Abstract
To determine whether vascular endothelial growth factor-C (VEGF-C) and its receptor (VEGFR-3) are involved in the glial reaction elicited by transplanted mesenchymal stem cells (MSCs), we examined the cellular localization of VEGF-C and VEGFR-3 proteins in the striatum of adult normal rats that received bone marrow-derived human MSCs. The MSC grafts were infiltrated with activated microglia/macrophages and astrocytes over a 2-week period post-transplantation, which appeared to parallel the loss of transplanted MSCs. VEGF-C/VEGFR-3 was expressed in activated microglia/macrophages recruited to the graft site, where the induction of VEGF-C protein was rather late compared with that of its receptor. VEGF-C protein was absent or very weak on day 3, whereas VEGFR-3 immunoreactivity was evident within the first three days. Furthermore, within three days, VEGF-C could be detected in the brain macrophages localized immediately adjacent to the needle track. At the same time, almost all the brain macrophages in both regions expressed VEGFR-3. Reactive astrocytes at the graft site expressed VEGFR-3, but not VEGF-C. These data demonstrated the characteristic time- and cell-dependent expression patterns for VEGF-C and VEGFR-3 within the engrafted brain tissue, suggesting that they may contribute to neuroinflammation in MSC transplantation, possibly through the recruitment and/or activation of microglia/macrophages and astrogliosis.
Keywords: VEGF-C, VEGFR-3, Brain macrophages, Astrocytes, Mesenchymal stem cells, Cell transplantation
Introduction
Mesenchymal stem cells (MSCs), a subset of non-hematopoietic stem cells of the bone marrow, have recently been investigated as a therapeutic approach for neurological disorders, including post-stroke deficits. Studies suggest that MSCs promote endogenous neuronal growth, decrease apoptosis, reduce levels of free radicals, encourage synaptic connections from damaged neurons, and regulate inflammation (Borlongan et al. 2011; Dharmasaroja 2009; Honmou et al. 2012; Huang et al. 2012; Joyce et al. 2010; Kan et al. 2007). Although the precise mechanism by which MSCs improve functional recovery still remains elusive, many experimental studies suggest that they stimulate endogenous repair mechanisms and improve functional deficits by increasing the levels of neurotrophic and growth factors rather than by integrating into the damaged cerebral tissue (Bao et al. 2011; Joyce et al. 2010; Lin et al. 2011; Wakabayashi et al. 2010).
However, a recent series of reports has demonstrated that glial cells regulate the brain’s innate immune response to cell grafts under both healthy and inflammatory conditions. Thus, only a limited number of stem cells can be stably engrafted into the brain (Bergwerf et al. 2011; De Vocht et al. 2013a; De Vocht et al. 2013b; Praet et al. 2012; Reekmans et al. 2012; Tambuyzer et al. 2009). These data suggest the interaction between cellular grafts and the microenvironment of the host brain, especially the glia, is important for graft survival. Furthermore, the glial reaction induced by engrafted MSCs in brain tissue is expected to have a profound influence on graft survival (Bergwerf et al. 2011; De Vocht et al. 2013b).
Vascular endothelial growth factor (VEGF)-C, a member of the VEGF family, primarily stimulates lymphangiogenesis (Karkkainen et al. 2004; Veikkola et al. 2001). It is also believed to act on neural stem/progenitor cells via the VEGF receptor 3 (VEGFR-3) and regulate neurogenesis in developing and adult brains (Calvo et al. 2011; Le Bras et al. 2006). In addition, VEGF-C/VEGFR-3 is induced in reactive astrocytes, perivascular-infiltrated macrophages, and activated microglia in rats with ischemic stroke and experimental autoimmune encephalomyelitis. Thus, VEGF-C/VEGFR-3 may be involved in the overarching glial reaction common to central nervous system (CNS) insults: astrogliosis, recruitment of monocytic macrophages, and modulation of inflammation and immunity (Park et al. 2013; Shin et al. 2010; Shin et al. 2008; Shin et al. 2013). Considered together, it is likely that the VEGF-C/VEGFR-3 system is implicated in the astroglial and microglial/macrophage responses to engrafted MSCs.
The purpose of the present study is to provide a standardized assessment of glial reaction induced by engrafted MSCs in normal adult rat brain and provide evidence that the VEGF-C/VEGFR-3 system is implicated in the astroglial and microglial responses to the engrafted MSCs in the normal adult brain. Therefore, we examined the temporal changes and cellular localization of VEGF-C and VEGFR-3 proteins within the graft site and its surrounding area after transplantation of human bone marrow-derived MSCs into the striatum of adult healthy rats. Particular attention was paid to their relationships with graft-induced glial responses over a 2-week time period post-transplantation. We also compared the expression profiles of VEGF-C and VEGFR-3 at the graft site with those occurring in the mechanical lesion created by needle insertion, which also induced a profound glial cell reaction.
Materials & Methods
Preparation and Stereotaxic Intrastriatal Transplantation of Human MSCs
All experimental procedures were conducted under and approved by the Laboratory Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Survival Surgery provided by the IACUC (Institutional Animal Care and Use Committee) in the College of Medicine, The Catholic University of Korea (Approval Number: CUMC-2014-0006-01).
Human bone marrow-derived mesenchymal stem cells (Catholic MASTER Cells) were obtained from the Catholic Institute of Cell Therapy (CIC; Seoul, Korea). Cultured MSCs were harvested with trypsin/EDTA, washed three times with normal saline, and resuspended at a concentration of 60,000 cells/µl in normal saline. MSCs at the fifth passage were used for experiments. Transplantation of human MSCs was carried out according to the transplantation methods described by Goto et al. (1997).
Adult male Sprague–Dawley rats (250–300 g) were anesthetized with chloral hydrate (400 mg/kg i.p.) and placed on a stereotaxic frame with a microinjector unit (KD Scientific Inc.; Holliston, MA). For transplantation of MSCs, animals were given 5 µl of suspended cells (3×105 cells in 5 µl saline) into the right side of dorsal striatum using a 26-gauge Hamilton syringe (Hamilton Company; Reno, NV) at the following coordinates, relative to the bregma: anterior-posterior, 0 mm; mediolateral, 3.0 mm; and dorsoventral, 4.0 mm. To prevent backflow along the needle track, cells were delivered at a rate of 0.5 µl/min. In addition, the needle was left in situ for 5 min prior to and after cell injection, and then slowly withdrawn. Rats in the control group were given 5 µl of saline instead of suspended MSCs. Animals were allowed to live for 1, 3, 7, or 14 days after transplantation. A total of 32 rats were injected with MSCs (n=5/time point) or saline (n=3/time point).
Immunocytochemistry
For immunocytochemistry assays, human MSCs (fifth passage) were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) for 1 hr at room temperature. The fixed cells were then incubated overnight at 4C with mouse monoclonal anti-alpha tubulin (1:8000; Sigma-Aldrich; St. Louis, MO) and one of either VEGFR-3 (1:100; Abnova; Taipei, Taiwan) or VEGF-C (1:300; Bioss Inc.; Woburn, MA) rabbit polyclonal antibodies. Antibody staining was visualized with Cy3-conjugated anti-rabbit (1:2000; Jackson ImmunoResearch; West Grove, PA) and Alexa Fluor-488 goat anti-mouse (1:300; Molecular Probes; Eugene, OR). The specificity of immunoreactivity was confirmed by the absence of any immunocytochemical reaction in cells from which either primary or secondary antibodies were omitted. Cell nuclei were counterstained with DAPI (1:1000; 4′,6-diamidino-2′-phenyindole, Roche, Germany). Slides were viewed with a confocal microscope (LSM 700; Carl Zeiss Co. Ltd.; Oberkochen, Germany) equipped with four lasers (Diode 405, Argon 488, HeNe 543, HeNe 633). Images were converted into TIFF format, and contrast levels were adjusted using Adobe Photoshop 7.0 (Adobe Systems Inc.; San Jose, CA).
Tissue Processing and Immunohistochemistry
Animals were deeply anesthetized with 16.9% urethane (10 ml/kg i.p), and killed by transcardial perfusion with a fixative containing 4% paraformaldehyde in 0.1 M PB (pH 7.4). The brains were removed, and the tissue block encompassing the injection site was taken and post-fixed in the same fixative for 2 hr at 4C. After treatment with 30% sucrose, the blocks were embedded in OCT compound, and then cut into 15-μm-thick coronal sections with a cryostat.
To identify transplanted MSCs, we used a monoclonal antibody specific to human nuclei (HuN; 1:100; Millipore; Billerica, MA), which stains the nuclei of all human cell types and reveals no cross-reactivity in rat or mouse nuclei (Sánchez-Pernaute et al. 2005). For double immunofluorescence labeling, sections were incubated at 4C overnight with a mix of monoclonal anti-HuN and either rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; 1:1500; Millipore, Temecula, CA) or anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:500; Wako Pure Chemical Industries Ltd.; Japan), well-established markers of astrocytes and microglia, respectively. In addition, another set of sections was incubated at 4C overnight with a mix of rabbit polyclonal antibodies to VEGFR-3 (1:100; Abnova) or VEGF-C (1:300; Bioss) and mouse monoclonal antibodies to GFAP (1:500; Millipore) or ED1 (1:100; Serotec; Oxford, UK), one of the most reliable markers for monocytes/macrophages and activated microglia (Damoiseaux et al. 1994). Antibody staining was visualized with Cy3-conjugated anti-mouse (1:2000; Jackson ImmunoResearch), Cy3-conjugated anti-rabbit (1:2000; Jackson ImmunoResearch), Alexa Fluor-488 goat anti-mouse (1:300; Molecular Probes), and Alexa Fluor-488 goat anti-rabbit (1:300; Molecular Probes). The specificity of VEGF-C immunoreactivity was confirmed by the dose-dependent decrease in the immunohistochemical reactions by overnight preadsorption with several concentrations (ranging from 0 to 1.8 µg/ml) of its immunizing protein (Bioss Inc.) at 4C (Supplementary Fig. S1). The specificity for VEGFR-3 has been described in detail in our previous study (Park et al. 2013), and was also confirmed by the significant decrease in VEGFR-3 protein levels in VEGFR-3-silenced C6 cells using small interfering RNA transfection (Park et al. 2014; Supplementary Fig. S2). For Tdt-dUTP terminal nick-end labeling (TUNEL), sections were stained by the TUNEL method according to the manufacturer’s protocol (Roche Diagnostics Corporation, Indianapolis, IN). The sections were then immunostained with monoclonal mouse anti-HuN antibody, followed by incubation with Alexa Fluor-488 goat anti-mouse (1:300; Molecular Probes) and Cy3-conjugated streptavidin (1:1500; Jackson ImmunoResearch) for 1 hr. DAPI was used to counterstain cell nuclei, and pictures acquired by the described confocal microscope setup above. Images were converted into TIFF format, and contrast levels were adjusted using Adobe Photoshop 7.0.
Results
Characterization of Bone Marrow-derived Human MSCs
To examine whether MSCs used for transplantation expressed VEGF-C or VEGFR-3, immunocytochemistry was done using some of the same passage cells used for transplantation (fifth passage). Microscopic inspection of the human bone marrow-derived MSCs that had been stained for α-tubulin, a well-established microtubule marker, showed an elongated spindle-shaped morphology (Fig. 1). Immunoreactivities for VEGF-C and VEGFR-3 were evident in nearly all MSCs, with nuclear staining more prominent than cytoplasmic staining in both cases.
Figure 1.
Expression of VEGF-C and VEGFR-3 protein by bone marrow-derived human mesenchymal stem cells (MSCs) in vitro. Double-labeling with VEGF-C (A) or VEGFR-3 (D) and α-tubulin (B, E), a well-established marker of microtubules. Note that MSCs display intense nuclear but weak cytoplasmic staining for VEGF-C and VEGFR-3. Cell nuclei become fluorescent blue after DAPI staining. (C) and (F) show merged images. Scale, 100 μm.
Glial Reaction Elicited by MSC Engraftment
After stereotactic intrastriatal injection of human MSCs, the rat striatal sections passing through the graft were examined at 1, 3, 7, and 14 days postoperatively. To define the relationship of grafted MSCs and astroglial and microglial responses, sequential coronal sections that included the graft sites were processed for double-labeling with HuN and well-established markers of astrocytes and microglia, GFAP and Iba1, respectively. On day 1 after transplantation, MSCs displaying HuN staining were visible as a mass of cells in the striatum (Fig. 2A, 2B). At this time point, only a few Iba1-positive brain macrophages were visible within the MSC graft and its surrounds, whereas no significant immunoreactivity for GFAP was detected. On day 3, many round, amoeboid-like brain macrophages positive for Iba1 had accumulated in the area surrounding the MSC graft, whereas some macrophages had infiltrated the graft itself (Fig. 2C, 2E, and Supplementary Fig. S3). Because infiltrating blood-borne monocytes or macrophages and reactive microglia are not distinguishable by morphological criteria due to a lack of discriminating specific markers (Guillemin and Brew 2004; Graeber and Streit 2010), these cells might be infiltrating macrophages or activated resident microglia. In contrast, GFAP-positive, thin glial processes were detected only in the surrounding area, and not within the graft itself (Fig. 2D, 2F). On day 7, grafts were infiltrated with Iba1-positive microglia/macrophages (Fig. 2G), whereas astroglial processes were preferentially localized along the graft border (Fig. 2H). At this time point, HuN staining within the MSC grafts appeared to decrease as compared with that noted on day 3. To determine whether the decreased HuN staining could be explained by cell death, we performed double labeling with TUNEL and anti-HuN antibody on days 1 and 3 after transplantation. TUNEL-positive cells appeared to increase in number within a large cluster of MSCs showing HuN immunoreactivity on day 3 as compared with those at day 1 (Fig. 3). On day 14 after transplantation, the latest time point examined, only a few HuN-positive nuclei were observed in the graft site, whereas there were many brain macrophages and marked astrogliosis (Fig. 2I, 2J). However, no HuN staining was observed in either the contralateral hemisphere or the injection site in sham controls (Supplementary Fig. S4).
Figure 2.
Spatial and temporal relationships between engrafted MSCs and astroglial and microglial responses in adjacent tissue sections from the striatum of MSC-grafted rats. Double-labeling was performed with the human nuclei-specific marker (HuN) and well-established markers of astrocytes or microglia, glial fibrillary acidic protein (GFAP) or Iba1, respectively. (A, B) On day 1 after transplantation, HuN-positive MSCs are visible as a mass of cells in the striatum. Note that virtually no glial reaction is observed at the graft site. (C–F) At 3 days (3d), brain macrophages have accumulated in the area surrounding the graft, with some infiltration into the graft (C, E), whereas GFAP-positive, thin glial processes are detected along the graft border (D, F). (E, F) Higher magnification views of the boxed areas in C and D, respectively. (G, H) At 7 days (7d), the graft is heavily infiltrated with brain macrophages (G), whereas GFAP-positive processes are still preferentially localized along the graft border (H). (I, J) At 14 days (14d), the graft is infiltrated with brain macrophages and reactive astrocytes. Note that HuN staining at the graft site appears to decrease by 7 days, with very little remaining at 14 days. Cell nuclei appear blue after DAPI staining. Scale (C, D), 200 µm; (A, B, E–J), 100 µm.
Figure 3.

Double labeling for human nuclei-specific marker (HuN) and TUNEL in the striatum of the MSC-grafted rats on days 1 (A–D) and 3 (E–H) after transplantation. TUNEL-positive cells appear to increase in number within the grafted MSCs showing HuN immunoreactivity on day 3 compared with those at day 1. (D, H) Higher magnification views of the boxed areas in C and G, respectively. Cell nuclei appear blue after DAPI staining. 1d, 1 day; 3d, 3 days. Scale (A–C, E–G), 100 µm; (D, H), 50 µm.
Spatiotemporal Patterning of VEGF-C in Engrafted Striatum
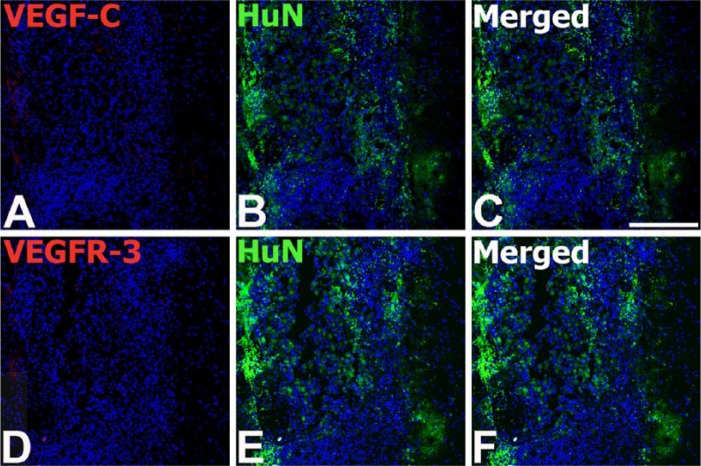
Based on the in vitro analysis, demonstrating that MSCs expressed VEGF-C or VEGFR-3 (Fig. 1), we examined whether engrafted MSCs still expressed VEGF-C or VEGFR-3. Double-labeling studies with HuN and VEGF-C or VEGFR-3 revealed that negligible immunoreactivity for VEGF-C or VEGFR-3 was observed in the engrafted MSCs at any time point after transplantation (Fig. 4).
Figure 4.
Double labeling for human nuclei-specific marker (HuN) and VEGF-C (A–C) or VEGFR-3 (D–F) in the serial tissue sections from the striatum of the MSC-grafted rats on day 1 after transplantation. HuN-positive MSCs are visible as a mass of cells in the striatum. Note that immunoreactivity for VEGF-C or VEGFR-3 is negligible or absent within the MSC graft. Cell nuclei appear blue after DAPI staining. Scale, 200 µm.
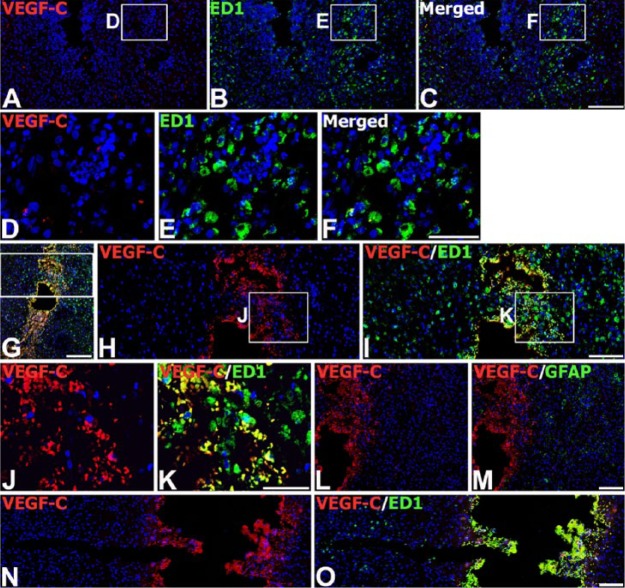
Next, we investigated the relationship between VEGF-C expression and glial responses in the engrafted striatum. On day 3 after transplantation, double-labeling with VEGF-C and ED1 (a monocytes/macrophages and activated microglia marker) at the graft site revealed that VEGF-C expression was absent or very weak in ED1-positive microglia/macrophages, despite their accumulation around and within the graft (Fig. 5A–5F). In addition, hardly any colocalization between VEGF-C and GFAP was detected around the graft (data not shown). However, at this time point, VEGF-C expression was evident in ED1-positive cells localized to a restricted area around the trajectory of the injection needle, but not in brain macrophages in areas distant from the needle track (Fig. 5G–5K). A similar expression pattern was observed in control animals that received only the vehicle: VEGF-C protein was induced in ED1-positive cells only near the needle track (Fig. 5N, 5O). However, GFAP staining was negligible or absent within the needle track in control and MSC-grafted animals (Fig. 5L, 5M).
Figure 5.
Expression of VEGF-C protein in brain macrophages in the striatum on day 3 after injection of MSCs or vehicle. (A–F) Double-labeling with VEGF-C and ED1 marker for microglia/macrophages at the graft site. Brain macrophages at the graft site exhibit negligible VEGF-C immunoreactivity. (D–F) Higher magnification views of the boxed areas in A–C, respectively. (G–K) Double-labeling with VEGF-C and ED1 in the trajectory of the injection needle after MSC engraftment. Note that brain macrophages localized in close vicinity to the needle track express VEGF-C. (H–K) Higher magnification views of the boxed area in G–I, respectively. (L, M) Double-labeling with VEGF-C and GFAP within the needle track after MSC engraftment, where GFAP staining is negligible or absent. (N, O) Double-labeling with VEGF-C and ED1 at a sham-injected site. Note that VEGF-C immunoreactivity is confined to brain macrophages within close proximity to the needle track. Cell nuclei appear blue after DAPI staining. Scale (G), 200 µm; (A–C, H, I, L–O), 100 µm; (D–F, J, K), 50 µm.
On day 7 after transplantation, intense VEGF-C immunoreactivity was observed within the graft, and virtually all of it was localized to ED1-positive brain macrophages (Fig. 6A–6C, 6G, 6H). In contrast, GFAP-positive astrocytes localized along the graft-host border were still devoid of VEGF-C immunoreactivity (Fig. 6D–6F, 6I, 6J). This expression pattern was maintained on day 14, but there was a notable decrease in the intensity of VEGF-C immunoreactivity in brain macrophages relative to that on day 7 (Fig. 6K–6M, 6Q, 6R). In addition, no specific immunoreactivity for VEGF-C was observed in reactive astrocytes, as identified by GFAP upregulation and hypertrophy, infiltrating the graft (Fig. 6N–6P, 6S, 6T).
Figure 6.
Spatiotemporal expression of VEGF-C protein in the striatum of MSC-grafted rats on days 7 and 14 after transplantation. (A–J) Double-labeling for VEGF-C (A, D) and ED1 (B) or GFAP (E) at 7 days. VEGF-C immunoreactivity is observed in ED1-positive brain macrophages infiltrating the graft, but not in the GFAP-positive processes enveloping the graft. (G–J) Higher magnification views of the boxed areas in A, C, D, and F, respectively. (K–T) Double-labeling for VEGF-C (K, N) and ED1 (L) or GFAP (O) at 14 days. The labeling pattern remains unchanged but the labeling intensity in ED1-positive cells appears to decrease relative to that seen on day 7. Note that GFAP-positive cells infiltrating the graft are still devoid of specific VEGF-C immunoreactivity. (Q–T) Higher magnification views of the boxed areas in K, M, N, and P, respectively. Cell nuclei appear blue after DAPI staining. Scale (A–F, K–P), 100 µm; (G–J, Q–T), 50 µm.
Spatiotemporal Induction Patterns of VEGFR-3 in Engrafted Striatum
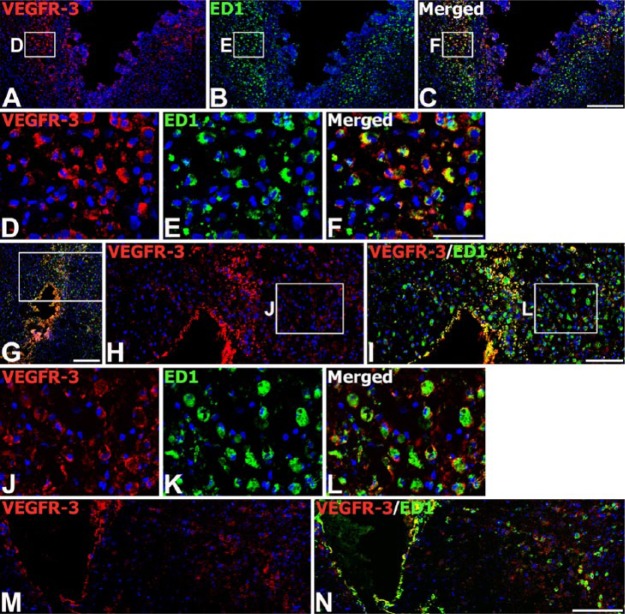
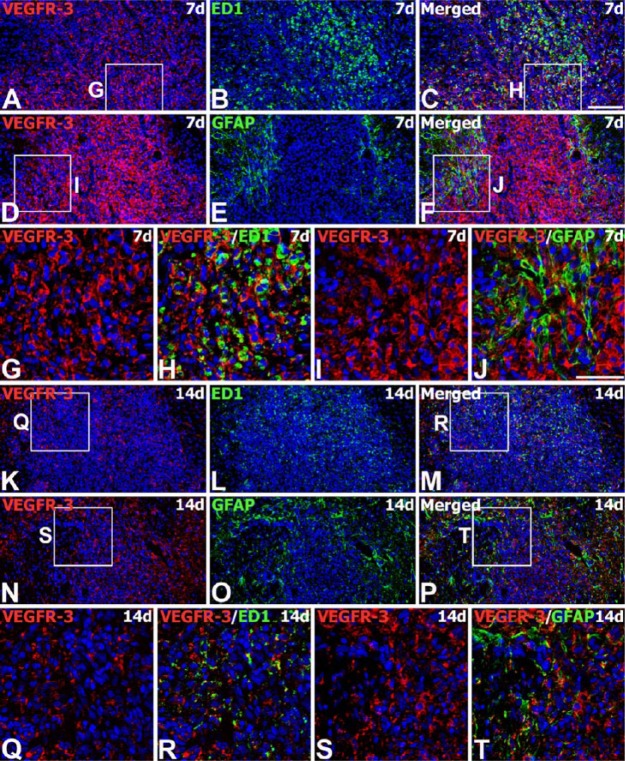
We next examined the expression of VEGFR-3 protein from the engrafted striatum. We used adjacent tissue sections for the labeling of VEGF-C and VEGFR-3. On day 3 after transplantation, VEGFR-3 immunoreactivity was evident in almost all ED1-positive brain macrophages. These cells were preferentially localized along the graft border, though some had infiltrated the graft (Fig. 7A–7F). In addition, VEGFR-3 protein could be detected in almost all of the brain macrophages recruited to the parenchyma adjacent to the needle track, as well as the surrounding area in the control (Fig. 7M, 7N) and MSC-grafted animals (Fig. 7G–7L). On day 7, intense immunoreactivity for VEGFR-3 was observed in nearly all brain macrophages infiltrating the graft (Fig. 8A–8C, 8G, 8H), and in reactive astrocytes forming an astroglial scar at the graft-host border (Fig. 8D–8F, 8I, 8J). On day 14, VEGFR-3 immunoreactivity was still evident in microglia/macrophages (Fig. 8K–8M, 8Q, 8R) and reactive astrocytes (Fig. 8N–8P, 8S, 8T), both of which had infiltrated the graft site. However, labeling intensity in these cells had declined in comparison to that on day 7.
Figure 7.
Expression of VEGFR-3 protein in brain macrophages on day 3 after injection of MSCs or vehicle. (A–L) Double-labeling with VEGFR-3 and ED1 at the graft site (A–F) and in the trajectory of the injection needle (G–L). Note that intense VEGFR-3 immunoreactivity is observed in nearly all of the ED1-positive brain macrophages within and surrounding the graft site/needle track. (D–F; H and I) Higher magnification views of the boxed areas in A–C and G, respectively. (J–L) Higher magnification views of the boxed areas in H and I, respectively. (M, N) Double-labeling with VEGFR-3 and ED1 at a sham-injected site. Note that VEGFR-3 protein can be detected in almost all of the brain macrophages within and surrounding the needle track. Cell nuclei appear blue after DAPI staining. Scale (A–C, G, M, N), 200 µm; (H, I), 100 µm; (D–F, J–L), 50 µm.
Figure 8.
Spatiotemporal expression of VEGFR-3 protein in the striatum of MSC-grafted rats on days 7 and 14 after transplantation. (A–J) Double-labeling for VEGFR-3 (A, D) and ED1 (B) or GFAP (E) at 7 days. VEGFR-3 immunoreactivity is observed in brain macrophages infiltrating the graft, and in astroglial processes enveloping the graft. (G–J) Higher magnification views of the boxed areas in A, C, D and F, respectively. (K–T) Double-labeling for VEGFR-3 (K, N) and ED1 (L) or GFAP (O) at 14 days. The grafts are heavily infiltrated with brain macrophages and reactive astrocytes, both of which express VEGFR-3 protein. Note that there is a notable decrease in the intensity of VEGFR-3 staining in these cells relative to that noted on day 7. (Q–T) Higher magnification views of the boxed areas in K, M, N and P, respectively. Cell nuclei appear blue after DAPI staining. Scale (A–F, K–P), 100 µm; (G–J, Q–T), 50 µm.
Discussion
The main finding of this study is that, within and around the engrafted brain tissue, VEGF-C and its tyrosine kinase receptor, VEGFR-3, are expressed in activated glial cells and macrophages with characteristic region- and cell-dependent expression patterns. Previous studies have suggested that allo- or xenogeneic stem cell grafts in both healthy and diseased brain can trigger robust microglial activation and severe astrogliosis. In fact, these glial reactions are the most prominent factors related to rejection of cell grafts in the CNS (Bergwerf et al. 2011; Coyne et al. 2007; Coyne et al. 2006; De Vocht et al. 2013a; De Vocht et al. 2013b; Praet et al. 2012; Reekmans et al. 2012; Ronsyn et al. 2007; Tambuyzer et al. 2009). In agreement with these previous reports, our observations demonstrated that the MSC grafts displayed massive infiltration by activated microglia, macrophages, and reactive astrocytes, whereas HuN staining at the graft site appeared to decrease over time. Thus, our data reinforce the idea that activated microglia/macrophages and astrogliosis play a central role in neuroinflammation following MSC transplantations, and provide new evidence for a role for VEGF-C/VEGFR-3 in MSC graft-induced glial reactions.
VEGF-C and VEGFR-3 proteins were expressed in ED1-positive brain macrophages accumulating within the graft site. These results agree well with the induction of VEGFR-3 expression in infiltrating monocytic macrophages within the injured CNS (Park et al. 2013; Shin et al. 2010; Shin et al. 2008). In addition, this study is the first to provide evidence that VEGF-C protein is indeed induced in activated microglia and macrophages. Several studies have demonstrated that VEGF-C or VEGFR-3 is expressed in bone marrow-derived cells, including macrophages, monocytes, and dendritic cells, and that VEGF-C affects macrophage migration via VEGFR-3 (Hamrah et al. 2004; Hamrah et al. 2003; Skobe et al. 2001; Stepanova et al. 2007). Thus, VEGF-C/VEGFR-3 may presumably be involved in the activation/recruitment of microglia and macrophages, perhaps via paracrine and/or autocrine mechanisms, in the engrafted striatum. This hypothesis was additionally supported by the observation that ED1-positive macrophages expressed VEGF-C and VEGFR-3 in experimental obliterative bronchiolitis occurring in rat tracheal allografts, suggesting that VEGF-C/VEGFR-3 signaling plays an important role in regulating innate and adaptive immune responses after lung transplantation (Krebs et al. 2012).
Interestingly, the expression of VEGF-C and VEGFR-3 proteins in brain macrophages at the graft site exhibited overlapping patterns, but VEGF-C expression was delayed relative to its receptor: VEGF-C protein was absent or very weak at 3 days, but increased by 7 days post-transplantation, whereas VEGFR-3 protein was induced within the first 3 days. Most brain macrophages accumulated in the area surrounding the MSC graft by 3 days after transplantation, with infiltration into the graft clearly observed at 7 days (compare Fig. 2C with 2G). In this regard, VEGF-C induction in these cells showed a close temporal relationship with their recruitment into the graft. In addition, brain macrophages showed decreased immunoreactivity for VEGF-C/VEGFR-3 by day 14, when most of the transplanted MSCs appeared to be eliminated from the graft and only a few showed HuN staining. This change has been noted not only in the present study, but also in previously published data (Coyne et al. 2007; Coyne et al. 2006). Thus, the temporal pattern of the expression for VEGF-C overlaps with the period during which brain macrophages are recruited into the graft core and they reject the transplanted MSCs. Relative to the graft site, the early induction (within 3 days) of VEGF-C protein could be detected in brain macrophages recruited to the parenchyma adjacent to the needle track in both MSC-engrafted and control animals, indicating that this may be a response to the traumatic injury rather than a specific reaction to the transplanted MSCs. Thus, it is likely that the time of induction of VEGF-C protein in brain macrophages depends on the experimental lesion type; that is, whether the lesion was from MSCs being engrafted or simply the mechanical lesion induced by needle insertion. In the latter, however, brain macrophages expressed VEGF-C only in the areas immediately adjacent to, but not away from, the needle track. Thus, VEGF-C protein was induced in a subset of brain macrophages, which were localized to the core region damaged by the mechanical lesion. In addition, VEGF-C-positive cells showed intense staining for ED1, a protein expressed in the membranes of phagolysosomes in microglia/macrophages and which is correlated with phagocytic activity (Damoiseaux et al. 1994). Thus, the spatiotemporal expression of VEGF-C protein within the graft/needle track implies that the induction of VEGF-C protein occurs in phagocytizing brain macrophages; although, the specific role of VEGF-C in these cells remains to be characterized.
Previous studies have proposed that reactive astrocytes form scar-like barriers surrounding the grafted MSCs, which sequesters them from immune effector cells, or that astrocytes infiltrating the graft may provide cellular and structural support for graft survival (Coyne et al. 2006; De Vocht et al. 2013a; De Vocht et al. 2013b; Praet et al. 2012). Our data demonstrated the induction of VEGFR-3 protein, but not its ligand, in reactive astrocytes at the graft site. These results agree well with previous findings that VEGFR-3 induction in reactive astrocytes is a generalized phenomenon during CNS lesions (Park et al. 2013; Shin et al. 2010; Shin et al. 2008; Zhang et al. 2012). However, they contrast with the finding of astroglial induction of VEGF-C mRNA in the post-ischemic hippocampus (Shin et al. 2008). Although the reason for this discrepancy is unclear, it could be due to differences in the experimental models, the brain area observed, or the expression of protein versus mRNA in the two studies. In addition, it is possible that the apparent absence of VEGF-C immunoreactivity is due to low VEGF-C protein levels that escaped detection by our techniques, because astroglial reaction in the engrafted brain is less prominent as compared with the activation of microglia and macrophages, as demonstrated by Reekmans et al. (Reekmans et al. 2012) and herein.
Another consideration is whether MSCs may secrete VEGF-C in situ in the engrafted striatum, given that MSCs expressed VEGF-C in vitro (see Fig. 1A). Interestingly, injection of VEGF-C into the intact brain activates astroglial and microglia, and disrupts the blood-brain barrier (Piltonen et al. 2011), and transplantation of human umbilical MSCs after complete transection of the rat spinal cord induces VEGFR-3 in the host spinal cord (Yang et al. 2008). However, our data showed that transplanted MSCs appeared to be rejected rapidly. This may be the reason why transplanted MSCs were devoid of specific VEGF-C staining in vivo after transplantation; although, low levels of VEGF-C expression in transplanted MSCs may escape detection. Furthermore, VEGF-C expression in the engrafted brain could be attributed instead to brain macrophages. Altogether, it is likely that the neuroinflammatory response in engrafted brain tissue is not induced by MSCs secreting VEGF-C but is rather a phenomenon secondary to cell death observed after allogeneic or xenogeneic stem cell transplantation into the CNS (De Vocht et al. 2013a). Previous studies have suggested that even autologous MSC grafts as well as allo- or xenogeneic stem cell grafts in the rat brain can trigger robust microglial activation and severe astrogliosis (Camp et al. 2009; Tambuyzer et al. 2009; Bergwerf et al. 2011; De Vocht et al. 2013a; De Vocht et al. 2013b). In addition, Khoo et al. (2011) reported that the glial reaction after transplantation of human MSCs into the rat brain occurs in the presence of cyclosporine immunosuppression, indicating that it is not specific to the transplanted cells. Thus, these data suggest that allo- or xenogeneic MSC grafts in the rat brain may elicit similar host responses and the consequent rejection of transplanted MSCs.
In summary, our results verified previous findings that human MSCs transplanted into the intact rat brain elicited a neuroinflammatory response mediated by activated microglia, macrophages, and astrogliosis, but also revealed several novel insights: (1) VEGF-C/VEGFR-3 was expressed in activated microglia/macrophages recruited to the graft site, where the induction of VEGF-C protein was rather late compared with its receptor; (2) brain macrophages in the mechanical lesion induced by needle insertion expressed VEGF/VEGFR-3 protein within 3 days; (3) VEGF-C protein could be detected immediately adjacent to, but not more distantly from, the needle track, whereas VEGFR-3 protein was expressed in both areas; (4) induction of VEGFR-3, but not VEGF-C, was noted in astrocytes surrounding and infiltrating the graft. Therefore, our data demonstrated that VEGF-C and its receptor showed characteristic time- and cell-dependent expression patterns within the engrafted brain tissue, suggesting that VEGF-C and VEGFR-3 may be involved in the recruitment/activation of microglia and macrophages, as well as in the astroglial reaction, and that they may contribute to the neuroinflammatory response elicited by MSC transplantation. However, further investigation is needed to determine whether the apparent expression of VEGF-C/VEGFR-3 in brain macrophages reflects its function in the recruitment of macrophages or is simply related to the functional status of microglia and macrophages.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Mid-career Researcher Program through the National Research Foundation of Korea (NRF) grant funded by the MEST (2011-0028319). The Catholic MASTER Cells supplied by Catholic Institute of Cell Therapy (CIC, Seoul, Korea) were derived from human bone marrow donated by healthy donors after informed consent.
References
- Bao X, Wei J, Feng M, Lu S, Li G, Dou W, Ma W, Ma S, An Y, Qin C, Zhao RC, Wang R. (2011). Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res 1367:103-113. [DOI] [PubMed] [Google Scholar]
- Bergwerf I, Tambuyzer B, De Vocht N, Reekmans K, Praet J, Daans J, Chatterjee S, Pauwels P, Van der Linden A, Berneman ZN, Ponsaerts P. (2011). Recognition of cellular implants by the brain’s innate immune system. Immunol Cell Biol 89:511-516. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. (2011). The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol 95:213-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, Barallobre MJ, Moutkine I, Lyytikka J, Tatlisumak T, Pytowski B, Zalc B, Richardson W, Kessaris N, Garcia-Verdugo JM, Alitalo K, Eichmann A, Thomas JL. (2011). Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev 25:831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Loeffler DA, Farrah DM, Borneman JN, LeWitt PA. (2009). Cellular immune response to intrastriatally implanted allogeneic bone marrow stromal cells in a rat model of Parkinson’s disease. J Neuroinflammation 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Reynolds K, Black IB, Woodbury D. (2007). Disparate host response and donor survival after the transplantation of mesenchymal or neuroectodermal cells to the intact rodent brain. Transplantation 84:1507-1516. [DOI] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB. (2006). Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells 24:2483-2492. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. (1994). Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 83:140-147. [PMC free article] [PubMed] [Google Scholar]
- De Vocht N, Lin D, Praet J, Hoornaert C, Reekmans K, Le Blon D, Daans J, Pauwels P, Goossens H, Hens N, Berneman Z, Van der Linden A, Ponsaerts P. (2013a). Quantitative and phenotypic analysis of mesenchymal stromal cell graft survival and recognition by microglia and astrocytes in mouse brain. Immunobiology 218:696-705. [DOI] [PubMed] [Google Scholar]
- De Vocht N, Praet J, Reekmans K, Le Blon D, Hoornaert C, Daans J, Berneman Z, Van der Linden A, Ponsaerts P. (2013b). Tackling the physiological barriers for successful mesenchymal stem cell transplantation into the central nervous system. Stem Cell Res Ther 4:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasaroja P. (2009). Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci 16:12-20. [DOI] [PubMed] [Google Scholar]
- Goto S, Yamada K, Yoshikawa M, Okamura A, Ushio Y. (1997). GABA receptor agonist promotes reformation of the striatonigral pathway by transplant derived from fetal striatal primordia in the lesioned striatum. Exp Neurol 147:503-509. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Banati RB, Streit WJ, Kreutzberg GW. (1989). Immunophenotypic characterization of rat brain macrophages in culture. Neurosci Lett 103:241-246. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. (2010). Microglia: biology and pathology. Acta Neuropathol 119:89-105. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. (2004). Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75:388-397. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana MR. (2004). Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res 79:553-561. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Chen L, Zhang Q, Dana MR. (2003). Novel Expression of Vascular Endothelial Growth Factor Receptor (VEGFR)-3 and VEGF-C on Corneal Dendritic Cells. The American Journal of Pathology 163:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD. (2012). Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med 18:292-297. [DOI] [PubMed] [Google Scholar]
- Huang B, Tabata Y, Gao JQ. (2012). Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release 162:464-473. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5:933-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan I, Melamed E, Offen D. (2007). Autotransplantation of bone marrow-derived stem cells as a therapy for neurodegenerative diseases. Handb Exp Pharmacol:219-242. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5:74-80. [DOI] [PubMed] [Google Scholar]
- Khoo ML, Tao H, Meedeniya AC, Mackay-Sim A, Ma DD. (2011). Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One 6:e19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R, Tikkanen JM, Ropponen JO, Jeltsch M, Jokinen JJ, Yla-Herttuala S, Nykanen AI, Lemstrom KB. (2012). Critical role of VEGF-C/VEGFR-3 signaling in innate and adaptive immune responses in experimental obliterative bronchiolitis. Am J Pathol 181:1607-1620. [DOI] [PubMed] [Google Scholar]
- Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL. (2006). VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci 9:340-348. [DOI] [PubMed] [Google Scholar]
- Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. (2011). Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke 42:2045-2053. [DOI] [PubMed] [Google Scholar]
- Park JH, Shin YJ, Riew TR, Lee MY. (2014). The Indolinone MAZ51 Induces Cell Rounding and G2/M Cell Cycle Arrest in Glioma Cells without the Inhibition of VEGFR-3 Phosphorylation: Involvement of the RhoA and Akt/GSK3β Signaling Pathways. PLoS One 9:e109055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Shin YJ, Cho JM, Choi JY, Jeun SS, Cha JH, Lee MY. (2013). Upregulation of vascular endothelial growth factor receptor-3 in the spinal cord of Lewis rats with experimental autoimmune encephalomyelitis. J Histochem Cytochem 61:31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen M, Planken A, Leskela O, Myohanen TT, Hanninen AL, Auvinen P, Alitalo K, Andressoo JO, Saarma M, Mannisto PT. (2011). Vascular endothelial growth factor C acts as a neurotrophic factor for dopamine neurons in vitro and in vivo. Neuroscience 192:550-563. [DOI] [PubMed] [Google Scholar]
- Praet J, Reekmans K, Lin D, De Vocht N, Bergwerf I, Tambuyzer B, Daans J, Hens N, Goossens H, Pauwels P, Berneman Z, Van der Linden A, Ponsaerts P. (2012). Cell type-associated differences in migration, survival, and immunogenicity following grafting in CNS tissue. Cell Transplant 21:1867-1881. [DOI] [PubMed] [Google Scholar]
- Reekmans K, De Vocht N, Praet J, Fransen E, Le Blon D, Hoornaert C, Daans J, Goossens H, Van der Linden A, Berneman Z, Ponsaerts P. (2012). Spatiotemporal evolution of early innate immune responses triggered by neural stem cell grafting. Stem Cell Res Ther 3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsyn MW, Daans J, Spaepen G, Chatterjee S, Vermeulen K, D’Haese P, Van Tendeloo VF, Van Marck E, Ysebaert D, Berneman ZN, Jorens PG, Ponsaerts P. (2007). Plasmid-based genetic modification of human bone marrow-derived stromal cells: analysis of cell survival and transgene expression after transplantation in rat spinal cord. BMC Biotechnol 7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Studer L, Ferrari D, Perrier A, Lee H, Viñuela A, Isacson O. (2005). Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells 23:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YJ, Choi JS, Choi JY, Hou Y, Cha JH, Chun MH, Lee MY. (2010). Induction of vascular endothelial growth factor receptor-3 mRNA in glial cells following focal cerebral ischemia in rats. J Neuroimmunol 229:81-90. [DOI] [PubMed] [Google Scholar]
- Shin YJ, Choi JS, Lee JY, Choi JY, Cha JH, Chun MH, Lee MY. (2008). Differential regulation of vascular endothelial growth factor-C and its receptor in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 116:517-527. [DOI] [PubMed] [Google Scholar]
- Shin YJ, Park JM, Cho JM, Cha JH, Kim SY, Lee MY. (2013). Induction of vascular endothelial growth factor receptor-3 expression in perivascular cells of the ischemic core following focal cerebral ischemia in rats. Acta Histochem 115:170-177. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. (2001). Concurrent Induction of Lymphangiogenesis, Angiogenesis, and Macrophage Recruitment by Vascular Endothelial Growth Factor-C in Melanoma. The American Journal of Pathology 159:893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova OI, Krylov AV, Lioudyno VI, Kisseleva EP. (2007). Gene expression for VEGF-A, VEGF-C, and their receptors in murine lymphocytes and macrophages. Biochemistry (Mosc) 72:1194-1198. [DOI] [PubMed] [Google Scholar]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. (2009). Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol 85:352-370. [DOI] [PubMed] [Google Scholar]
- Thomas WE. (1992). Brain macrophages: evaluation of microglia and their functions. Brain Res Rev 17:61-74. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. (2001). Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo j 20:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. (2010). Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res 88:1017-1025. [DOI] [PubMed] [Google Scholar]
- Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. (2008). Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One 3:e3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CQ, Shu HF, Yin Q, An N, Xu SL, Yin JB, Song YC, Liu SY, Yang H. (2012). Expression and cellular distribution of vascular endothelial growth factor-C system in cortical tubers of the tuberous sclerosis complex. Brain Pathol 22:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.