Abstract
BACKGROUND
Contemporary data on temporal trends in incidence and survival after atrial fibrillation are scarce.
METHODS
Olmsted County, MN residents with a first-ever atrial fibrillation or atrial flutter event between 2000–2010 were identified. Age- and sex-adjusted incidence rates were standardized to the 2010 US population and the relative risk of AF in 2010 vs. 2000 was calculated using Poisson regression. Standardized mortality ratios of observed vs. expected survival were calculated, and time trends in survival were examined using Cox regression.
RESULTS
We identified 3344 incident atrial fibrillation/atrial flutter events (52% male, mean age 72.6, 95.7% white). Incidence did not change over time (age- and sex-adjusted rate ratio (95% CI): 1.01 (0.91–1.13) for 2010 vs. 2000). Within the first 90 days, the risk of all-cause mortality was greatly elevated compared to individuals of a similar age and sex distribution in the general population (standardized mortality ratios (95% CI): 19.4 (17.3–21.7) and 4.2 (3.5–5.0) for the first 30 days and 31–90 days after diagnosis, respectively). Survival within the first 90 days did not improve over the study period (adjusted hazard ratio (HR) (95% CI): 0.96 (0.71–1.32) for 2010 vs. 2000); likewise, no difference in mortality between 2010 and 2000 was observed among 90 day survivors (HR (95% CI): 1.05 (0.85–1.31)).
CONCLUSIONS
In the community, atrial fibrillation incidence and survival have remained constant over the last decade. A dramatic and persistent excess risk of death was observed in the 90 days after atrial fibrillation diagnosis, underscoring the importance of early risk stratification.
Keywords: atrial fibrillation, epidemiology, incidence, survival
INTRODUCTION
Atrial fibrillation is the most common sustained cardiac arrhythmia, affecting 2.7–6.1 million Americans1, 2 with annual healthcare costs between $6–26 billion.3–5 Atrial fibrillation was designated as a new epidemic near the end of the 20th century.6 The aging population and increased survival after myocardial infarction7–12 and heart failure13–16 amplifies the public health impact of atrial fibrillation. Despite its major clinical and public health impact, contemporary data on the atrial fibrillation epidemic are scarce. While there have been reports of an increase in atrial fibrillation incidence between 1980–2000 in Olmsted County, MN,2 incidence rates were stable in the Atherosclerosis Risk in Communities (ARIC) study between 1987–200417 and among Medicare beneficiaries between 1993–2007.18 In addition, the preferential reliance on hospitalized atrial fibrillation17 or claims databases18 may limit the generalizability of previous studies, underscoring the need for current data to improve our understanding of the atrial fibrillation epidemic.
The outcomes of atrial fibrillation are of major clinical consequence as atrial fibrillation causes substantial morbidity and confers an excess risk of death.19 No changes in short-term or long-term survival were observed after atrial fibrillation from 1980–2000 in Olmsted County, MN residents20 or between 1993–2007 in Medicare beneficiaries.18 Nevertheless, whether the risk of death has decreased in more contemporary times among atrial fibrillation patients of all ages is unknown. Therefore, we undertook this study to provide contemporary insights into the atrial fibrillation epidemic by examining decade long trends in incidence and survival of atrial fibrillation in a community study of patients diagnosed with a first atrial fibrillation or atrial flutter event from 2000 to 2010.
METHODS
Study Population
This study was conducted in Olmsted County, Minnesota, an area relatively isolated from other urban centers and where only a few providers, mainly Mayo Clinic, Olmsted Medical Center, and their affiliated hospitals, deliver most health care to local residents. The retrieval of all health care related events occurring in Olmsted County is made possible by the records-linkage system of the Rochester Epidemiology Project (REP).21, 22 This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Validation of Incident Atrial Fibrillation
International Classification of Diseases, Ninth Revision (ICD-9) codes 427.31 and 427.32 from all providers in the REP, as well as Mayo Clinic electrocardiograms indicating atrial fibrillation or atrial flutter were obtained from inpatient and outpatient encounters among adults aged ≥18 from 2000–2010. Patients with electrocardiographic evidence of atrial fibrillation or atrial flutter prior to 2000 were considered prevalent and excluded. Among the possible incident cases, the entire medical record(s) were manually reviewed. First-ever evidence of atrial fibrillation or atrial flutter on one or more of the following were required to validate the incident events: 1) on electrocardiogram or rhythm strip, 2) on Holter monitor, event monitor, or telemetry, 3) on monitor during an emergency department visit or hospitalization, 4) on the electrocardiogram during an echocardiogram, 5) on pacemaker interrogation, or 6) a physician diagnosis.
If the first evidence of atrial fibrillation occurred within 30 days of cardiothoracic surgery, these events were considered post-op and were not counted as incident. For those with post-op atrial fibrillation, we continued to review the medical record for another episode of atrial fibrillation not associated with a surgery (or occurring more than 30 days after a surgery); in this scenario, the episode not associated with surgery was classified as the incident date of atrial fibrillation. Those with post-op atrial fibrillation but no future atrial fibrillation unrelated to surgery were exluded.
Ascertainment of All-Cause Mortality
Deaths through June 30, 2013 were obtained from inpatient and outpatient medical records, death certificates from the state of Minnesota, and obituaries and notices of death in the local newspapers. The underlying cause of death from the death certificate, when available, was categorized as cardiovascular or non-cardiovascular based on American Heart Association classifications.23
Clinical Data Collection
Height, weight, and smoking status (current, former, never) were abstracted at the time of incident AF. Body mass index was calculated as weight (in kg) divided by height (in meters) squared. The remaining covariates were ascertained by retrieving ICD-9 codes from inpatient and outpatient encounters at all providers indexed in the REP (Supplemental Table 1). Two occurrences of a code (either the same or two different codes within the code set for a given disease) within the 5 years prior to index were required. These code sets and the 2-hit rule have been validated in cohorts of myocardial infarction and heart failure patients; the agreement with manually abstracted data was >80% for all variables in myocardial infarction patients and >90% for all variables in heart failure patients.
Statistical Analysis
Analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC). Characteristics of the patients by year of diagnosis (2000–2003, 2004–2007, 2008–2010) were compared using chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Annual incidence rates were calculated using the atrial fibrillation events as the numerators and the Olmsted County, MN population aged ≥18 from the 2000 and 2010 US census, with linear interpolation for the inter-censal years, as the denominator. Incidence rates were standardized to the age and sex distribution of the US 2010 population. To assess a temporal trend in the incidence of atrial fibrillation, Poisson regression was used to calculate the rate ratio (RR) for 2010 vs. 2000 after adjustment for age and sex. In the Poisson regression model with year, age, and sex, all 2-way and 3-way interactions were tested and an age*age and age*sex interaction were found to be significant (both p-values <0.01). Thus, we categorized age into <60 years, 60–69 years, 70–79 years, and ≥80 years and ran a Poisson regression model with the age*sex interaction terms to provide RRs for men compared to women within each age group.
Standardized mortality ratios of observed vs. expected survival were based on the age and sex of the study population and death rates from the Minnesota life tables. A dramatic excess risk of death was observed in the first 90 days. Thus, Cox proportional hazards regression was used to determine the association of year of atrial fibrillation diagnosis with all-cause mortality within the first 90 days and among 90-day survivors in separate models. The models were adjusted for age, sex, body mass index, smoking status, and prior diagnoses of hypertension, diabetes, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, dementia, rheumatologic disease, renal disease, liver disease, hemiplegia/paraplegia, malignancy, and metastatic solid tumor. All 2-way and 3-way interactions involving year of atrial fibrillation diagnosis, age, and sex were tested in both models. In ancillary analyses, additional adjustment for ischemic stroke and heart failure as time-dependent variables was conducted. Due to missing data on covariates in the fully-adjusted models, which was less than 1% for all variables, 3292 individuals were included in the all-cause mortality models. Population attributable fractions (PAFs) for risk factors of death within 90 days were calculated using the formula PAF=pdi[(RRi−1)/RRi], where pdi is the proportion of those who died who had the ith exposure level and RRi is the relative risk in the adjusted model comparing the ith exposure level to the unexposed.24 PAFs were not calculated for protective factors.
RESULTS
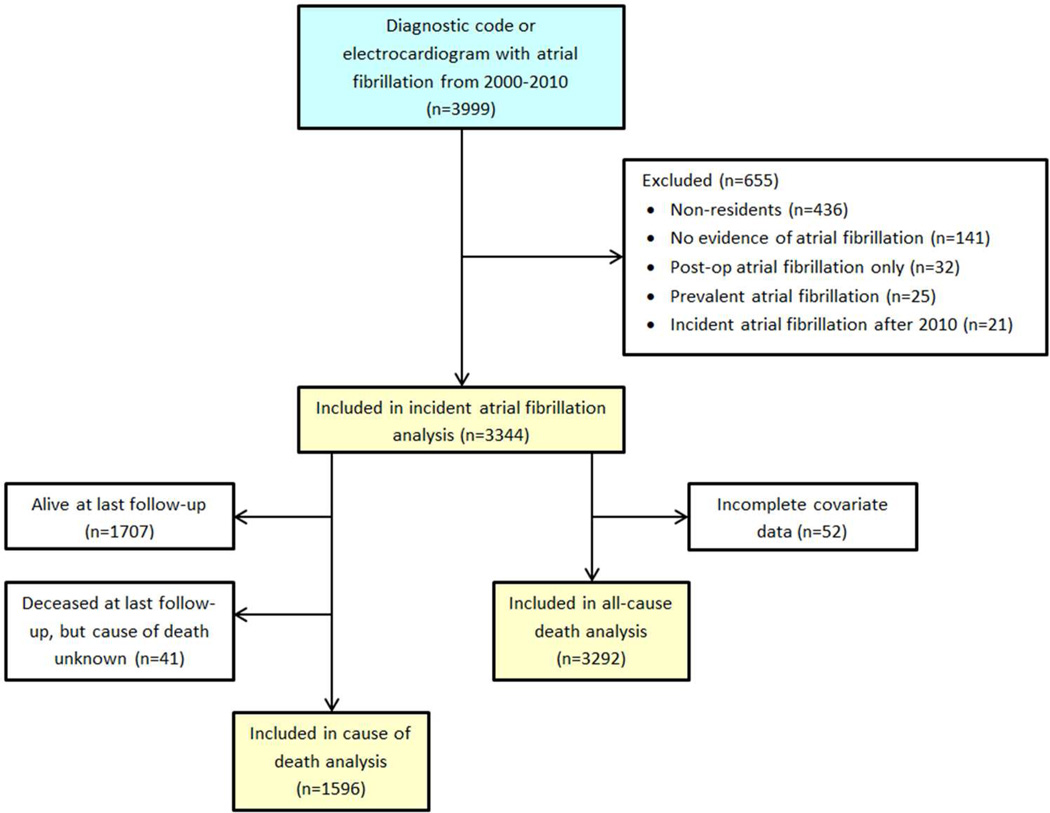
Between 2000–2010, 3344 incident atrial fibrillation patients (52% male, mean (SD) age 72.6 (14.7), 95.7% white) were identified (Figure 1). Among these, 88% had a documented electrocardiogram with evidence of atrial fibrillation or atrial flutter, 4% had evidence from a Holter monitor, event monitor, or telemetry, 4% from a monitor during a hospitalization or emergency department visit, and the remaining patients had evidence on pacemaker interrogation, on an electrocardiogram during an echocardiogram, or a physician diagnosis only. An increasing prevalence of hypertension, diabetes, and dementia and decreasing prevalence of heart failure and chronic pulmonary disease were observed across years of atrial fibrillation diagnosis (Table 1). A higher proportion of males and slightly higher body mass index were observed among patients diagnosed with atrial fibrillation in 2008–2010.
Figure 1.
Identification and validation of incident atrial fibrillation in Olmsted County, Minnesota between 2000 and 2010.
Table 1.
Characteristics of incident atrial fibrillation patients by year of diagnosis
| 2000–2003 (N=1092) |
2004–2007 (N=1247) |
2008–2010 (N=1005) |
p value | |
|---|---|---|---|---|
| Age, years | 72.8 (14.5) | 72.7 (14.9) | 72.2 (14.6) | 0.61 |
| Male | 549 (50.3%) | 621 (49.8%) | 555 (55.2%) | 0.02 |
| Body mass index, kg/m2 | 29.1 (7.3) | 28.9 (7.0) | 29.9 (7.5) | <0.01 |
| Smoking status | 0.68 | |||
| Never | 532 (49.0%) | 597 (48.2%) | 459 (45.9%) | |
| Current | 113 (10.4%) | 124 (10.0%) | 108 (10.8%) | |
| Former | 441 (40.6%) | 518 (41.8%) | 432 (43.2%) | |
| Hypertension | 686 (63.1%) | 857 (69.6%) | 715 (71.7%) | <0.01 |
| Diabetes | 201 (18.5%) | 263 (21.4%) | 255 (25.6%) | <0.01 |
| Myocardial infarction | 148 (13.6%) | 150 (12.2%) | 123 (12.3%) | 0.55 |
| Heart failure | 261 (24.0%) | 239 (19.4%) | 186 (18.7%) | <0.01 |
| Cerebrovascular disease | 174 (16.0%) | 199 (16.2%) | 151 (15.1%) | 0.79 |
| Peripheral vascular disease | 78 (7.2%) | 96 (7.8%) | 88 (8.8%) | 0.37 |
| Chronic pulmonary disease | 212 (19.5%) | 177 (14.4%) | 119 (11.9%) | <0.01 |
| Renal disease | 74 (6.8%) | 67 (5.4%) | 71 (7.1%) | 0.22 |
| Liver disease | 11 (1.0%) | 22 (1.8%) | 15 (1.5%) | 0.29 |
| Dementia | 26 (2.4%) | 52 (4.2%) | 53 (5.3%) | <0.01 |
| Rheumatologic disease | 54 (5.0%) | 60 (4.9%) | 47 (4.7%) | 0.97 |
| Hemiplegia/paraplegia | 27 (2.5%) | 26 (2.1%) | 28 (2.8%) | 0.57 |
| Malignancy | 225 (20.7%) | 200 (16.2%) | 203 (20.4%) | 0.01 |
| Metastatic solid tumor | 47 (4.3%) | 52 (4.2%) | 52 (5.2%) | 0.48 |
| CHADS2 score | 1.8 (1.4) | 1.9 (1.4) | 1.9 (1.3) | 0.53 |
Values are N (%) for categorical variables and mean (SD) for continuous variables.
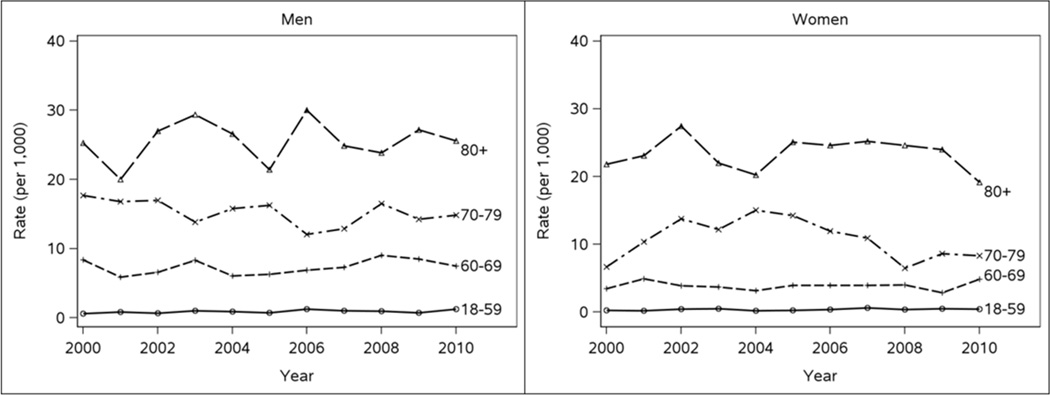
The atrial fibrillation incidence rates (95% confidence intervals (CI)) per 1,000 population standardized to the age and sex distribution of the 2010 US population were 2.99 (2.61–3.38), 3.23 (2.86–3.60), and 3.23 (2.89–3.58) for 2000, 2005, and 2010, respectively. No temporal trend in atrial fibrillation incidence was observed, with an age- and sex-adjusted RR (95% CI) of 1.01 (0.91–1.13) for 2010 vs. 2000. As expected, the incidence of atrial fibrillation increased exponentially with increasing age and was higher in men than women (Figure 2). The relative difference in sex was greatest in the youngest ages, attenuated with increasing age, and was no longer apparent among those ≥80 years of age (Table 2). The incidence of atrial fibrillation was 2.4-fold greater in men compared to women among those 18–59 years of age. Among those 60–69, a nearly two-fold higher rate of atrial fibrillation was observed in men, whereas among the 70–79 year olds, only a 40% higher incidence of AF was observed in men.
Figure 2.
Incidence of atrial fibrillation by age and sex strata in Olmsted County, Minnesota between 2000 and 2010.
Table 2.
Age-specific incidence rates and rate ratios for incidence of atrial fibrillation in men compared to women, 2000–2010
| Incidence Rates* | Rate Ratio (95% CI) | ||
|---|---|---|---|
| Men | Women | Men vs. Women | |
| 18–59 | 0.9 (0.8–1.0) | 0.4 (0.3–0.4) | 2.42 (2.03–2.88) |
| 60–69 | 7.4 (6.7–8.2) | 3.9 (3.4–4.4) | 1.90 (1.61–2.23) |
| 70–79 | 15.2 (13.9–16.6) | 10.7 (9.7–11.8) | 1.42 (1.24–1.61) |
| ≥80 | 25.6 (23.3–28.1) | 23.4 (21.8–25.1) | 1.10 (0.98–1.23) |
Unadjusted incidence rates per 1,000 (95% CI).
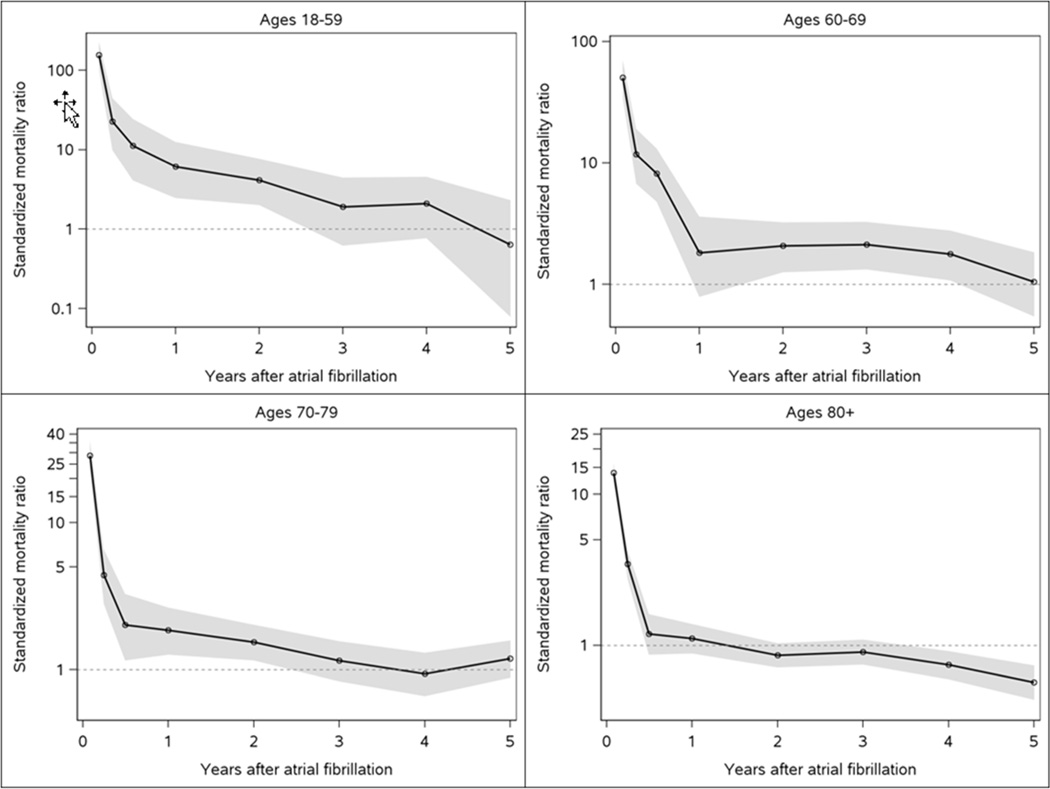
Over a mean (standard deviation) follow-up of 4.6 (3.5) years, 1615 deaths occurred. A dramatic excess risk of death was observed within the first 90 days after atrial fibrillation diagnosis. Standardized mortality ratios (95% CI) comparing the risk of mortality for atrial fibrillation patients to the Minnesota population of a similar age and sex distribution for the first 30 days, 31–90 days, and 91–365 days after diagnosis were 19.4 (17.3–21.7), 4.2 (3.5–5.0), and 1.5 (1.3–1.7), respectively. Atrial fibrillation patients exhibited an elevated risk of death through 1 year after diagnosis, but no increased mortality thereafter. The dramatic excess risk of death within the first 90 days was observed for all age groups; however, those <60 exhibited more than a 4-fold increased risk of death through 2 years after diagnosis (Figure 3).
Figure 3.
Standardized mortality ratios by age group after incident atrial fibrillation Shaded area indicates 95% confidence intervals.
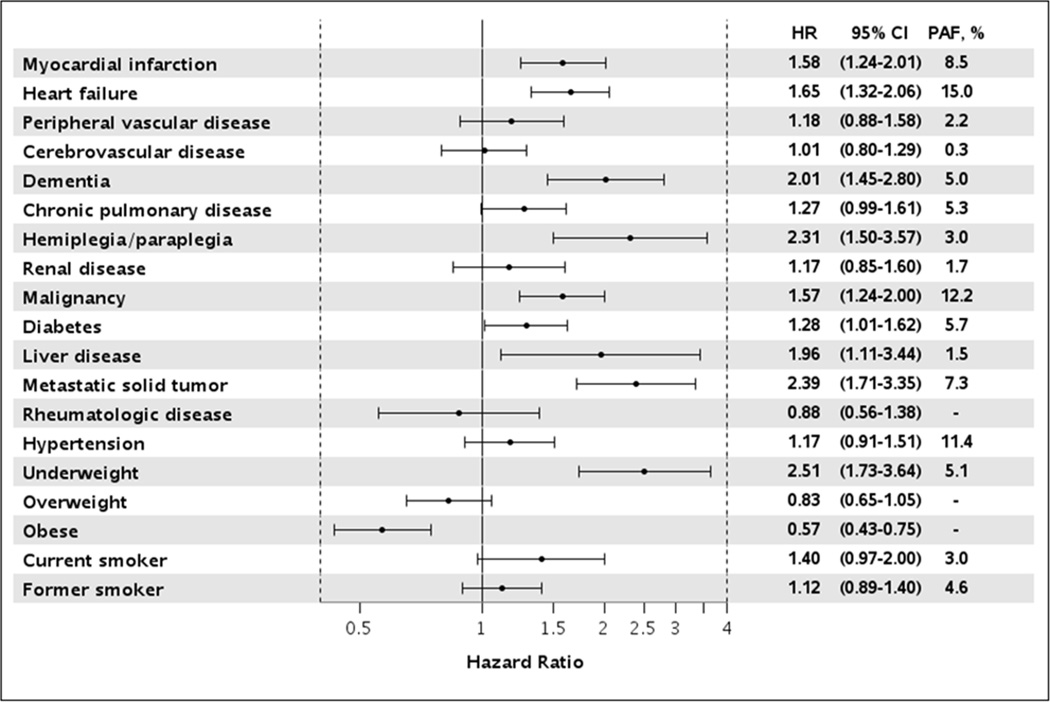
Within the first 90 days, 433 deaths occurred. Overall, the risk of death did not differ over the study period with an adjusted hazard ratio (HR) (95% CI) of 0.96 (0.71–1.32) for 2010 vs. 2000. However, a borderline sex*year of atrial fibrillation diagnosis interaction was observed (p=0.07). Women exhibited a slightly increased risk of death for 2010 vs. 2000 (HR 1.24, 95% CI 0.82–1.87) while men observed a slightly decreased risk (HR 0.70, 95% CI 0.44–1.11), although both estimates were nonsignificant. Results were similar after further adjustment for ischemic stroke and heart failure as time-dependent covariates (overall: HR 0.94, 95% CI 0.69–1.28). Predictors of death within 90 days included increasing age, underweight (body mass index <18.5), current smoking status, prior myocardial infarction, heart failure, diabetes, chronic pulmonary disease, liver disease, dementia, hemiplegia/paraplegia, and cancers including metastatic solid tumors (Figure 4). Risk factors with the largest PAF of death within 90 days were heart failure (15.0%), malignancy (12.2%), and hypertension (11.4%). For example, assuming a causal relationship, if heart failure were eliminated, 15% of the deaths within 90 days would have been avoided.
Figure 4.
Predictors of death within 90 days after diagnosis of atrial fibrillation Hazard ratios are adjusted for year of atrial fibrillation diagnosis, age, sex, and all other variables presented.
HR, hazard ratio; CI, confidence interval; PAF, population attributable fraction
Among 90-day survivors, the observed difference in all-cause mortality for 2010 vs. 2000 was not significant (HR 1.05, 95% CI 0.85–1.31), and no significant 2-way or 3-way interactions between year of atrial fibrillation, age, and sex were observed. Results were similar after further adjustment for time-dependent ischemic stroke and heart failure (HR 1.00, 95% CI 0.79–1.27). Finally, the proportion of deaths due to cardiovascular vs. non-cardiovascular causes did not vary significantly across year of atrial fibrillation diagnosis, with 38.2% of all deaths within 90 days and 40.6% of deaths after 90 days attributed to an underlying cardiovascular cause.
DISCUSSION
In this geographically-defined community, the incidence of atrial fibrillation remained constant between 2000–2010. A large excess risk of all-cause mortality was observed in the first 90 days following diagnosis. Survival has not improved over the study period for the first 90 days after atrial fibrillation or among 90 day survivors.
Contemporary community data on atrial fibrillation incidence are sparse. In Olmsted County, MN the incidence of atrial fibrillation increased between 1980–2000, de facto indicative of a true epidemic.2 Herein, we report the epidemic has leveled off as the incidence of atrial fibrillation remained constant in this community between 2000–2010. Our findings are consistent with data from the ARIC study between 1987–200417 and among Medicare beneficiaries between 1993–2007,18 which reported a constant rate of atrial fibrillation over time. Our incidence rates are similar to the rates for whites in the ARIC study,17 despite differences in the populations studied and methods employed for ascertainment of atrial fibrillation, which relied mainly on hospital discharge diagnoses in ARIC. In contrast, we observed lower incidence rates compared to Medicare beneficiaries;18 however, the reliance on Medicare claims data likely resulted in an overestimation of atrial fibrillation incidence since patients may receive diagnostic codes for atrial fibrillation for rule out and suspect scenarios.
We observed no significant decline in all-cause mortality after atrial fibrillation, and while a small decrease in mortality was observed over time in the Medicare study,18 our results are consistent with a prior study in Olmsted County from 1980–2000, which also found no improvement in survival after atrial fibrillation over time.20 Importantly, our data support previous findings of a large risk of death early after development of atrial fibrillation18, 20 and a nearly 20-fold increased risk of death within the first 30-days after diagnosis compared to individuals of similar age and sex in the general population.18 Interestingly, our data showed that the excess risk of mortality among atrial fibrillation patients was restricted to the first year after diagnosis and, thereafter, patients who survived the first year experienced similar mortality compared to the general population. These findings suggest that improvements in early survival after atrial fibrillation are needed; however, the majority of atrial fibrillation patients are elderly and have multiple comorbidities, which add complexity to their clinical management.
Clinical Implications and Future Directions
We have described the burden of atrial fibrillation in the community and shown that no improvements have been made in incidence or survival over the last decade. This finding has important clinical relevance given the increased focus on primary prevention of atrial fibrillation25, 26 and the many trials implemented over the last decade designed to improve treatment strategies in atrial fibrillation patients. In fact, no survival differences have been reported between rate and rhythm control treatment strategies.27–33 Furthermore, little survival benefit for the new oral anticoagulants compared to warfarin has been observed. Compared to warfarin, participants randomized to rivaroxaban in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial experienced similar mortality,34 and a borderline lower risk of all-cause mortality was observed among participants in the Apixaban for Reduction In Stroke and Other ThromboemboLic Events in Atrial Fibrillation (ARISTOTLE) trial receiving apixaban (HR 0.89, 95% CI 0.80–0.998).35 A lower risk of mortality was observed among those in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial receiving dabigatran at a dose of 150 mg (HR 0.89, 95% CI 0.79–1.01), but no survival benefit was observed at a dose of 110 mg.36 Nevertheless, a meta-analysis comparing novel oral anticoagulants to warfarin found a decrease in mortality for those on the novel agents (odds ratio (95% CI): 0.88 (0.82–0.95)).37
Collectively, the lack of improvement in incidence and survival over the last decade and the limited survival benefit of new therapies suggest the need for continued prevention efforts and management of atrial fibrillation risk factors. In addition, it is increasingly important to consider comorbid conditions in the management of patients with atrial fibrillation and to gain a better understanding of the role comorbidities play in the outcomes of these patients. Finally, continued research is warranted to identify additional factors associated with early death after atrial fibrillation and to identify optimal treatment strategies that will improve survival in these patients.
Limitations and Strengths
Limitations of our study, which are common to all studies on atrial fibrillation, include potentially missing atrial fibrillation in patients who were asymptomatic or who did not seek medical care for their symptoms, and misclassification of some prevalent asymptomatic or paroxysmal atrial fibrillation as incident. We may have missed patients who had their atrial fibrillation outside of Olmsted County and we may have missed some deaths that occurred outside of Minnesota. In addition, while the population of Olmsted County, MN is representative of the state of Minnesota and the Midwest region of the US,38 results may not be generalizable to the entire US. However, broad disease trends and age- and sex-specific mortality rates in Olmsted County are similar to national data,38 emphasizing the applicability of our results. Finally, as in any observational study, causality cannot be inferred from our results.
Despite these limitations, our study has many strengths, including the defined community setting, linkage of medical records allowing complete ascertainment from multiple sources of care, and inclusion of adults of all ages. In addition, following recommendations outlined by an expert panel to advance the understanding of the epidemiology of atrial fibrillation,25 our study has identified events occurring in both inpatient and outpatient settings and rigorous manual validation of each incident atrial fibrillation event was employed.
CONCLUSION
In the community, atrial fibrillation incidence has remained constant over the last decade. An excess risk of mortality was observed early after atrial fibrillation onset, yet survival did not improve. Thus, the atrial fibrillation epidemic remains largely unabated. These findings underscore the importance of management of atrial fibrillation risk factors, identification of prognostic factors, continued surveillance of outcomes, as well as a better understanding of how to optimize the management of atrial fibrillation to reduce the burden of atrial fibrillation in the community.
Supplementary Material
Clinical Significance.
In a geographically-defined community, AF incidence has remained constant between 2000 and 2010.
Likewise, no improvement has been made over time in survival after AF.
An excess risk of mortality among AF patients was observed in the 90 days after diagnosis. However, those who survived the first year experienced similar mortality compared to the general population, suggesting that improvements in early survival after AF are needed.
ACKNOWLEDGEMENTS
We thank Kay Traverse and Susan Stotz for assistance in data collection and Deborah Strain for secretarial assistance.
Funding: This work was supported by grants from the American Heart Association (11SDG7260039) and the National Institute on Aging (R01 AG034676). Dr. Roger is an Established Investigator of the American Heart Association. The funding sources played no role in the design, conduct, or reporting of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Authorship: All authors had access to the data and played a role in writing the manuscript.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu B-C, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11(2):281–298. doi: 10.3111/13696990802063425. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 7.Engstrom G, Goransson M, Hansen O, et al. Trends in long-term survival after myocardial infarction: less favourable patterns for patients from deprived areas. J Intern Med. 2000;248(5):425–434. doi: 10.1046/j.1365-2796.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 8.Floyd KC, Yarzebski J, Spencer FA, et al. A 30-year perspective (1975–2005) into the changing landscape of patients hospitalized with initial acute myocardial infarction: Worcester Heart Attack Study. Circ Cardiovasc Qual Outcomes. 2009;2(2):88–95. doi: 10.1161/CIRCOUTCOMES.108.811828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125(15):1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, et al. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353(9164):1547–1557. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 12.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Heywood JT, Heidenreich PA, et al. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153(6):1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Joffe SW, Webster K, McManus DD, et al. Improved survival after heart failure: a community-based perspective. J Am Heart Assoc. 2013;2(3):e000053. doi: 10.1161/JAHA.113.000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 16.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 17.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49(9):986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.St. Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin EJ, Chen P-S, Bild DE, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119(4):606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes NAM, 3rd, Sacco RL, Al-Khatib SM, et al. American Heart Association atrial fibrillation research summit: a conference report from the American Heart Association. Circulation. 2011;124(3):363–372. doi: 10.1161/CIR.0b013e318224b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41(10):1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 28.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356(9244):1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 29.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126(2):476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 30.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 31.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 32.Testa L, Biondi-Zoccai GGL, Dello Russo A, et al. Rate-control vs. rhythm-control in patients with atrial fibrillation: a meta-analysis. Eur Heart J. 2005;26(19):2000–2006. doi: 10.1093/eurheartj/ehi306. [DOI] [PubMed] [Google Scholar]
- 33.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 34.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 35.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 36.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 37.Capodanno D, Capranzano P, Giacchi G, et al. Novel oral anticoagulants versus warfarin in non-valvular atrial fibrillation: a meta-analysis of 50,578 patients. Int J Cardiol. 2013;167(4):1237–1241. doi: 10.1016/j.ijcard.2012.03.148. [DOI] [PubMed] [Google Scholar]
- 38.St. Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.