Abstract
During an immune response against a microbial pathogen, activated naïve T lymphocytes give rise to effector cells that provide acute host defense and memory cells that provide long-lived immunity. It has been shown that T lymphocytes can undergo asymmetric division, enabling the daughter cells to inherit unequal amounts of fate-determining proteins and thereby acquire distinct fates from their inception. Here, we show that the absence of the atypical protein kinase C (aPKC) isoforms, PKCζ and PKCλ/ι, disrupts asymmetric CD8+ T lymphocyte division. These alterations were associated with aberrant acquisition of a ‘pre-effector’ transcriptional program, detected by single-cell gene expression analyses, in lymphocytes that had undergone their first division in vivo and enhanced differentiation toward effector fates at the expense of memory fates. Together, these results demonstrate a role for aPKC in regulating asymmetric division and the specification of divergent CD8+ T lymphocyte fates early during an immune response.
INTRODUCTION
The generation of effector and memory CD8+ T lymphocyte subsets is a key function of an adaptive immune response against microbial pathogen. Short-lived, terminally differentiated effector T (TSLE) cells provide acute host defense, while memory lymphocytes provide long-term protection against reinfection (1). Among long-lived memory lymphocytes, effector memory T (TEM) cells patrol the peripheral tissues and execute immediate effector functions after re-encountering microbe, while central memory T (TCM) cells patrol the lymphoid tissues and retain the capacity to proliferate upon rechallenge (2). Recent reports have suggested that another lymphocyte subset, so-called ‘long-lived effector’ T cells, may persist into the memory phase and mediate a potent protective response upon re-infection, but these cells seem to lack the same capacity for long-term survival as TEM or TCM cells (3, 4).
While it has been shown that a single activated naïve CD8+ T cell can generate all of the diverse cellular fates necessary for a robust immune response (5, 6), it remains unclear when the differentiation pathways leading to these disparate cellular fates diverge. One possibility is that the progeny of an activated CD8+ T lymphocyte progress along a linear differentiation pathway, initially becoming effector cells, with a subset of these cells later acquiring the memory fate (7). Another possibility is that an activated CD8+ T cell might undergo asymmetric division, thereby enabling lymphocyte fates to diverge early during an immune response (8–10). During asymmetric division, cellular components and fate determinants are unequally partitioned into the two daughter cells, which may subsequently acquire distinct fates as a result of differences in size, morphology, gene expression, or protein abundance (11). In T lymphocytes, potential fate determinants that undergo asymmetric partitioning during the first division include the transcription factor T-bet and the IL-2 and IFN-γ receptors (8–10). Because signals downstream of these pathways have been implicated in effector CD8+ T cell differentiation (12–17), these observations suggest a key role for asymmetric division in regulating CD8+ T lymphocyte fate specification.
Asymmetric division has been shown to control fate specification of many different cell types and tissues in C. elegans embryos and Drosophila neuroblasts (11, 18, 19). In these model systems, evolutionarily conserved polarity proteins, most notably atypical protein kinase C (aPKC), have been shown to regulate asymmetric cell division and, in turn, control the balance between terminal differentiation and self-renewal (20–22). Chemical inhibition and siRNA knockdown approaches have suggested a similar role for aPKC in the regulation of asymmetric division by CD8+ T cells (9, 23), but the extent and specific role of each aPKC isoform, PKCζ or PKCλ/ι, remains unanswered. Additionally, while both PKCζ and PKCλ/ι have been implicated in CD4+ T cell differentiation (24, 25), it remains to be seen whether the aPKC isoforms regulate differentiation of CD8+ T cells. Here, we show that PKCζ and PKCλ/ι regulate asymmetric localization of effector fate-associated factors during the first CD8+ T cell division in vivo, thereby influencing the specification of T lymphocyte fates.
MATERIALS AND METHODS
Mice
All animal work was done in accordance with the Institutional Animal Care and Use Guidelines of the University of California, San Diego. All mice were housed in specific pathogen-free conditions prior to use. Prkcz−/− mice were obtained from the European Conditional Mouse Mutagenesis Program (EUCOMM). Prckifl/fl mice (26) were bred to Cd4Cre mice. Prkcz−/− and Cd4CrePrkcifl/fl mice were bred with OT-I TCR transgenic mice that recognize chicken ovalbumin peptide SIINFEKL (residues 257–264)/Kb. Wild-type C57BL/6J recipient mice were purchased from the Jackson Laboratory.
CFSE labeling and in vitro cell culture
Splenocytes were isolated from OT-I mice and labeled with 5μM CFSE for 9 min at 37°C. Reactions were quenched with FBS, and CD8+ T cells were isolated with a negative selection magnetic microbeads kit (Miltenyi Biotec), according to the manufacturer’s protocol. Splenocytes were harvested from wild-type mice and irradiated for 15 minutes at 3000 rads. T cells were depleted using magnetic microbeads (Miltenyi Biotec) and the remaining splenocytes were pulsed with 1μM OT-I peptide (SIINFEKL). Cells were cultured together for 3 days and analyzed on an Accuri C6 (BD Biosciences) flow cytometer with FlowJo software (Treestar).
Immune synapse assay
Wild-type mice were injected intraperitoneally with 106 Flt3-ligand secreting B16 tumor cells (27), which were provided by Dr. Glenn Dranoff (Dana Farber Cancer Institute, Boston, MA). 10 days post-injection, splenocytes were harvested and CD11c+ dendritic cells were isolated with a positive selection magnetic microbeads kit (Miltenyi Biotec), according to the manufacturer’s protocol. Following isolation, cells were labeled with 1μM CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin) Dye for 30 min at 37°C. Reactions were quenched with FBS, and cells were pulsed with 1μM OT-I peptide for 1 hr at 37°C. Splenocytes were isolated from OT-I mice, and CD8+ T cells were isolated as above. CD11c+ cells and CD8+ T cells were incubated together for 30 min at 37°C and then prepared for immunofluorescence as described below. Dendritic cell-T cell conjugates were identified by the presence of an unlabeled cell (T cell) contacting a CMAC labeled cell (dendritic cell).
Adoptive cell transfers and infections
For primary infections, 5 × 103 OT-I CD45.1+CD8+ T cells were adoptively transferred into wild-type CD45.2+ recipients, followed by infection intravenously one day later with 5 × 103 CFU of Listeria monocytogenes expressing full-length chicken ovalbumin (Lm-OVA). Blood was collected from mice at 7, 14, 35, or 50 days post-infection in 5mM EDTA solution. Blood samples were lysed with Red Cell Lysing Buffer (Sigma) for 15 minutes before staining for indicated markers. Splenocytes were isolated from recipient mice at 7, 14, 35, or 50 days post-infection. For rechallenge experiments, 104 memory OT-I CD45.1+CD8+ T cells were adoptively transferred into wild-type CD45.2+ recipients, followed by infection intravenously one day later with 105 CFU of Lm-OVA. Blood was collected on day 8 post-rechallenge and analyzed as above. To measure bacterial burden, spleens were removed on day 5 post-rechallenge and homogenized. Duplicate serial dilutions were plated on brain-heart infusion (BD Biosciences) agar plates containing 1μg/mL erythromycin, and bacterial colonies were counted following incubation for ~36 hrs at 37°C. To isolate cells that had undergone their first division, 2 × 106 OT-I CD8+ T cells were first labeled with CFSE, as above, prior to adoptive transfer, and splenocytes from recipient mice were harvested at 48 hours post-infection. Lymphocyte fate-tracking experiments were performed as previously described (8). Briefly, 500 sorted IL-2Rαhi or IL-2Rαlo first division cells were adoptively transferred into infection-matched recipients. At day 50 post-transfer, recipients were rechallenged with 105 CFU Lm-OVA, and population expansion was tracked in the blood, as above. Cells were sorted on a MoFlo (Beckman Coulter) or FACS Aria II (BD Biosciences) flow cytometer.
T lymphocyte confocal microscopy
Immunofluorescence of T cells was performed as previously described (10) with the following antibodies: anti-β-tubulin (T8328) (Sigma); anti-IL-2Rα (PC61.5), anti-T-bet (4B10), anti-LFA-1 (M17/4) (eBioscience); anti-proteasome 20S C2 (ab3325) (Abcam); anti-IFNγR (2E2) (Biolegend); and anti-mouse Alexa Fluor 488, anti-rat Alexa Fluor 488, anti-mouse Alexa Fluor 647, anti-rabbit Alex Fluor 647, streptavidin Alexa Fluor 647, and anti-rat Alexa Fluor 647 (Life Technologies). DAPI (Life Technologies) was used to detect DNA. Pre-mitotic cells were identified by a single microtubule organizing center (MTOC) with β-tubulin staining, mitotic blasts were identified by the presence of two MTOCs, and cells undergoing cytokinesis were identified by dual nuclei and pronounced cytoplasmic cleft by brightfield. Acquisition of image stacks was performed as previously described (10) using a FV1000 laser scanning confocal microscope (Olympus). The volume of 3D pixels (voxels) containing the designated protein fluorescence was quantified within each hemisphere or within nascent daughter in cytokinetic cells as previously described (10) using ImageJ software.
Antibodies and flow cytometry
The following antibodies were used: T-bet (4B10), Eomes (Dan11mag), BCL2 (BCL/10C4), IRF4 (IRF4.3E4), CD27 (LG.7F9), CD8a (53–6.7), CD45.1 (A20), CD62L (MEL-14), KLRG1 (2F1), IL-7R (A7R34), CD44 (1M7), Vα2 (B20.1), CD4 (RM4-5), IFNγ (XMG1.2), TNFα (MP6-XT22), IL-2 (JES6-5H4) and F(ab′)2 anti-rabbit anti-IgG and were obtained from Biolegend or eBioscience. Rabbit anti-TCF-1 (C63D9) antibody was obtained from Cell Signaling Technology. Anti-human PE-conjugated Granzyme B (GB11) was obtained from Life Technologies. For intracellular staining of T-bet, Eomes, BCL2, TCF-1, IRF4, and Granzyme B, FoxP3/Transcription Factor Staining Buffer Kit was used (eBioscience). For intracellular detection of IFNγ, TNFα, and IL-2, CD8+ T cells were stimulated for 6 hrs at 37 °C ex vivo with 1μM OT-I peptide in the presence of brefeldin A (Sigma); cells were fixed in 4% paraformaldehyde (Electron Microscopy Services) and permeabilized before staining. All samples were analyzed on an Accuri C6 or FACSCanto (BD Biosciences) flow cytometer with FlowJo software (Treestar).
Single-cell gene expression assays, data processing, and analysis
Single CD8+ T cells were sorted and analyzed in 96.96 Dynamic Arrays on a BioMark system (Fluidigm) as previously described (8). BioMark data processing and principal component analysis were performed as previously described (8).
RESULTS
Loss of PKCζ or PKCλ/ι does not affect CD8+ T cell activation
To study the role of PKCζ and PKCλ/ι in CD8+ T cell asymmetric division and differentiation, we used Prkcz+/+ (wild-type) and Prkcz−/− (PKCζ-deficient) mice or Prckifl/fl (wild-type) and Cd4CrePrkcifl/fl (PKCλ/ι-deficient) mice. PKCζ-deficient mice have been shown to develop a normal immune system (28), whereas conditional Prkcifl/fl mice (26), which were used to overcome the previously reported embryonic lethality of germline Prkci−/− mice (29), have yet to be characterized. To assess T cell development and homeostasis, thymus, spleen, and lymph nodes from pairs of wild-type and PKCζ-deficient mice or wild-type and PKCλ/ι-deficient mice were analyzed. We observed similar percentages of double-negative, double-positive, and single-positive CD4 and CD8 T cells in the thymus (Fig. 1A) and similar percentages of CD4+ and CD8+ T cells in the spleen and lymph nodes (Fig. 1B). Previous activation of CD8+ T cells to environmental antigens was assessed by staining for CD44 and CD62L to delineate between naïve (CD44loCD62Lhi) and antigen-experienced (CD44hi) cells. Compared to wild-type controls, PKCζ- and PKCλ/ι-deficient mice exhibited normal percentages of naïve and antigen-experienced CD8+ T cells in the spleen and lymph nodes (Fig. 1C).
Figure 1.
PKCζ- and PKCλ/ι-deficient mice develop normal immune systems. (A) Frequencies of double-negative, double-positive, and single-positive CD4 and CD8 T cells from thymi of wild-type, PKCζ-deficient, and PKCλ/ι-deficient mice. (B) Frequencies of CD4+ and CD8+ T cells from spleens and lymph nodes of wild-type, PKCζ-deficient, and PKCλ/ι-deficient mice. (C) Frequencies of naïve CD8+ T cells (CD62LhiCD44lo) and effector memory or central memory CD8+ T cells (CD62LloCD44hi and CD62LhiCD44hi, respectively) from spleens and lymph nodes of wild-type, PKCζ-deficient, and PKCλ/ι-deficient mice. (D) Confocal microscopy of LFA-1 (green) and β-tubulin (red) in wild-type, PKCζ-deficient, or PKCλ/ι-deficient OT-I CD8+ T cells interacting with ovalbumin-pulsed CD11c+ dendritic cells labeled with CellTracker Blue CMAC Dye (blue) after 30 minutes of incubation. (E) Incidence of polarized localization of LFA-1 or the microtubule organizing center (MTOC) in wild-type (n=21), PKCζ-deficient (n=22), or PKCλ/ι-deficient (n=21) T cells toward a dendritic cell, as shown in (D). (F) CFSE dilution of unstimulated (gray filled), wild-type (solid black), and PKCζ-deficient (dashed black) CD8+ OT-I TCR transgenic T cells (left) or unstimulated (gray filled), wild-type (solid black), and PKCλ/ι-deficient (dashed black) CD8+ OT-I TCR transgenic T cells (right) after 3 days in vitro culture with IL-2 and irradiated, T cell-depleted splenocytes pulsed with 1μM ovalbumin peptide. Data are representative of three experiments.
Previous reports have suggested that aPKC activity is required for effective scanning of dendritic cells (30) and polarization of surface receptors and T cell secretory machinery toward the immunological synapse in activated T cells (31–33), raising the possibility that loss of PKCζ or PKCλ/ι might affect immunological synapse formation, activation, or proliferation of naïve CD8+ T cells. We bred wild-type, PKCζ-deficient, and PKCλ/ι-deficient mice with OT-I TCR transgenic mice to generate mice with CD8+ T cells that recognize amino acids 257–264 (SIINFEKL) of chicken ovalbumin (34). Expression of the OT-I TCR did not affect T cell development in PKCζ- or PKCλ/ιdeficient mice (Supplemental Fig. 1). To investigate the formation of immunological synapses, purified wild-type, PKCζ-deficient, or PKCλ/ι-deficient naïve CD8+ T cells were incubated in vitro for 30 minutes with purified ovalbumin peptide-pulsed CD11c+ dendritic cells labeled with CellTracker Blue CMAC Dye. Examination via confocal microscopy of LFA-1 and the microtubule-organizing center, polarization of which indicate a fully formed immunological synapse (35), revealed no deficiency in the ability of PKCζ- or PKCλ/ι-deficient CD8+ T cells to polarize either component toward the interface shared with a dendritic cell, compared to wild-type controls (Fig. 1D, 1E). This finding suggests that PKCζ and PKCλ/ι are not required for the initial engagement of dendritic cells or the establishment of a polarized immunological synapse by naïve CD8+ T cells. To assess the ability of naïve PKCζ- or PKCλ/ι-deficient CD8+ T cells to respond to antigen following dendritic cell engagement, purified CD8+ T cells of each genotype were labeled with CFSE and cultured in vitro with ovalbumin peptide-pulsed splenocytes. We observed that PKCζ- and PKCλ/ι-deficient CD8+ T cells proliferated comparably to wild-type controls (Fig. 1F), suggesting that antigen-induced activation of naïve CD8+ T cells is not affected by the loss of either isoform. Together, these data show that loss of PKCζ or PKCλ/ι does not alter development, immunological synapse formation, or activation of naïve CD8+ T cells.
Loss of PKCζ or PKCλ/ι impairs asymmetric CD8+ T cell division
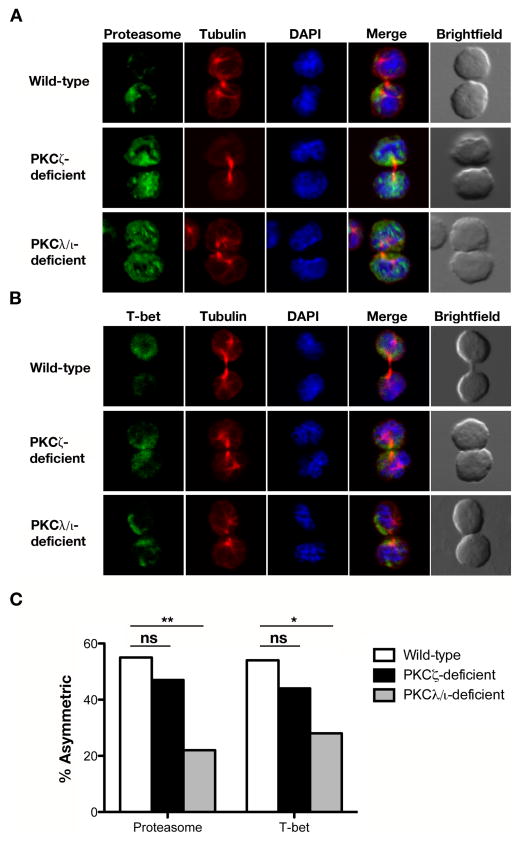
Previous reports have implicated aPKC in the regulation of asymmetric CD8+ T cell division (9, 23). It has been previously shown that asymmetric segregation of the proteasome degradation machinery during mitosis enables localized destruction of T-bet within a dividing CD8+ T lymphocyte, yielding daughter cells ‘proximal’ or ‘distal’ to the immunological synapse that inherit different amounts of T-bet and the proteasome. Knockdown or pseudosubstrate inhibition of PKCζ resulted in a loss of proteasome asymmetry during mitosis, leading to the loss of preferential T-bet localization to the proximal daughter cell (9). To investigate the individual roles of PKCζ and PKCλ/ι in the localization of these two factors during asymmetric CD8+ T cell division, we used a previously established in vivo method to isolate activated naïve CD8+ T cells undergoing their first division in response to a microbial pathogen (10). OT-I CD8+ T cells of each genotype were labeled with CFSE and adoptively transferred into mice that had been infected 24 hours prior with recombinant Listeria monocytogenes expressing ovalbumin (Lm-OVA). Undivided donor CD8+ T cells were isolated by flow cytometry 36 hours after adoptive transfer and examined by confocal microscopy. We observed a modest, but statistically insignificant, decrease in proteasome and T-bet asymmetry in cytokinetic PKCζ-deficient CD8+ T cells and a more prominent defect in asymmetry of these two components in cytokinetic PKCλ/ι-deficient CD8+ T cells (Fig. 2). This result raises the possibility that the prior experimental approach (9) targeting aPKC may have affected both isoforms, which is unsurprising given the high degree of homology between PKCζ and PKCλ/ι (36).
Figure 2.
Loss of PKCζ or PKCλ/ι impairs asymmetric segregation of proteasome and T-bet during the first division of an activated naïve CD8+ T cell. Confocal microscopy of (A) proteasome or (B) T-bet (green), β-tubulin (red), and DNA (blue; stained with the DNA-intercalating dye DAPI) in sorted wild-type, PKCζ-deficient, or PKCλ/ι-deficient OT-I CD8+ T cells undergoing their first division after adoptive transfer into Lm-OVA infected recipient mice. (C) Incidence of asymmetric protein localization from T cells shown in (A) and (B). The number of dividing cells from two experiments is indicated in parenthesis as follows (wild-type, PKCζ-deficient, PKCλ/ι-deficient): proteasome (58, 30, 27) and T-bet (28, 34, 43). ns, not significant; *P < 0.05 and **P < 0.01 (one-tailed unconditional Fisher’s exact test).
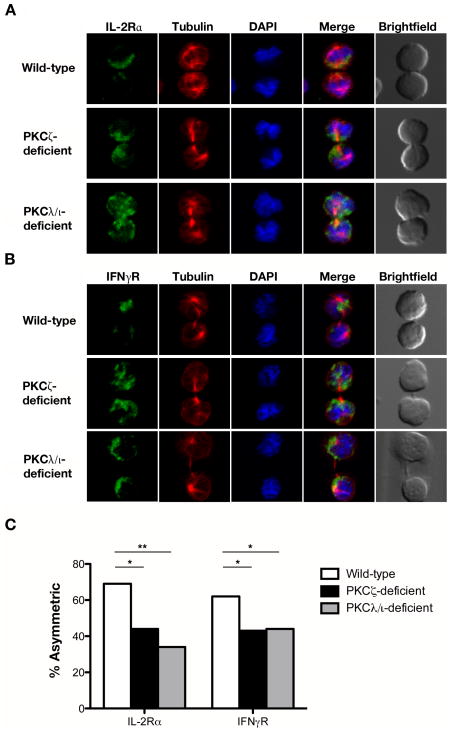
Because aPKC has been established as a key regulator of asymmetric cell division in other model systems (37), we hypothesized that PKCζ and PKCλ/ι might regulate the segregation of other T cell components that are known to localize asymmetrically during the first division of an activated naïve T cell (8, 10). IL-2Rα and IFNγR are two such components and were selected for investigation because they have been shown to mediate signals that influence T cell fate decisions (14–17). Examination of these two cytokine receptors in pre-mitotic cells showed that both receptors were polarized similarly in wild-type, PKCζ-deficient, and PKCλ/ι-deficient CD8+ T cells early during activation, prior to division (Supplemental Fig. 2). During the first CD8+ T cell division, however, a decrease in the asymmetric localization of both proteins in PKCζ- and PKCλ/ι-deficient CD8+ T cells was observed (Fig. 3), indicating that PKCζ and PKCλ/ι are important for maintaining polarity through the late stages of CD8+ T cell activation. Taken together, these results suggest that the function of aPKC as a regulator of asymmetric division is conserved in CD8+ T cells, but PKCζ and PKCλ/ι may have some non-redundant roles in segregating specific proteins unequally into the proximal or distal daughter cells.
Figure 3.
PKCζ and PKCλ/ι regulate asymmetric localization of IL-2Rα and IFNγR during the first division of an activated naïve CD8+ T cell. Confocal microscopy of (A) IL-2Rα or (B) IFNγR (green), β-tubulin (red), and DNA (blue) in sorted wild-type, PKCζ-deficient, or PKCλ/ι-deficient OT-I CD8+ T cells prepared as in Figure 2. (C) Incidence of asymmetric protein localization from dividing T cells shown in (A) and (B). The number of dividing cells from two experiments is indicated in parentheses as follows (wild-type, PKCζ-deficient, PKCλ/ι-deficient): IL-2Rα (36, 36, 32) and IFNγR (78, 30, 41). *P < 0.05 and **P < 0.01 (one-tailed unconditional Fisher’s exact test).
PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes exhibit reduced differentiation into putative memory precursor T cells
Because signals downstream of T-bet, IL-2Rα, and IFNγR facilitate the differentiation of effector T cells (12–17), we hypothesized that the symmetric distribution of these determinants during the first division of an activated naïve CD8+ T cell, would, as a result of PKCζ or PKCλ/ι deficiency, alter subsequent differentiation into effector and memory T cells. To investigate this possibility, we adoptively transferred wild-type, PKCζ-deficient, or PKCλ/ι-deficient OT-I CD45.1+CD8+ T cells into separate CD45.2+ wild-type recipients, which were infected 24 hours later with Lm-OVA. Compared to wild-type CD8+ T lymphocytes, we observed no difference in the kinetics of the PKCζ- or the PKCλ/ι-deficient CD8+ T cell response following infection (Fig. 4A and Supplemental Fig. 3A).
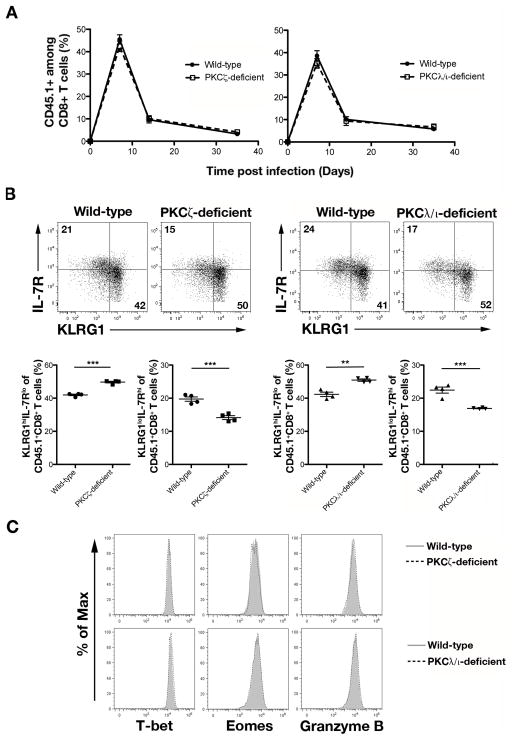
Figure 4.
PKCζ- and PKCλ/ι-deficient CD8+ T cells give rise to reduced KLRG1lo IL-7Rhi T cells at day 7 post-infection. (A) Percentages of CD45.1+ cells of CD8+ T cells on days 7, 14, and 35 in the blood of mice that received 5×103 wild-type (closed circles) or PKCζ-deficient (open squares) OT-I CD45.1+CD8+ T cells (left) or wild-type (closed circles) or PKCλ/ι-deficient (open squares) OT-I CD45.1+CD8+ T cells (right) and were infected with Lm-OVA; points represent mean ± SEM; n ≥ 4/group. (B) Expression of KLRG1 and IL-7R by CD45.1+CD8+ T cells in the spleen on day 7 post-infection (top). Frequencies of KLRG1hiIL-7Rlo and KLRG1loIL-7Rhi cells (bottom); each point represents an individual mouse and lines indicate the mean ± SEM; n = 4/group. **P < 0.01 and ***P < 0.001 (two-tailed unpaired t-test). (C) Expression of T-bet, Eomes, and Granzyme B by wild-type (gray filled) and PKCζ-deficient (dashed black) CD45.1+CD8+ T cells (top) or wild-type (gray filled) and PKCλ/ι-deficient (dashed black) CD45.1+CD8+ T cells (bottom) in the spleen on day 7 post-infection. Data are representative of three experiments.
It has been previously shown that CD8+ T lymphocytes responding to microbial infection can be divided into TSLE cells and putative memory precursor (TMP) cells at 7 days post-infection (38). TSLE cells can be identified by high expression of the lectin-like receptor, KLRG1, and low expression of IL-7R (KLRG1hiIL-7Rlo), while TMP cells can be identified by reciprocally high expression of IL-7R and low expression of KLRG1 (KLRG1loIL-7Rhi) (38). We observed that mice that received PKCζ- or PKCλ/ι-deficient CD8+ T cells exhibited an increase in the percentage of TSLE cells and a decrease in the percentage of TMP cells in both the blood and spleen at day 7 post-infection. (Fig. 4B and Supplemental Fig. 3B). Additionally, increased numbers of TSLE cells and decreased numbers of TMP cells continued to be observed through day 35 post-infection in mice that received PKCζ- or PKCλ/ι-deficient CD8+ T cells (Supplemental Fig. 3C, 3D). Despite these alterations in the differentiation patterns of PKCζ- and PKCλ/ι-deficient CD8+ T cells, examination of key transcription factors, T-bet and Eomes, and the cytotoxic molecule, Granzyme B, revealed no differences between PKCζ- or PKCλ/ι-deficient CD8+ T cells and wild-type controls (Fig. 4C). Together these results show that aPKC regulates CD8+ T lymphocyte differentiation without affecting proliferation or expression of key effector-associated molecules.
PKCζ and PKCλ/ι regulate differentiation into long-lived CD8+ T lymphocyte fates
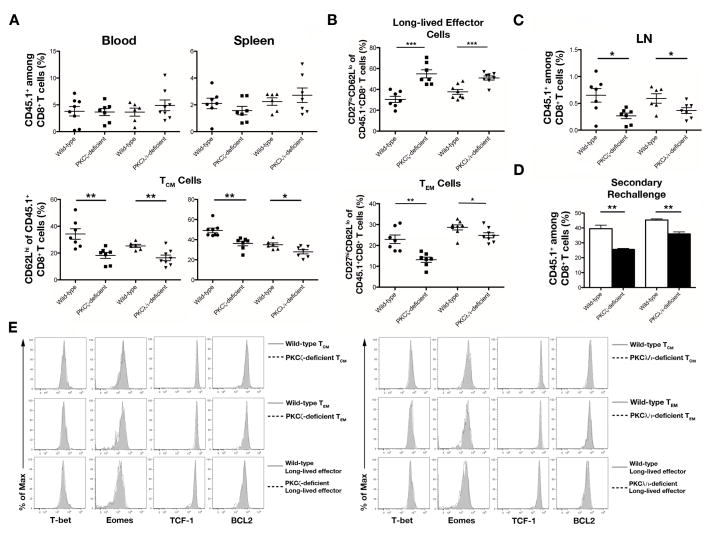
Next, we investigated the differentiation of CD8+ T cells into memory lymphocytes. The decreased numbers of TMP cells through day 35 post-infection in mice that received PKCζ- and PKCλ/ι-deficient CD8+ T cells (Supplemental Fig. 3C) suggested that there might be a corresponding decrease in the formation of memory lymphocytes at later timepoints. Indeed, at day 50 post-infection, mice that received CD8+ T cells lacking PKCζ or PKCλ/ι displayed a 1.5- to 2-fold reduction of CD62Lhi TCM cells in the blood and spleen with no changes in the percentages of total CD8+ T lymphocytes surviving into the memory phase (Fig. 5A and Supplemental Fig. 4A). Recent reports describing an additional subset of effector-like CD8+ T lymphocytes, termed ‘long-lived effector’ T cells, that survives into the memory phase (3, 4) raised the possibility that the defective ability of PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes to become TCM cells might be a consequence of enhanced differentiation toward this long-lived effector fate. Long-lived effector T cells are phenotypically KLRG1hiCD127loCD27loCD62Llo (3, 4). We confirmed that KLRG1hi lymphocytes at day 50 post-infection in our system were also CD62Llo and CD27lo (Supplemental Fig. 4B, 4C) and therefore used CD27 expression to distinguish between the long-lived effector (CD27loCD62Llo) and TEM (CD27hiCD62Llo) cell subsets. Assessment of these CD8+ T lymphocyte populations revealed that mice receiving PKCζ- or PKCλ/ι-deficient CD8+ T lymphocytes had increased percentages of long-lived effector T cells and decreased percentages of TEM cells (Fig. 5B).
Figure 5.
PKCζ and PKCλ/ι regulate central and effector memory CD8+ T cell differentiation. (A) Frequencies of CD45.1+CD8+ T cells (top) and CD62LhiCD45.1+CD8+ TCM cells (bottom) in the blood and spleen on day 50 post-infection; each point represents an individual mouse and lines indicate the mean ± SEM; n = 6–7/group. (B) Frequencies of CD27loCD62LloCD45.1+CD8+ long-lived effector T cells (top) and CD27hiCD62LloCD45.1+CD8+ TEM cells (bottom) in the spleen on day 50 post-infection; each point represents an individual mouse and lines indicate the mean ± SEM; n = 7–8/group. (C) Frequencies of CD45.1+CD8+ T cells in the lymph nodes on day 50 post-infection; each point represents an individual mouse and lines indicate the mean ± SEM; n = 6–7/group. (D) Frequencies of CD45.1+CD8+ T cells on day 8 in the blood of mice that received 104 wild-type, PKCζ-deficient, or PKCλ/ι-deficient CD45.1+CD8+ T cells on day 50 post-infection and were subsequently challenged with 105 CFU Lm-OVA. Bars indicate the mean ± SEM; n = 4/group. (E) Expression of T-bet, Eomes, TCF-1, and BCL2: left, wild-type (gray filled) and PKCζ-deficient (dashed black) CD45.1+CD8+ TCM cells (top), TEM cells (middle), and long-lived effector T cells (bottom); right, wild-type (gray filled) and PKCλ/ι-deficient (dashed black) CD45.1+CD8+ TCM cells (top), TEM cells (middle), and long-lived effector T cells (bottom) in the spleen on day 50 post-infection. For (A–D) *P < 0.05, **P < 0.01, and ***P < 0.001 (two-tailed unpaired t-test). Data are representative of two (D) or three (A–C, E) experiments.
As a result of this altered differentiation pattern, we observed a 2-fold reduction of PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes in lymph nodes compared to wild-type controls (Fig. 5C), consistent with the prior observation that CD62L expression is required for entry into secondary lymphoid organs (39). Moreover, because long-lived effector T cells mediate a more potent protective response while exhibiting minimal proliferation upon re-exposure to antigen (3, 4), we hypothesized that PKCζ- and PKCλ/ι deficient CD8+ T lymphocytes surviving into the memory phase would maintain an ability to clear pathogen but generate an impaired secondary proliferative response to reinfection. Indeed, PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes did not display any changes in their ability to produce cytokine compared to wild-type controls (Supplemental Fig. 4D), and upon adoptive transfer of equal numbers of CD45.1+CD8+ T cells followed by secondary rechallenge, PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes displayed similar abilities to clear bacteria in the spleen at day 5 post-rechallenge compared to wild-type controls (Supplemental Fig. 4E). However, PKCζ- and PKCλ/ι-deficient CD8+ T lymphocytes failed to proliferate in response to rechallenge as well as wild-type control cells (Fig. 5D). Taken together, these results demonstrate that in the absence of PKCζ or PKCλ/ι, CD8+ T lymphocytes differentiate toward a long-lived effector fate at the expense of the TCM and TEM cell fates.
We hypothesized that the altered differentiation patterns of PKCζ- and PKCλ/ι deficient CD8+ T lymphocytes might be due to a defect in the expression of key transcription factors and cell components within one or more of the long-lived T cell subsets. To investigate this possibility, we examined the expression of T-bet and Eomes in TCM, TEM, and long-lived effector T cells. We also assessed the expression of T cell factor 1 (TCF-1), which is important for differentiation and persistence of memory CD8+ T cells (40), and B cell lymphoma 2 (BCL2), an anti-apoptotic molecule thought to be important for T cell survival (41). Expression of these factors was similar between PKCζ- or PKCλ/ι-deficient cells of each subset and their wild-type counterparts (Fig. 5E), suggesting that the increased differentiation of aPKC-deficient T lymphocytes into the long-lived effector fate was not caused by changes in transcriptional profiles at later timepoints.
PKCζ and PKCλ/ι influence the transcriptional program of cells that have undergone their first division
The alterations in the types of effector and memory cells that differentiated in the absence of aPKC did not appear to be the result of defective expression of key effector- and memory-associated transcription factors at days 7 or 50 post-infection (Figs. 4, 5). These findings raised the possibility that disruptions to the transcriptional programs of aPKC-deficient T cells might be occurring early during the immune response. We hypothesized that the impaired asymmetric segregation of effector-associated molecules we observed in mitotic PKCζ- and PKCλ/ι-deficient T lymphocytes (Figs. 2, 3) might result in a subsequent alteration in the transcriptional programs following the first division. To address this possibility, we performed single-cell gene expression analyses with Fluidigm 96.96 Dynamic Arrays in individual naïve PKCζ- or PKCλ/ι-deficient CD8+ T cells and cells that had completed their first division in vivo.
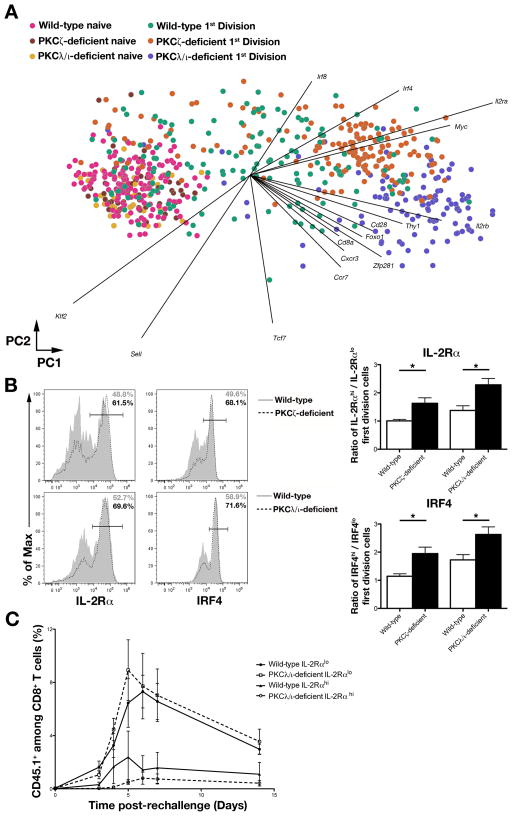
We used principal component analysis (PCA) to analyze the gene expression patterns of single naïve and first division PKCζ- and PKCλ/ι-deficient CD8+ T cells compared to their wild-type counterparts. PCA is an unsupervised dimensionality reduction method that projects data into two dimensions by its coordinates in the first two principal components (PC1 and PC2). These principal components are linear combinations of the genes that account for the largest variations in the data. PCA revealed that naïve T cells from each genotype clustered together (Fig. 6A), suggesting that any differences observed in the eventual fates of PKCζ- and PKCλ/ι-deficient CD8+ T cells were not due to differences present prior to activation. Wild-type first division cells exhibited substantial heterogeneity (Fig. 6A), reflective of distinct predispositions towards the effector and memory fates (8). In contrast, the majority of PKCζ- and PKCλ/ι-deficient cells that had undergone their first division formed clusters that shared minimal overlap (Fig. 6A). This observation suggested that most of these cells lacking PKCζ or PKCλ/ι were molecularly homogeneous and that the aPKC isoforms might have non-redundant roles in regulating asymmetric division.
Figure 6.
PKCζ and PKCλ/ι regulate early molecular heterogeneity in cells that have undergone their first division in vivo. (A) PC projections (PC1, horizontal axis; PC2, vertical axis) for single-cell gene-expression data derived from individual lymphocytes from populations of naïve wild-type (pink), naïve PKCζ-deficient (brown), naïve PKCλ/ι deficient (yellow), first division wild-type (teal), first division PKCζ-deficient (orange), and first division PKCλ/ι-deficient (purple) OT-I CD8+ T cells sorted before or after adoptive transfer into Lm-OVA infected recipient mice. Each circle represents an individual cell; each vector emanating from the origin represents an individual gene. PC1 and PC2 account for 20% and 5% of the variance, respectively. (B) Left, expression of IL-2Rα and IRF4 by wild-type (gray filled) and PKCζ-deficient (dashed black) first division OT-I CD8+ T cells (top) or wild-type (gray filled) and PKCλ/ι-deficient (dashed black) first division OT-I CD8+ T cells (bottom) after adoptive transfer into recipient mice and subsequent infection with Lm-OVA. Right, ratios of IL-2Rαhi and IL-2Rlo (top) or IRF4hi and IRF4lo (bottom) first division OT-I CD8+ T cells; bars represent mean ± SEM; n = 3–4/group; *P < 0.05 (two-tailed unpaired t-test). (C) Population expansion of CD45.1+CD8+ T cells on days 3, 4, 5, 6, 7, and 14 post-rechallenge in blood obtained from Lm-OVA infected CD45.2+ mice that received 500 sort-purified wild-type IL-2Rαlo (closed circles), wild-type IL-2Rαhi (closed triangles), PKCλ/ι-deficient IL-2Rαlo (open squares), or PKCλ/ι-deficient IL-2Rαhi (open circles) first division CD45.1+CD8+ OT-I T cells on day 2 post-infection and were rechallenged with 105 CFU Lm-OVA on day 50 post-transfer; points represent mean ± SEM; n = 3–4/group.
This loss of molecular heterogeneity was driven, in part, by increased mRNA expression of Irf4, Irf8, and Il2ra (Fig. 6A), which encode molecules related to effector T cell differentiation (14, 16, 17, 42–45). These changes in mRNA expression were accompanied by increased protein expression of IL-2Rα and interferon regulatory factor 4 (IRF4) by PKCζ- and PKCλ/ι-deficient first division CD8+ T cells (Fig. 6B). As we have previously shown that IL-2Rα, in particular, may represent an early molecular switch in the differentiation pathway of an activated naïve CD8+ T cell towards an effector fate (8), the finding of an increased proportion of IL-2Rαhi cells at the expense of IL-2Rαlo cells provides a potential mechanism underlying the impaired TEM and TCM differentiation observed in the setting of aPKC deficiency.
To test whether PKCζ or PKCλ/ι deficiency might also alter cell fate once T lymphocytes have already diverged with respect to IL-2Rα expression, sorted first division IL-2Rαlo or IL-2Rαhi cells from wild-type or PKCλ/ι-deficient donor mice were adoptively transferred into infection-matched recipients, followed by a secondary rechallenge at day 50 post-transfer. Regardless of genotype, IL-2Rαlo cells exhibited robust proliferation in response to rechallenge whereas IL-2αhi cells failed to do so (Fig. 6C). These results suggest that deletion of one aPKC isoform does not alter the fate of T lymphocytes once they have acquired differential amounts of IL-2Rα. Instead, PKCζ and PKCλ/ι appear to specifically regulate asymmetric division, which may allow each isoform to influence CD8+ T lymphocyte fate specification by controlling the relative proportion of IL-2Rαhi and IL-2Rαlo cells that are ultimately fated toward the effector and memory lineages.
DISCUSSION
Asymmetric cell division has been previously observed in activated naïve and memory lymphocytes (8–10, 23, 46–48), yet evidence for a functional role of asymmetric division in lymphocyte differentiation has been limited (49, 50). Our data suggest that asymmetric division by activated naïve CD8+ T cells responding to a microbial infection may serve to exclude effector fate-promoting factors from distal daughter cells, thereby enabling them to initiate a ‘pre-memory’ transcriptional program. In the setting of impaired asymmetric division, owing to the absence of either aPKC isoform, symmetric inheritance of key effector fate-promoting molecules seems to result in the acquisition of a ‘pre-effector’ transcriptional program, detectable by single-cell gene expression analyses, by both daughter cells. This reduction in cells that would otherwise have acquired a pre-memory transcriptional program appears to yield an increased proportion of long-lived effector T cells at the expense of TCM and TEM cells.
Our results show that PKCζ and PKCλ/ι are regulators of asymmetric CD8+ T cell division and indicate that each isoform may have some non-redundant roles. Regulation of proteasome and T-bet asymmetry during the first division appears to be PKCλ/ι specific. Nonetheless, loss of either isoform yields similar functional consequences with respect to CD8+ T lymphocyte differentiation during an immune response. Both PKCζ and PKCλ/ι influence asymmetric partitioning of IL-2Rα and IFNγR in activated naïve CD8+ T cells undergoing their first division in vivo. Moreover, the absence of PKCζ or PKCλ/ι in first division CD8+ T cells seems to result in similar alterations in their transcriptional programs with a gain of genes that promote effector differentiation. Taken together, these results suggest that the shared functions of PKCζ and PKCλ/ι may be more significant in determining CD8+ T lymphocyte fate than any unique role of either isoform.
Through these shared functions in regulating asymmetric division, PKCζ and PKCλ/ι may enable the generation of first division CD8+ T lymphocytes that are molecularly heterogeneous at the single-cell level. Our recent work suggested that this molecular heterogeneity may be indicative of ‘pre-effector’ and ‘pre-memory’ transcriptional programs that are predictive of the eventual fates of the cells (8). Lymphocytes that transition through a pre-effector state can undergo further differentiation to acquire the TSLE fate, whereas cells transitioning through a pre-memory state can further diverge to give rise to TCM or TEM cells (8). The present findings suggest that disruption of asymmetric division by the absence of aPKC results in an increased proportion of first division cells expressing high levels of IL-2Rα and exhibiting a pre-effector transcriptional program.
This increased proportion of pre-effector cells was associated with an increased percentage of KLRG1hi cells, both at the peak of the immune response and in the memory phase. Because both TSLE and long-lived effector T cells express high levels of KLRG1, one potential interpretation of these results is that a subset of TSLE cells present at day 7 post-infection subsequently gives rise to long-lived effector T cells. Commitment to the TSLE fate, however, is thought to correlate with increased proliferation of CD8+ T cells (51) and increased apoptosis following clearance of an infection (13). The lack of an alteration in the kinetics of the CD8+ T cell response in the absence of PKCζ or PKCλ/ι suggests that differentiation into TSLE cells is unaffected by the loss of either aPKC isoform. An alternative possibility, therefore, is that cells transitioning through the pre-effector state (8) diverge in fate early during an immune response, giving rise to long-lived effector T cells as well as TSLE cells that together comprise the KLRG1hi population at day 7 post-infection. The modest increase in the KLRG1hi population at day 7 post-infection may, therefore, represent a 2-fold change in long-lived T lymphocyte fates that has already occurred during an aPKC-deficient CD8+ T cell response.
Although aPKC-deficient CD8+ T lymphocytes exhibited increased differentiation towards the effector (TSLE and long-lived effector) fates, the absence of aPKC did not result in a complete loss of memory (TCM and TEM) cells. The continued generation of aPKC-deficient TCM and TEM cells is likely due to the continued presence of aPKC-deficient IL-2Rαlo pre-memory cells, which, unlike IL-2Rαhi pre-effector cells, maintain the capacity to differentiate into the memory fates following the first division in vivo. These pre-memory cells may be the product of asymmetric divisions still exhibited by some PKCζ- and PKCλ/ι-deficient cells, as the absence of either aPKC isoform did not completely disrupt the unequal segregation of IL-2Rα. As such, PKCζ- and PKCλ/ι deficient naïve CD8+ T cells appear to give rise to reduced frequencies of IL-2Rαlo pre-memory cells, which subsequently reduces, rather than completely eliminates, memory differentiation. These findings indicate that the high and low expression of IL-2Rα resulting from an asymmetric division may be important for the initial predisposition of CD8+ T lymphocytes to adopt either an effector fate or a memory fate. However, despite acquiring a tendency toward either the effector or the memory fates at the first division, specific commitment into a particular CD8+ T cell subset likely requires additional input, signals which may include further exposure to cytokines (52), additional antigen encounter (53, 54), or migration into specific tissue sites during the immune response (55). Nonetheless, our results suggest that disruption of asymmetric division, via deletion of PKCζ or PKCλ/ι, alters the ratio of pre-effector and pre-memory cells, thereby influencing the transcriptional programming of first division CD8+ T lymphocytes and the specification of effector and memory CD8+ T cell fates.
The finding that CD8+ T cells undergo asymmetric division (9, 10) suggested the possibility that lymphocyte fates may diverge early during an immune response to a microbial infection. Single-cell gene expression analyses revealed distinct molecular patterns, predictive of eventual fates, within lymphocytes that had undergone their first division in vivo (8). Here we provide some of the first experimental evidence that an evolutionarily conserved regulator of asymmetric cell division influences CD8+ T lymphocyte fate specification by controlling unequal partitioning of fate-influencing molecules during mitosis. Disruption of asymmetric CD8+ T cell division, as a result of aPKC deficiency, was associated with striking changes in the transcriptional patterns exhibited by first division lymphocytes and appeared to alter the balance of effector- and memory-fated progeny. These findings provide new evidence in support of the proposal that asymmetric division mediates a divergence in lymphocyte fates at the initiation of an adaptive immune response to microbial infection. Although the role of each CD8+ T cell subset in maintaining a long-term protective response is still being explored and debated (4, 56), the finding that asymmetric division plays a functional role in lymphocyte fate specification is likely to be important in understanding how to generate robust immunological protection against a variety of infectious diseases.
Supplementary Material
Acknowledgments
We thank members of the Chang and Yeo labs for helpful comments and suggestions.
This work was supported by US National Institutes of Health (DK093507, OD008469, and AI095277 to J.T.C. and HG004659 and NS075449 to G.W.Y.), the UCSD Digestive Diseases Research Development Center Grant DK80506, California Institute for Regenerative Medicine grants (RB1-01413 and RB3-05009 to G.W.Y.), and UCSD Neuroscience Microscopy Shared Facility Grant P30 NS047101. B.K. is a National Science Foundation graduate research fellow. G.W.Y. is a recipient of the Alfred P. Sloan Research Fellowship. J.T.C. is a Howard Hughes Medical Institute Physician-Scientist Early Career Awardee.
Abbreviations used in this article
- aPKC
atypical protein kinase C
- BCL2
B cell lymphoma 2
- IRF4
interferon regulatory factor 4
- KLRG1
killer-cell lectin like receptor G1
- Lm-OVA
Listeria monocytogenes-OVA
- MTOC
microtubule organizing center
- PCA
principal component analysis
- TCF-1
T cell factor 1
- TCM
central memory T cell
- TEM
effector memory T cell
- TMP
putative memory precursor T cell
- TSLE
short-lived effector T cell
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, Zehn D, Bevan MJ, Schepers K, Schumacher TN. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. 2010;207:1235–1246. doi: 10.1084/jem.20091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15:365–372. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JT, Ciocca ML, Kinjyo I, Palanivel VR, McClurkin CE, Dejong CS, Mooney EC, Kim JS, Steinel NC, Oliaro J, Yin CC, Florea BI, Overkleeft HS, Berg LJ, Russell SM, Koretzky GA, Jordan MS, Reiner SL. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 11.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doe CQ, Bowerman B. Asymmetric cell division: fly neuroblast meets worm zygote. Curr Opin Cell Biol. 2001;13:68–75. doi: 10.1016/s0955-0674(00)00176-9. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita YM, Yuan H, Cheng J, Hunt AJ. Polarity in stem cell division: asymmetric stem cell division in tissue homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a001313. doi: 10.1101/cshperspect.a001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atwood SX, Prehoda KE. aPKC phosphorylates Miranda to polarize fate determinants during neuroblast asymmetric cell division. Curr Biol. 2009;19:723–729. doi: 10.1016/j.cub.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 23.Oliaro J, Van Ham V, Sacirbegovic F, Pasam A, Bomzon Z, Pham K, Ludford-Menting MJ, Waterhouse NJ, Bots M, Hawkins ED, Watt SV, Cluse LA, Clarke CJ, Izon DJ, Chang JT, Thompson N, Gu M, Johnstone RW, Smyth MJ, Humbert PO, Reiner SL, Russell SM. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J Immunol. 2010;185:367–375. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin P, Villares R, Rodriguez-Mascarenhas S, Zaballos A, Leitges M, Kovac J, Sizing I, Rennert P, Marquez G, Martinez AC, Diaz-Meco MT, Moscat J. Control of T helper 2 cell function and allergic airway inflammation by PKCzeta. Proc Natl Acad Sci U S A. 2005;102:9866–9871. doi: 10.1073/pnas.0501202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JQ, Leitges M, Duran A, Diaz-Meco MT, Moscat J. Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc Natl Acad Sci U S A. 2009;106:1099–1104. doi: 10.1073/pnas.0805907106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koike C, Nishida A, Akimoto K, Nakaya MA, Noda T, Ohno S, Furukawa T. Function of atypical protein kinase C lambda in differentiating photoreceptors is required for proper lamination of mouse retina. J Neurosci. 2005;25:10290–10298. doi: 10.1523/JNEUROSCI.3657-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 28.Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 29.Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM. Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol. 2004;173:3250–3260. doi: 10.4049/jimmunol.173.5.3250. [DOI] [PubMed] [Google Scholar]
- 30.Real E, Faure S, Donnadieu E, Delon J. Cutting edge: Atypical PKCs regulate T lymphocyte polarity and scanning behavior. J Immunol. 2007;179:5649–5652. doi: 10.4049/jimmunol.179.9.5649. [DOI] [PubMed] [Google Scholar]
- 31.Bertrand F, Esquerre M, Petit AE, Rodrigues M, Duchez S, Delon J, Valitutti S. Activation of the ancestral polarity regulator protein kinase C zeta at the immunological synapse drives polarization of Th cell secretory machinery toward APCs. J Immunol. 2010;185:2887–2894. doi: 10.4049/jimmunol.1000739. [DOI] [PubMed] [Google Scholar]
- 32.Bertrand F, Muller S, Roh KH, Laurent C, Dupre L, Valitutti S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci U S A. 2013;110:6073–6078. doi: 10.1073/pnas.1218640110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO, Russell SM. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110–117. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 35.Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 37.Prehoda KE. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect Biol. 2009;1:a001388. doi: 10.1101/cshperspect.a001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 39.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 42.Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, Nutt SL, Kallies A. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 43.Miyagawa F, Zhang H, Terunuma A, Ozato K, Tagaya Y, Katz SI. Interferon regulatory factor 8 integrates T-cell receptor and cytokine-signaling pathways and drives effector differentiation of CD8 T cells. Proc Natl Acad Sci U S A. 2012;109:12123–12128. doi: 10.1073/pnas.1201453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raczkowski F, Ritter J, Heesch K, Schumacher V, Guralnik A, Hocker L, Raifer H, Klein M, Bopp T, Harb H, Kesper DA, Pfefferle PI, Grusdat M, Lang PA, Mittrucker HW, Huber M. The transcription factor Interferon Regulatory Factor 4 is required for the generation of protective effector CD8+ T cells. Proc Natl Acad Sci U S A. 2013;110:15019–15024. doi: 10.1073/pnas.1309378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH, Sun J. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciocca ML, Barnett BE, Burkhardt JK, Chang JT, Reiner SL. Cutting edge: Asymmetric memory T cell division in response to rechallenge. J Immunol. 2012;188:4145–4148. doi: 10.4049/jimmunol.1200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thaunat O, Granja AG, Barral P, Filby A, Montaner B, Collinson L, Martinez-Martin N, Harwood NE, Bruckbauer A, Batista FD. Asymmetric segregation of polarized antigen on B cell division shapes presentation capacity. Science. 2012;335:475–479. doi: 10.1126/science.1214100. [DOI] [PubMed] [Google Scholar]
- 49.Hawkins ED, Oliaro J, Kallies A, Belz GT, Filby A, Hogan T, Haynes N, Ramsbottom KM, Van Ham V, Kinwell T, Seddon B, Davies D, Tarlinton D, Lew AM, Humbert PO, Russell SM. Regulation of asymmetric cell division and polarity by Scribble is not required for humoral immunity. Nat Commun. 2013;4:1801. doi: 10.1038/ncomms2796. [DOI] [PubMed] [Google Scholar]
- 50.King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37:709–720. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plumlee CR, Sheridan BS, Cicek BB, Lefrancois L. Environmental cues dictate the fate of individual CD8+ T cells responding to infection. Immunity. 2013;39:347–356. doi: 10.1016/j.immuni.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 54.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol. 2010;185:5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.