Abstract
Objective
To determine if higher subcutaneous adipose tissue lipoprotein lipase activity (AT-LPLA) is associated with greater triglyceride (TG) storage in subcutaneous adipose tissue (SAT), thereby reducing visceral adipose tissue (VAT) accumulation and metabolic dysfunction.
Design and Method
Obese postmenopausal women (60±1 yrs; mean±SEM; N=101) had body composition by DXA and CT, fat aspirations for fat-cell weight (FCW) and AT-LPLA. Women were ranked by visceral to total abdominal fat ratio (VAT/TAF), and the lowest and highest groups (n=24) matched for % fat and age.
Results
The prevalence of metabolic dysfunction was 7–10 fold higher in women with high VAT/TAF (P’s<0.01). Women with low VAT/TAF had 11% and 6% lower abdominal and gluteal FCWs, but 28% and 54% higher AT-LPLA/106 cells in abdominal and gluteal fat, respectively. Abdominal FCW correlated with AT-LPLA in women with low (r=0.63, P<0.01), but not high (r=0.14, P=0.52) VAT/TAF, and these lines differed in slope (P<0.05) and intercept (P<0.01), suggesting greater capacity for TG storage with low VAT/TAF. There were no relationships between gluteal FCW and AT-LPLA. The relationship between SAT and abdominal AT-LPLA (r=0.39, P<0.01) suggests that higher AT-LPLA promotes TG storage.
Conclusions
These results suggest that higher AT-LPLA is associated with SAT adipocyte hypertrophy, which reduces visceral adiposity and metabolic risk in obese, older women.
Keywords: lipoprotein lipase activity, adipocyte hypertrophy, visceral adipose tissue, metabolic syndrome
Introduction
The accumulation of fat in visceral adipose tissue (VAT) is strongly associated with insulin resistance, impaired glucose tolerance (IGT), and metabolic syndrome (MSyn) (1, 2). Several mechanisms are proposed to explain the increased uptake and storage of triglycerides (TG) in VAT. One theory posits that the impaired expandability of subcutaneous adipose tissue (SAT) during the development of obesity results in the overflow of lipid into VAT and ectopic sites, such as skeletal muscle, liver, and pancreas (3, 4). This is supported by studies that show that reduced lipogenesis and adipogenesis in SAT are associated with greater fat deposition in VAT and metabolic dysfunction in adolescent obesity (5), and in response to weight gain during high fat overfeeding in adults (6).
Larger adipocytes accumulate more TG and release more free fatty acids (FFA) than smaller adipocytes (7, 8). The uptake and storage of TG in adipocytes is primarily regulated by adipose tissue lipoprotein lipase (AT-LPL), the rate limiting enzyme in the clearance of circulating TG-rich lipoproteins. Insulin is a key regulator of AT-LPL, and the hyperinsulinemia and insulin resistance of obesity is associated with decreased sensitivity of adipocyte lipolysis to insulin, higher AT-LPL activity (AT-LPLA) and greater fat cell size (9, 10). The higher AT-LPLA and lower basal lipolysis in gluteal (GLT) and abdominal (ABD) SAT in postmenopausal compared to perimenopausal women suggests that regional differences in AT-LPLA may contribute to the higher body weight, central adiposity, and metabolic abnormalities associated with menopause (11, 12). This is supported by studies that show that age-associated regional differences in fat deposition correlate with regional AT-LPLA (13, 14). The purpose of this study was to determine the relationship of subcutaneous AT-LPLA to subcutaneous adipocyte size, visceral adiposity, and metabolic dysfunction in obese, older Caucasian women. We hypothesized that the ability of adipocytes in SAT to hypertrophy, reduces visceral adiposity and cardiometabolic risk in obese, older women.
Methods
Subjects
One hundred one healthy, obese (body fat >35% (15)) Caucasian postmenopausal women who had previously provided University of Maryland IRB approved informed consent and participated in weight loss studies (16, 17) were included if they had a DXA scan for body composition, a computerized axial tomography (CT) scan to measure SAT and VAT, and a fat aspiration to measure AT-LPLA in ABD and GLT sites. The women were sedentary (<20 minutes of aerobic exercise two times/wk) and weight stable (<2 kg weight change) over the prior six months. Women with diabetes (fasting glucose >7 mmol/L or 2hr glucose tolerance test glucose >11 mmol/L) on oral agents or insulin, triglycerides >400 mg/dl, overt cardiovascular, renal, or liver disease, or unstable medical conditions were excluded. Women were ranked by their VAT/TAF ratio and 24 women with the lowest VAT/TAF were matched for % body fat (±2%) and age (±5yrs) to 24 women with the highest VAT/TAF. Approximately 23% of women in each group took ACE inhibitors, none took a diuretic, and none were on a lipid lowering medication. The diagnosis of MSyn was based on the presence of three or more of the following criteria: central obesity (waist >88 cm), impaired glucose metabolism (fasting glucose >5.6 mmol/L), elevated blood pressure (>130/85 mmHg or antihypertensive treatment), and dyslipidemia (TG >1.7 mmol/L or HDL-C <1.3 mmol/L) (18).
Body Composition
Body mass index (kg/m2) was calculated from height and weight measured using a stadiometer and electric scale, respectively. Percent body fat and fat-free mass (FFM) were measured by dual energy x-ray absorptiometry scan (DXA: DPX-IQ or Prodigy; LUNAR Radiation Corp., Madison, Wisconsin, USA). CT scans were performed with a PQ 6000 scanner (Marconi Medical Systems, Cleveland, OH) to quantify VAT, SAT, and mid-thigh low density lean tissue areas. For SAT and VAT, a single 5-mm scan was taken at the L4–L5 region while the subject was supine, with arms stretched overhead. A second scan performed at the level of the mid-thigh quantified low-density lean tissue area, and data for the right leg are reported (19). CT data are expressed as cross-sectional area of tissue (cm2), where muscle area is considered 30–80 Hounsfield units (HU), adipose tissue −190 to −30 HU, and low density lean tissue 0–29 HU (20).
Metabolic Testing
To minimize the effects of diet composition on metabolism, a registered dietitian weight stabilized the women on a Step I American Heart Association diet (21) for ~4 weeks prior to metabolic testing. Dietary intake compliance was verified by food records, and counseling was provided when saturated fat exceeded 10% of calories and desirable fat intake was >35%. For two-days prior to all metabolic tests the women were given a weight maintaining diet composed of 50–55% carbohydrate, ~30% fat, and 15–20% protein, based on their food records. Body weight was stable (±0.5 kg) during metabolic tests. Blood was drawn following a 12 hour fast for measurement of lipoprotein lipids, as previously described (22), on two separate occasions a week apart and averaged. A 2-hour oral glucose tolerance test was performed with measures of glucose and insulin at fasting and 30 minute increments following ingestion of 75g glucose. Plasma glucose concentrations were measured using the glucose oxidase method (2300 STAT Plus; YSI, Yellow Springs, OH) and immunoreactive insulin by radioimmunoassay (Linco Research Inc., St. Charles, MO). Subjects were classified as having normal (fasting glucose <7 mmol/L) or IGT (2-hour glucose of 7.8 to 11.0 mmol/L); none were diabetic (23).
Adipose Tissue Lipoprotein Lipase
After an overnight fast, subcutaneous adipose tissue was aspirated under local anesthesia (0.5% xylocaine) from both the ABD and GLT regions using a 10 mm mini-cannula and adipocytes were isolated by collagenase digestion (1 mg/mL) and fat cell weights (FCW) and surface areas (FCSA) were calculated from fat cell diameter (10, 24). AT-LPLA was measured as heparin elutable LPL in 40–50 mg pieces, as previously described (9). To evaluate substrate quality and variability within each assay, 20 μL of a human postheparin plasma pool (stored at −80°C) was incubated with each substrate for 30 minutes and the % of 14C-FFA counts hydrolyzed was calculated. The inter-assay pool variability was 13±4%, and those assays with a pool activity ≤5% or ≥20% were excluded. One GLT AT-LPLA assay was excluded for methodological reasons. AT-LPLA was not corrected for the pool activity.
Statistics
Standard methods were used to compute means and standard errors of the means (SEM). Analysis of covariance and chi-square tests compared differences in variables of interest between low versus high VAT/TAF. Pearson and Spearman correlation coefficients were calculated after log transformation of plasma TG, AT-LPLA, FCW, and SA, as appropriate. Linear regression predicting FCW and FCSA from a model including the log of AT-LPLA, an indicator variable for low and high VAT/TAF, and a log AT-LPLA*indicator variable interaction was used to determine if the slope or intercept differed between the two groups. None of these data met criteria for outliers and high leverage data points as calculated using Cook’s distance (25) or were >2 standard deviations from the mean. HOMA-IR was calculated as [(fasting insulin (μU/ml) × fasting glucose [mmol/l])/22.5] (26). Data were analyzed using SPSS Version 20 and SAS 9.3. All tests were two-tailed, and P-values <0.05 were considered statistically significant.
Results
Subject Characteristics (Table 1)
Table 1.
Physical characteristics of subjects with low and high VAT/TAF
| Low VAT/TAF (N=24) | High VAT/TAF (N=24) | P-value | |
|---|---|---|---|
| Age (years) | 58±1 | 62±2 | 0.07 |
| BMI (kg/m2) | 30±1 | 33±1 | 0.02 |
| Waist Circumference (cm) | 89±2 | 101±3 | <0.01 |
| Body Fat (%) | 46±1 | 47±1 | 0.31 |
| Fat-Free Mass (kg) | 42±1 | 44±1 | 0.04 |
| SAT area (cm2) | 459±19 | 421±17 | 0.08 |
| VAT area (cm2) | 113±6 | 214±12 | <0.01 |
| VAT/TAF | 0.20±0.01 | 0.34±0.01 | <0.01 |
| Mid-thigh LDLT area (cm2) | 16.5±1.5 | 24.7±1.9 | <0.01 |
| Systolic Blood Pressure (mm Hg) | 122±3 | 126±2 | 0.25 |
| Diastolic Blood Pressure (mm Hg) | 70±3 | 76±2 | 0.03 |
Values are expressed as means±SEM. SAT= subcutaneous abdominal adipose tissue; VAT= visceral abdominal adipose tissue; TAF=total abdominal fat (SAT+VAT); LDLT: low density lean tissue.
The 24 women with high VAT/TAF were of comparable age, % body fat, and systolic blood pressure, but had higher BMI and waist circumference (P<0.05) than those with low VAT/TAF. As expected, women with high VAT/TAF tended to have 9% lower SAT (P=0.08) and 89% higher VAT (P<0.01), and thus a higher VAT/TAF (P<0.01). Women with high VAT/TAF had a higher mid-thigh low density lean tissue area (P<0.01), evidence of ectopic fat distribution in muscle.
Glucose and Lipid Metabolism (Table 2)
Table 2.
Metabolic measurements of subjects with low and high VAT/TAF
| Low VAT/TAF | High VAT/TAF | P-value | |
|---|---|---|---|
| Glucose Metabolism | |||
| Fasting glucose (mmol/L) | 5.2±0.1 | 5.6±0.1 | <0.01 |
| 2h glucose (mmol/L) | 6.4±0.5 | 8.0±0.4 | <0.01 |
| HOMA-IR | 2.5±0.3 | 4.4±0.3 | <0.01 |
| Lipid Metabolism | |||
| Triglycerides (mmol/L) | 1.2±0.1 | 1.8±0.1 | <0.01 |
| Total Cholesterol (mmol/L) | 5.1±0.2 | 5.4±0.2 | 0.24 |
| LDL-C (mmol/L) | 3.1±0.1 | 3.4±0.2 | 0.35 |
| HDL-C (mmol/L) | 1.4±0.1 | 1.2±0.1 | <0.01 |
| HDL2-C (mmol/L) | 0.24±0.04 | 0.14±0.03 | 0.05 |
| HDL3-C (mmol/L) | 1.18±0.03 | 1.07±0.04 | <0.01 |
| Metabolic Risk | |||
| IGT Prevalence (%) | 8 | 58 | <0.01 |
| MSyn Prevalence (%) | 8 | 83 | <0.01 |
Values are expressed as means±SEM.
The women with high VAT/TAF had higher mean fasting and 2-hr glucose and ~2-fold higher HOMA-IR than women with a low ratio (all P’s<0.01). Plasma TG levels were higher and HDL-C, HDL2-C, and HDL3-C were lower (P<0.05) in women with high VAT/TAF; however, total cholesterol and LDL-C did not differ between the groups. The prevalence of IGT was seven times (58 vs. 8%, P<0.01) and metabolic syndrome 10 times (83 vs. 8%, P<0.01) higher in women with high compared to those with lower VAT/TAF. VAT/TAF correlated positively with mid-thigh low density lean tissue area (r=0.58), fasting (r=0.45) and 2-hr (r=0.40) glucoses, HOMA-IR (r=0.51), and TG (r=0.56) (all P’s<0.01) and negatively with HDL-C (r=−0.29, P<0.05) across the groups (N=48); however, there were no significant relationships within groups.
Fat Cell Weight, Surface Area and Adipose Tissue LPL Activity by VAT/TAF Group
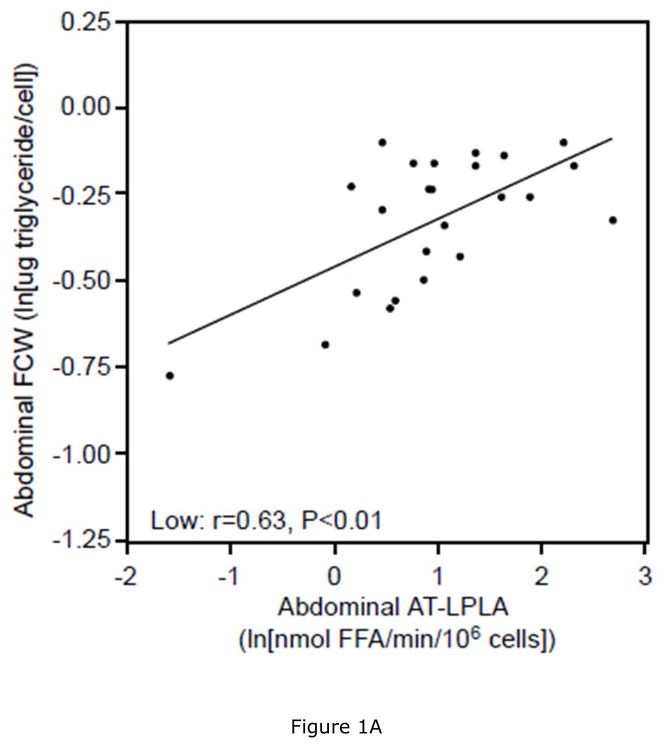
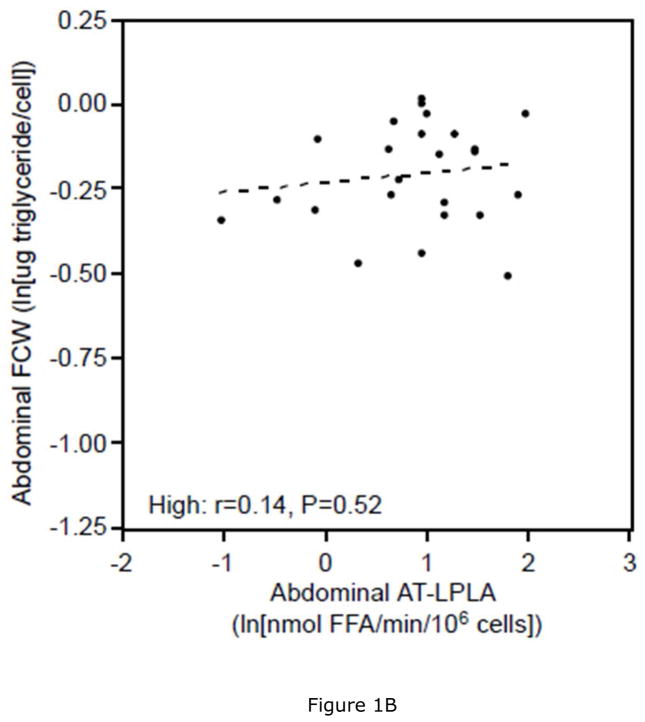
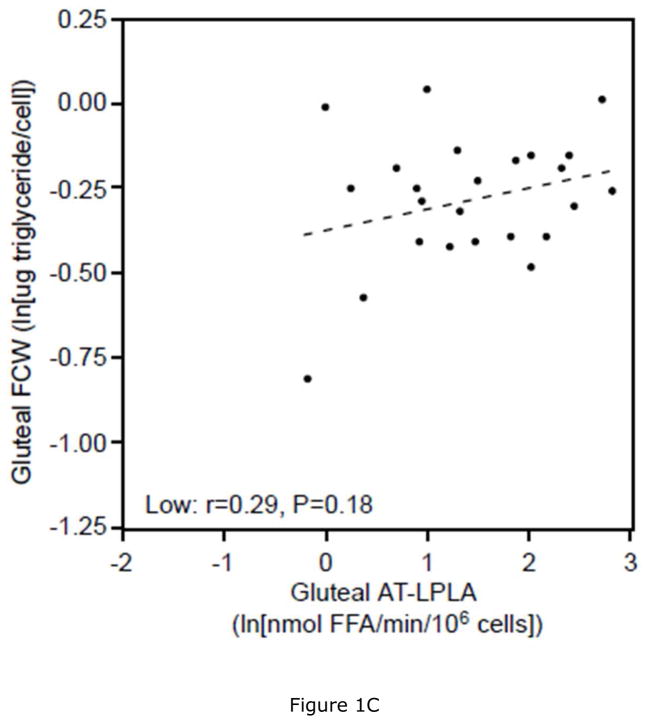
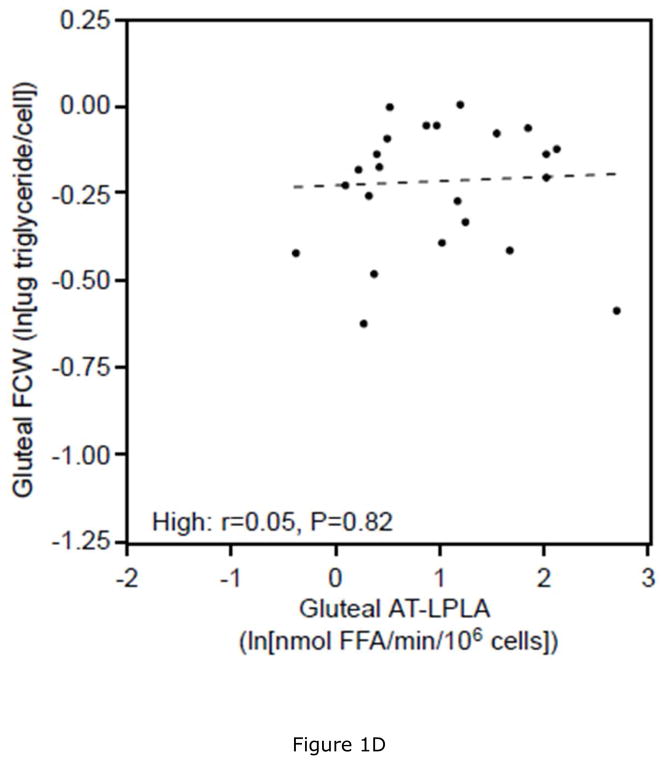
Women with low VAT/TAF had 11% lower ABD (P<0.05) and 6% lower GLT (P=0.22) FCW, as well as 7% lower ABD (P<0.05) and 4% lower GLT (P=0.23) FCSA than women with high VAT/TAF. This was associated with 28% and 54% higher AT-LPLA when expressed per 106 cells and 40% and 50% higher AT-LPLA when expressed per FCSA in both ABD and GLT subcutaneous tissue, respectively, despite comparable % body fat (Table 3). In the women with low VAT/TAF, increasing FCW (and FCSA- data not shown) correlated with ABD AT-LPLA (rcell= 0.63, P<0.01; rFCSA= 0.33, P<0.05, Figure 1A), suggesting that with increasing subcutaneous adipocyte size these women have higher AT-LPLA and greater capacity to store fat and expand their adipocytes. In contrast, women with high VAT/TAF have higher FCW (and FCSA- data not shown) at each AT-LPLA and the relationship (slope) with AT-LPLA is flat (rcell=0.14, P=0.52; rFCSA=−0.09, P=0.67, Figure 1B), suggesting less adipocyte capacity to store more TG and expand. The significant difference in the lines depicting these relationships in the ABD site by slope (P=0.05) and y-intercept (P<0.01) whether AT-LPLA is expressed per 106 cells or FCSA (data not shown) suggests that adipocytes of women with low VAT/TAF have greater potential to hypertrophy due to higher AT-LPLA/cell than women with high VAT/TAF. The relatively flat relationship of GLT FCW to AT-LPLA in both the low (rcell=0.29, P=0.18 [Figure 1C]; rFCSA=−0.21, P=0.34) and high (rcell=0.05, P=0.82 [Figure 1D]; rFCSA=−0.32, P=0.14) VAT/TAF groups suggests that GLT stores may not be able to store more TG in these obese women. HOMA-IR did not correlate with FCW, FCSA, or regional AT-LPLA. However, there was a positive relationship between SAT area and ABD AT-LPLA, expressed per 106 cells (r=0.39, P<0.01) and FCSA (r=0.31, P<0.05), but not for VAT area, supporting our hypothesis that higher AT-LPLA may enhance TG storage in SAT, thereby potentially limiting TG “spillover” into VAT.
Table 3.
Adipose tissue FCW and AT-LPLA by lowest and highest VAT/(TAF)
| Lowest VAT/TAF | Highest VAT/TAF | P-value | |
|---|---|---|---|
| Abdominal FCW (μg triglyceride/cell) | 0.73±0.03 | 0.82±0.02 | 0.02 |
| Gluteal FCW (μg triglyceride/cell) | 0.76±0.03 | 0.81±0.03 | 0.22 |
| Abdominal adipocyte surface area (μm2) | 2.4±0.06 | 2.6±0.05 | 0.02 |
| Gluteal adipocyte surface area (μm2) | 2.5±0.06 | 2.6±0.06 | 0.23 |
| Abdominal AT-LPLA (nmol FFA/min/106 cells) | 3.7±0.7 | 2.9±0.4 | 0.32 |
| Gluteal AT-LPLA (nmol FFA/min/106 cells) | 5.7±0.9 | 3.7±0.7 | 0.09 |
| Abdominal AT-LPLA (nmol FFA/min/μm2) | 2.0±0.3 | 1.4±0.2 | 0.19 |
| Gluteal AT-LPLA (nmol FFA/min/μm2) | 3.0±0.4 | 2.0±0.5 | 0.16 |
Values are expressed as means±SEM.
Figure 1.
There is a significant linear relationship between abdominal fat cell weight (FCW) and adipose tissue-lipoprotein lipase activity (AT-LPLA) in women with low VAT/TAF (FCW=(0.14)(AT-LPLA)−0.46; r=0.63, P<0.01, Figure 1A (solid line)), but not in the high VAT/TAF group (FCW=(0.03)(AT-LPLA)−0.23; r=0.14, P=0.52, Figure 1B (dashed line)). The line in women with high VAT/TAF differed significantly from those with low VAT/TAF in y-intercept (P<0.01) and slope (P=0.05). The relationship of gluteal FCW to AT-LPLA was not significant in either the low (FCW=(0.06)(AT-LPLA)−0.38; r=0.29, P=0.18, Figure 1C) or high (FCW=(0.01)(AT-LPLA)−0.24; r=0.05, P=0.82, Figure 1D) VAT/TAF groups. The solid line represents a significant relationship, while the dashed lines represent non-significant relationships.
Discussion
Lipoprotein lipase is the rate-limiting enzyme in the clearance of circulating TG-rich lipoproteins and the uptake and storage of energy stores in adipose tissue (27). AT-LPLA increases with feeding and weight gain, and high levels of AT-LPLA are associated with abdominal (central) body fat distribution and weight regain after diet-induced weight loss (27, 28). The results of this study suggest that obese postmenopausal women with a lower ratio of VAT/TAF have greater capacity for adipocyte hypertrophy, and this is evidenced by their higher AT-LPLA and smaller adipocytes than women with high VAT/TAF. The significantly steeper slope of the relationship between ABD FCW and AT-LPLA in women with low compared to high VAT/TAF is compatible with their smaller ABD fat cells having greater lipogenic capacity to store more lipid than women with high VAT/TAF, who seem to have saturated their subcutaneous adipocyte TG stores. The flat relationship of GLT FCW to AT-LPLA in both low and high VAT/TAF groups suggests the GLT storage depot already may be saturated in these obese women. Collectively, these data support the theory that molecular mechanisms (5) regulate the ability of subcutaneous adipocytes to hypertrophy (3, 4), increasing their capacity to store more lipid subcutaneously and prevent the metabolic consequences of lipid spillover to visceral and other ectopic sites. The lower VAT area, muscle fat infiltration, and cardiometabolic risk profile of obese women with low VAT/TAF further supports our hypothesis that higher AT-LPLA in SAT prevents ectopic lipid accumulation and metabolic risk factors associated with visceral adiposity. The congruent findings in obese adolescents (5) suggest that, across the age-span, obese individuals may remain metabolically healthy as long as their subcutaneous adipose depots can expand to more efficiently store excess lipid and prevent its accumulation in visceral tissues (29).
The number of preadipocytes, heterogeneity in adipogenesis, lipogenesis, and adipocyte functionality, and the vascular capacity of adipose tissue for denovo angiogenesis can enhance an obese person’s ability to store fat subcutaneously (30, 31), thereby avoiding the adverse health consequences of visceral fat accumulation. While we did not examine the contribution of adipocyte hyperplasia to subcutaneous lipid storage capacity, the higher FCW in women with lower compared to higher VAT/TAF makes it likely that there also are more small subcutaneous adipocytes available to hypertrophy in response to weight gain or fat overfeeding, as suggested by Alligier et al. (6) and Kashiwagi et al. (32). However, despite the larger peak diameter of subcutaneous ABD adipocytes in adolescents with high vs. low VAT/TAF, Kursawe et al. (5) found similar fat cell number and a reduced fraction of large fat cells in the high VAT/TAF group. The notion that the ability to expand subcutaneous adipocytes could prevent or treat type 2 diabetes and metabolic dysfunction is supported by the fact that thiazolidinedione (TZD) administration is associated with SAT hypertrophy, reductions in VAT, and improvements in lipid and glucose metabolism in type 2 diabetes. The ability of TZDs to increase PPARγ and other lipogenic gene expression in SAT suggests they stimulate subcutaneous, not visceral, adipocyte hypertrophy and would likely reduce VAT accumulation in obese women with high VAT/TAF (33). This observation suggests that in contrast to visceral fat, subcutaneous fat has beneficial effects on metabolism.
Weight loss and aerobic exercise have tissue specific effects on fat and muscle AT-LPLA. While weight loss does not appear to affect skeletal muscle LPLA (34), the effects of weight loss on AT-LPLA appears to be heterogeneous, with some studies showing increases (28, 35), decreases (36), or both (12). This variability may be due to differences in study populations (i.e. gender, pre- vs. postmenopausal women, and moderate vs. morbid obesity) and the amount and duration of weight loss. Further, the amount of weight regained following weight loss appears to be greater in women who increase AT-LPLA with weight loss (12). In contrast, exercise reduces adipose and increases skeletal muscle AT-LPLA, decreasing lipid storage in adipose tissue and increasing muscle fat oxidation (37, 38). The effects of these lifestyle interventions on the lipogenic capacity of adipose tissue and muscle, adipogenesis and angiogenesis warrants further investigation in obese individuals with high VAT who are at risk for diabetes and cardiometabolic complications.
Although these results suggest that the impaired ability to store lipid subcutaneously may increase lipid accumulation in VAT and skeletal muscle, similar findings in adipose tissue biopsies from VAT would be conclusive, but this is not possible without biopsies during abdominal surgery. Unfortunately, we did not have enough tissue to measure additional mechanisms regulating fat cell expandability, such as adipocyte hyperplasia, capillarization and biomarkers of angiogenesis and lipogenesis. These results also should be tempered by the cross-sectional nature of this study and female population; it will be valuable to study the effects of exercise and weight loss on adipose tissue metabolism in comparably obese men who tend to have twice as much VAT and are at higher risk for type 2 diabetes and MSyn than obese women (39), as well as African Americans who tend to be more insulin resistant than Caucasian women, despite less visceral fat (40). These limitations are, however, somewhat balanced by our strong study design that controlled for obesity, age, and race by matching Caucasian women with low and high VAT/TAF for % body fat and age, studied obese postmenopausal women at high risk for type 2 diabetes and cardiovascular disease, monitored diet compliance, and excluded subjects with metabolic dysfunction such as diabetes, hypothyroidism, and renal disease, which could alter adipose metabolism and AT-LPLA (27).
In sum, these results support the hypothesis that the enhanced ability to store TG in SAT reduces lipid accumulation in VAT and skeletal muscle and their associated metabolic abnormalities (1, 6). The finding that higher AT-LPLA was associated with a greater ability to expand adipocytes in women with low VAT/TAF, provides a potential mechanism by which excess circulating TG-FFA can be stored, thereby preventing VAT accumulation and metabolic dysfunction. Studies to determine the effects of exercise, weight loss and pharmacotherapy on the mechanisms regulating fat accumulation in subcutaneous and visceral adipose tissue could reveal therapies that could expand subcutaneous adipocytes, thereby preventing lipid accumulation in ectopic tissues and its cardiometabolic consequences.
What is already known
The accumulation of fat in visceral adipose tissue (VAT) is strongly associated with insulin resistance and metabolic syndrome.
The uptake and storage of triglyceride (TG) in adipocytes is primarily regulated by adipose tissue lipoprotein lipase activity (AT-LPLA).
Adolescents with high VAT have lower lipogenic gene expression (e.g. LPIN1, PPARγ2, etc.) in subcutaneous adipose tissue (SAT) and adults with low lipogenic gene expression in SAT tend to store more lipid in VAT, suggesting that the inability to accumulate TG in SAT leads to lipid spillover into VAT.
What this study adds
Older women with lower VAT/total abdominal fat (TAF) have lower fat cell weight, higher AT-LPLA, and a lower prevalence of metabolic syndrome and impaired glucose tolerance.
Abdominal FCW and SAT area correlate with AT-LPLA in women with low, but not high VAT/TAF, suggesting greater capacity to store TG in subcutaneous adipocytes.
These data support the hypothesis that the ability of adipocytes in SAT to hypertrophy and store more lipid, reduces visceral adiposity and cardiometabolic risk in older, obese women.
Acknowledgments
Our appreciation is extended to the women who participated in this study. We are grateful to the nurses, exercise physiologists, and registered dietitians of the University of Maryland School of Medicine Division of Gerontology and Geriatric Medicine and Baltimore VA GRECC for their assistance in this project. M.S. was involved in data analysis and manuscript development, A.R. body composition analysis and manuscript review, J.S. statistical support and manuscript review, K.F. statistical support, and A.G. in the study design, collection of research data, data interpretation, and manuscript development. This study was supported by funds from: National Institute on Aging (NIA) grants: R01-AG18408, R01-AG20116, and 5T32AG000219-18; NIDDK Mid-Atlantic Nutrition Obesity Research Center (NIH P30 DK072488); Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC); VA Advanced Health Postdoctoral Fellowship; the Baltimore VA Medical Research Service, VA Research Career Scientist Award.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Reaven G. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1998;37:1559–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Annals of the New York Academy of Sciences. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 4.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 5.Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59:2288–2296. doi: 10.2337/db10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alligier M, Gabert L, Meugnier E, Lambert-Porcheron S, Chanseaume E, Pilleul F, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab. 2013;98:802–810. doi: 10.1210/jc.2012-3289. [DOI] [PubMed] [Google Scholar]
- 7.Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. The American Journal of Physiology. 1993;265:E374–379. doi: 10.1152/ajpendo.1993.265.3.E374. [DOI] [PubMed] [Google Scholar]
- 8.Farnier C, Krief S, Blache M, Diot-Dupuy F, Mory G, Ferre P, et al. Adipocyte functions are modulated by cell size change: potential involvement of an integrin/ERK signalling pathway. Int J Obes Relat Metab Disord. 2003;27:1178–1186. doi: 10.1038/sj.ijo.0802399. [DOI] [PubMed] [Google Scholar]
- 9.Berman DM, Nicklas BJ, Ryan AS, Rogus EM, Dennis KE, Goldberg AP. Regulation of lipolysis and lipoprotein lipase after weight loss in obese, postmenopausal women. Obesity Research. 2004;12:32–39. doi: 10.1038/oby.2004.6. [DOI] [PubMed] [Google Scholar]
- 10.Fried SK, Tittelbach T, Blumenthal J, Sreenivasan U, Robey L, Yi J, et al. Resistance to the antilipolytic effect of insulin in adipocytes of African-American compared to Caucasian postmenopausal women. Journal of Lipid Research. 2010;51:1193–1200. doi: 10.1194/jlr.P000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002;87:4166–4170. doi: 10.1210/jc.2001-012034. [DOI] [PubMed] [Google Scholar]
- 12.Nicklas BJ, Rogus EM, Berman DM, Dennis KE, Goldberg AP. Responses of adipose tissue lipoprotein lipase to weight loss affect lipid levels and weight regain in women. Am J Physiol Endocrinol Metab. 2000;279:E1012–1019. doi: 10.1152/ajpendo.2000.279.5.E1012. [DOI] [PubMed] [Google Scholar]
- 13.Raison J, Basdevant A, Sitt Y, Guy-Grand B. Regional differences in adipose tissue lipoprotein lipase activity in relation to body fat distribution and menopausal status in obese women. Int J Obes. 1988;12:465–472. [PubMed] [Google Scholar]
- 14.Rebuffe-Scrive M, Enk L, Crona N, Lonnroth P, Abrahamsson L, Smith U, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. The Journal of Clinical Investigation. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2003;58:181–189. doi: 10.1093/gerona/58.2.m181. [DOI] [PubMed] [Google Scholar]
- 17.Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302:E145–152. doi: 10.1152/ajpendo.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 21.Dietary guidelines for healthy American adults. A statement for physicians and health professionals by the Nutrition Committee, American Heart Association. Circulation. 1988;77:721A–724A. [PubMed] [Google Scholar]
- 22.Nicklas BJ, Katzel LI, Bunyard LB, Dennis KE, Goldberg AP. Effects of an American Heart Association diet and weight loss on lipoprotein lipids in obese, postmenopausal women. Am J Clin Nutr. 1997;66:853–859. doi: 10.1093/ajcn/66.4.853. [DOI] [PubMed] [Google Scholar]
- 23.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 (Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibel RL, Hirsch J. Site- and sex-related differences in adrenoreceptor status of human adipose tissue. J Clin Endocrinol Metab. 1987;64:1205–1210. doi: 10.1210/jcem-64-6-1205. [DOI] [PubMed] [Google Scholar]
- 25.Cook RD. Influential observations in linear regression. Journal of the American Statistical Association. 1979;74:169–174. [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 28.Kern PA, Ong JM, Saffari B, Carty J. The effects of weight loss on the activity and expression of adipose-tissue lipoprotein lipase in very obese humans. The New England Journal of Medicine. 1990;322:1053–1059. doi: 10.1056/NEJM199004123221506. [DOI] [PubMed] [Google Scholar]
- 29.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Current Opinion in Lipidology. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 31.Veilleux A, Caron-Jobin M, Noel S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60:1504–1511. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwagi A, Mott D, Bogardus C, Lillioja S, Reaven GM, Foley JE. The effects of short-term overfeeding on adipocyte metabolism in Pima Indians. Metabolism: Clinical and Experimental. 1985;34:364–370. doi: 10.1016/0026-0495(85)90226-4. [DOI] [PubMed] [Google Scholar]
- 33.Defronzo RA, Mehta RJ, Schnure JJ. Pleiotropic effects of thiazolidinediones: implications for the treatment of patients with type 2 diabetes mellitus. Hosp Pract (1995) 2013;41:132–147. doi: 10.3810/hp.2013.04.1062. [DOI] [PubMed] [Google Scholar]
- 34.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz RS, Brunzell JD. Increase of adipose tissue lipoprotein lipase activity with weight loss. The Journal of Clinical Investigation. 1981;67:1425–1430. doi: 10.1172/JCI110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imbeault P, Almeras N, Richard D, Despres JP, Tremblay A, Mauriege P. Effect of a moderate weight loss on adipose tissue lipoprotein lipase activity and expression: existence of sexual variation and regional differences. Int J Obes Relat Metab Disord. 1999;23:957–965. doi: 10.1038/sj.ijo.0801025. [DOI] [PubMed] [Google Scholar]
- 37.Seip RL, Angelopoulos TJ, Semenkovich CF. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. The American Journal of Physiology. 1995;268:E229–236. doi: 10.1152/ajpendo.1995.268.2.E229. [DOI] [PubMed] [Google Scholar]
- 38.Ortmeyer HK, Ryan AS, Blumenthal JB, Joseph LJ, Goldberg AP. Exercise and weight loss (WL), but not WL alone, affects skeletal muscle and adipose tissue lipoprotein lipase actvity in postmenopausal women. Diabetes. 2006;55:A5. [Google Scholar]
- 39.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]