Abstract
Objective
Metabolic syndrome (MetS) risk increases significantly during menopause and remains elevated post-menopause. Several botanicals, including blueberries (BB), have been shown to delay MetS progression, but few studies have been conducted in postmenopausal animal models. Here, we examined the effects of BB supplementation on obese postmenopausal mice using a chemically-induced menopause model.
Design and Methods
After induction of menopause, mice were fed a high-fat diet or the same diet supplemented with 4% BB powder for 12 weeks. Body weight and body composition were measured, and mice were subjected to glucose and insulin tolerance tests. Serum triglycerides and adiponectin were measured, and liver histology and hepatic gene expression were assessed. Results: Menopausal and BB-supplemented mice had significantly higher body weights and fat mass than control mice, while menopausal mice had impaired glucose tolerance and higher serum triglycerides when compared with control and BB-supplemented mice. Menopausal mice also had hepatic steatosis that was prevented by BB supplementation and correlated with expression of genes involved in hepatic fatty acid oxidation.
Conclusions
We conclude that BB supplementation prevents the glucose intolerance and hepatic steatosis that occur in obese postmenopausal mice, and that these effects are independent of body weight.
Keywords: menopause, blueberries, glucose tolerance, female
Introduction
Menopause, the permanent cessation of menstruation and fertility, occurs in American women at a mean age of 51 years (1). Approximately 49 million women in the U.S. are currently considered postmenopausal. Metabolic syndrome (MetS) affects 40% of people over age 60 and is a significant risk factor for the development of type 2 diabetes (1). Women of reproductive age are considered to be at lower risk for MetS in comparison to men of similar age; however, MetS risk increases significantly during the perimenopausal period and remains elevated post-menopause (2). The estrogen decline observed during menopause was once thought to increase MetS risk; however, it is now known that a progressive increase in the ‘androgenicity’ of the hormonal milieu is the primary culprit, not estrogen loss per se (2, 3).
The most common animal model for menopause research is ovariectomy, where the ovaries are removed surgically. More recently, a menopausal mouse model with intact ovaries was developed using 4-vincylcyclohexene diepoxide (VCD), an occupational chemical that accelerates the natural process of follicular atresia (4, 5, 6). With this model, repeated daily dosing allows the gradual onset of ovarian failure while the residual ovarian tissue remains intact (4, 5, 6). Similar to humans, the residual ovarian tissue of VCD-injected mice produces androgens, which contribute to the altered estrogen/androgen ratio thought to increase MetS risk. For these reasons, the VCD model is more representative of the natural progression of menopause (4, 5, 6).
Notably, there has been a recent shift of research and public interest towards the potential of whole foods to mitigate a variety of health conditions, including MetS. Several botanicals have been shown to delay MetS progression, but these types of studies are lacking in postmenopausal animals. One whole food that has been shown to attenuate obesity, dyslipidemia, and/or insulin resistance in several preclinical models of MetS is the blueberry (BB; Vaccinium species)(7, 8, 9, 10), which therefore may be a feasible option for the natural treatment of this disorder.
The purpose of this study was to assess the effects of BB supplementation in high-fat diet-fed postmenopausal mice. We examined parameters associated with MetS in cycling control, menopausal, and BB-supplemented menopausal mice fed a high-fat diet over 12 weeks. Measured parameters included: weight gain, body composition, fasting blood glucose levels, glucose and insulin tolerance, serum triglycerides, and hepatic steatosis. Our results demonstrate that ovarian failure/menopause, in combination with a high-fat diet, in the C57BL/6J mouse leads to obesity, glucose intolerance, and hepatic steatosis, and that BB-supplementation prevents the development of the glucose intolerance and hepatic steatosis in this animal model.
Methods
Animals and diets
Four-week old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were housed in a temperature (22° ± 2 degrees Celsius)- and humidity-controlled (45–55%) room under a 12-hour light/dark cycle (lights on from 0700h – 1900h). All animals were housed in groups of 3–4 for the duration of the study. Mice were allowed ad libitum access to food and water, and body weights were measured weekly. All experiments were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee. Three groups of mice were used in this study. Groups were: high fat diet (HFD) cycling (C; n=4), HFD+VCD injections (V; n=6), HFD+BB+VCD (VB; n=8).
A complete study timeline appears in Table 1. Briefly, immediately upon arrival to the animal facility, mice were placed on a purified low-fat pelleted control diet (D12450J; Research Diets, New Brunswick, NJ). Two days after VCD injections were completed, all animals were switched to a purified high-fat pelleted diet (HFD, D12429; Research Diets, New Brunswick, NJ). All animals were maintained on HFD until confirmation of menopause (approximately 60 days following the first VCD injection; study week 8). Once menopause was confirmed, animals in the VB group were switched to the same pelleted HFD with 4% w:w highbush BB powder (batch T-10711) from the Tifblue (Vaccinium virgatum (ashei)) cultivar added; the BB was incorporated into the HFD by Research Diets as a custom formulation. Diets were isocaloric at 5.1 kcal/gram and each contained 18% kcal from protein, 21% kcal from carbohydrate, and 61% kcal from fat. The BB powder was a kind gift from the United States Highbush Blueberry Council. Mice were maintained on their respective diets for 12 additional weeks. Fresh diet was provided every other day to minimize fat oxidation and deterioration of BB active ingredients, and diet was stored at −80 degrees Celsius until use. Diet compositions are indicated in Table 2, and the nutrient composition of the BB powder used appears in Table 3.
Table 1.
Study timeline.
| Week | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization | ● | ||||||||||||||||||||
| Injections (17 days) | ● | ● | |||||||||||||||||||
| High fat diet | ● | ● | ● | ● | ● | ||||||||||||||||
| Vaginal cytology | ● | ● | ● | ● | ● | ● | |||||||||||||||
| High fat diet −/+ BB | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||
| Food Intake & Body Weight | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Body Composition | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||
| GTT | ● | ||||||||||||||||||||
| ITT | ● |
Abbreviations used: BB, blueberry; GTT, glucose tolerance test; ITT, insulin tolerance test.
Table 2.
Compositions of high-fat and blueberry-supplemented high-fat diets. Ingredients are presented as grams per kilogram of diet.
| Ingredient | High Fat Diet | High Fat Diet + 4% BB |
|---|---|---|
| Casein | 200 | 199.2 |
| L-Cystine | 3 | 3 |
| Maltodextrin | 125 | 125 |
| Sucrose | 68.8 | 51 |
| Cellulose | 50 | 42.5 |
| Soybean oil | 25 | 25 |
| Lard | 245 | 245 |
| Mineral Mix | 10 | 10 |
| DiCalcium Phosphate | 13 | 13 |
| Calcium Carbonate | 5.5 | 5.5 |
| Potassium Citrate | 16.5 | 16.5 |
| Vitamin Mix | 10 | 10 |
| Choline Bitartrate | 2 | 2 |
| Blueberry Powder | 0 | 31.2 |
Table 3.
Nutritional information for blueberry powder (per 100 g).
| Calories (kcal) | 390 |
| Protein (g) | 2.18 |
| Carbohydrates (g) | 93.5 |
| Fat (g) | 0.83 |
| Saturated Fat (g) | 0.16 |
| Total Sugars (g) | 55.9 |
| Fructose (g) | 28.5 |
| Glucose (g) | 27.4 |
| Sucrose (g) | <0.1 |
| Maltose (g) | <0.1 |
| Lactose (g) | 10 |
| Dietary Fiber (g) | 24.0 |
| Insoluble Fiber (g) | 20.9 |
| Soluble Fiber (g) | 3.1 |
| Cholesterol (mg) | 0 |
| Total beta carotene (IU) | 0 |
| Vitamin C (mg) | 9.83 |
| Calcium (mg) | 31.4 |
| Iron (mg) | 1.96 |
| Potassium (mg) | 468 |
| Sodium (mg) | 8.84 |
Abbreviations used: g, grams; kcal, kilocalories; IU, international units; mg, milligrams.
Induction of menopause
After a 1-week acclimation to the animal room, 5-week old mice were weighed and injected intraperitoneally (i.p.) each day with VCD, at a concentration of 160 mg/kg for 17 consecutive days; this dosing regimen has been optimized by the creators of the VCD menopause model (5, 11). Control mice were injected daily with sesame oil vehicle. After completion of injections, vaginal cytology was monitored daily to assess estrous cycle cessation. Briefly, vaginal lavage was performed on each mouse (between 7 a.m. and 9 a.m. daily) using 20 ul of sterile 0.9% saline. A drop of fluid from each lavage was placed onto a slide, allowed to air dry, stained with 0.25% methylene blue, and viewed under a microscope to determine cycle stage. Mice were considered acyclic after 10 days of consecutive diestrus (5, 11). After mice were sacrificed, ovaries were removed, and periovarian fat was trimmed away and stored for later analysis. A piece of liver from each animal was removed and frozen at −80 degrees Celsius for later analysis. Another piece of liver from each animal was formalin-fixed, paraffin-embedded, and sectioned at a thickness of 3 um; sections were placed on slides and stained with hematoxylin and eosin for histologic evaluation.
Body composition measurements and blood collection
Non-fasting body composition was analyzed by NMR (Bruker LF50, Bruker Optics, Germany) at baseline and every other week on all mice for the duration of the study. Body weights were obtained weekly. At baseline and study end, mice were fasted for 4 hours, and tail blood glucose was measured using a Bayer Breeze 2 glucometer. At baseline and study end, submandibular venous blood was collected from fasted mice (4 hours). Mice were anesthetized with isoflurane, and the submandibular vein punctured with a sterile lancet. Several drops of blood were collected into sterile serum separator tubes. Blood was centrifuged at 3500 rpm for 15 minutes at 4 degrees Celsius, and serum was removed and stored at −80 degrees Celsius. .
Insulin and glucose tolerance testing
Insulin tolerance tests and glucose tolerance tests (ITT and GTT, respectively) were performed on mice after a 4-hour light phase fast (8 a.m. to noon, for ITT) or a 12-hour overnight fast (8 p.m. to 8 a.m., for GTT). ITT was performed at noon two weeks prior to sacrifice and GTT was performed at 8 a.m. one week prior to sacrifice. Tail glucose was measured at baseline (time 0) prior to the administration of insulin or glucose. I.p. injections of insulin (0.8 mU/g body weight, Sigma Aldrich, USA) or D-glucose (1.6 mg/g body weight, Fisher Scientific, USA) were given, and tail glucose measured at 20, 45, 60, 90, and 120 minutes post-injection.
Gene expression analyses
Total RNA from approximately 100 mg of tissue was isolated by column purification (Qiagen) and yield determined by spectrophotometry (NanoDrop Technologies). From each RNA sample, 200 ng was reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Relative quantification of mRNA expression was analyzed using an ABI Prism 7900 Sequence Detection System (Applied Biosystems). PPIA was used as a ‘housekeeping’ control gene. Mouse primers came from Integrated DNA Technologies, and the sequences were as follows: PPIA (cyclophilin A) forward, CACTGTCGCTTTTCGCCGC; PPIA (cyclophilin A) reverse, TGCAAACAGCTCGAAGGAGACGC; Cs (citrate synthase) forward, GTCCATGCAGTCCTCATAGATG; Cs (citrate synthase) reverse, CTCAACAGTGAAAGCAACTTCG; Hadha (long-chain hydroxyacyl-CoA dehydrogenase) forward, GCTATTCTGTATTGGCATGCTATG; Hadha (long-chain hydroxyacyl-CoA dehydrogenase) reverse, CGACTCTCACAGGAAGGTCAGAG; Cd36 (fatty acid translocase) forward, GGCATCATTGGGCACTCCTT; Cd36 (fatty acid translocase) reverse, ACCAACAGCTGCCATGGATC.
Serum and tissue analyses
Serum triglycerides and serum adiponectin were assayed using kits from Sigma (St. Louis, MO) and Millipore (Billerica, MA), respectively, according to manufacturer’s instructions. Quantification of liver triglycerides was performed using a kit from Abcam (Cambridge, MA) according to manufacturer’s instructions.
Statistical Analyses
For results over time, repeated measures ANOVA was performed. Student’s t-tests were performed on NMR, GTT area under the curve, liver triglycerides and serum triglycerides. Analysis of covariance (ANCOVA) was performed on serum adiponectin values with group and fat mass as covariants. Differences in results were considered significant when p<0.05.
Results
Body weight and body composition
Body weights of postmenopausal (V) or postmenopausal with BB supplementation (VB) mice were significantly greater than those of control (C) mice beginning at study week 6 and continuing through study end (Figure 1A). Both groups of menopausal mice had significantly greater fat mass, but no changes in fat free mass, compared with control mice (Figure 1B). There were no differences in body weight, fat mass, or fat free mass between the V and VB groups, indicating that BB supplementation did not affect these parameters. In support of these data, cumulative food intake estimates per cage over study weeks 8–20 did not differ between the V and VB groups, although both groups consumed more than the C group (data not shown).
Figure 1. Menopause-induced changes in body weight and body composition are not altered by BB supplementation.
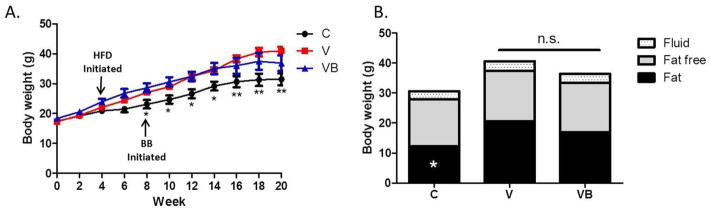
A) Body weights for all animals throughout study duration. Body weights of VCD-injected mice and BB-supplemented VCD-injected mice were not significantly different from each other, but were significantly different from those of control mice from weeks 8–20 of the study. B) Mean body composition data for various groups at study end (20 weeks). Control mice had significantly less fat mass than V or VB mice, but no changes in fat free mass or fluid were noted. *p<0.05, **p<0.01, C group vs. other two groups. HFD = high fat diet, BB = blueberry, C = cycling HFD control group, V = HFD+VCD, VB = HFD+BB+VCD, g = grams.
Blood glucose measurements
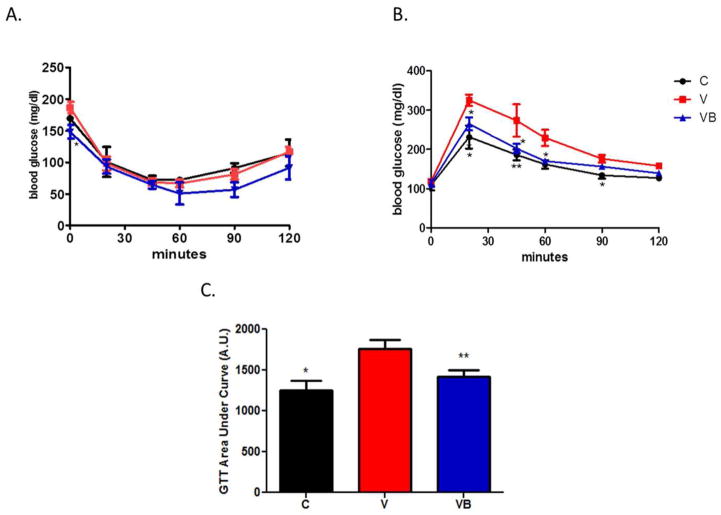
Compared to VB mice, mice in the V group had significantly higher blood glucose levels after a 4-hour fast (p = 0.0259). There were no significant differences among blood glucose levels at any other time points during an ITT (Figure 2A). In contrast with the ITT results, both the C and VB groups had significantly lower blood glucose levels than V mice for most time points throughout an GTT (Figure 2B) and significantly lower GTT area under the curve values (Figure 2C), suggesting that BB supplementation may prevent the development of glucose intolerance in obese postmenopausal mice.
Figure 2. BB supplementation does not improve insulin tolerance, but does improve glucose tolerance, in obese postmenopausal mice.
A) Graphical depiction of insulin tolerance time following an i.p. insulin injection. At baseline, V mice had higher blood glucose levels than VB or C groups; however, no changes in blood glucose levels over time were noted among any of the groups in response to an insulin injection. B) Graphical depiction of glucose tolerance over time following an i.p. bolus of glucose. V mice demonstrated impaired glucose tolerance at 30, 45, 60, and 90 minutes post-injection. C) GTT results expressed as area under the curve *p<0.05, **p<0.01 vs. V group. C = cycling HFD control group, V = HFD+VCD, VB = HFD+BB+VCD, mg = milligrams, dl = deciliter, A.U. = arbitrary units.
Serum measurements
Serum triglycerides were significantly higher in obese postmenopausal mice when compared to C and VB mice (Figure 3A). Interestingly, serum adiponectin was higher in both the V and VB mice than in the control mice, although these differences were not significant. However, the increased fat mass may account for the increased adiponectin production observed in both groups of menopausal animals (Figure 3B). When ANCOVA was performed using group and fat mass as covariants, fat mass was found to have a significant effect on serum adiponectin (p=0.0094).
Figure 3. BB supplementation improves serum triglycerides in obese postmenopausal mice.
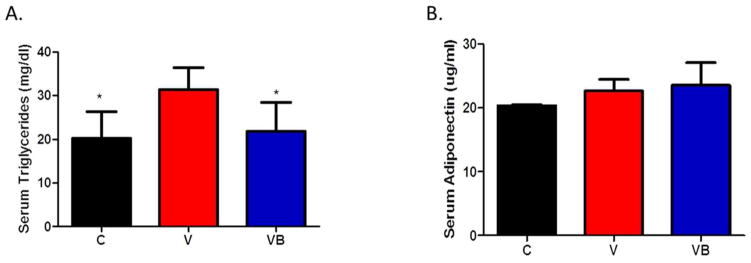
A) Serum triglyceride levels at study end. B) Serum adiponectin levels at study end. *p<0.05 vs. V group. C = cycling HFD control group, V = HFD+VCD, VB = HFD+BB+VCD, mg = milligrams, dl = deciliter, ug = micrograms, ml = milliliter.
Liver histology, triglyceride content, and gene expression
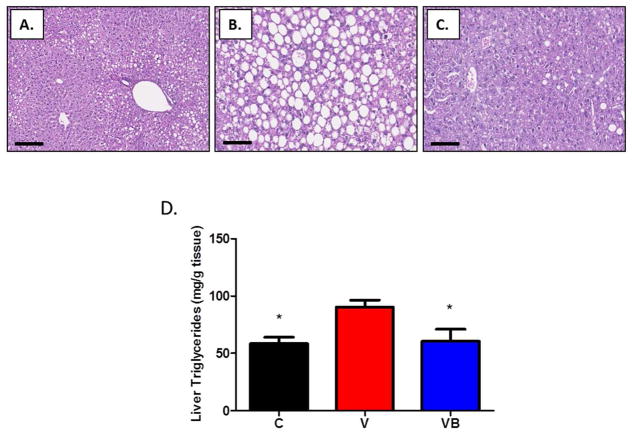
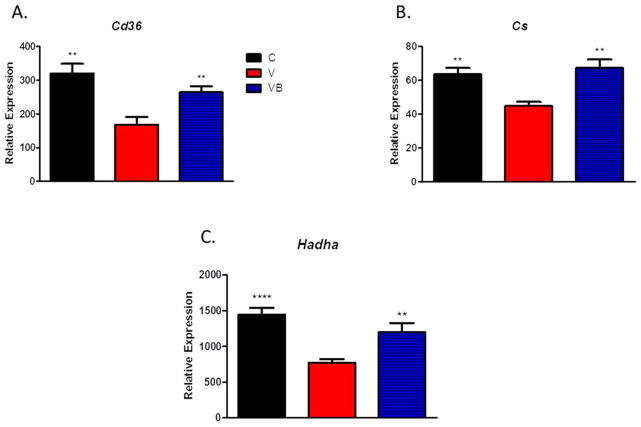
As expected with the high fat diet, some lipid deposition was observed in livers from animals in all groups, but was much more severe in V mice than in control mice (Figures 4A–C). Livers from VB mice had less hepatic steatosis than livers from V mice, indicating that BB supplementation may prevent the increased lipid deposition present in the livers of obese postmenopausal mice. Results from the liver triglyceride quantification assay supported our histology results, with significantly higher liver triglyceride levels in the V group when compared to the C and VB groups (Figure 4D). In addition, hepatic gene expression analyses revealed significant increases in citrate synthase, long-chain hydroxyacyl-CoA dehydrogenase, and fatty acid translocase/CD36 in VB animals when compared to V animals, suggesting enhanced fatty acid oxidation in the livers of BB-treated obese postmenopausal mice (Figures 5A-C).
Figure 4. BB supplementation prevents hepatic steatosis in obese postmenopausal mice.
Representative photomicrographs of H&E-stained liver sections at 100x magnifications. Scale bars = 50 um. The livers of V mice (panel B) had more severe lipid deposition than C mice (panel A), and BB supplementation prevented the lipid deposition seen in the menopausal liver (panel C). These qualitative histologic results were verified with a quantitative method for tissue triglyceride measurement; results appear in D. *p<0.05 vs. V group. mg=milligrams, g=grams.
Figure 5. Menopause decreases expression of genes associated with hepatic fatty acid oxidation and BB supplementation prevents these changes.
Relative expression levels of hepatic A) CD36, B) Cs, and C) Hadha in livers after 12 weeks of HFD or HFD supplemented with BB . **p<0.01, ****p<0.0001 vs. V group. C = cycling HFD control group, V = HFD+VCD, VB = HFD+BB+VCD, Cd36 = fatty acid translocase/CD36, Cs = citrate synthase, and Hadha = long-chain hydroxyacyl-CoA dehydrogenase.
Discussion
The purpose of this study was to assess the effects of BB supplementation on body composition, glucose tolerance, and lipid handling in obese postmenopausal mice. We used the VCD injection model of ovarian failure that causes gradual follicle loss and allows for retention of residual ovarian tissue that produces androgens that may contribute to postmenopausal metabolic dysfunction (4, 5). Here, we combined the VCD menopause model with a 60% high-fat diet to induce obesity. MetS studies are generally conducted in high-fat fed male C57BL/6J mice since they gain more weight, are obesity-prone, and become insulin-resistant (12, 13), while high-fat diet-fed female mice of reproductive age do not tend to become insulin-resistant (13). Very few studies have examined the effects of high-fat diet feeding in postmenopausal C57BL/6J mice, and our study is the first to examine the effects of BB supplementation in these mice. Our data demonstrate that high-fat diet-fed VCD-injected mice become obese and glucose intolerant and develop hepatic steatosis, and that BB supplementation can prevent the glucose intolerance and hepatic steatosis observed in these animals.
Menopause is associated with gains in body weight and fat mass in both humans and animals (4, 14, 15, 16, 17, 18, 19). In line with previous results in VCD-injected B6C3F1 mice (4) and ovariectomized mice (15, 18, 19), our menopausal animals had significantly greater body weights and fat mass than cycling control animals; interestingly, BB supplementation did not significantly affect either parameter. Previous studies in ovariectomized female rats have also demonstrated no differences in body weights between control- or BB-fed groups, although the diets used were not high-fat (20, 21). Also, no changes in fat mass or body weight were reported in BB-supplemented high-fat-fed male mice (9, 22) or obese male Zucker rats (8). These results are supported by the lack of any substantial changes in food intake between the menopausal and BB-supplemented menopausal mice in our study (data not shown). Conversely, increases in energy intake, fat mass, and body weight have been reported in male rodents with BB supplementation. The study reporting increased energy intake in HFD-fed male mice provided 10% BB, which is 250% more BB than we provided (10). In this study, the increased levels of BB may have increased diet palatability, as BB-supplemented animals consumed approximately 12% more energy per day; this could have led to increased body fat. In the aforementioned Zucker rat study, an increase in body weight, but not adiposity, was only observed in the low-fat-fed lean control Zucker rats with 2% BB supplementation, and not in the high-fat-fed obese Zucker rats (8). Nonetheless, our current results suggest that the metabolically beneficial effects of BB supplementation in obese postmenopausal mice are independent of alterations in body weight and body composition.
Estrogen is known to improve glucose homeostasis in mice and humans (23, 24, 25, 26, 27). The elevated blood glucose levels and impaired glucose tolerance seen in our obese postmenopausal mice support previous results demonstrating the effects of menopause/estrogen loss on glucose homeostasis in vivo (4, 15, 18, 23, 27, 28, 29, 30). In VCD-injected B6C3F1 mice, significantly higher blood glucose levels after a 4-hour fast and impaired glucose tolerance were observed after 16 weeks on a high-fat diet (4). Similar effects have been shown in ovariectomized high-fat diet-fed mice (15, 28, 29, 30). Our BB-supplemented postmenopausal mice had significantly lower blood glucose levels after a 4-hour fast and improved glucose tolerance when compared to non-supplemented menopausal mice. These results demonstrate metabolically favorable, weight-independent effects of BB on glucose metabolism in the post-menopausal state. Interestingly, although 4-hour fasting blood glucose was elevated in menopausal mice compared to BB-supplemented menopausal mice, there were no differences in blood glucose after a 12-hour fast. Our results suggest that liver may contribute to this effect. If the menopausal mice had hepatic insulin resistance, the mild 4-hour fast could result in increased hepatic glucose output in excess of the glucose demands of other tissues, thereby leading to elevated blood glucose. However, the severity of the 12-hour fast would likely have led to an increase in tissue demand for glucose to match hepatic glucose output, and would have likely led to a normalization of glucose levels as we observed in our study. Overall, additional studies utilizing hyperinsulinemiceuglycemic clamping are required to provide more detailed information regarding the tissues responsible for the improved glucose tolerance in these animals.
Alterations in lipid homeostasis are common in menopausal female rodents and humans; specifically, increased cholesterol, triglycerides, and free fatty acids are often present (3, 4, 27, 31). Hepatic steatosis is also a common feature in menopausal animal models (27, 31, 32). As expected, compared to control mice, our postmenopausal mice had increased serum triglycerides and hepatic steatosis, while BB supplementation prevented these effects. Supplementation of HFD with BB or its active components has been previously shown to mitigate the changes in lipid levels seen in several male rodent models of MetS (22, 33, 34, 35), but until now, no studies have examined BB effects on serum lipids or hepatic steatosis in obese postmenopausal mice. Our results support previous findings regarding circulating and hepatic lipid levels in BB-supplemented rodents but now extend these findings to females.
One possible explanation for the prevention of hepatic steatosis in the BB-supplemented mice is an enhancement in hepatic fatty acid oxidation. In liver, menopause/estrogen loss is associated with a decrease in the partitioning of triglyceride into oxidative pathways (36). Consistent with this observation, expression levels of key hepatic fatty oxidation genes (citrate synthase, long-chain hydroxyacyl-CoA dehydrogenase, and fatty acid translocase/CD36) were decreased in our menopausal mice when compared to controls. Expression levels of all three genes in BB-supplemented mice were similar to control levels, suggesting an improvement in hepatic fatty acid oxidation and lipid handling, which may explain the vast improvements in liver histology and the decrease in serum triglycerides seen in these animals. Decreased hepatic citrate synthase, which indicates an overall decline in total mitochondrial oxidative capacity, has been demonstrated with high-fat diet feeding in male mice (37), but no evidence exists in female mice. Our results are the first to demonstrate the beneficial effects of BB supplementation on hepatic fatty acid oxidation and hepatic lipid deposition in the postmenopausal mouse liver. In addition to its possible effects on hepatic steatosis, the improvement in hepatic fatty acid oxidative gene expression also suggests a route by which glucose tolerance may be improved in our BB-supplemented mice.
Although our data in liver are compelling, it is worth noting that some of the beneficial effects of BB supplementation we observed in this study may not be directly due to liver effects, but instead due to improved adipose tissue function. It is well-accepted that in obesity and insulin resistance, adipose tissue lipolysis provides fatty acids that contribute to hepatic triglyceride deposition and development of hepatic steatosis (38). Thus, if insulin resistance was prevented in the BB-supplemented animals, an increase in adipose lipolytic rate may have been avoided, allowing for improved triglyceride sequestration and decreased ectopic lipid deposition, which would have also decreased hepatic steatosis and improved glucose tolerance in these animals. More detailed studies are warranted in order to test these hypotheses.
Overall, our results highlight the metabolically beneficial roles of BB supplementation in VCD-injected C57BL/6J mice fed a high-fat diet. After 12 weeks on the BB-supplemented diet, postmenopausal mice exhibited no alterations in body weight or fat mass compared to their non-supplemented counterparts, but were more glucose tolerant, had lower serum triglycerides, and had less hepatic steatosis paired with increased expression of key hepatic fatty acid oxidation genes. Ours is the first study to demonstrate these effects in a postmenopausal mouse model. These results highlight the potential utility of a natural product in preventing the metabolic derangements observed with menopause. It will be important to determine if BB supplementation can convey the same benefits in a natural menopause rodent model, or if the benefits conferred are unique to young animals in which menopause has been chemically- or surgically-induced. Further investigations will be required to determine the molecular mechanisms by which BB exert these positive effects.
What is already known about this subject
Metabolic syndrome risk in women increases after menopause
Blueberries have been shown to improve metabolic syndrome risk in preclinical studies
What this study adds
Blueberries improve glucose tolerance in obese postmenopausal mice
Blueberries improve serum and liver lipids in obese postmenopausal mice
The effects of blueberries are independent of changes in body weight
Acknowledgments
The authors gratefully acknowledge Samuel Marion and Patricia Hoyer for their constructive input regarding use of the VCD model and Michael Pellizzon for formulating the diets. This work was supported by a NCAAM- and ODS-funded grant to Pennington Biomedical Research Center (2P50AT002776). CME was supported by an NIH postdoctoral training fellowship granted to Pennington Biomedical Research Center (T32AT004094). This work was partially supported by a NIDDK-funded NORC Center Grant (2P30DK072476) entitled “Nutritional Programming: Environmental and Molecular Interactions”.
Footnotes
CONFLICTS OF INTEREST STATEMENT
Competing interests: the authors have no competing interests.
Author contributions: CME conceived and carried out experiments, analyzed and interpreted data, and wrote the paper. JDT carried out experiments. DKI and JMS assisted with study design and analyzed and interpreted data. All authors gave final approval of the submitted manuscript.
References
- 1.CDC. Women's Reproductive Health: Menopause. U.S. Department of Health and Human Services; Jul 25, 2012. Available from: http://www.cdc.gov/reproductivehealth/womensrh/menopause.htm. [Google Scholar]
- 2.Carr MC. The Emergence of the Metabolic Syndrome with Menopause. Journal of Clinical Endocrinology & Metabolism. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 3.Korytkowski MT, Krug EI, Daly MA, DeRiso L, Wilson JW, Winters SJ. Does androgen excess contribute to the cardiovascular risk profile in postmenopausal women with type 2 diabetes? Metabolism. 2005;54:1626–1631. doi: 10.1016/j.metabol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297:R587–R592. doi: 10.1152/ajpregu.90762.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The Follicle-Deplete Mouse Ovary Produces Androgen. Biology of Reproduction. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 6.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comparative Medicine. 2005;55:523–527. [PubMed] [Google Scholar]
- 7.Vuong T, Benhaddou-Andaloussi A, Brault A, Harbilas D, Martineau LC, Vallerand D, et al. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKAy mice. Int J Obes. 2009;33:1166–1173. doi: 10.1038/ijo.2009.149. [DOI] [PubMed] [Google Scholar]
- 8.Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, et al. Blueberry Intake Alters Skeletal Muscle and Adipose Tissue Peroxisome Proliferator-Activated Receptor Activity and Reduces Insulin Resistance in Obese Rats. J Med Food. 2011;14:1511–1518. doi: 10.1089/jmf.2010.0292. [DOI] [PubMed] [Google Scholar]
- 9.DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS, et al. Dietary Blueberry Attenuates Whole-Body Insulin Resistance in High Fat-Fed Mice by Reducing Adipocyte Death and Its Inflammatory Sequelae. The Journal of Nutrition. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole Berries versus Berry Anthocyanins: Interactions with Dietary Fat Levels in the C57BL/6J Mouse Model of Obesity. Journal of Agricultural and Food Chemistry. 2008;56:647–653. doi: 10.1021/jf071993o. [DOI] [PubMed] [Google Scholar]
- 11.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reproductive Toxicology. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of Sex, Strain and Age Factors in High Fat Diet-Induced Obesity in C57BL/6J and BALB/cA Mice. Experimental Animals. 2007;56:263–272. doi: 10.1538/expanim.56.263. [DOI] [PubMed] [Google Scholar]
- 13.Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beranger GE, Pisani DF, Castel J, Djedaini M, Battaglia S, Amiaud J, et al. Oxytocin Reverses Ovariectomy-Induced Osteopenia and Body Fat Gain. Endocrinology. 2014;155:1340–1352. doi: 10.1210/en.2013-1688. [DOI] [PubMed] [Google Scholar]
- 15.Kanaya N, Vonderfecht S, Chen S. Androgen (dihydrotestosterone)–mediated regulation of food intake and obesity in female mice. The Journal of Steroid Biochemistry and Molecular Biology. 2013;138:100–106. doi: 10.1016/j.jsbmb.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers NH, Perfield JW, II, Strissel KJ, Obin MS, Greenberg AS. Reduced Energy Expenditure and Increased Inflammation Are Early Events in the Development of Ovariectomy-Induced Obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, et al. Estrogen Receptor-β-selective Ligands Alleviate High-fat Diet- and Ovariectomy-induced Obesity in Mice. Journal of Biological Chemistry. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanaya N, Chen S. Conjugated linoleic acid reduces body weight gain in ovariectomized female C57BL/6J mice. Nutrition Research. 2010;30:714–721. doi: 10.1016/j.nutres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Lazarenko OP, Blackburn ML, Shankar K, Badger TM, Ronis MJJ, et al. Feeding Blueberry Diets in Early Life Prevent Senescence of Osteoblasts and Bone Loss in Ovariectomized Adult Female Rats. PLoS ONE. 2011;6:e24486. doi: 10.1371/journal.pone.0024486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devareddy L, Hooshmand S, Collins JK, Lucas EA, Chai SC, Arjmandi BH. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. The Journal of Nutritional Biochemistry. 2008;19:694–699. doi: 10.1016/j.jnutbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Prior RLE, Wilkes SR, Rogers T, Khanal RC, Wu X, Howard LR. Purified Blueberry Anthocyanins and Blueberry Juice Alter Development of Obesity in Mice Fed an Obesogenic High-Fat Diet. Journal of Agricultural and Food Chemistry. 2010;58:3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 23.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen Regulation of Adiposity and Fuel Partitioning: EVIDENCE OF GENOMIC AND NON-GENOMIC REGULATION OF LIPOGENIC AND OXIDATIVE PATHWAYS. Journal of Biological Chemistry. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 24.Camporez JPG, Jornayvaz FR, Lee H-Y, Kanda S, Guigni BA, Kahn M, et al. Cellular Mechanism by Which Estradiol Protects Female Ovariectomized Mice From High-Fat Diet-Induced Hepatic and Muscle Insulin Resistance. Endocrinology. 2013;154:1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–558. doi: 10.1038/nrendo.2009.166. [DOI] [PubMed] [Google Scholar]
- 26.Whitcroft S, Herriot A. Insulin resistance and management of the menopause: a clinical hypothesis in practice. Menopause International. 2011;17:24–28. doi: 10.1258/mi.2011.011003. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, et al. Estrogen Treatment After Ovariectomy Protects Against Fatty Liver and May Improve Pathway-Selective Insulin Resistance. Diabetes. 2013;62:424–434. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeAngel RE, Berrigan D, Nunez NP, Hursting SD, Perkins SN. Dietary Calcium Source Influences Body Composition, Glucose Metabolism and Hormone Levels in a Mouse Model of Postmenopausal Obesity. In Vivo. 2009;23:527–535. [PubMed] [Google Scholar]
- 29.Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, et al. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Molecular Metabolism. 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbins R, Holcomb V, Hong J, Núñez N. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 31.Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1031–G1043. doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- 32.Jackson KC, Wohlers LM, Valencia AP, Cilenti M, Borengasser SJ, Thyfault JP, et al. Wheel running prevents the accumulation of monounsaturated fatty acids in the liver of ovariectomized mice by attenuating changes in SCD-1 content. Applied Physiology, Nutrition, and Metabolism. 2011;36:798–810. doi: 10.1139/h11-099. [DOI] [PubMed] [Google Scholar]
- 33.Elks CM, Francis J, Stull AJ, Cefalu WT, Shukitt-Hale B, Ingram DK. Bioactives in Fruit. John Wiley & Sons, Ltd; 2013. Overview of the Health Properties of Blueberries; pp. 251–271. [Google Scholar]
- 34.Vendrame S, Daugherty A, Kristo AS, Klimis-Zacas D. Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. British Journal of Nutrition. 2014;111:194–200. doi: 10.1017/S0007114513002390. [DOI] [PubMed] [Google Scholar]
- 35.Roopchand DE, Kuhn P, Rojo LE, Lila MA, Raskin I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacological Research. 2013;68:59–67. doi: 10.1016/j.phrs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paquette A, Chapados NA, Bergeron R, Lavoie JM. Fatty Acid Oxidation is Decreased in the Liver of Ovariectomized Rats. Horm Metab Res. 2009;41:511–515. doi: 10.1055/s-0029-1202348. [DOI] [PubMed] [Google Scholar]
- 37.Enos RT, Velázquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. The Journal of Nutritional Biochemistry. 2014;25:600–612. doi: 10.1016/j.jnutbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]