lungs peaked on day 3 p.i.; macrophage and dendritic cell numbers peaked on day 5 p.i. and declined thereafter (Figure 1D), indicating an active myeloid-lineage cell infiltration. Both CD4+ and CD8+ T cell numbers peaked on day 14 p.i in the lungs (Figure 1E). To assess the infiltration of C. trachomatis-specific CD4+ T cells into the lungs, we transferred C. trachomatis-specific CD4+ TCR transgenic T cells (NR1) into naïve mice and challenged these mice intranasally. NR1 cell numbers in the mediastinal lymph nodes peaked around day 5 p.i. (Figure 1F). Similar to bulk CD4+ T cells, pathogen-specific CD4+ T cells peaked on day 14 p.i. in the lungs (Figure 1F). Overall, these data indicate that C. trachomatis can infect the respiratory tract and induce an immune response that controls the infection.
Intranasal immunization with C. trachomatis confers protection against secondary challenge in the genital tract
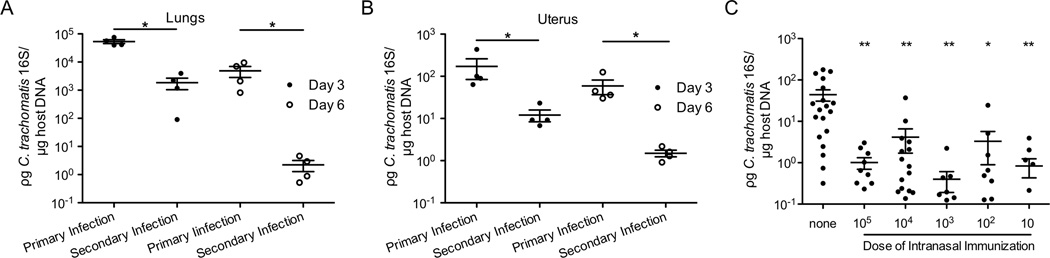
To determine whether intranasal infection confers protection against secondary intranasal infection, we rechallenged intranasally-inoculated mice a month later in the lung. These mice demonstrated significantly less C. trachomatis burden in their lungs than non-immunized mice (Figure 2A), indicating that intranasal infection conferred protection against secondary challenge in the same tissue. We then examined whether intranasal immunization confers cross-mucosal protection against C. trachomatis genital infection. Three and 6 days after secondary transcervical infection, intranasally-immunized mice had less C. trachomatis burden than non-immunized mice (Figure 2B), indicating that intranasal immunization conferred cross-mucosal protection against genital infection. To determine whether a lower intranasal immunization dose would be sufficient to confer cross-mucosal protection, we immunized mice intranasally with 105, 104, 103, 102 and 10 IFU of C. trachomatis. Three days after secondary challenge, all the intranasal-immunized mice had significantly less bacterial burden than the non-immunized mice (Figure 2C). Taken together, these data indicate that intranasal immunization of C. trachomatis, with as few as 10 IFU, confers protection against genital infection.
Figure 2.
Intranasal immunization with C. trachomatis confers protection against secondary challenge. Groups of 4 C57BL/6 mice were intranasally infected with C. trachomatis. Four weeks later, these mice and naïve mice were rechallenged (A) intranasally or (B) transcervically. Three and six days after rechallenge, tissues were harvested and bacterial burden was determined by quantitative PCR. (C) Goups of 5–19 C57BL/6 mice were intranasally immunized with indicated doses of C. trachomatis. Four weeks after immunization, these mice and naïve mice were challenged in the genital tract. Five days after genital challenge, bacterial burden was determined by quantitative PCR. The data are representative of 3 independent experiments.*: p ≤ 0.05. **: p ≤ 0.01.
Antibodies are not necessary for cross-mucosal protection against C. trachomatis
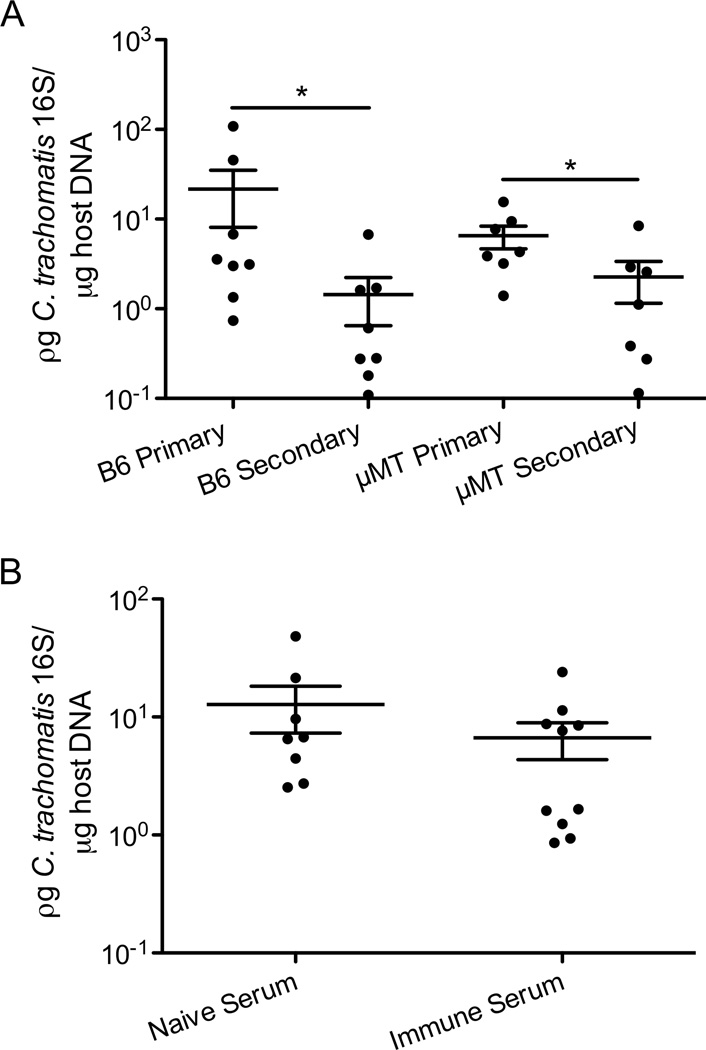
It has been shown that in B-cell-deficient (µMT) mice, C. muridarum clearance after secondary vaginal challenge is slightly delayed (21, 36). Furthermore, previous studies have demonstrated that the cross-mucosal protection against C. muridarum genital tract infection following intranasal immunization is associated with elevated antibody titers (12, 37). To test whether the cross-mucosal protection is antibody dependent, intranasally-immunized wt and µMT mice were rechallenged transcervically with C. trachomatis. Interestingly, both wt and µMT mice had lower bacterial burden in their uteri than non-immunized mice (Figure 3A), demonstrating that mature B cells are not required for cross-mucosal protection. To further confirm these results, we transferred serum from intranasally-immunized mice into naïve mice before transcervical challenge. Similar levels of C. trachomatis were detected in mice that received immune serum vs. naïve serum (Figure 3B), indicating that immune serum from intranasally-immunized mice is not sufficient to confer cross-mucosal protection. Together these data showed that B cells did not mediate the cross-mucosal protection against C. trachomatis.
Figure 3.
Mature B cells are not necessary for cross-mucosal protection against C. trachomatis. (A) Groups of 7–8 C57BL/6 and µMT mice were intranasally immunized with C. trachomatis. Four weeks after immunization, these mice, naïve C57BL/6, and naïve µMT mice were challenged in the genital tract. Five days later, bacterial burden was determined by quantitative PCR. (B) Groups of 7–8 C57BL/6 mice were intranasally immunized. Four weeks later, serum was extracted from peripheral blood of these mice and naïve mice. Immune or naïve sera were injected i.v. into naïve mice. These mice were then infected in the genital tract and 5 days later bacterial burden was determined by quantitative PCR. These data are representative of 2 independent experiments. *: p ≤ 0.05.
T cells are required for cross-mucosal protection against C. trachomatis, and either CD4+ or CD8+ T cells are sufficient
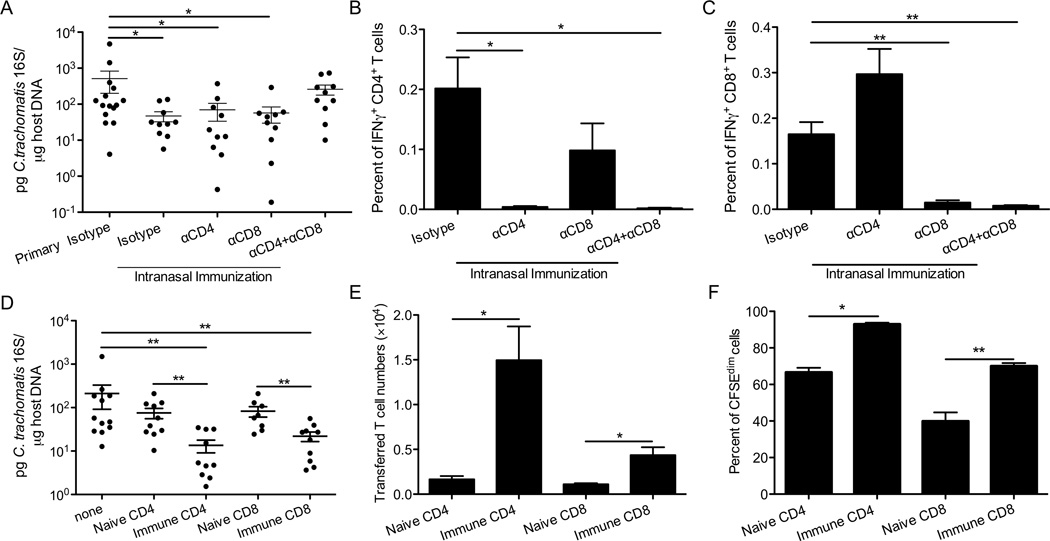
Since CD4+ T cells are required and sufficient for protection against C. trachomatis rechallenge in the uterus (29), we hypothesized that CD4+ T cells might also be responsible for the cross-mucosal protection. To test this, we treated intranasally-immunized mice with isotype control antibodies or antibodies to deplete CD4+ T cells both before and throughout the transcervical challenge. Surprisingly, CD4+ T cell-depleted mice remained protected against C. trachomatis (Figure 4A). These results suggested that unlike priming in the genital tract, priming in the respiratory tract stimulates a protective T cell response in the genital mucosa that is independent of CD4+ T cells. Since IFNγ plays a pivotal role in C. trachomatis growth restriction and both CD4+ T and CD8+ T cells in the uteri are independently capable of producing IFNγ (Figure 4B and 4C), it was possible that CD8+ T cells were compensating for the lack of CD4+ T cells and mediating cross-mucosal protection. To test this, mice were primed intranasally and treated with neutralizing antibodies for both CD4+ and CD8+ T cells. These mice had significantly more C. trachomatis burden compared with isotype-control-treated mice (Figure 4A), indicating that a T cell population was required for the cross-mucosal protection against C. trachomatis.
Figure 4.
T cells are required and sufficient for cross-mucosal protection against C. trachomatis. Groups of 10–15 C57BL/6 mice were intranasally immunized with C. trachomatis, rested for 4 weeks, and then treated with the neutralizing or isotype control antibodies as indicated. These mice and naïve mice that received isotype control antibodies (primary isotype) were then challenged in the genital tract. Six days after challenge, (A) bacterial burden was determined by quantitative PCR. (B) The percent of IFNγ+CD4+CD3+ or (C) IFNγ+CD8+CD3+ T cells among live cells from uterine tissues was determined by intracellular staining and flow cytometry. (D-F) CD90.1+ mice were immunized intranasally with C. trachomatis. A month later, CD4+ or CD8+ T cells were purified from SLOs of these mice or naïve control mice, labeled with CFSE, and transferred into groups of 8–12 CD90.2+ mice. These mice and mice that did not receive donor cells (none) were then challenged in the genital tract. Five days after challenge, (D) bacterial burden was determined by quantitative PCR. (E) Absolute number of donor cells and (F) percent of donor cells that diluted CFSE were determined by flow cytometry. These data are representative of at least 3 independent experiments. *: p ≤ 0.05. **: p ≤ 0.01.
Since mice that received CD4+ or CD8+ T cell depletion treatment but not both were protected against secondary genital challenge (Figure 4A), we hypothesized that CD4+ or CD8+ T cells alone are sufficient to confer cross-mucosal protection. To test this, CD4+ or CD8+ T cells were isolated from intranasally-immunized mice and naïve mice, CFSE labeled, and transferred into naïve hosts 4 hours before C. trachomatis transcervical challenge. We observed a significant reduction of C. trachomatis burden in mice that received immune but not naïve CD4+ or CD8+ T cells compared with control mice that did not receive any T cells (primary) (Figure 4D). These data indicate that CD4+ or CD8+ T cells alone from intranasally-primed mice are sufficient to mediate protection, and this protection is not simply due to increased T cell numbers since naïve T cells have no protective effect (Figure 4D). Similar numbers of naïve and immune T cells were recovered from draining lymph nodes (data not shown), and a similar proportion of naïve and immune T cells in the lymph nodes proliferated as indicated by CFSE dilution (data not shown). Interestingly, significantly more intranasally-primed CD4+ or CD8+ T cells reached the upper genital tract (Figure 4E) and were actively proliferating compared to naïve T cells (Figure 4F). These results suggest that intranasal immunization enables both CD4+T and CD8+T cells to more efficiently enter the upper genital tract and engage in proliferation and protection. In addition, when mice were immunized in the respiratory tract with a lower dose, 100 IFU, immune CD4+ or CD8+ T cells were still sufficient to confer protection against transcervical infection (data not shown).
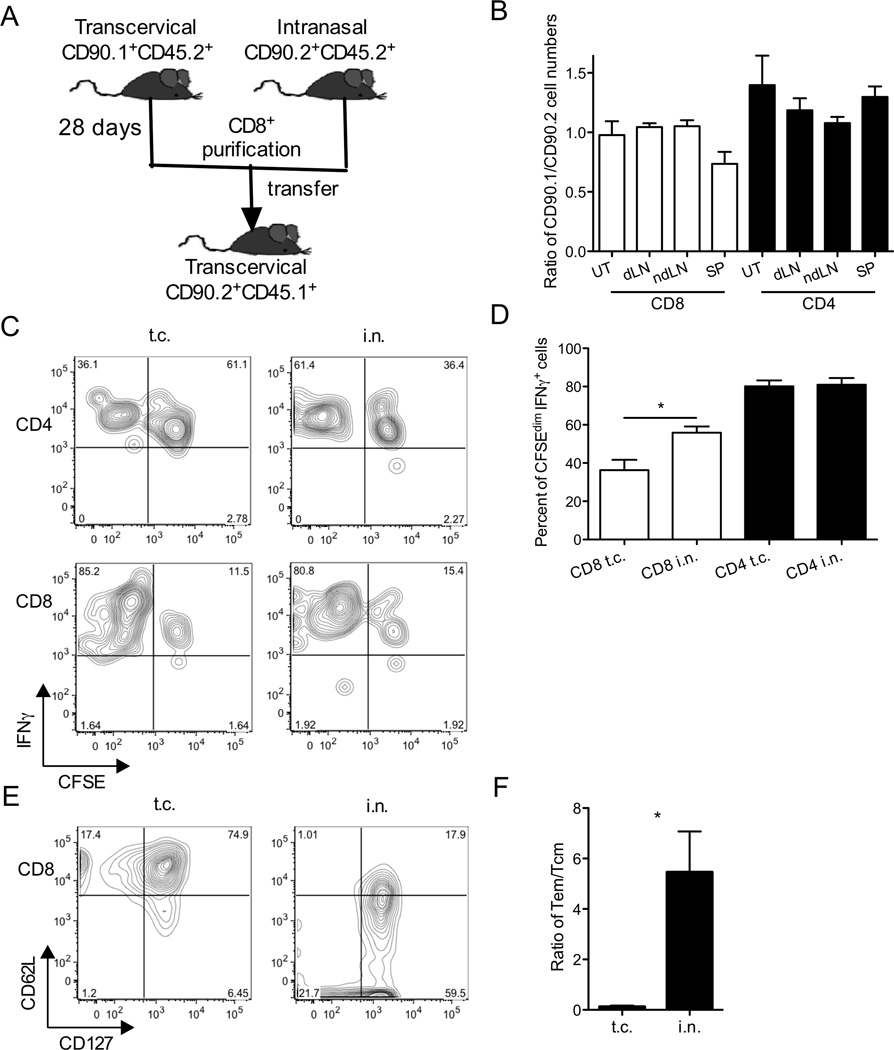
Based on previous T cell depletion and transfer studies, CD8+ T cells from mice immunized in the genital tract with C. trachomatis were not required or sufficient to confer protection (27, 29). However, as shown here, CD8+ T cells from C. trachomatis intranasally-primed mice are sufficient to confer protection (Figure 4D). As a first step towards understanding the differential protective phenotypes of transcervically- vs. intranasally-primed CD8+ T cells, we compared the ability of immune CD8+ T cells primed transcervically or intranasally to migrate to the genital mucosa and proliferate in a sensitive co-transfer experiment (Figure 5A). CD8+ T cells were isolated from SLOs of CD90.1/CD45.2 transcervically-primed or CD90.2/CD45.2 intranasally-primed mice, CFSE-labeled, and mixed 1:1 before transfer into CD45.1 hosts, which were then transcervically infected. A control group received 1:1 mixture of intranasally-primed and transcervically-primed CD4+ T cells. Five days p.i., we recovered similar numbers of intranasally-primed and transcervically-primed T cells from all examined organs (Figure 5B). These results suggested that T cells from SLOs of intranasally-primed and transcervically-primed donors have a similar ability to migrate to the genital tract following transcervical infection. A similar proportion of CD4+ T cells from transcervically-primed or intranasally-primed donors proliferated (CFSEdim) and was able to secrete IFNγ (Figure 5C and 5D). Interestingly, significantly more intranasally-primed CD8+ T cells proliferated and secreted IFNγ compared to their transcervically-primed counterparts (Figure 5C and 5D). These results suggest that compared with transcervical priming, intranasal priming engages a larger proportion of CD8+ T cells to proliferate and secrete IFNγ in response to transcervical C. trachomatis infection. When we compared C. trachomatis-specific memory CD8+ T cells primed either intranasally or transcervically, we observed an enrichment of effector memory T cells (Tem) in intranasally primed CD8+T cells when compared to transcervically primed CD8+T cells (Figure 5E and 5F). Since Tem cells are thought to provide immediate effector function at the portal of pathogen entry (38), the downregulation of CD62L on intranasally-primed pathogen-specific memory CD8+ T cells might contribute to their better effector function and superior protective capacity upon secondary challenge in the distal mucosa.
Figure 5.
Intranasally-primed CD8+ T cells proliferate more and produce more IFNγ than transcervically-primed CD8+ T cells in the genital tract. (A) Experimental scheme of co-transfer experiment. A control group received a 1:1 mixture of transcervialy-primed (CD90.1+) and intranasally-primed (CD90.2+) CD4+ T cells. B-D) Five days after challenge, uteri (UT), spleens (SP), draining iliac lymph nodes (dLNs), and inguinal non-draining lymph nodes (ndLNs) were harvested. (B) Ratios of CD90.1+ and CD90.2+ donor cells among organs, (C) representative flow plots showing CFSE and IFNγ gating, and (D) percent of CFSEdim IFNγ+ T cells among live donor cells in the uterine tissues were determined by intracellular cytokine staining and flow cytometry. (E) CD127 and CD62L expression and (F) ratio of Tem(CD127+CD62Llow)/Tcm(CD127+CD62Lhigh) among CrpA63–71+ CD8+ T cells from spleens of transcervically and intranasally immunized mice were determined on day 22 p.i. These data are representative of 2 independent experiments.*: p ≤ 0.05.
CXCR3 and CCR5 are dispensable for cross-mucosal protection against C. trachomatis
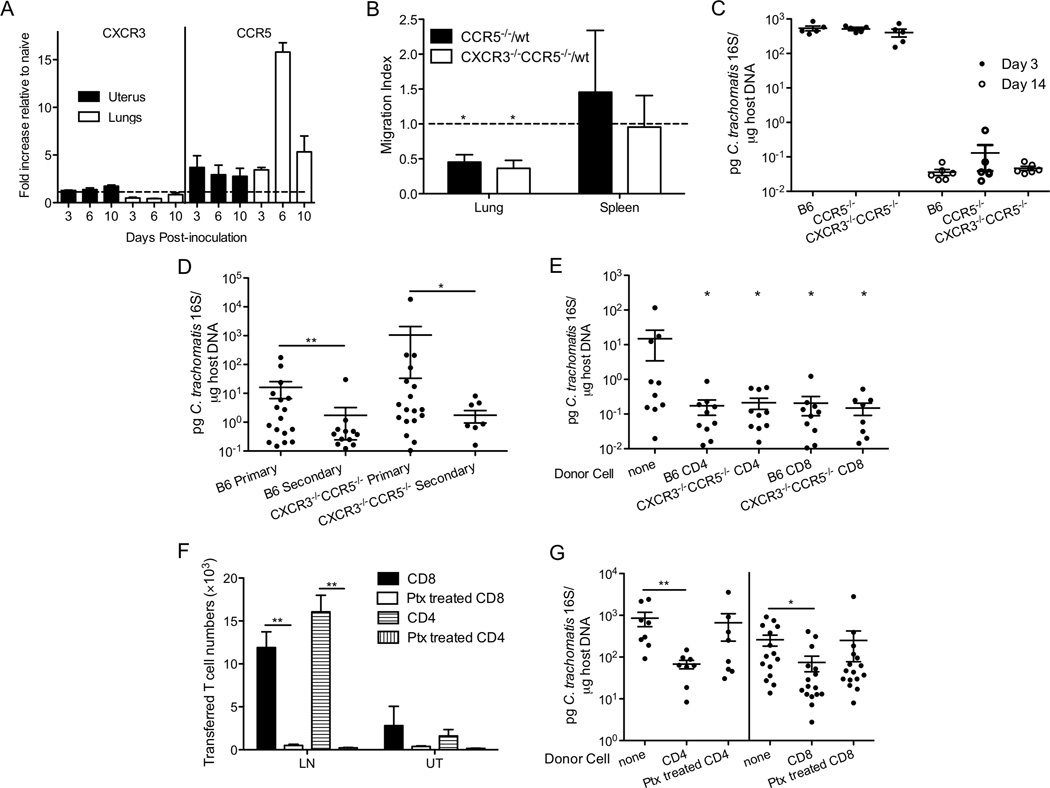
A previous study from our lab has shown that CXCR3 and CCR5 cooperatively direct Chlamydia-specific CD4+ T cell into the genital mucosa (30). Interestingly, CXCR3 but not CCR5 directs antigen-specific CD4+ T cells into the lungs during parainfluenza virus infection (39). To determine whether CXCR3 and CCR5 contribute to T cell homing during C. trachomatis intranasal infection and whether they are involved in cross-mucosal protection, we tested whether CXCR3 and CCR5 are up-regulated on leukocytes during C. trachomatis respiratory tract infection. Consistent with the previous study, both CXCR3 and CCR5 were up-regulated on uterine leukocytes following C. trachomatis genital infection (Figure 6A) (30). In contrast, only CCR5 but not CXCR3 expression was induced on lung leukocytes following C. trachomatis infection (Figure 6A). We did not observe significant up-regulation of other chemokine receptors, including CCR4 that was recently identified to be involved in lung-homing of T cells (data not shown) (40). To determine whether CCR5 contributes to the homing of C. trachomatis-specific CD4+ T cells to the lungs during intranasal infection, we conducted a sensitive competitive homing assay that monitors the ability of wt and chemokine-receptor-deficient transgenic T cells to home to the lungs under the same conditions. We transferred 1:1 mixture of NR1 T cells from wt and chemokine-receptor-deficient transgenic mice before intranasal infection. At the peak of T cell recruitment, we determined the migration index of the transferred T cells in the lungs. Statistically, the migration index was 1 in the spleens. However, in the lungs, the migration indices of both CCR5−/− and CXCR3−/−CCR5−/− NR1 T cells were significantly lower than 1 (Figure 6B), indicating that CCR5 contributes to the homing of C. trachomatis-specific CD4+ T cells into the lungs. Moreover, since the migration index of CCR5−/− and CCR5−/−CXCR3−/− T cells were similar, CXCR3 does not seem to have an additive or antagonistic impact on the contribution of CCR5 to T cell trafficking. Although CCR5 contributes to the migration of C. trachomatis-specific CD4+ T cells into the lungs during C. trachomatis respiratory infection, CCR5−/− and CXCR3−/−CCR5−/− mice had similar bacterial burden in their lungs compared to wt mice (Figure 6C), suggesting that these chemokine receptors are not required for C. trachomatis clearance in the lungs.
Figure 6.
CCR5 contributes to Chlamydia-specific CD4+ T cell recruitment to the lungs but is not required for T cell trafficking during cross-mucosal protection. (A) CXCR3 and CCR5 mRNA levels in the lungs or uteri of infected mice were determined by real-time PCR and shown as fold increase relative to naïve tissues (n=2–3). (B) Chlamydia-specific CD45.2+CD90.1+ wt NR1 CD4+ T cells were mixed 1:1 with CD45.2+CD90.2+ chemokine receptor deficient (CCR5−/− or CXCR3−/−CCR5−/−) NR1 CD4+ T cells, and transferred into groups of 5 CD45.1+ mice. The following day these mice were intranasally infected with C. trachomatis. On day 14 p.i., the ratio of transferred wt/knockout cells was determined by flow cytometry and shown as a migration index. (C) Groups of 5–6 C57BL/6, CCR5−/−, and CXCR3−/−CCR5−/− mice were intranasally inoculated and bacterial burden in the lungs was determined 3 and 14 days p.i. (D) Groups of 11–18 C57BL/6 and CXCR3−/−CCR5−/− mice were intranasally immunized with C. trachomatis. A month later, these mice and naïve mice were challenged in the genital tract. Five days after challenge, bacterial burden was determined by quantitative PCR. (E) C57BL/6 and CXCR3−/−CCR5−/− mice were intranasally immunized with C. trachomatis. A month later, CD4+ or CD8+ T cells were sorted from their SLOs and transferred into groups of 8–10 CD90.1+ naïve mice. These mice were then challenged transcervically and bacterial burden was determined 5 days post-challenge by quantitative PCR. (F, G) CD90.1+ mice were intranasally immunized with C. trachomatis. A month later, CD4+ or CD8+ T cells were sorted from the SLOs of these mice, left untreated or treated with pertussis toxin, and transferred into groups of 8–16 CD90.2+ naïve mice. These mice were then challenged transcervically. Three days later, (F) the number of transferred T cells in the uterus (UT) and lymph node (LN) was determined by flow cytometry and (G) bacterial burden was determined by quantitative PCR. These data are representative of at least 3 independent experiments. *: p ≤ 0.05. **: p ≤ 0.01.
Since CXCR3 and CCR5 are required for T-cell-mediated protection against C. trachomatis primary transcervical infection (30), we examined whether these chemokine receptors are also involved in cross-mucosal protective immunity. To test this, intranasally-immunized wt and CXCR3−/−CCR5−/− mice were subsequently challenged in the genital tract. These mice were all protected against genital challenge (Figure 6D), indicating that CXCR3 and CCR5 are dispensable for cross-mucosal protection against C. trachomatis. Although chemokine-receptor-deficient mice efficiently reduced C. trachomatis transcervical burden following intranasal immunization, it is still possible that only one of the T cell populations requires these chemokine receptor to mediate cross-mucosal protection considering that CD4+ or CD8+ T cells alone are sufficient to mediate protection (Figure 4D). To address this, we transferred CD4+ and CD8+ T cells from intranasally-immunized wt and CXCR3−/−CCR5−/− mice into naïve hosts before transcervical challenge. Five days after challenge, CXCR3−/−CCR5−/− CD4+ and CD8+ T cells migrated to the genital tract and proliferated as efficiently as wt T cells (data not shown). Similar to wt CD4+ and CD8+T cells, transferred CXCR3−/−CCR5−/− CD4+ and CD8+ T cells conferred cross-mucosal protection (Figure 6E). Taken together these data suggest that CD4+ and CD8+ T cells mediate cross-mucosal protective immunity against C. trachomatis in a CXCR3 and CCR5-independent manner.
To determine whether chemokine receptors other than CXCR3 and CCR5 contribute to the ability of intranasally-primed T cells to mediate cross-mucosal protection, we isolated T cells from intranasally-immunized mice and treated these cells with pertussis toxin (Ptx) to block Gi-receptor-coupled signaling downstream of most chemokine receptors. Treated or untreated immune T cells were transferred into naïve mice, which were then challenged transcervically with C. trachomatis. Since Ptx treatment efficiently blocks Gi-receptor-coupled signaling for 3 days (34), we determined the number of transferred T cells and bacterial burden 3 days after transcervical challenge. We recovered almost no Ptx-treated donor cells in the draining lymph nodes, suggesting that the ex vivo Ptx treatment was efficient in blocking the chemokine receptor signaling necessary for T cell trafficking to the lymph nodes (Figure 6F). Although the accumulation of treated cells in the uterine tissues was not statistically different from the accumulation of untreated cells, we did observe a trend towards fewer Ptx-treated cells than untreated cells (Figure 6F), suggesting that a Ptx-sensitive chemokine receptor(s) might contribute to the trafficking of intranasally-primed T cell into genital mucosa. Moreover, mice that received Ptx-treated T cells were no longer protected against C. trachomatis challenge in the genital tract (Figure 6G), indicating that some Ptx-sensitive Gi-receptor-coupled signaling is required for cross-mucosal protection.
IFNγ produced by CD4+ and CD8+ T cells is required for cross-mucosal protection against C. trachomatis
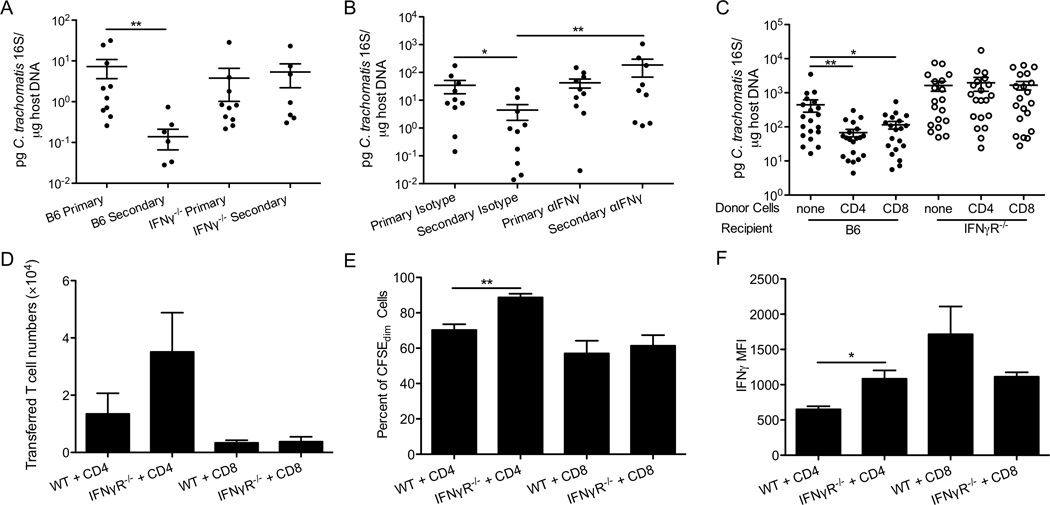
Since IFNγ plays a key role in controlling Chlamydia genital infection and activated CD4+ and CD8+ T cells are both potent producers of IFNγ (24–26, 41), we hypothesized that IFNγ produced by intranasally-primed T cells is responsible for cross-mucosal protection. To test this, we immunized wt and IFNγ−/− mice intranasally with 100 IFU of C. trachomatis and then challenged these mice transcervically. We chose this immunization dose because IFNγ−/− mice survive this challenge dose and yet this dose is sufficient to confer protection in the wt mice (Figure 2C). Immunized IFNγ−/− mice were not protected from challenge, showing similar levels of bacterial burden to non-immunized IFNγ−/− mice (Figure 7A). To additionally explore the role of IFNγ during secondary challenge while allowing normal development of immunity during priming, we treated intranasally-immunized mice before and throughout the secondary genital challenge with antibodies to neutralize IFNγ. Immunized mice that received neutralizing antibodies were not protected against C. trachomatis since the bacterial levels were similar to non-immunized mice and higher than immunized mice treated with the isotype control antibody (Figure 7B). Taken together these data demonstrate that IFNγ is required for cross-mucosal protective immunity against C. trachomatis.
Figure 7.
T cell-mediated cross-mucosal protection against C. trachomatis is dependent on IFNγ. (A) Groups of 6–10 C57BL/6 and IFNγ−/− mice were intranasally immunized with 100 IFU of C. trachomatis. A month later, these mice and naïve mice were challenged in the genital tract. Bacterial burden was determined 3 days post-challenge by quantitative PCR. (B) Groups of 9–10 C57BL/6 mice were intranasally immunized with C. trachomatis. A month later, these mice and naïve mice were treated with IFNγ-neutralizing or isotype control antibodies, and then challenged in the genital tract. Bacterial burden was determined by quantitative PCR 5 days after challenge. (C-F) Groups of 19–20 CD90.1+ mice were intranasally immunized with C. trachomatis. A month later, CD4+ or CD8+ T cells were purified from their SLOs, labeled with CFSE, and transferred into CD90.2+ wt or IFNγR−/− mice. These mice and control mice that did not receive any T cells were then challenged in the genital tract. Five days later, (C) bacterial burden was determined by quantitative PCR. (D) Absolute number of donor cells, identified as CD90.1+CD4+ or CD90.1+CD8+, (E) percent of donor cells that were CFSEdim, and (F) IFNγ MFI of donor cells in the uterine tissues were determined by intracellular staining and flow cytometry. These data are representative of 2 independent experiments. *: p ≤ 0.05. **: p ≤ 0.01
To determine whether IFNγ produced by T cells is required for cross-mucosal protection, CD4+T and CD8+T cells from intranasally-immunized mice were transferred into wt or IFNγR−/− hosts. We then challenged the mice to determine their ability to clear genital infection. As expected, transferred immune T cells were able to significantly reduce C. trachomatis burden in wt mice (Figure 7C). In contrast, the bacterial levels in IFNγR−/− mice that received immune CD4+ or CD8+ T cells were similar to mice that did not receive any T cells (Figure 7C). Thus, the protective effect of transferred CD4+ and CD8+ T cells requires that the host is able to respond to IFNγ. The lack of protection observed in IFNγR−/− mice was not due to a deficit of CD4+T or CD8+T cell infiltration or function since similar amount of transferred T cells accumulated, proliferated, and produced similar levels of IFNγ in the uteri of wt and IFNγR−/− mice (Figures 7D-F). The increase in CD4+ T cell proliferation and IFNγ secretion in IFNγR−/− mice might be due to higher bacterial burden in these mice. Overall these results suggest that IFNγ produced by intranasally-primed CD4+ and CD8+ T cells is required for cross-mucosal protection against genital C. trachomatis infection.
DISCUSSION
Antibody-response-associated cross-mucosal protection is well documented (10, 12, 13). Here we took advantage of the ability of C. trachomatis to infect both the murine respiratory and genital tracts to ask whether cross-mucosal protection can be elicited using a pathogen where protection is mainly T-cell-mediated. Our findings demonstrate that intranasal immunization with C. trachomatis induces cross-mucosal protection against a subsequent transcervical challenge. Although we cannot completely rule out the role of B cells in fine-tuning this process, antibodies are dispensable for the cross-mucosal protection against transcervical C. trachomatis infection. In contrast, T cells are necessary for cross-mucosal protection. Moreover, either CD4+ T cells or CD8+ T cells are sufficient to mediate this protection through IFNγ secretion. Although a previous report has shown that TNFα secreted by CD8+ T cells contributes to pathology in mice intravaginally inoculated with C. muridarum (42) and we did observe TNFα production by CD8+ T cells, we did not observe increased pathology 30 days following secondary genital challenge with C. trachomatis (data not shown).
Previous studies have shown that intranasal immunization with either adjuvanted Chlamydia proteins or live bacteria confers protection against a genital challenge, and that protection was correlated with heightened antibody titers and IFNγ secretion (12, 37, 43). However, none of these studies tested specifically which arm of the adaptive immune system (antibodies or T cells) is required or sufficient for the cross-mucosa protection. Only with our experiments using neutralizing reagents specifically during secondary challenge and T cell transfer into IFNγ-receptor-deficient animals can we clearly demonstrate the requirement and sufficiency of T-cell-secreted IFNγ for cross-mucosal protection. The obvious question arises as to what enables intranasally-primed T cells to mediate protection in the genital tract. The upper genital tract and lower respiratory tract resemble the liver and central nervous systems where even commensal bacteria cannot be tolerated. Moreover, in contrast to the gut mucosa, the uterus and lung lack organized lymphoid elements in healthy individuals (44–46). Therefore, the induction of immunity to respiratory and genital tract by pathogens must occur outside of the tissue followed by recruitment of recirculating cells into infected sites. Previous studies have shown that T cells activated through systemic or mucosal vaccination have the capacity to traffic widely to mucosal tissues and differentiate into protective memory T cells (38, 47–49). However, these studies used viral vaccines that were either given systemically or later disseminated systemically. This suggests that antigen spread might be critical in these cases to form protective T cell populations across mucosal sites. Although the L2 serotype of C. trachomatis used in this study has the ability to spread via the lymphatic system to draining lymph nodes in humans (50), we detected almost no C. trachomatis 16S DNA in systemic organs or lymph nodes of intranasally challenged mice. We did detect minimal levels of C. trachomatis 16S DNA in the lung-draining lymph nodes on day 3 p.i. but not later (Figure 1C and 1D). However, we believe that this is due to antigen presenting cells transiently carrying bacterial antigens to the local lymph nodes. This suggests that systemic spread might not be required for the cross-mucosal protection observed in our study. There have been previous studies showing that CD8+ T cell responses induced in one tissue can bridge different mucosal compartments. For instance, oral rotavirus infection, Sendai virus lung infection, and Listeria monocytogenes oral infection all result in pathogen-specific CD8+ T cells detectable at nonlymphoid sites beyond the initial site of antigen encounter (49). These and our observations suggest that the anatomic site of initial T cell activation may not predict the subsequent tissue-specific migration of these T cells. Therefore antigen-experienced T cells that disseminate systemically might not only protect against systemic spread of the primary infection but also may serve as key sentinels, protecting distant mucosal surfaces against secondary infection.
The ability of C. trachomatis to efficiently replicate in both the lung and uterus allowed us to study the tissue specific contribution of chemokine signaling to the migration of clonal T cell populations in the context of the same pathogen challenge. Although both CXCR3 and CCR5 are up-regulated on uterine leukocytes during C. trachomatis genital infection (30), only CCR5 is up-regulated on leukocytes during C. trachomatis lung infection. CCR5 contributes to C. trachomatis-specific CD4+ T cell trafficking to the lung, but seems to be dispensable for the accumulation of C. trachomatis-specific CD8+ T cells in the lung (data not shown). Nevertheless, the CCR5−/− and CXCR3−/−CCR5−/− T cells that migrate to the lungs are sufficient to reduce C. trachomatis burden at similar rates to wild-type T cells. This is different from influenza virus lung infection where CXCR3 deficiency rescues the lethal infection in CCR5−/− mice caused by phagocyte-mediated immunopathology (51). This is also different from genital C. trachomatis infection where both CXCR3 and CCR5 are required for pathogen-specific CD4+ T cell recruitment and efficient bacterial clearance (30). Overall these observations suggest that both tissue-specific and pathogen-specific factors may result in the different chemokine signals mediating migration to different infected tissues. Intranasally-primed memory CD4+ or CD8+ T cells do not require CXCR3 or CCR5 to migrate to the upper genital tract. Some Ptx-sensitive chemokine receptors are required for intranasally-primed memory CD4+ and CD8+ T cells to confer protection in the genital tract. Ptx-treated donor CD4+ or CD8+ cells were completely absent from the lymph nodes draining the genital tract. Therefore, lymph node priming guided by Ptx-sensitive chemokine receptor(s), possibly CCR7, seems to be necessary for the T cells to mediate protection. Future studies will focus on delineating the exact Gi-coupled-receptor signaling critical for the protective capacity of intranasally-primed T cells against genital tract infection.
Although it is well accepted that CD4+ T cells are both necessary and sufficient for secondary protection against C. trachomatis genital tract infection (20, 28, 29), previous studies have demonstrated that a number of C. trachomatis antigens gain access to the host cell cytosol where they can also stimulate CD8+ T cells (33, 52). Moreover, transfer of CD8+ T cell lines specific for these antigens or immunization with a recombinant virus expressing these antigens provides protection (33). Intriguingly, for a pathogen that replicates primarily in epithelial cells that express MHC-I but not high levels of MHC-II, a protective CD8+ T cell response does not seem to develop during natural genital infection. However, we showed that priming in the respiratory tract stimulates a CD8+ T cell population that is sufficient for cross-mucosal protection in the genital tract. These observations suggest that some tissue-specific factors may contribute to differential priming outcomes of CD8+ T cells. Previous reports on the differential impact of IL17 on Th1 immune responses and C. muridarum clearance in the respiratory tract vs. genital tract are consistent with this concept (53, 54). Since dendritic cells are key to priming T cells, it is possible that differential expression of stimulatory and/or inhibitory molecules on these tissue-specific dendritic cells may affect priming and/or memory development of CD8+ T cells. For instance, we observed lower PDL1 expression on lung dendritic cells from intranasally-primed mice as compared to PDL1 expression on uterine dendritic cells from mice primed transcervically (data not shown). PD1-PDL1 signaling has been shown to hinder the CD8+ T cell response, specifically Tem cell formation, following C. trachomatis infection in the genital tract (27). Since the lower PDL1 induction in the lungs correlates with an enrichment of Tem cells in the SLOs of intranasally-primed mice and their superior protective capacity, we speculate that differential induction of PD1-PDL1 signaling might contribute to the differential priming outcomes in the lung and in the genital tract. This hypothesis will be tested in future studies where we will also compare DC populations and other factors that might be involved in CD8+T cell priming at these two mucosal surfaces. These studies will shed light on what factors are critical for shaping protective CD8+ T cell responses to C. trachomatis and other human pathogens that depend on CD8+ T cells for protection.
C. trachomatis and other sexually transmitted pathogens are a worldwide public health concern. Vaccines against these pathogens would significantly reduce the reproductive damage caused by these organisms. This study demonstrates that intranasal immunization with C. trachomatis can confer cross-mucosal protection against genital infection, showing that T-cell-mediated cross-mucosal protection can be induced. Our findings provide proof-of-concept that intranasal immunization can be used as a route of immunization for genital pathogens that depend on T cells for protection, such as C. trachomatis and HIV.
ACKNOWLEDGMENTS
We thank Sarah Fankhauser and other members of the Starnbach lab for helpful discussions and their assistance with animal experiments. We thank the NIH tetramer core facility for providing the tetramers.
This work was supported by the National Institutes of Health grants AI39558 (M.N.S.), AI062827 (M.N.S), Fundação para a Ciência e Tecnologia, Portugal (SFRH/BPD73739/2010) (C.V.N), and by a postdoctoral fellowship supported by the Kaneb family (C.V.N.).
Abbreviations used in this article
- IFU
inclusion-forming unit
- SLO
secondary lymphoid organs
- p.i
post-infection
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa J, Edghill-Smith Y, Moniuszko M, Hel Z, Belyakov IM, Berzofsky JA, Parks RW, Markham PD, Letvin NL, Tartaglia J, Franchini G. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006;80:3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 6.Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189:1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudak S, Hagen M, Liu Y, Catron D, Oldham E, McEvoy LM, Bowman EP. Immune surveillance and effector functions of CCR10(+) skin homing T cells. J Immunol. 2002;169:1189–1196. doi: 10.4049/jimmunol.169.3.1189. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plante M, Jerse A, Hamel J, Couture F, Rioux CR, Brodeur BR, Martin D. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J Infect Dis. 2000;182:848–855. doi: 10.1086/315801. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Zeng H, Li Z, Lei L, Yeh IT, Wu Y, Zhong G. Protective immunity against mouse upper genital tract pathology correlates with high IFNgamma but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway. Vaccine. 2012;30:475–485. doi: 10.1016/j.vaccine.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007;75:666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DA, Latif AS, Ndowa F. WHO global strategy for the prevention and control of sexually transmitted infections: time for action. Sex Transm Infect. 2007;83:508–509. doi: 10.1136/sti.2007.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol. 2004;2:530–531. doi: 10.1038/nrmicro931. [DOI] [PubMed] [Google Scholar]
- 16.Beatty WL, Byrne GI, Morrison RP. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 17.Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect. 1999;75:21–24. doi: 10.1136/sti.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mpiga P, Ravaoarinoro M. Chlamydia trachomatis persistence: an update. Microbiol Res. 2006;161:9–19. doi: 10.1016/j.micres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Chawla R, Bhalla P, Sachdev HP. A pilot study of Chlamydia trachomatis pneumonia in infants. Indian J Med Microbiol. 2004;22:185–187. [PubMed] [Google Scholar]
- 20.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondek DC, Roan NR, Starnbach MN. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J Immunol. 2009;183:1313–1319. doi: 10.4049/jimmunol.0900295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry LL, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell HD. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 25.Roan NR, Starnbach MN. Antigen-specific CD8+ T cells respond to Chlamydia trachomatis in the genital mucosa. J Immunol. 2006;177:7974–7979. doi: 10.4049/jimmunol.177.11.7974. [DOI] [PubMed] [Google Scholar]
- 26.Starnbach MN, Bevan MJ, Lampe MF. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 27.Fankhauser SC, Starnbach MN. PD-L1 limits the mucosal CD8+ T cell response to Chlamydia trachomatis. J Immunol. 2014;192:1079–1090. doi: 10.4049/jimmunol.1301657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol. 2012;189:2441–2449. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive AJ, Gondek DC, Starnbach MN. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2011;4:208–216. doi: 10.1038/mi.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard L, Orenstein NS, King NW. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974;27:102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starnbach MN, Loomis WP, Ovendale P, Regan D, Hess B, Alderson MR, Fling SP. An inclusion membrane protein from Chlamydia trachomatis enters the MHC class I pathway and stimulates a CD8+ T cell response. J Immunol. 2003;171:4742–4749. doi: 10.4049/jimmunol.171.9.4742. [DOI] [PubMed] [Google Scholar]
- 34.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein-Hanley I, Coers J, Balsara ZR, Taylor GA, Starnbach MN, Dietrich WF. The p47 GTPases Igtp and Irgb10 map to the Chlamydia trachomatis susceptibility locus Ctrq-3 and mediate cellular resistance in mice. Proc Natl Acad Sci U S A. 2006;103:14092–14097. doi: 10.1073/pnas.0603338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DM, Grubbs BG, Pack E, Kelly K, Rank RG. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igietseme JU, Uriri IM, Kumar SN, Ananaba GA, Ojior OO, Momodu IA, Candal DH, Black CM. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 39.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med. 2013;210:1855–1869. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun. 2011;79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson EM, You JZ, Motin V, de la Maza LM. Intranasal immunization with Chlamydia trachomatis serovar E, protects from a subsequent vaginal challenge with the homologous serovar. Vaccine. 1999;17:2901–2907. doi: 10.1016/s0264-410x(99)00131-0. [DOI] [PubMed] [Google Scholar]
- 44.Pabst R, Tschernig T. Bronchus-associated lymphoid tissue: an entry site for antigens for successful mucosal vaccinations? Am J Respir Cell Mol Biol. 2010;43:137–141. doi: 10.1165/rcmb.2010-0152RT. [DOI] [PubMed] [Google Scholar]
- 45.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suvas PK, Dech HM, Sambira F, Zeng J, Onami TM. Systemic and mucosal infection program protective memory CD8 T cells in the vaginal mucosa. J Immunol. 2007;179:8122–8127. doi: 10.4049/jimmunol.179.12.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 50.Schachter J. Infection and disease epidemiology. Washington DC: ASM Press; 1999. [Google Scholar]
- 51.Fadel SA, Bromley SK, Medoff BD, Luster AD. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur J Immunol. 2008;38:3376–3387. doi: 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grotenbreg GM, Roan NR, Guillen E, Meijers R, Wang JH, Bell GW, Starnbach MN, Ploegh HL. Discovery of CD8+ T cell epitopes in Chlamydia trachomatis infection through use of caged class I MHC tetramers. Proc Natl Acad Sci U S A. 2008;105:3831–3836. doi: 10.1073/pnas.0711504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 54.Scurlock AM, Frazer LC, Andrews CW, Jr, O'Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]