Abstract
DNA replication occurs during S-phase in spermatogonia and preleptotene spermatocytes during spermatogenesis. 5-Bromo-2’-deoxyuridine (BrdU) is incorporated into synthesized DNA and is detectable in the nucleus by immunohistochemistry (IHC). To identify BrdU-labeled spermatogenic cells, the spermatogenic stages must be determined by visualizing acrosomes and detecting cell type-specific marker molecules in the seminiferous tubules. However, the antibody reaction with BrdU routinely requires denaturation of the DNA, which is achieved by pretreating tissue sections with hydrochloric acid; however, this commonly interferes with further histochemical approaches. Therefore, we examined optimal methods for pretreating paraffin sections of the mouse testis to detect incorporated BrdU by an antibody and, at the same time, visualize acrosomes with peanut agglutinin (PNA) or detect several marker molecules with antibodies. We found that the use of heat-induced antigen retrieval (HIAR), which consisted of heating at 95C in 20 mM Tris-HCl buffer (pH 9.0) for 15 min, was superior to the use of 2 N hydrochloric acid for 90 min at room temperature in terms of the quality of subsequent PNA-lectin histochemistry with double IHC for BrdU and an appropriate stage marker protein. With this method, we identified BrdU-labeled spermatogenic cells during mouse spermatogenesis as A1 spermatogonia through to preleptotene spermatocytes.
Keywords: 5-bromo-2’-deoxyuridine, DNA replication, spermatogenesis, heat-induced antigen retrieval, immunohistochemistry, lectin histochemistry
Introduction
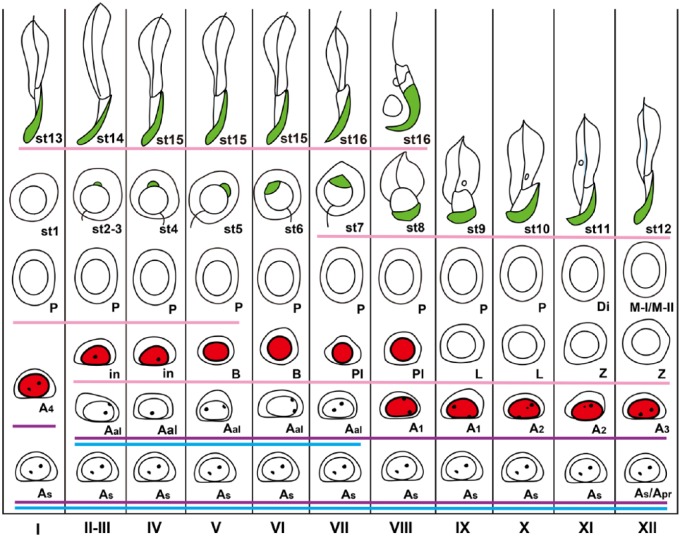
Spermatogenesis is a complex process consisting of the mitosis of spermatogonia, meiosis of spermatocytes, and transformation of spermatids (spermiogenesis), and occurs within the seminiferous tubules (Kerr et al. 2006). Spermatogenic cells represent well-defined cell associations called ‘stages’ during spermatogenesis, of which there are 12 in the mouse (Oakberg 1956). Spermatogenic cells in the seminiferous tubules of the adult testis and some of the interstitial cells have the potential to proliferate. The spermatogenic cells that are capable of replicating DNA are spermatogonia and preleptotene spermatocytes, which are located in the basal compartment of the seminiferous tubules. Collectively, spermatogonia comprise undifferentiated and differentiated spermatogonia, with the former containing A-single (As), A-paired (Apr) and A-aligned (Aal) types of spermatogonia and the latter, A1, A2, A3, A4, intermediate, and B spermatogonia types (de Rooij 2001). Some undifferentiated spermatogonia are believed to constitute the stem cell population. Spermatogonia enter the S-phase of the cell cycle during mitosis whereas preleptotene spermatocytes do so during meiosis. Undifferentiated spermatogonia, i.e., As–Aal, randomly proliferate during stages X–II, stop proliferation thereafter, and Aal spermatogonia finally differentiate without dividing into A1 spermatogonia in stages VII–VIII (de Rooij 2001). Differentiated spermatogonia, i.e., A1-B, divide in a highly synchronized manner in particular spermatogenic stages; for example, A1 spermatogonia in stages VIII–IX and B spermatogonia in stages V–VI (Grasso et al. 2012).
In order to classify the stages of mouse spermatogenesis, it is important to identify the steps of spermiogenesis, which are defined by the morphological features of spermatids based on the acrosomal formation and shape of the nucleus (Oakberg 1956). Periodic acid Schiff (PAS)-hematoxylin staining of paraffin-embedded testis sections has been commonly used for this purpose (Meistrich and Hess 2013). The use of fluorescent dye-conjugated lectins, such as peanut agglutinin (PNA), which is derived from Arachis hypogaea (peanut) and reacts specifically with acrosomal components, has recently been established to visualize acrosomal formation during spermiogenesis under fluorescence microscopy (Aviles et al. 1997; Szász et al. 2000).
Determining the specific stages of spermatogenesis in histological sections with lectin histochemistry (LHC) allows specific types of spermatogenic cells, which are known to be present in each stage, to be identified in the seminiferous tubules. In addition, the visualization of various marker proteins with immunohistochemistry (IHC) also helps identify the specific types of spermatogenic and somatic cells in the seminiferous tubules. For example, GFRA1 (Glial cell-derived neurotrophic factor receptor alpha 1) is a membrane receptor that is expressed in undifferentiated A spermatogonia (Meng et al. 2000; Yomogida et al. 2003). Promyelocytic leukemia zinc-finger (PLZF) (official designation ZBTB16: zinc finger and BTB domain containing 16) is a transcription factor that is localized in the nuclei of undifferentiated and differentiated A spermatogonia (Buaas et al. 2004). cKIT is a membrane receptor that is expressed in differentiating spermatogonia (Yoshinaga et al. 1991). Cell adhesion molecule-1 (CADM1) is a cell adhesion molecule of the immunoglobulin superfamily that is localized in the plasma membranes of intermediate spermatogonia through to early pachytene spermatocytes as well as in step 7 to step 16 spermatids (Wakayama et al. 2003, 2007; Nakata et al. 2012). Synaptonemal complex protein 3 (SCP3) is a nuclear protein that is expressed in spermatocytes (Dobson et al. 1994; Lammers et al. 1994). Tyrosine tubulin is a component of microtubules (Wenz and Hess 1998) and vimentin is an intermediate filament protein (Mali et al. 1987), both of which are expressed in Sertoli cells. Alpha smooth muscle actin (αSMA) is a contractile protein that is expressed in peritubular myoid cells (Maekawa et al. 1996). 3β-hydroxysteroid dehydrogenase (3βHSD) is a steroidogenic enzyme that is expressed in Leydig cells (Baker et al. 1999). GATA4 is a transcription factor that is localized in the nuclei of Sertoli, Leydig, and peritubular myoid cells (Imai et al. 2004).
Labeling with 5-bromo-2’-deoxyuridine (BrdU), an analogue of thymidine, has been successfully used to visualize proliferating cells in histological sections (Nagashima et al. 1985). BrdU is incorporated into newly synthesized DNA during the S-phase of the cell cycle and is detectable by IHC using specific antibodies against BrdU. The one-shot, repeated, or continuous administration of BrdU into the veins or peritoneal cavity has been commonly used to analyze relative proliferative activity, the length of the cell cycle, and the percentage of S-phase cells in tissues in vivo. Previous studies suggested that BrdU-labeled cells in seminiferous tubules are preleptotene spermatocytes (Salmand et al. 2008; Koruji et al. 2012) and/or A2–A4 spermatogonia (Blanco-Rodríguez et al. 2003; Muñoz-Velasco et al. 2013).
The combination of the fluorescence techniques—IHC for BrdU, LHC for PNA, and IHC for marker proteins—is needed to determine precisely which spermatogenic cell types, especially spermatogonia, are labeled with BrdU. However, because the monoclonal antibody for BrdU only recognizes BrdU that is exposed on single-stranded DNA, BrdU immunohistochemistry commonly requires the denaturation of double-stranded DNA by the adequate pretreatment of tissue sections, typically involving hydrochloric acid (HCl) (Nagashima et al. 1985). The low pH of HCl is considered to remove some histones from DNA and partially cut the hydrogen bonds between DNA strands, thereby allowing access of the anti-BrdU antibody. However, pretreatment with HCl also reduces antigenicity of many proteins as well as the binding activity of DNA for dyes such as Hoechst and 4’,6’-diamidino-2-phenylindole (DAPI), and is, thus, inappropriate for the purpose of combining BrdU staining with other histochemical tools.
Methods have been developed in IHC for the pretreatment of tissue sections for “antigen retrieval” (Pileri et al. 1997), and include the digestion of proteins by proteases, solubilization of proteins by detergents, and degeneration of proteins by heat. Of these, the wet heat treatment at temperatures greater than 95C, designated as heat-induced antigen-retrieval (HIAR), has been the most successful for detecting antigens in formalin-fixed, paraffin-embedded specimens (Shi et al. 1991; Yamashita 2007; Krenacs et al. 2010). HIAR is believed to disrupt the aldehyde cross-links that occur in proteins during formalin fixation and restore the original conformation of antigenic epitopes. Heating in a weak acidic buffer, such as 10 mM citrate buffer at pH 6.0, is very common in HIAR. However, Emoto et al. (2005) demonstrated that HIAR in an alkaline condition, such as 121C in 20 mM Tris-HCl buffer (pH 9.0), was a more effective method for antigen retrieval. Furthermore, Tang et al. (2007) demonstrated in the developing mouse brain that HIAR at 99C in 10 mM citrate buffer (pH 6.0) gave superior results to the HCl pretreatment for detecting BrdU-labeled cells, retrieving several neural antigens, and preserving the stainability of nuclei for the DNA-binding dyes Hoechst and DAPI.
In the present study, we applied HIAR to paraformaldehyde-fixed, paraffin-embedded mouse testis specimens in order to identify proliferating cell populations in the seminiferous tubules. We found that HIAR with heating at 95C in 20 mM Tris-HCl buffer (pH 9.0) for 15 min was the optimal pretreatment for this purpose because it markedly reinforced the detection of both BrdU and marker proteins with double-IHC while preserving the visualization of acrosomes with PNA-LHC.
Materials & Methods
Mice, BrdU Administration, and Tissue Preparation
Mouse experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals in Kanazawa University. C57BL6 strain male mice were purchased from Nippon SLC, Inc. (Hamamatsu, Japan) and raised until the age of 10–15 weeks under standard laboratory conditions with a 12-hr light/dark cycle and free access to food and water.
Mice were given an intraperitoneal injection of BrdU (Sigma-Aldrich; St. Louis, MO) dissolved in physiological saline at 50 mg/kg body weight. After 2 hr, the mice were anaesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg body weight), sacrificed by bleeding from the right atrium, and perfused transcardially with cold physiological saline followed by cold 4% paraformaldehyde in phosphate buffer (pH 7.4). The testes were then removed and further fixed overnight by immersion in the same fixative at 4C. Specimens were embedded in paraffin after washing, and 4-μm-thick sections were prepared using a microtome and mounted on silanized glass slides.
Pretreatment of Tissue Sections for BrdU Detection
HCl pretreatment
Deparaffinized sections were treated with 2 N HCl (Wako Pure Chemical Industries; Tokyo, Japan) for 90 min at room temperature and then neutralized with two washes in PBS for 3 min each.
HIAR
Deparaffinized sections were immersed in 20 mM Tris-HCl buffer (pH 9.0), heated at 95C for 15 min using an autoclave apparatus (ACV-3167N; Asahi Techno Glass, Funabashi, Japan), and allowed to cool to room temperature. In one experiment, 10 mM citrate buffer (pH 6.0) was used instead of 20 mM Tris-HCl buffer (pH 9.0).
IHC and LHC
After pretreatment (HCl or HIAR) or no treatment, sections of the mouse testis were subjected to IHC and/or LHC. In IHC with enzyme detection, the sections were first treated with 3% hydrogen peroxide in methanol to inactivate intrinsic peroxidase activity, and then with 5% normal goat serum to prevent non-specific antibody binding. They were then incubated overnight at 4C with a mouse monoclonal anti-BrdU antibody (Clone Bu20a; DakoCytomation, Glostrup, Denmark; Magaud et al. 1989) at 1:150 dilution. After washing with PBS, the site of the immunoreaction was visualized by incubating the sections successively with a biotinylated anti-mouse IgG antibody (1:250; Vector Laboratories, Burlingame, CA) for 1 hr, horseradish peroxidase-conjugated streptavidin (1:300; DakoCytomation) for 1 hr, and ImmPact DAB (Vector Laboratories) for 1 min. Normal mouse IgG (10 μg/ml; Vector Laboratories) was used instead of the anti-BrdU antibody as a negative control. In IHC with fluorescence detection, the sections were treated with 1% bovine serum albumin alone and then incubated overnight at 4C with the mouse monoclonal anti-BrdU antibody (1:150), either alone for single immunofluorescence or in combination with a goat polyclonal anti-rat GFRA1 antibody (1:200; Neuromics, Edina, MN) (Naughton et al. 2006; Grasso et al. 2012), rabbit polyclonal anti-mouse PLZF antibody (1: 200, Sigma-Aldrich) (Meistrich and Hess 2013), rabbit polyclonal anti-mouse CADM1 antibody developed in our laboratory (0.5 μg/ml) (Wakayama et al. 2003, 2007; Nakata et al. 2012), or goat anti-mouse Gata4 antibody (1: 400; Santa Cruz Biotechnology, Dallas, TX) (Imai et al. 2004) for double immunofluorescence microscopy. After washing, the immunoreactive sites were visualized by incubating the sections for 1 hr at room temperature with the anti-mouse IgG antibody conjugated with Alexa Fluor 594 (1:400; Molecular Probes, Eugene, OR) alone for single immunofluorescence or in combination with the anti-rabbit or anti-goat IgG antibodies conjugated with Alexa Fluor 488 (1:400; Molecular Probes) for double immunofluorescence. The fluorescence detection of IHC for BrdU in combination with LHC using PNA was also performed. To achieve this, the sections were incubated successively with the mouse monoclonal anti-BrdU antibody and anti-mouse IgG antibody conjugated with Alexa Fluor 594. After washing, the sections were then incubated with PNA conjugated with Alexa Fluor 488 (1:500; Molecular Probes) for 30 min at room temperature. All sections for fluorescence microscopy were counterstained in the nucleus with BisBenzimide H33258 (Hoechst 33258, Sigma-Aldrich) at 100 ng/ml for 5 min at room temperature. The sections were then observed with an immunofluorescence microscope (BX50/BX-FLA; Olympus, Tokyo, Japan).
Quantification of BrdU-labeled Cells
Under fluorescence microscopy of BrdU-IHC combined with PNA-LHC, the stages of spermatogenesis were determined in cross sections of seminiferous tubules based on acrosomal morphology, and the number of seminiferous tubules at each stage containing more than one BrdU-labeled cell in the epithelium was counted and expressed as a percentage of all seminiferous tubules at each stage. More than 20 seminiferous tubules in each stage were counted per section, and the mean values in three different sections from the same animal were calculated. Differences between two values from multiple mean values were examined by a one-factor analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. A value of p<0.05 (n=3) was considered significant. All experiments were repeated in at least 5 animals, with essentially the same results.
Results
We examined 4-μm paraffin sections of the mouse testis 2 hr after an intraperitoneal injection of BrdU solution into male mice.
Effects of Pretreatments with HIAR and HCl for BrdU-IHC, PNA-LHC, and marker protein-IHC
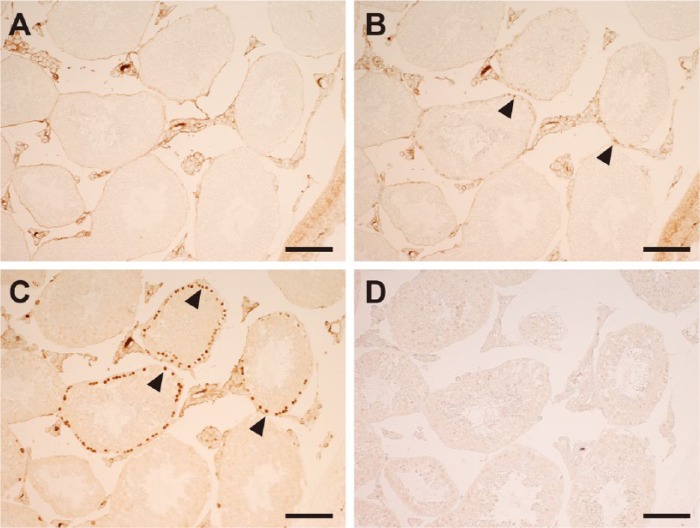
The results of IHC for BrdU, as detected by the peroxidase method, were compared after pretreatment with HIAR, HCl, or no treatment (Fig. 1). In non-treated sections, reactivity was undetectable in the seminiferous tubules and interstitial tissue, except for some background staining in the latter (Fig. 1A). In HCl-pretreated and HIAR-pretreated sections, BrdU immunoreactivity was detected in the nuclei nearest to the basement membrane in the seminiferous tubules (Fig. 1B, 1C). Although the population of immunoreactive cells was unchanged between the two sections, the intensity of the reactivity was markedly stronger in HIAR-pretreated than in HCl-pretreated sections. When the anti-BrdU antibody was replaced with normal mouse IgG, reactivity in HIAR-pretreated sections was eliminated completely in the seminiferous tubules and interstitial tissue (Fig. 1D).
Figure 1.
Light microscopic images of the mouse testis showing the effects of different pretreatments for 5-bromo-2’-deoxyuridine (BrdU) immunohistochemistry as detected using the enzyme method. Paraffin sections of the testis were left untreated (A) or subjected to a pretreatment with HCl (B) or heat-induced antigen retrieval (HIAR; C, D) and then immunostained with the mouse monoclonal anti-BrdU antibody (A–C) or normal mouse IgG (D). In (B) and (C), BrdU was localized in the nuclei (arrowheads) of the seminiferous tubules. In (A) and (D), no nuclear staining was observed. Scale, 100 μm.
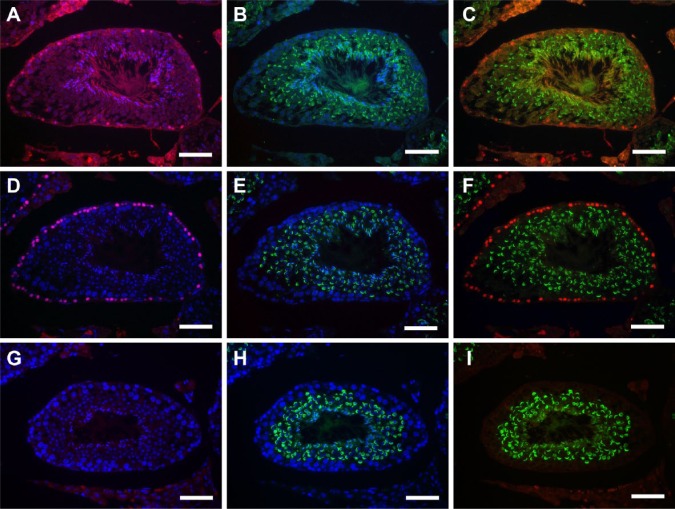
Double fluorescence microscopy for BrdU-IHC and PNA-LHC was performed in sections pretreated with HCl or HIAR (Fig. 2). When the anti-BrdU antibody was replaced with normal mouse IgG, only PNA-stained acrosomes, not BrdU-labeled nuclei, were detected with HIAR pretreatment, (Fig. 2G–2I). After HCl or HIAR pretreatment, BrdU reactivity was detected in the nuclei of preleptotene spermatocytes in stage VIII seminiferous tubules, for example, as identified by the pattern of acrosome staining with PNA lectin (Fig. 2A–2F). Consistent with the results of the peroxidase detection, the population of immunopositive cells was unchanged, whereas the intensity of BrdU immunofluorescence in these cells was stronger in HIAR-pretreated (Fig. 2D–2F) than in HCl-pretreated sections (Fig. 2A–2C). Furthermore, BrdU immunofluorescence in HCl-pretreated sections was not restricted to preleptotene spermatocytes; it was also diffusely detected in the lumen of seminiferous tubules and in the interstitial tissue as non-specific or background signals. The fluorescence of PNA lectin was also not restricted to acrosomes in HCl-pretreated sections; it was also observed in the flagella of step 16 spermatids as a non-specific signal. In contrast, both BrdU and PNA lectin signals were clearly localized to the nuclei of preleptotene spermatocytes and to acrosomes of spermatids in HIAR-pretreated sections, respectively. The intensity of the staining of nuclei with Hoechst 33258 was markedly weaker in HCl-pretreated sections, whereas it was as strong in HIAR-pretreated sections as that in non-treated sections.
Figure 2.
Fluorescence images of mouse seminiferous tubules showing the effects of different pretreatments on the simultaneous use of IHC for 5-bromo-2’-deoxyuridine (BrdU;red) and lectin histochemistry (LHC) for peanut agglutinin (PNA; green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the testis were pretreated with HCl (A-C) or heat-induced antigen retrieval (HIAR; D–I). They were then immunostained with the mouse monoclonal anti-BrdU antibody (A–F) or normal mouse IgG (G–I) and, at the same time, were stained with PNA-LHC. Pictures in different combinations of wavelengths are shown. Scale, 25 μm.
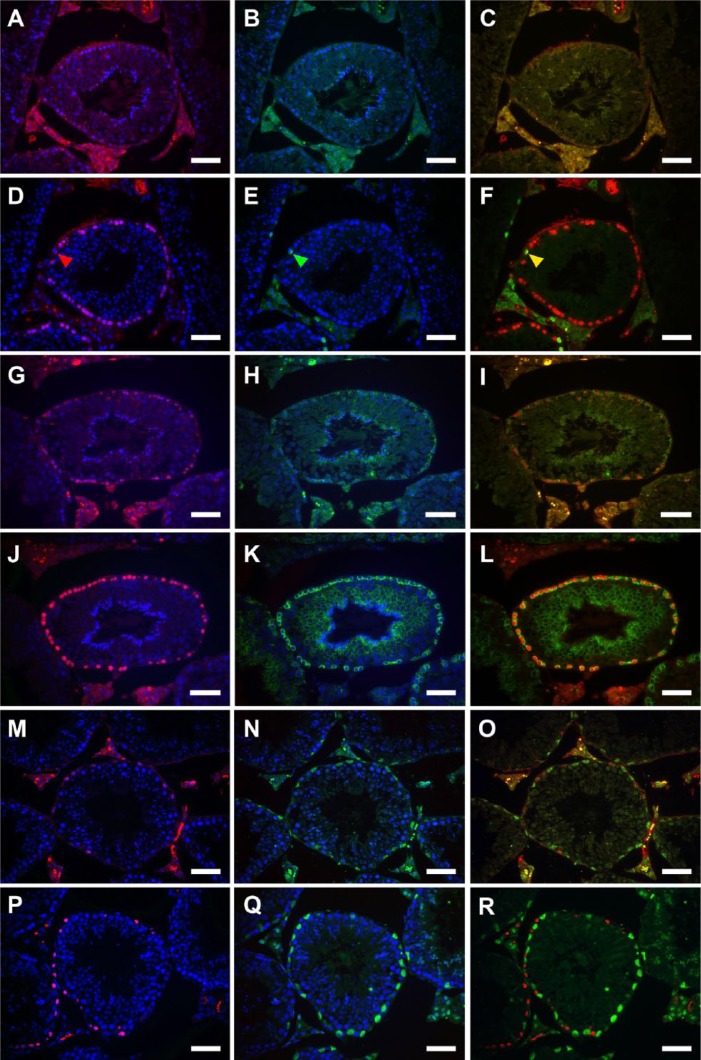
We then examined whether the pretreatment methods affected the results of IHC for the following markers: PLZF, as the nuclear marker for undifferentiated and differentiated A spermatogonia; CADM1, as the plasma membrane marker for intermediate spermatogonia through to early pachytene spermatocytes as well as for step 7 and later spermatids; and GATA4, as the nuclear marker for Sertoli cells in the seminiferous epithelium. Double immunofluorescence microscopy for BrdU and each marker was performed in sections pretreated with HCl or HIAR (Fig. 3). In all stages, the intensity of nuclear immunoreactivity in BrdU-labeled cells was stronger in HIAR-pretreated (Fig. 3D–3F, 3J–3L, 3P–R) than in HCl-pretreated sections (Fig. 3A–3C, 3G–3I, 3M–3O), as already demonstrated in Figures 1 and 2. Furthermore, the reactivities of PLZF (Fig. 3A–3F), CADM1 (Fig. 3G–3L), and GATA4 (Fig. 3M–3R) were all stronger in HIAR-pretreated than in HCl-pretreated sections, and the background signal was markedly lower in the former than in the latter sections.
Figure 3.
Fluorescence images of mouse seminiferous tubules showing the effects of different pretreatments on double immunostaining of BrdU (red) and various marker proteins (green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the testis were pretreated with HCl (A–C, G–I, M–O) or heat-induced antigen retrieval (HIAR; D–F, J–L, P–R), and then immunostained with the mouse monoclonal anti-BrdU antibody together with the rabbit polyclonal anti-PLZF antibody (A–F), rabbit polyclonal anti-CADM1 antibody (G–L), or goat polyclonal anti-GATA4 antibody (M–R). Pictures in different combinations of wavelengths are shown. One cell was simultaneously immunopositive for BrdU (red arrowhead, D) and PLZF (green arrowhead, E) when the photographs were merged (yellow arrowhead, F). The immunoreactivity of BrdU (red; G, J) and CADM1 (green; H, K) mostly overlapped (yellow; I, L). The immunoreactivity of BrdU (red; M, P) and GATA4 (green; N, Q) never overlapped (O, R). Both the immunoreactivity of BrdU (A, D, G, J, M, P) and marker proteins (B, E, H, K, N, Q) were much stronger and clearer in HIAR-pretreated sections (D-F, J-L, P-R) than in HCl-pretreated sections (A-C, G-I, M-O). Scale, 25 μm.
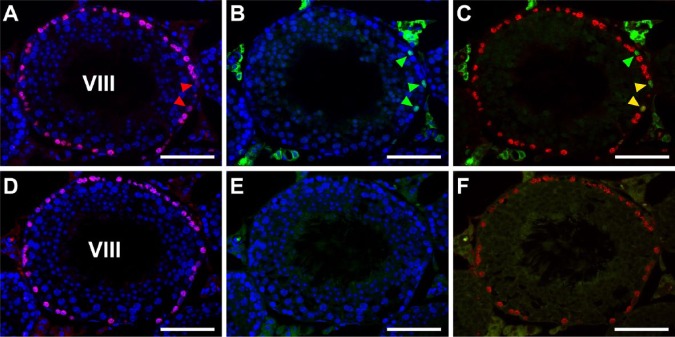
The present HIAR adopted 20 mM Tris-HCl buffer (pH 9.0). However, more acidic conditions, such as HIAR using 10 mM citrate buffer (pH 6.0), are also commonly used. Therefore, we compared both conditions of HIAR for their effects on PNA-LHC, BrdU-IHC, and marker protein-IHC in seminiferous tubules (Fig. 4). The effects on PNA-LHC were similar between the two buffer conditions; i.e., neither buffer caused a decrease in the intensity of acrosomal staining (data not shown). However, whereas the enhancing effects on BrdU-IHC were also similar between the two buffer conditions, the immunoreactivity for PLZF and GFRA1(i.e., two of the 4 marker proteins used in the present study), appeared to be markedly stronger in the pH 9.0 condition than in the pH 6.0 condition. For example, PLZF-positive cell nuclei in stage VIII seminiferous tubules were clearly detected at pH 9.0 (Fig. 4A–4C), but were difficult to detect with a higher non-specific background, at pH 6.0 (Fig. 4D–4F).
Figure 4.
Fluorescence images of mouse seminiferous tubules showing the effects of different pH conditions of heat-induced antigen retrieval (HIAR) on double immunostaining of BrdU (red) and PLZF (green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the testis were pretreated with HIAR with 20 mM Tris-HCl buffer, pH 9.0 (A–C) or 10 mM citrate buffer, pH 6.0 (D–F), and were then immunostained with mouse monoclonal anti-BrdU antibody together with the rabbit polyclonal anti-PLZF antibody. (A–C) and (D–F) represent two serial sections of the same stage VIII seminiferous tubule. Pictures in different combinations of wavelengths are shown. The immunoreactivity of BrdU (red) was localized in the nuclei of numerous cells (including two cells indicated by red arrowheads) that were in contact with the basement membrane, with similar intensities observed between the pretreatment in pH 9.0 (A-–C) and that in pH 6.0 (D–F). With pH 9.0, the immunoreactivity of PLZF (green) was clearly detected in the nuclei of three cells (green arrowheads, B). Two of the PLZF-positive cells were simultaneously positive for BrdU (yellow arrowheads), whereas the other was negative for BrdU (green arrowhead) in the merged picture (C). However, at pH 6.0, the immunoreactivity of PLZF was hardly detected in any cells, and there was high background fluorescence in the cytoplasm of elongated spermatids (E, F). Scale, 50 μm.
These results indicated that the present HIAR pretreatment was the method of choice for the combined analyses with BrdU-IHC for proliferating cells, PNA-LHC for spermatogenic stages, and marker protein-IHC for specific cell types in the sections of paraformaldehyde-fixed, paraffin-embedded testis specimens.
Identification of BrdU-labeled Cells in Testes Pretreated with HIAR
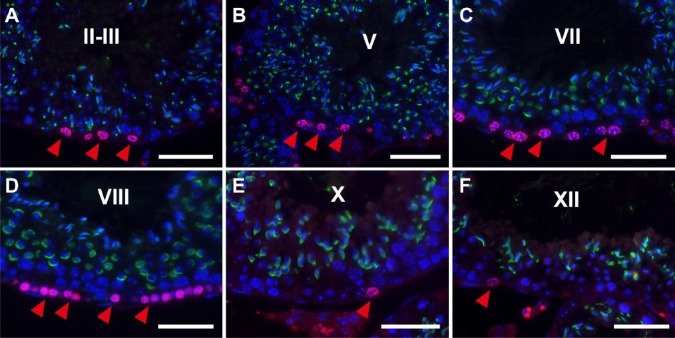
We analyzed individual BrdU-labeled cells at defined stages in the seminiferous tubules in testis sections pretreated with HIAR using double fluorescence microscopy for BrdU and PNA lectin (Fig. 5). We successfully identified 12 stages of mouse spermatogenesis with PNA-LHC based on the morphological criteria for acrosomes in spermatids originally defined by PAS staining. In stage II-III (Fig. 5A) and IV (not shown) seminiferous tubules, the nuclear immunoreactivity for BrdU was detected in cells in contact with the basement membrane, which are known to be mostly intermediate spermatogonia or undifferentiated A spermatogonia. In stage V (Fig. 5B) and VI (not shown) seminiferous tubules, reactivity was detected in cells with small, round nuclei and also in those in contact with the basement membrane, which presumably were B spermatogonia or undifferentiated A spermatogonia. In stage VII (Fig. 5C) and VIII (Fig. 5D) seminiferous tubules, reactivity was detected in cells with oval to round nuclei and located slightly above the basement membrane, which presumably were preleptotene spermatocytes, undifferentiated or differentiated A spermatogonia. In stage IX (not shown), X (Fig. 5E), XI (not shown), XII (Fig. 5F) and I (not shown) seminiferous tubules, reactivity was detected in a small number of cells that were in contact with the basement membrane, which presumably were undifferentiated or differentiated A spermatogonia. Throughout these stages, leptotene and later spermatocytes (with large round nuclei that were located apart from the basement membrane) showed no nuclear reactivity for BrdU.
Figure 5.
Fluorescence images of mouse seminiferous tubules showing the simultaneous IHC of BrdU (red) and peanut agglutinin-lectin (PNA-LHC; green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the mouse testis were pretreated with heat-induced antigen retrieval (HIAR) and then immunostained with mouse monoclonal anti-BrdU antibody and PNA lectin. The merged images of different wavelengths are shown. At stages II-III (A), V (B), VII (C), VIII (D), X (E), and XII (F), the immunoreactivity of BrdU was localized in the nuclei of cells that are in contact with or close to the basement membrane (red arrowheads). Scale, 25 μm.
We measured the number of seminiferous tubules containing more than one BrdU-labeled spermatogonium or preleptotene spermatocyte in stage I to XII seminiferous tubules (Fig. 6). In stages VII and VIII, during which undifferentiated A spermatogonia, preleptotene spermatocytes, and also differentiated A1 spermatogonia (only in stage VIII) are present, all seminiferous tubules (100%) contained BrdU-labeled cells. In stages II to VI, during which intermediate spermatogonia (in stages II to IV), B spermatogonia (in stages V and VI), and undifferentiated A spermatogonia (in stages II to VI) exist, approximately 70%–80% of seminiferous tubules contained BrdU-labeled cells. In stages IX to I, during which undifferentiated A spermatogonia and differentiated A1-A4 spermatogonia exist, approximately 10%–30% of seminiferous tubules contained BrdU-labeled cells (p<0.5 among VII-VIII, II-IV, and IX-I).
Figure 6.
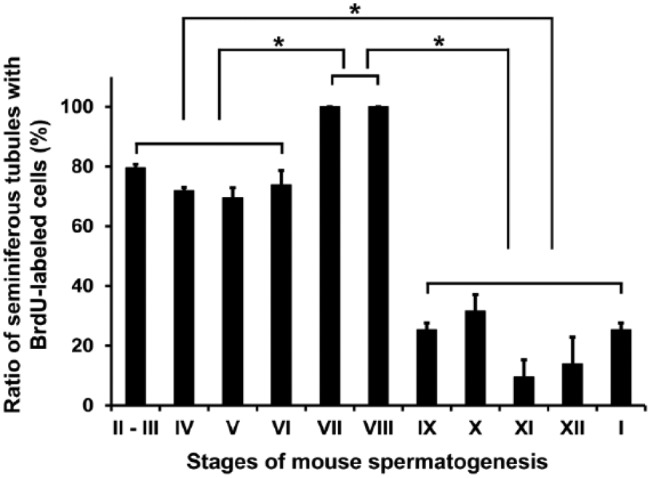
The ratio (%) of seminiferous tubules with BrdU-labeled spermatogenic cells in different spermatogenic stages. The number of seminiferous tubules at each stage containing more than one BrdU-labeled spermatogenic cell against that of the total number of seminiferous tubules at the same stage per section was calculated in three different sections from the same animal. Each value represents the mean ± SD of three sections. *p<0.05 indicates a significant difference.
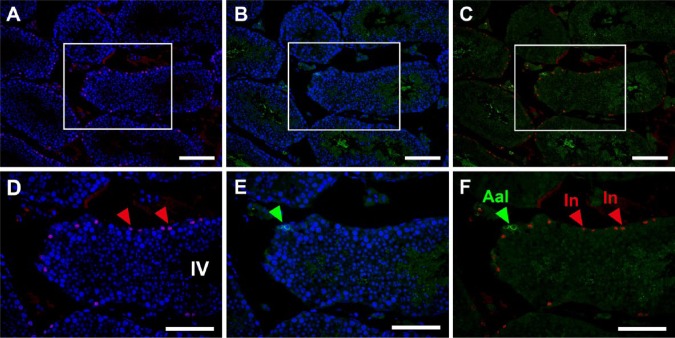
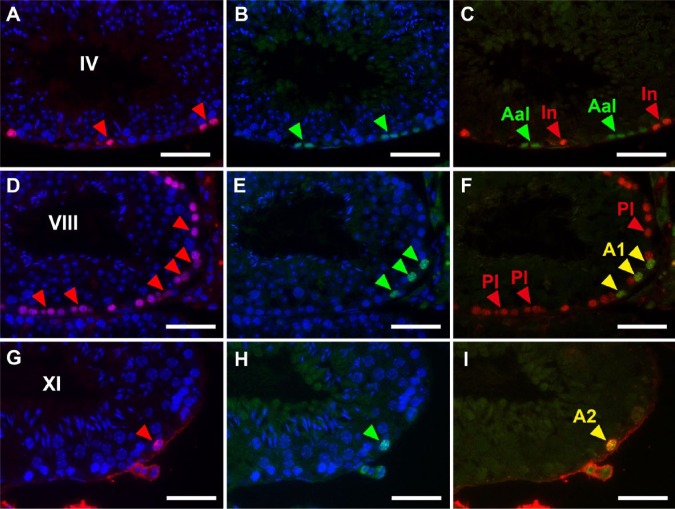
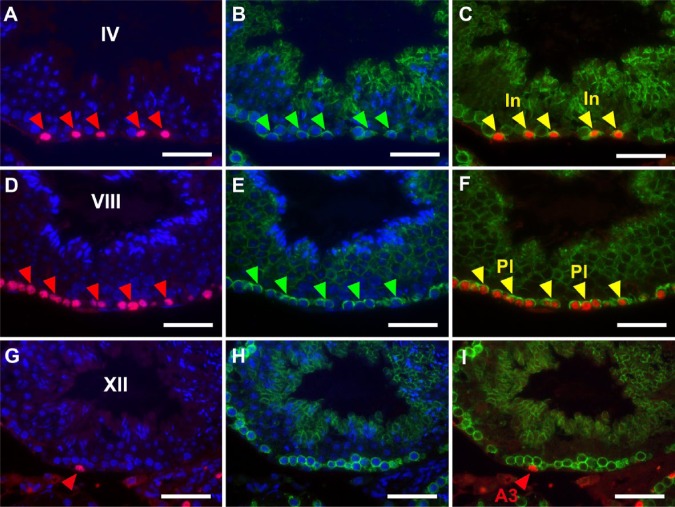
Thus, although the determination of stages with PNA-LHC enabled us to narrow down the candidates of BrdU-labeled spermatogenic cells, the exact determination of labeled cell types still requires the identification of cells with marker proteins. Therefore, we performed double immunofluorescence microscopy using the anti-BrdU antibody in combination with antibodies for marker proteins. Preliminary experiments indicated that BrdU-labeled cells never overlapped with GATA4-labeled Sertoli cells at any stage of the seminiferous tubules (Fig. 3P–3R); therefore, we only analyzed the overlap of BrdU-labeled cells with spermatogenic cells labeled by GFRA1 (Fig. 7), PLZF (Fig. 8) or CADM1 (Fig. 9) in detail. GFRA1 is known to be expressed in the plasma membrane of undifferentiated A (As, Apr, Aal) spermatogonia (Meng et al. 2000; Yomogida et al. 2003), whereas PLZF is known to be expressed in the nuclei of both undifferentiated A and differentiated A (A1, A2, A3, A4) spermatogonia (Buaas et al. 2004). Although the absolute number of GFRA1-expressing cells was very small throughout the stages, a GFRA1-positive Aal spermatogonia found in stage IV seminiferous tubules was negative for BrdU, and none of the BrdU-positive cells were positive for GFRA1 (Fig. 7A–7F). We examined more than 100 seminiferous tubules containing all stages, but undifferentiated A spermatogonia expressing GFRA1 showed no nuclear BrdU reactivity. Therefore, in stages II–IV seminiferous tubules (Fig. 8A–8C), the cells that were labeled with BrdU but did not express PLZF were clearly identified as intermediate spermatogonia. Similarly, in stages V and VI, BrdU-labeled cells not expressing PLZF were identified as B spermatogonia (not shown). In stage VIII (Fig. 8D–8F), most BrdU-labeled cells not expressing PLZF were identified as preleptotene spermatocytes. However, some cells were weakly labeled with BrdU and expressed PLZF in the same stage, which suggested that they were differentiated A1 spermatogonia. In stages IX–XII and I, most BrdU-labeled cells expressed PLZF and were identified as differentiated A1-A4 spermatogonia (Fig. 8G–8I). The other marker protein, CADM1, is known to be expressed in the membranes of intermediate spermatogonia through to early pachytene spermatocytes as well as in step 7 and later spermatids (Wakayama et al. 2003, 2007; Nakata et al. 2012). In stages II-III and IV (Fig. 9A–9C), all BrdU-labeled cells also expressed CADM1, which confirmed that they were intermediate spermatocytes. In stages VII and VIII (Fig. 9D–9F), most BrdU-labeled cells also expressed CADM1, suggesting that they are preleptotene spermatocytes. In stage XII (Fig. 9G–9I), BrdU-labeled cells did not express CADM1, which indicated that they are differentiated A3 spermatogonia and not zygotene spermatocytes. Figure 10 summarizes the spatiotemporal distribution of BrdU-labeled cells at a time point 2 hr after the injection of BrdU, and these were identified as differentiated spermatogonia (A1, A2, A3, A4, intermediate, and B spermatogonia) and preleptotene spermatocytes.
Figure 7.
Fluorescence images of mouse seminiferous tubules showing double immunostaining of BrdU (red) and GFRA1 (green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the mouse testis were pretreated with heat-induced antigen retrieval (HIAR) and then immunostained with the mouse monoclonal anti-BrdU antibody together with the goat polyclonal anti-GFRA1 antibody. Pictures in different combinations of wavelengths are shown. (A–C) At a lower magnification, a small number of undifferentiated A spermatogonia expressing GFRA1 were distributed in some seminiferous tubules. (D–F) At a higher magnification of stage IV seminiferous tubule in the rectangular area shown in (A–C), the immunoreactivity of BrdU was localized in intermediate spermatogonia (In; red arrowheads). Note that undifferentiated Aal spermatogonia (Aal), as identified with GFRA1 (green arrowhead), were devoid of BrdU. Scale, (A–C) 100 μm, and (D–F) 50 μm.
Figure 8.
Fluorescence images of mouse seminiferous tubules showing double immunostaining of BrdU (red) and PLZF (green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the mouse testis were pretreated with heat-induced antigen retrieval (HIAR) and then immunostained with the mouse monoclonal anti-BrdU antibody together with the rabbit polyclonal anti-PLZF antibody. Pictures in different combinations of wavelengths are shown. (A–C) At stage IV, BrdU was localized in intermediate spermatogonia (In; red arrowheads). Note that undifferentiated Aal spermatogonia (Aal), as identified with PLZF (green arrowheads) were devoid of BrdU. (D–F) At stage VIII, BrdU was localized in differentiated A1 spermatogonia (A1) and preleptotene spermatocytes (Pl) (red arrowheads in D). Of these, differentiated A1 spermatogonia (A1) were simultaneously positive for PLZF (green arrowheads in E and yellow arrowheads in F), whereas preleptotene spermatocytes (Pl) were negative for PLZF (red arrowheads in F). (G–I) At stage XI, BrdU was localized in a differentiated A2 spermatogonium (A2) that was simultaneously positive for PLZF (red arrowhead in G, green arrowhead in H, and yellow arrowhead in I). Scale, 25 μm.
Figure 9.
Fluorescence images of mouse seminiferous tubules showing double immunostaining of BrdU (red) and CADM1 (green). Nuclei were counterstained with Hoechst 33258 (blue). Paraffin sections of the mouse testis were pretreated with heat-induced antigen retrieval (HIAR) and then immunostained with the mouse monoclonal anti-BrdU antibody together with the rabbit polyclonal anti-CADM1 antibody. Pictures in different combinations of wavelengths are shown. (A–C) At stage IV, BrdU was localized in intermediate spermatogonia (In) that were simultaneously positive for CADM1 (red arrowheads in A, green arrowheads in B, and yellow arrowheads in C). (D–F) At stage VIII, BrdU was localized in preleptotene spermatocytes (Pl) that were simultaneously positive for CADM1 (red arrowheads in D, green arrowheads in E, and yellow arrowheads in F). (G–I) At stage XII, BrdU was localized in a differentiated A3 spermatogonium (A3) that was negative for CADM1 (red arrowhead in G and I). Scale, 25 μm.
Figure 10.
Schematic figure showing the distribution of BrdU-labeled spermatogenic cells in the different stages of mouse spermatogenesis. With the present method, BrdU (red) was detected in the nuclei of A1 spermatogonia through to preleptotene spermatocytes. PNA (green) was detected in the acrosomes in spermiogenesis. GFRA1 was expressed in undifferentiated A spermatogonia (drawn with a light blue line), PLZF was expressed in undifferentiated and differentiated A spermatogonia (drawn with a purple line), and CADM1 was expressed in intermediate spermatogonia through to early pachytene spermatocytes and step 7 and later spermatids (drawn with a pink line). I–XII, stages of spermatogenesis; As: A single, Apr: A paired, Aal: A aligned, A1, A2, A3, A4: differentiated A spermatogonia; In: Intermediate spermatogonium; B: B spermatogonium; Pl: preleptotene spermatocyte; L: leptotene spermatocyte; Z: zygotene spermatocyte; P: pachytene spermatocyte; Di: diplotene spermatocyte; M-I/M-II: first and second meiotic divisions; st1-16: step 1-16 spermatids.
Discussion
In the present study, we demonstrated that the pretreatment of sections from paraformaldehyde-fixed, paraffin-embedded mouse testis specimens with HIAR was adequate for identifying BrdU-labeled cells in spermatogenesis. Our HIAR pretreatment conditions were 20 mM Tris-HCl, pH 9.0, at 95C for 15 min, which was optimal for IHC for BrdU-labeled nuclei in combination with PNA-LHC for acrosomes and IHC for marker proteins specific for spermatogenic cell types. The purpose of the pretreatment in the detection of BrdU was to denature DNA so that BrdU molecules incorporated into single-stranded DNA become exposed and could be accessed by the monoclonal anti-BrdU antibody. The double-stranded structure of DNA is most stable at neutral pH, and both alkalines and acids, especially a strong acid such as HCl, will cause the partial denaturation of DNA. Pretreatments with 2 N HCl at room temperature for 90 min (Koruji et al. 2012) or 4 N HCl at room temperature for 30 min (Salmand et al. 2008) have previously been used to detect BrdU-labeled spermatogenic cells in the testis, and these studies reported that preleptotene spermatocytes were the main type of BrdU-labeled spermatogenic cell. However, they never combined the detection of BrdU with specific marker proteins in spermatogenic cells, and this may have been because the pretreatment with HCl is known to reduce the antigenicity of many proteins.
High temperatures above the melting temperature of DNA, which is 80–85C depending on the nucleotide components of DNA, also cause the denaturation of DNA. However, heating has been used for the purpose of antigen retrieval rather than DNA denaturation in the field of histochemistry. HIAR pretreatments may cut or weaken the cross-linkages in antigens that are formed by the aldehyde fixative and recover the molecular structure of antigens so that many epitopes in the molecules can react with the antibody (Shi et al. 1992; Cattoretti et al. 1993; Shi et al. 1999; Emoto et al. 2005; Yamashita and Okada 2005; Yamashita 2007). Tang et al. (2007) reported for the first time the application of HIAR to the immunohistochemical detection of BrdU-labeled proliferating cells in the developing rat brain. They also showed that HIAR retrieved the immunoreactivity of several neural proteins while preserving the stainability of nuclei for DNA-binding dyes in the same sections. The use of HIAR to detect BrdU has also been recently reported in human skin (Petersen et al. 2012). In the present study, we confirmed that HIAR was superior to the HCl pretreatment in the mouse testis because it increased the intensity of the immunoreactions for both BrdU and several marker proteins, while preserving the stainability of the nuclei for Hoechst 33258. Moreover, the present study demonstrated that acrosomal staining with PNA-LHC was preserved better by HIAR than by HCl pretreatment.
Several previous studies have used 10 mM citrate buffer (pH 6.0) for HIAR; however, many proteins are refractory to HIAR with this buffer (Kim et al. 2004). More recently, an alkaline pretreatment with 20 mM Tris-HCl (pH 9.0) was shown to be effective for antigen retrieval in many nuclear, cytoplasmic, and membrane proteins (Yamashita 2007). The present study indicated that HIAR using this alkaline buffer at pH 9.0 was superior to the conventional near-neutral buffer at pH 6.0 in detecting at least two of the four marker proteins examined. Heating at temperatures higher than 100C in an autoclave has often been adopted in HIAR, not only in methods using near-neutral buffers but also in those using alkaline buffers (Yamashita and Okada 2005; Yamashita 2007). However, in the present study, we found that heating at 121C for 10 min markedly reduced the staining intensity of nuclei with DNA-binding dyes such as Hoechst 33258 and hematoxylin in preliminary experiments. This was attributed to this temperature causing excessive denaturation and partial fragmentation of the DNA. Tang et al. (2007) also demonstrated that HIAR at more than 100C causes a reduction in the affinity of DAPI, Hoechst 33342, and Syto24. In the present study, we showed that the condition of heating at 95C for 15 min followed by its gradual restoration to room temperature sufficiently maintained the dye affinity in the nuclei; this method is achievable using a temperature-controllable autoclave apparatus.
Proteases have also been used to detect BrdU-labeled spermatogenic cells (Blanco-Rodríguez et al. 2003). In this study, testis sections were pretreated with HCl followed by trypsin digestion, and BrdU-labeled A2-A4 spermatogonia were detected with high sensitivity. However, acrosomes were not stained by PAS-hematoxylin or fluorescence-conjugated PNA lectin, and the immunohistochemical detection of marker proteins specific for spermatogenic cells in the same pretreated sections was not performed. We confirmed in our preliminary experiments that the combination of trypsin digestion and HCl pretreatment was as effective as the present HIAR pretreatment for visualizing BrdU-labeled nuclei, but, as expected, the former procedures made it difficult to apply the sections to further histochemical analyses for acrosomes and marker proteins because of a reduction in lectin-responsiveness and antigenicity. To avoid the strong DNA-denaturing pretreatments necessary for detecting BrdU, the use of 5-ethynyl-2’-deoxyuridine (EdU) instead of BrdU was recently reported (Salic and Mitchison, 2008). EdU incorporated in DNA is detectable chemically with a fluorescent azide without preceding tissue fixation or DNA denaturation. Whereas EdU may be highly useful for detecting DNA-synthesizing cells in whole-mount preparations of fresh large tissue and organs, it is a very expensive compound and antigen retrieval is still necessary to analyze the marker proteins in combination with EdU. Given the remarkable effects of HIAR on the detection of not only BrdU but also marker proteins, as shown in the present study, BrdU appears to be the first choice for general use.
In the present study, we attempted to accurately identify the types of BrdU-labeled cells present 2 hr after the administration of BrdU by combining BrdU immunohistochemical staining with staining of several cell marker proteins within particular stages of adult mouse spermatogenesis, as defined by the acrosome staining. We examined four marker proteins, i.e., GFRA1 for undifferentiated A spermatogonia, PLZF for undifferentiated and differentiated A spermatogonia, CADM1 for intermediate spermatogonia through to early pachytene spermatocytes as well as step 7 and later spermatids, and GATA4 for Sertoli, Leydig and peritubular myoid cells. BrdU-labeled cells never overlapped with GATA4-labeled somatic cells in the seminiferous tubules, which suggested that BrdU-labeled cells were exclusively spermatogenic cells. Furthermore, leptotene, zygotene, pachytene and diplotene spermatocytes as well as round and elongated spermatids, which were easily recognized by their size, shape, and position in the seminiferous epithelium, were never labeled with BrdU. These results suggest that BrdU-labeled cells may be exclusively spermatogonia and preleptotene spermatocytes.
According to the known distribution of various types of spermatogenic cells in particular stages of mouse spermatogenesis (Fig. 10), stages II–VI contain undifferentiated A (As and Aal), intermediate and B spermatogonia. Therefore, the BrdU-labeled cells in these stages that were immunopositive for CADM1, but immunonegative for PLZF, were identified as intermediate or B spermatogonia, and those that were positive for PLZF, but negative for CADM1, would have been identified as undifferentiated A (As and Aal) spermatogonia, even though we never encountered the latter cells in the present study. Stage VII contained undifferentiated A (As and Aal) spermatogonia and preleptotene spermatocytes, and stage VIII contained undifferentiated A (As) and differentiated A (A1) spermatogonia and preleptotene spermatocytes. Therefore, BrdU-labeled cells that were positive for CADM1 in stages VII and VIII were identified as preleptotene spermatocytes. Assuming that the BrdU-labeled cells positive for GFAR1 could be neglected, the BrdU-labeled cells that were positive for PLZF in stage VIII were identified as A1 spermatogonia. Stages IX to XII and I contained undifferentiated A (As, Apr and Aal) and differentiated A (A1 to A4) spermatogonia that were negative for CADM1. Therefore, the BrdU-labeled cells in these stages that were positive for PLZF were identified as differentiated A spermatogonia. The results obtained in the present study for the simultaneous immunostaining of BrdU and GFRA1, PLZF or CADM1, in combination with the determination of stages with acrosome staining, were consistent with this theoretical prediction. Taken together, the results of the present study have clarified that only differentiated spermatogonia and preleptotene spermatocytes are labeled with BrdU.
Only a few undifferentiated A (As, Apr and Aal) spermatogonia expressing GFRA1 were found in each section of the seminiferous tubules; however, none of them overlapped with BrdU-labeled cells. This suggested that very few, if any, undifferentiated spermatogonia are synthesizing DNA. The number of undifferentiated A (As, Apr and Aal) spermatogonia is known to be markedly smaller than that of differentiated (A1-A4, intermediate and B) spermatogonia. Furthermore, the former proliferate at random, whereas the latter proliferate in a highly synchronized manner, dividing during particular stages of spermatogenesis; that is, A1 spermatogonia in stages VIII and IX; A2 spermatogonia in stages X and XI; A3 spermatogonia in stage XII; A4 spermatogonia in stage I; intermediate spermatogonia in stages II-III and IV; and B spermatogonia in stages V and VI (Grasso et al. 2012). Undifferentiated A spermatogonia have a markedly longer cell cycle and G1 phase than those of differentiated spermatogonia (Huckins 1971a, 1971b), which markedly reduces the probability of incorporating BrdU into S-phase nuclei during the 2-hr labeling time. This may explain why BrdU-labeled undifferentiated A spermatogonia were never encountered in the present study.
The length of each stage of spermatogenesis is not constant, with stages VII and VIII containing preleptotene spermatocytes for a longer period of time than the other stages (Oakberg 1956). Spermatogenic cells proliferate synchronously to make clones connected by intercellular bridges, which suggests that the same population of differentiated spermatogonia or preleptotene spermatocytes may synthesize DNA at the same time in particular stages of spermatogenesis. The number of preleptotene spermatocytes is estimated to be 64-fold that of A1 spermatogonia, indicating that preleptotene spermatocytes represent the majority of spermatogenic cells in the basal portions of stage VII and VIII seminiferous tubules. This appears to account for the ratio of the seminiferous tubules with BrdU-labeled spermatogenic cells being 100% in stages VII and VIII in the present results. The ratio in other stages, i.e., 70%–80% in stages II–IV containing intermediate spermatogonia and in stages V–VI containing B spermatogonia, and 10%–30% in stages IX to I containing A1 to A4 spermatogonia, may also be interpreted in terms of the number of DNA-synthesizing cells and the length of their cell cycle. Figure 10 summarizes the distribution of BrdU-labeled spermatogenic cells in the different stages of mouse spermatogenesis revealed in the present study.
The precise identification and quantification of particular types of spermatogenic cells is essential for analyzing cell kinetics in spermatogenesis, and the present method of detection of BrdU-labeled cells will contribute to this goal. This method can also be applied to an analysis of abnormal spermatogenesis in transgenic or knockout mice and the pursuit of transplanted spermatogenic cells by spermatogonial stem cell transplantation.
Acknowledgments
The authors thank Mr. Shuichi Yamazaki for his histological work and Mr. Yuki Nukii for his assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work described herein was supported in part by grants from the Japanese Ministry of Education, Science and Culture to TW (25460241).
References
- Aviles M, castells MT, Martinez-Menarguez JA, Abascal I, Ballesta J. (1997). Localization of penultimate carbohydrate residues in zona pellucida and acrosomes by means of lectin cytochemistry and enzymatic treatments. Histochemical J 29:583-592. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Sha JA, McBride MW, Peng L, Payne AH, O’Shaughnessy PJ. (1999). Expression of 3beta-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur J Biochem 260:911-917. [DOI] [PubMed] [Google Scholar]
- Blanco-Rodríguez J, Martínez-García C, Porras A. (2003). Correlation between DNA synthesis in the second, third and fourth generations of spermatogonia and the occurrence of apoptosis in both spermatogonia and spermatocytes. Reproduction 126:661-668. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36:647-652. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E. (1993). Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 171:83-98. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. (2001) Proliferation and differentiation of spermatogonial stem cells. Reproduction 121:347-354. [DOI] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB. (1994). Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci 107:2749-2760. [DOI] [PubMed] [Google Scholar]
- Emoto K, Yamashita S, Okada Y. (2005). Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens? J Histochem Cytochem 53:1311-1321. [DOI] [PubMed] [Google Scholar]
- Grasso M, Fuso A, Dovere L, de Rooij DG, Stefanini M, Boitani C, Vicini E. (2012). Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reproduction 143:325-332. [DOI] [PubMed] [Google Scholar]
- Huckins C. (1971a). Cell cycle properties of differentiating spermatogonia in adult Sprague-Dawley rats. Cell Tissue Kinet 4:139-154. [DOI] [PubMed] [Google Scholar]
- Huckins C. (1971b). The spermatogonial stem cell population in adult rats II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet 4:313-334. [DOI] [PubMed] [Google Scholar]
- Imai T, Kawai Y, Tadokoro Y, Yamamoto M, Nishimune Y, Yomogida K. (2004). In vivo and in vitro constant expression of GATA-4 in mouse postnatal Sertoli cells. Mol Cell Endocrinol 214:107-115. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Loveland KL, O’Bryan MK, de Kretser DM. (2006). ‘Cytology of the testis and intrinsic control mechanisms’ in Neill JD. (ed.) Knobil and Neill’s Physiology of Reproduction, 3rd edition, New York: Academic Press, pp. 827-947. [Google Scholar]
- Kim SH, Myeong CK, Hyung GS. (2004). Optimal conditions for the retrieval of CD4 and CD8 antigens in formalin-fixed, paraffin-embedded tissues. J Mol Histol 35:403-408. [DOI] [PubMed] [Google Scholar]
- Koruji M, Movahedin M, Mowla SJ, Gourabi H, Pour-Beiranvand S, Jabbari Arfaee A. (2012). Autologous transplantation of adult mice spermatogonial stem cells into gamma irradiated testes. Cell J 14:82-89. [PMC free article] [PubMed] [Google Scholar]
- Krenacs L, Krenacs T, Stelkovics E, Raffeld M. (2010). Heat-induced antigen retrieval for immunohistochemical reactions in routinely processed paraffin sections. Methods Mol Biol 588:103-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, Heyting C. (1994). The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol 14:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. (1996). Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 59:1-13. [DOI] [PubMed] [Google Scholar]
- Magaud JP, Sargent I, Clarke PJ, Ffrench M, Rimokh R, Mason DY. (1989). Double immunocytochemical labeling of cell and tissue samples with monoclonal anti-bromodeoxyuridine. J Histochem Cytochem 37:1517-1527. [DOI] [PubMed] [Google Scholar]
- Mali P, Virtanen I, Parvinen M. (1987). Vimentin expression in spermatogenic and Sertoli cells is stage-related in rat seminiferous epithelium. Andrologia 19:644-653. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Hess RA. (2013). ‘Assessment of spermatogenesis through staging of seminiferous tubules’ in Carrell DT, Aston KI. (Eds.) Methods in Molecular Biology Series. Spermatogenesis Methods and Protocols, New York: Humana Press; pp 299-307. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489-1493. [DOI] [PubMed] [Google Scholar]
- Muñoz-Velasco I, Ortíz R, Echeverría OM, Escobar ML, Vázquez-Nin GH. (2013). Characterization of the pre-meiotic S phase through incorporation of BrdU during spermatogenesis in the rat. J Histochem Cytochem 61:680-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima T, DeArmond SJ, Murovic J, Hoshino T. (1985). Immunocytochemical demonstration of S-phase cells by anti-bromodeoxyuridine monoclonal antibody in human brain tumor tissues. Acta Neuropathol 67:155-159. [DOI] [PubMed] [Google Scholar]
- Nakata H, Wakayama T, Adthapanyawanich K, Nishiuchi T, Murakami Y, Takai Y, Iseki S. (2012). Compensatory upregulation of myelin protein zero-like 2 expression in spermatogenic cells in cell adhesion molecule-1-deficient mice. Acta Histochem Cytochem 45:47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. (2006) Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 74:314-321. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. (1956). A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat 99:391-413. [DOI] [PubMed] [Google Scholar]
- Petersen M, Davids LM, Kidson SH. (2012). Simultaneous immunofluorescent labeling using anti-BrdU monoclonal antibody and a melanocyte-specific marker in formalin-fixed paraffin-embedded human skin samples. Appl Immunohistochem Mol Morphol 20:614-617. [DOI] [PubMed] [Google Scholar]
- Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sab-attini E, Ascani S. (1997). Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol 183:116-123. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. (2008). A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 105:2415-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmand PA, Jungas T, Fernandez M, Conter A, Christians ES. (2008). Mouse heat-shock factor 1 (HSF1) is involved in testicular response to genotoxic stress induced by doxorubicin. Biol Reprod 79:1092-1101. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. (1991). Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748. [DOI] [PubMed] [Google Scholar]
- Shi SR, Coté C, Kalra KL, Taylor CR, Tandon AK. (1992). A technique for retrieving antigens in formalin-fixed, routinely acid-decalcified, celloidin-embedded human temporal bone sections for immunohistochemistry. J Histochem Cytochem 40:787-792. [DOI] [PubMed] [Google Scholar]
- Shi SR, Coté RJ, Hawes D, Thu S, Shi Y, Young LL, Taylor CR. (1999). Calcium-induced modification of protein conformation demonstrated by immunohistochemistry: What is the signal? J Histochem Cytochem 47: 463-470. [DOI] [PubMed] [Google Scholar]
- Szász F, Sirivaidyapong S, Cheng FP, Voorhout WF, Marks A, Colenbrander B, Solti L, Gadella BM. (2000). Detection of calcium ionophore induced membrane changes in dog sperm as a simple method to predict the cryopreservability of dog semen. Mol Reprod Dev 55:289-298. [DOI] [PubMed] [Google Scholar]
- Tang X, Falls DL, Li X, Lane T, Luskin MB. (2007). Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J Neurosci 27:5837-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Koami H, Ariga H, Kobayashi D, Sai Y, Tsuji A, Yamamoto M, Iseki S. (2003). Expression and functional characterization of the adhesion molecule spermatogenic immunoglobulin superfamily in the mouse testis. Biol Reprod 68:1755-1763. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Sai Y, Ito A, Kato Y, Kurobo M, Murakami Y, Nakashima E, Tsuji A, Kitamura Y, Iseki S. (2007). Heterophilic binding of the adhesion molecules poliovirus receptor and immunoglobulin superfamily 4A in the interaction between mouse spermatogenic and Sertoli cells. Biol Reprod 76:1081-1090. [DOI] [PubMed] [Google Scholar]
- Wenz JR, Hess RA. (1998) Characterization of stage-specific tyrosinated alpha-tubulin immunoperoxidase staining patterns in Sertoli cells of rat seminiferous tubules by light microscopic image analysis. Tissue Cell 30:492-501. [DOI] [PubMed] [Google Scholar]
- Yamashita S. (2007). Heat-induced antigen retrieval: Mechanisms and application to histochemistry. Prog Histochem Cytochem 41:141-200. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Okada Y. (2005). Mechanisms of heat-induced antigen retrieval: analyses in vitro employing SDS-PAGE and immunohistochemistry. J Histochem Cytochem 53:13-21. [DOI] [PubMed] [Google Scholar]
- Yomogida K, Yagura Y, Tadokoro Y, Nishimune Y. (2003). Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse Sertoli cells. Biol Reprod 69:1303-1307. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113:689-699. [DOI] [PubMed] [Google Scholar]