Abstract
Background
Cardiac troponin levels offer prognostic information for patients with heart failure. Highly sensitive assays detect levels of cTn much lower than the 99th percentile of standard cTn assays. We hypothesize that cardiac troponin (cTn) levels measured by a high sensitivity assay provide better prognostic value compared to cTn levels measured by a standard assay in patients with chronic heart failure.
Methods
We measured high sensitivity cTnT (hs-cTnT) and standard cTnI levels, as well as aminoterminal pro B-type natriuretic peptide (NT-proBNP) in 504 sequential stable patients with a history of heart failure who underwent elective coronary angiography, without acute coronary syndrome, and with 5-year follow-up of all-cause mortality.
Results
The median hs-cTnT level was 21.2 [interquartile range 12.3, 40.9] ng/L and 170 subjects died over 5-years. In a head-to-head overall comparison, hs-cTnT provided increased prognostic utility compared to cTnI (area under the curve [AUC] 66.1% and AUC 69.4%, respectively, p=0.03; 9.0% integrated discrimination improvement, p<.001; and 13.6% event-specific reclassification, p<.001), and was independent of NT-proBNP and renal function. Even within the subset of patients where cTn levels by both assays were above the limit of quantification, higher hs-cTnT is associated with a 2-fold increase in 5-year mortality risk after adjusting for traditional risk factors (tertile 1 vs. 3: Hazard ratio [95% confidence interval] 2.0 [1.3-3.2]; p=0.0002).
Conclusion
Cardiac troponin can be detected by the high sensitivity assay in more patients with chronic heart failure than the standard assay, and may yield independent and better prognostic accuracy for mortality prediction than standard assay.
Keywords: Heart Failure, high sensitivity cardiac troponin, cardiac troponin, prognosis
INTRODUCTION
Increasing levels of circulating cardiac troponin (cTn) are highly specific for ongoing myocardial damage and are traditionally utilized as markers for defining myocardial infarction.(1) Circulating cTn levels can also be elevated in other cardiac conditions such as acute and advanced chronic heart failure (2-3) where they may be related to to acute or chronic supply and demand mismatch (4) and may signify increased cardiomyocyte turnover in the setting of progressive myocardial dysfunction.(5)
With technological advances, cTn levels measured by high sensitivity assays have recently been developed, and can detect levels nearly one-tenth that of standard assays.(6) High sensitivity cTn assays are well-suited for detecting sub-clinical cardiac structural abnormalities and are frequently detected in patients with chronic heart failure than standard assays.(7) In patients with heart failure, circulating hs-cTn is associated with adverse cardiovascular events and with both cardiac and all-cause mortality.(7-11) High sensitivity assays expand the range of cTn detection and there is likely significant overlap with standard assays in patients with heart failure. Yet, there are few head-to-head comparisons of the prognostic utility of these two assays. As such, we hypothesize that circulating high sensitivity cTn will be associated with mortality and have increased prognostic accuracy compared to circulating cTn measured by a standard assay in patients with chronic stable heart failure.
METHODS
Study Population
We enrolled 504 consecutive subjects with a medical history of chronic heart failure who were undergoing elective diagnostic coronary angiography at the Cleveland Clinic between 2001 and 2007. We excluded patients who had an acute coronary syndrome, recent (<30 days) coronary revascularization, or history of heart transplantation. All participants gave their written informed consent and the study was approved by the Cleveland Clinic Institutional Review Board.
Study Design
Arterial blood samples were collected at the time of coronary angiography, after an overnight fast, after arterial sheath placement, but prior to the catheterization procedure or any therapies that were administered (including anticoagulation medications). Estimated glomerular filtration rate (eGFR) was calculated via the Modification of Diet in Renal Disease equation.(12) Left ventricle ejection fraction was determined via transthoracic echocardiography via biplane Simpson’s method by the Cleveland Clinic echocardiography lab and the results were collected via chart review of the electronic medical record, EPIC (EPIC, Verona, WI). Heart failure with preserved or reduced ejection was defined as left ventricular ejection fraction ≥40% or <40%, respectively. Adjudicated outcomes including mortality, death, myocardial infarction, and stroke, were prospectively collected over the 5 years by dedicated research personnel and by Social Security Death Index after enrollment for all cohort subjects.
Cardiac Biomarkers Measurement
All biomarkers were measured at a central core laboratory. High-sensitivity cardiac troponin T (hs-cTnT) was measured by a high sensitivity (5th generation) assay on a Roche Cobas e411 platform (Roche Diagnostics, Basel, Switzerland). The limit of detection (LOD) was 3 ng/L and there were no values measured below this level in this cohort. The 99th percentile cutoff was 14 ng/L with an average coefficient of variation <10% at 13 ng/L. Aminoterminal pro B-type natriuretic peptide (NT-proBNP) was measured on the same Roche platform. Cardiac troponin I (cTnI) was measured by a standard sensitivity assay on the Abbott Architect platform (STAT Troponin I, Abbott Laboratories, Abbott Park, IL, USA) with analytical sensitivity at 0.01ng/mL. Troponin I values below the LOD were considered “undetectable.” Creatinine and fasting lipid profiles were measured on the same Abbott platform.
Statistical Analysis
Statistical analyses were performed using JMP Pro version 10 (SAS Institute, Inc, Cary, North Carolina) and R software, version 3.0.2. Continuous variables were expressed as either mean ± standard deviation or median [interquartile range] and analyzed by the Student’s unpaired t-test or the Wilcoxon or Kruskal-Wallis tests where appropriate. Categorical variables were expressed as percentage (%) and analyzed by Fisher’s Exact test. Spearman’s correlations were performed to assess relationship between hs-cTnT and clinical characteristics characterized by continuous variables. This cohort wass split into two groups, split by the LOD of cTnI in a normal reference population: subjects with cTnI < 0.01 ng/mL (“undetectable cTnI”) or with cTnI ≥ 0.01 ng/mL (“detectable cTnI”). The subgroups above and below the cTnI LOD were each split into tertiles of hs-cTnT levels. Independent variables were cTnI ≥ or < 0.01 ng/mL (n=302 and n=202, respectively), hs-cTnT tertiles overall, and hs-cTnT tertiles in each cTnI subgroup. Dependent variables were mortality at 5 years. Two-sided p-values of ≤ 0.05 were considered significant to reject the null hypothesis that there are no differences in mortality at 5 years of follow-up between cTn levels. Survival analyses were completed via the Kaplan-Meier method and log-rank analysis to compare survival curves between cTnI and hs-cTnT groups. Cox proportional hazards models were used to compare time-to-event analysis to determine hazard ratios (HR) and 95% confidence intervals (CI) for 5-year mortality across tertiles of cTnI and hs-cTnT. Multivariate adjustment (base model for mortality) was for age, sex, systolic blood pressure, diabetes mellitus, smoking history, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol. In contrast to the area under the curve (AUC), which is a measure of discrimination for the predictive separation of a model based on risk, we included the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) as methods to compare the relative performance of two prognostic models.(13) NRI reflects the proportion of cases that are reclassified to a higher risk category between models. The term IDI is based on the difference of average predicted risks for the cases and controls between models. Risk prediction and net-reclassification methods were used to compare Cox hazard models for mortality by the Pencina method.(14)
RESULTS
Baseline Characteristics
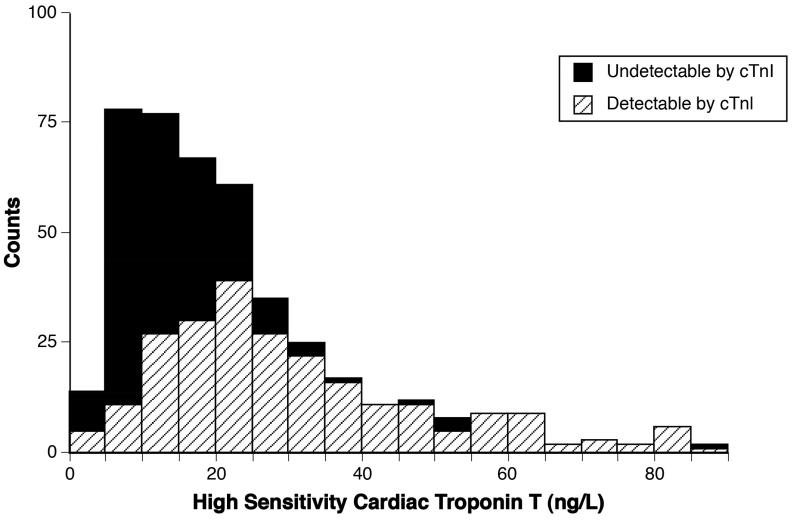
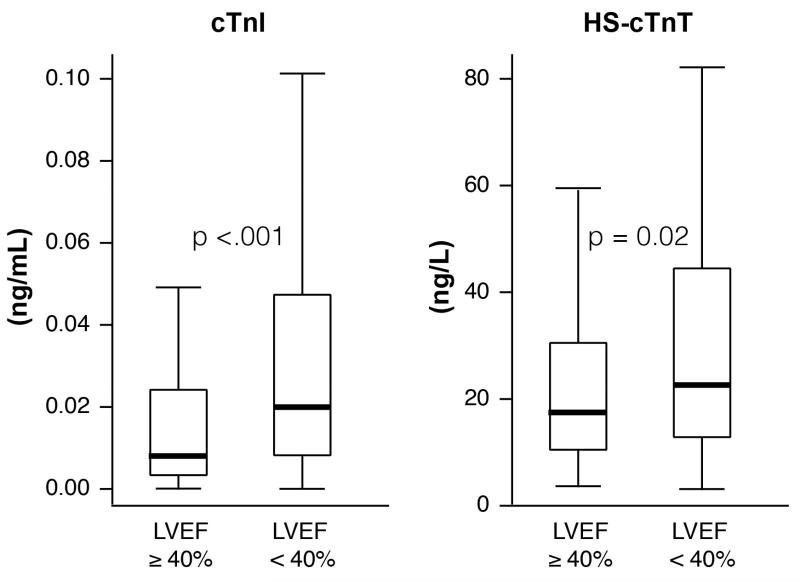
Baseline characteristics for our cohort (all with detectable hs-cTnT and 302 (59.9%) with detectable cTnI) were representative of a patient population with chronic heart failure and are described in Table 1. High sensitivity cTnT levels were non-parametrically distributed with a right skew (Figure 1). The median hs-cTnT level was 21.2 [12.3, 40.9] ng/L. Median hs-cTnT levels across increasing tertiles of hs-cTnT for the whole cohort were 9.6 [7.1, 12.2], 21.1 [17.9, 24.6], and 63.2 [40.7, 189.9] ng/L, respectively. Both cTnI and hs-cTnT levels were higher in subjects with left ventricular ejection fraction<40% in comparison to subjects with an left ventricular ejection fraction≥40% (p<.001 and p=0.02, respectively (Figure 2)). Median hs-cTnT levels were higher in men than women (23 [14, 49] ng/L and 18 [10, 31] ng/L, p=0.0005, respectively).
Table 1. Baseline Characteristics (n=504).
| Variable | Total Cohort (n=504) |
Detectable cTnI (n=302) |
Undetectable cTnI (n=202) |
p-value |
|---|---|---|---|---|
| Age [years] | 68 ± 10 | 69 ± 11 | 66 ± 10 | 0.01 |
| Male (%) | 63.1 | 67.2 | 56.9 | 0.02 |
| Diabetes (%) | 36.5 | 43.1 | 26.5 | 0.0001 |
| Coronary Artery Disease (%) |
78.0 | 83.3 | 70.0 | 0.0006 |
| Hypertension (%) | 76.4 | 79.7 | 71.6 | 0.04 |
| eGFR [mL/min/1.73 m2] | 74.8 ± 26.2 | 69.3 ± 27.5 | 83.3 ± 21.7 | <.0001 |
| Ever Smoker (%) | 70.2 | 68.5 | 72.8 | 0.3 |
| BMI [kg/m2] | 29 ± 6 | 29 ± 7 | 30 ± 7 | 0.1 |
| Beta-Blocker (%) | 63.3 | 61.3 | 66.3 | 0.3 |
| ACEI / ARB (%) | 68.3 | 65.9 | 71.8 | 0.2 |
| Loop Diuretic (%) | 63.9 | 67.2 | 58.9 | 0.06 |
| Left ventricular ejection fraction [%] |
40 [27, 55] | 35 [25, 50] | 45 [35, 55] | <.0001 |
| NT-proBNP [pg/mL] | 1057 [400, 2715] |
1787 [798, 4720] |
531 [197, 1110] |
<.0001 |
| hs-cTnT [ng/L] | 21.2 [12.3, 40.9] |
32.7 [20.3, 75.2] |
12.5 [8.6, 18.1] |
<.0001 |
Abbreviations: eGFR: estimated glomerular filtration rate, MDRD; BMI: body mass index; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; NT-proBNP: amino terminus - pro B-type natriuretic peptide; cTnI: cardiac troponin I measured by standard assay; hs-cTnT: cardiac troponin T measured by a highly sensitive assay; and DASI: Duke Activity Status Index.
Continuous values are expressed mean ± standard deviation or median [interquartile range].
For comparison between cTnI levels ≥ or < 0.01 ng/mL, P-values calculated via Student’s T-test or Wilcoxon for continuous variables and Fisher’s Exact Test for categorical variables.
Figure 1. Distribution of High Sensitivity Cardiac Troponin T Levels.
All values are ≥ the limit of detection for the high sensitivity cardiac troponin T assay, 3 ng/L and 302 (59.9%) subjects had detectable cTnI.
Figure 2. Cardiac Troponin Levels Stratified by Left Ventricular Ejection Fraction.
p-value calculated by Wilcoxon Test. Left Ventricular Ejection Fraction: left ventricular ejection fraction; cTnI: cardiac troponin I; hs-cTnT: high sensitivity cardiac troponin T.
Circulating hs-cTnT and Mortality in the Overall Cohort
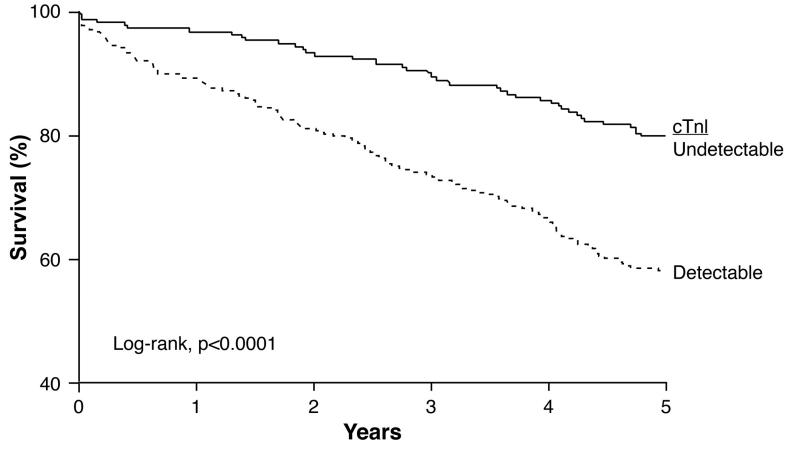
At 5 years, there were a total of 170 deaths with an estimated cohort 5-year survival of 66%. Detectable cTnI was associated with higher incident mortality than undetectable cTnI (57% versus 80%%, respectively; log-rank chi-square 28.0 and p<0.0001, Figure 3a).
Figure 3a. Kaplan-Meier Estimates of 5-Year Survival Rates According to cTnI Levels Above or Below the Limit of Detection (n=504).
cTnI = cardiac troponin I. P-value calculated by the log-rank test.
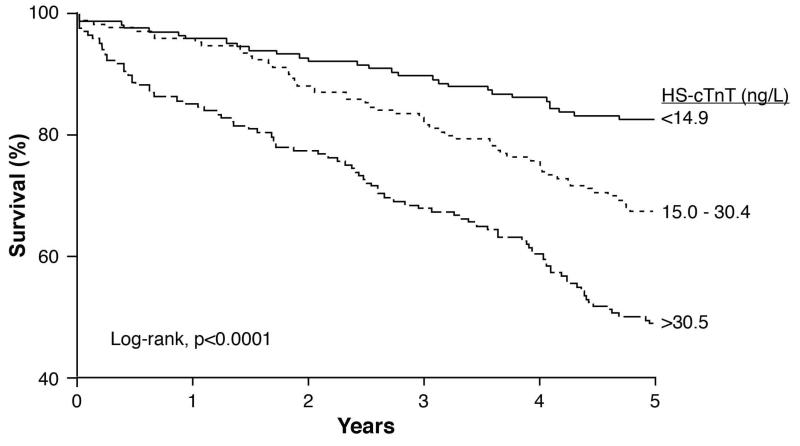
Grouped by hs-cTnT level, hs-cTnT tertiles 1, 2, and 3 had 29, 55, and 86 deaths, respectively, with significant decrements in survival for increasing tertiles (Figure 3b, log-rank chi-square 44.9 and p<.0001). Increased hs-cTnT was associated with nearly a 3.7-fold increase in 5-year mortality (tertile 1 vs. 3, HR 3.74, 95% CI 2.49-5.79, p<.0001). After adjustment for traditional risk factors in addition to hypertension history, and coronary artery disease history, eGFR, NT-proBNP, angiotensin converting enzyme inhibitor/angiotensin receptor blocker use, beta-blocker use, history of chronic obstructive pulmonary disease, serum sodium, left ventricular ejection fraction, and blood urea nitrogen, increased hs-cTnT remained independently associated with 5-year mortality (tertile 1 vs. 3: HR 2.14, 95% CI 1.24-3.79, p=0.006).
Figure 3b. Kaplan-Meier Estimates of 5-Year Survival Rates According to hs-cTnT Levels (n=504).
hs-cTnT = high sensitivity cardiac troponin T. P-value calculated by the log-rank test.
In comparison to the base model for mortality with cTnI the prognostic accuracy of the model with hs-cTnT (Table 2) was modestly improved (AUC 66.1% and AUC 69.4%, respectively, p=0.03) with a 9.0% IDI (p<.001) and 13.6% NRI (p<.001). In contrast, there was no increase in prognostic accuracy when cTnI and hs-cTnT were both added to the base model for mortality (AUC 69.4% and AUC 69.2%, respectively, p=0.9) although there was continued IDI (9.0%, p<.001) and NRI (3.6%, p<.001).
Table 2. Risk Prediction and Net Reclassification for Cox Multivariate Hazard Models for Mortality.
| Whole Cohort (n=504) | Detectable cTnI (n=302) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrated Discrimination Improvement |
Event-Specific Reclassification |
ROC Analysis | Integrated Discrimination Improvement |
Event-Specific Reclassification |
ROC Analysis | |||||||
| Covariate | IDI (%) | p- value |
NRI (%) | p- value |
AUC (%) |
p- value |
IDI (%) | p- value |
NRI (%) | p- value |
AUC (%) |
p- value |
| With HS- cTnT* |
9.0 | <.001 | 13.6 | <.001 | 69.4 | 0.03 | 11.0 | <.001 | 8.1 | <.001 | 71.2 | 0.8 |
| With HS- cTnT over cTnI* |
9.0 | <.001 | 3.6 | <.001 | 69.2 | 0.9 | 11.0 | <.001 | 6.8 | <.001 | 70.9 | 0.9 |
Base model for 5-year mortality includes: age, gender, systolic blood pressure, low-density lipoprotein cholestero, high-density lipoprotein cholesterol, smoking, and diabetes
p-values for comparison to base model + cTnl
Base model + cTnl AUC: 66.1 and 70.8%, for whole cohort and detectable cTnl, respectively
Abbreviations: IDI: integrated discrimination improvement; NRI: net reclassification improvement; AUC: area under the curve; ROC: receiver operating curve; cTnl: cardiac troponin I; HS-cTnT: cardiac troponin T measured by a highly sensitive assay; and ref: reference model.
Circulating hs-cTnT and Mortality in the Detectable cTnI Subgroup
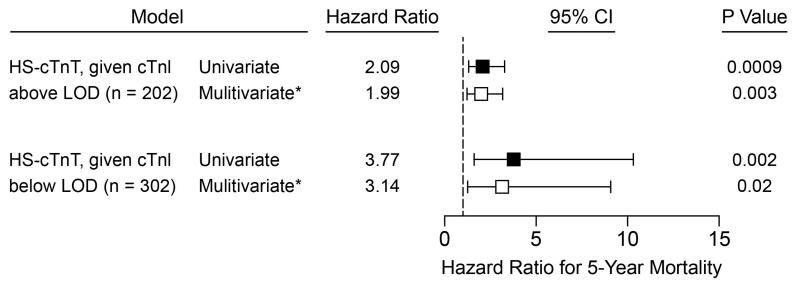
In the subgroup with detectable cTnI (n=302), cTnI and hs-cTnT were correlated (Spearman’s rho 0.74, p<.0001).The highest hs-cTnT tertile was associated with a 2.1-fold increase in 5-year mortality risk when compared to the lowest hs-cTnT tertile (HR 2.1, 95% CI 1.4-3.3, p=0.0009, Figure 4). After multivariate adjustment for traditional risk factors, the association between high hs-cTnT and mortality was persisted (HR 2.0, 95% CI 1.3-3.2, p=0.003). In a sensitivity analysis with additional adjustment for hypertension history and coronary artery disease history, the association between high hs-cTnT and mortality persisted (HR 1.96, 95% CI 1.22-3.20, p=0.005). In furthersensitivity analyses with additional adjustment for eGFR to traditional risk factors, there was a similar association with high hs-cTnT and mortality (HR 2.1, 95% CI 1.1-4.0, p=0.03), but the effects were not significant when NT-proBNP was further added to the model.
Figure 4. Cox Proportional Hazards Models and Forest Plot for Risk of 5-Year Mortality.
Tertiles 1 vs 3. For the detectable cTnI subgroup, hs-cTnT tertiles 1-3 were < 23.6, 23.6-52.1, and >52.1 ng/L. For the undetectable cTnI subgroup, hs-cTnT tertiles 1-3 were < 9.7, 9.7-12.4, and >12.4 ng/L.
*adjustment for age, sex, systolic blood pressure, diabetes, smoking history, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. †LOD: limit of detection; for cTnI = 0.01.
In comparison to the base model for mortality with cTnI (AUC 70.8%), the prognostic accuracy of base model with hs-cTnT or with both hs-cTnT and cTnI was not different (AUC 71.2%, p=0.8 and AUC 70.9%, p=0.9; respectively; Table 2). However, both models had sustained IDI at 11.0% (p<.001, respectively) and had 8.1% (p<.001) and 6.8% (p<.001) event-specific NRI, respectively, when compared to the base model for mortality with cTnI.
Circulating hs-cTnT Mortality in the Undetectable cTnI Subgroup
High sensitivity cTnT levels in this subgroup were modestly correlated with age (r=0.30, p<0.0001) and NT-proBNP (r=0.25, r=0.0001) but negatively correlated with eGFR (r=−0.33, p<.0001). For the undetectable cTnI subgroup (n=202), tertiles of circulating hs-cTnT were < 9.7, 9.7-12.4, and >12.4 ng/L. In Cox proportional hazards models, the highest hs-cTnT tertile was associated with a 3.8-fold increase in 5-year mortality compared to the lowest hs-cTnT tertile (HR 3.8, 95% CI 1.6-10.3, p=0.002, Figure 4). This relationship persisted after multivariate adjustment for traditional risk factors (HR 3.1, 95% CI 1.2-9.1, p=0.02). In a sensitivity analysis with additional adjustment for hypertension history and coronary artery disease history, the association between high hs-cTnT and mortality persisted (HR 3.12, 95% CI 1.21-9.16, p=0.02). In further sensitivity analysess with additional adjustment for eGFR to traditional risk factors, higher hs-cTnT had a similar association with mortality (HR 3.6, 95% CI 1.4-10.4, p=0.008), yet the effects were also not significant when NT-proBNP was further added to the model.
DISCUSSION
This head-to head comparative study of cTn assays has several novel findings that improve our understanding of the clinical utility of cardiac troponin levels measured by highly sensitive assays in chronic stable heart failure. First, circulating cTn was detectable in more (33.1%) patients via the high sensitivity assay compared to the standard assay. Second, patients with heart failure and reduced ejection fraction had higher cTn levels compared to patients with heart failure and preserved ejection fraction. Third, hs-cTnT yielded independent and incremental prognostic information to traditional risk factors and even to NT-proBNP and eGFR. However, while the analytical performance of hs-cTnT appeared superior, there was overlap in prognostic accuracy of hs-cTnT in subjects with detectable cTnI. These findings highlight the prognostic value of highly sensitive cTn assays in the setting of heart failure, yet also point to the need for future studies to better determine whether the improved sensitivity of cTn assays can translate into incremental clinical benefits.
In contrast to the general population,(15-16) patients with chronic heart failure have more prevalent detectable cTn. The etiology of cTn release in chronic heart failure patients is unclear and likely multifactorial. It may be triggered by acute and chronic myocardial stress, chronic sub-clinical sub-endocardial ischemia, or direct cardiomyocyte injury.(17) It may also result from increased apoptosis in heart failure,(5) thus representing increased cardiomyocyte turnover which may be indicative of progressive myocardial dysfunction. In heart failure patients, circulating cTn levels have prognostic value independent of renal function (18) and natriuretic peptide levels,(19) in either heart failure with reduced or preserved left ventricular ejection fraction,(20) and in the elderly.(11)
Our results support and add to the growing body of evidence that detectable cTn at any level of assay sensitivity has strong prognostic utility in patients with heart failure.(7-11, 21-23) As hypothesized, increased circulating hs-cTnT was independently and incrementally associated with incident 5-year mortality after multivariate adjustment for strong heart failure risk factors. Previously, a retrospective analysis of the Valsartan Heart Failure Trial (Val-HeFT) found a similarly high portion (92.0% (3728/4053)) had detectable hs-cTn by an older assay.(7) In this and another analysis which pooled the Val-HeFT and Gruppo Italiano per lo Studio della Sproavvivenza nell’Insufficienza Cardiaca-Heart Failure cohorts,(9) higher circulating hs-cTn was associated with both incident heart failure hospitalization and mortality and improved the prognostic accuracy of subjects’ clinical risk factors in addition to B-type natriuretic peptide. Unlike our analysis, however, no head-to-head comparisons were made between circulating cTn by both assays.
What is unclear from previous chronic heart failure cohorts, where circulating cTn is measured by both assays,(7, 9) is determining whether there is a clinical advantage to prognosticate by measuring hs-cTn when cTn may be detectable by a standard assay; and if such an advantage is related to the increased sensitivity of the hs-cTn assay. In our cohort, circulating hs-cTnT had higher prognostic accuracy when compared to circulating cTnI, thus supporting the use of measuring cTn by high sensitivity assays instead of standard assays in chronic heart failure. However, there was overlap in incident mortality discrimination of measuring circulating hs-cTnT if cTnI was detectable, which questions the use of measuring cTn by a high sensitivity assay if cTn is in the detectable range of the standard assay. Therefore, these results suggest that circulating hs-cTn may have a higher prognostic accuracy primarily as a result of their increased sensitivity.(6) In other words, when patients present with a quantifiable cTn level based on standard assay, there appears to be an incremental benefit to stratify risk, although a limited change in endpoint prediction (discrimination) with the addition of a highly sensitive cTn test based on our findings. Risk calibration and endpoint discrimination are often discordant for major disease factors and excessive reliance on the AUC has been previously discouraged. (24)
One of the biggest advantages of utilizing a more sensitive assay is to expand the lower range of quantifiable cTn. From head-to-head comparisons in non-heart failure populations, circulating cTn measured by high sensitivity assays identifies a larger population with cardiac risk factors, structural cardiac abnormalities, increased risk of incident heart failure, and adverse cardiac events than circulating cTn measured by standard assays.(15-16, 25) In the undetectable cTnI subgroup, very low levels of circulating hs-cTnT were still associated with 5-year mortality (Figure 4). This suggests that very low circulating hs-cTnT, well below the assay’s 99th percentile cut-off, yields prognostically important information in patients with heart failure and are supported by similar findings in previous heart failure cohorts.(7, 9, 11) Taken in aggregate, there appears to beclinically important information embedded in very low cTn levels, thusquestioning the clinical utility of using a 99th percentile cut-off for “normal” in patients with heart failure. Although we describe associations of very low cTn levels with age, NT-proBNP, and renal function, further studies are needed to determine the etiology of cTn release and whether similarly low-risk chronic heart failure populations need further risk stratification.
Furthermore, high sensitivity cTn levels may be viable therapeutic targets for medication titration in similarly low-risk patients with chronic heart failure. In patients with non-ST-segment elevation acute coronary syndromes, antiplatelet therapy escalation guided by circulating cTn has been shown to favorably influence treatment outcomes.(26) Indeed, detectable cTn levels in the setting of receiving high-dose chemotherapy have already demonstrated the ability to identify a patient population with risk of progressive deterioration of cardiac dysfunction that may be ameliorated by initiation of angiotensin-converting enzyme inhibitors.(27) In heart failure, however, whether adjusting chronic heart failure therapy (beta-blockers, renin-angiotensin blockers, or mineralocorticoid receptor antagonists) affects serial hs-cTn levels is unknown. Yet, because changing high sensitivity cTn levels are associated with prognosis in chronic heart failure,(9, 28) future studies to assess associations of medical therapy adjustments and changes high sensitivity cTn levels are therefore warranted.
These results must be interpreted in the context of several limitations in our study design. Because cTn levels were only measured at one point in time, we were unable to examine the variability and prognostic value of changing cTn levels by two cTn assays over time or the impact of different therapies in the interim. We cannot exclude the presence of selection bias for those undergoing coronary angiography for further evaluation and management of heart failure at a tertiary care center, even though based on baseline clinical characteristics, our cohort is relatively representative of a contemporary patient population with chronic heart failure with both preserved and reduced left ventricular ejection fraction and we excluded all patients with any suspicion or clinical history of acute coronary syndromes. However, limitations to external validity include a large proportion of patients in this analysis with ischemic cardiomyopathy. Indeed, many non-cardiac conditions are associated with detectable circulating troponin (29) such as sepsis, pulmonary embolism, chronic kidney disease, and myocarditis. With the exception of renal dysfunction, the incidence of these and other acute conditions where troponin is associated with mortality was likely very low as subjects in this study were included prior to elective coronary angiography .Nevertheless, based on these analyses and because we are in the era when recognizing the potential harms for excessive diagnostics are at the forefront, further investigations continuing to clarify clinical utilities of cTn measured by highly sensitive assays are warranted.
CONCLUSION
In patients with chronic heart failure, when compared to standard assays, high sensitivity assays identify more patients with detectable circulating cTn. Although plasma hs-cTnT levels provide incremental and independent prognostic value and increased prognostic accuracy in patients with chronic heart failure, there is overlap in this value when both assays measure cTn in the detectable range.
CLINICAL SIGNIFICANCE.
-
-
Circulating cardiac troponin (cTn) was detectable in more stable heart failure patients via the high sensitivity assay compared to the standard assay.
-
-
Heart failure with reduced ejection fraction was associated with higher cTn levels than heart failure with preserved ejection fraction.
-
-
Although high sensitivity cTn levels can risk-stratify lower-risk heart failure patients, there is no added prognostic value when circulating cTn is detectable by a standard assay.
Acknowledgments
FUNDING SOURCE(S):
Dr. Tang is supported by National Institutes of Health (NIH) grants R01HL103931, P20HL113452 (with Office of Dietary Supplements), P01HL076491, P01HL098055, R01HL103931, and UL1TR 000439.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT:
Dr. Hazen is named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott Diagnostics, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., P&G, and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., P&G, and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens.
All other authors (JLG, SN, and WHWT) have no relationships to disclose.
All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Peacock WFt, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 3.Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56(14):1071–8. doi: 10.1016/j.jacc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Wu AH. Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med. 2001;27(6):959–61. doi: 10.1007/s001340100970. [DOI] [PubMed] [Google Scholar]
- 5.Narula J, Pandey P, Arbustini E, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999;96(14):8144–9. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58(11):1574–81. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 7.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116(11):1242–9. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan V, Hernandez AV, Tang WH. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart. 2012;98(24):1778–86. doi: 10.1136/heartjnl-2012-301779. [DOI] [PubMed] [Google Scholar]
- 9.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125(2):280–8. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 10.Egstrup M, Schou M, Tuxen CD, et al. Prediction of outcome by highly sensitive troponin T in outpatients with chronic systolic left ventricular heart failure. Am J Cardiol. 2012;110(4):552–7. doi: 10.1016/j.amjcard.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Gravning J, Askevold ET, Nymo SH, et al. Prognostic Effect of High-Sensitive Troponin T Assessment in Elderly Patients With Chronic Heart Failure: Results From the CORONA Trial. Circ Heart Fail. 2014;7(1):96–103. doi: 10.1161/CIRCHEARTFAILURE.113.000450. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48(12):1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113(16):1958–65. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 16.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Schaus BJ, Fallavollita JA, et al. Preload induces troponin I degradation independently of myocardial ischemia. Circulation. 2001;103(16):2035–7. doi: 10.1161/01.cir.103.16.2035. [DOI] [PubMed] [Google Scholar]
- 18.Orea-Tejeda A, Sanchez-Gonzalez LR, Castillo-Martinez L, et al. Prognostic value of cardiac troponin T elevation is independent of renal function and clinical findings in heart failure patients. Cardiol J. 2010;17(1):42–8. [PubMed] [Google Scholar]
- 19.Jungbauer CG, Riedlinger J, Buchner S, et al. High-sensitive troponin T in chronic heart failure correlates with severity of symptoms, left ventricular dysfunction and prognosis independently from N-terminal pro-b-type natriuretic peptide. Clin Chem Lab Med. 2011;49(11):1899–906. doi: 10.1515/CCLM.2011.251. [DOI] [PubMed] [Google Scholar]
- 20.Macin SM, Perna ER, Cimbaro Canella JP, et al. Increased levels of cardiac troponin-T in outpatients with heart failure and preserved systolic function are related to adverse clinical findings and outcome. Coron Artery Dis. 2006;17(8):685–91. doi: 10.1097/01.mca.0000236287.56435.14. [DOI] [PubMed] [Google Scholar]
- 21.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108(7):833–8. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 22.Hudson MP, O’Connor CM, Gattis WA, et al. Implications of elevated cardiac troponin T in ambulatory patients with heart failure: a prospective analysis. Am Heart J. 2004;147(3):546–52. doi: 10.1016/j.ahj.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Healey JS, Davies RF, Smith SJ, et al. Prognostic use of cardiac troponin T and troponin I in patients with heart failure. Can J Cardiol. 2003;19(4):383–6. [PubMed] [Google Scholar]
- 24.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 25.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamm CW, Heeschen C, Goldmann B, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N Engl J Med. 1999;340(21):1623–9. doi: 10.1056/NEJM199905273402103. [DOI] [PubMed] [Google Scholar]
- 27.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara C, Tsutamoto T, Sakai H, et al. Prognostic value of serial measurements of highly sensitive cardiac troponin I in stable outpatients with nonischemic chronic heart failure. Am Heart J. 2011;162(4):639–45. doi: 10.1016/j.ahj.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60(23):2427–63. doi: 10.1016/j.jacc.2012.08.969. [DOI] [PubMed] [Google Scholar]