Abstract
Tumor necrosis factor-alpha-induced protein-8 like-3 (TNFAIP8L3, TIPE3) is a newly discovered member of TNFAIP8 family and regarded as a lipid second messenger transfer protein that promotes cancer. Yet the nature of the cells and tissues that express TIPE3 protein has not been determined. In this study, we examined TIPE3 expression in various murine and human tissues by immunohistochemistry and quantitative PCR. We found that TIPE3 expression was almost identical in most organs from human and mice. TIPE3 is a cytoplasmic protein expressed preferentially in epithelial-derived cells with secretory functions. Furthermore, TIPE3 protein is highly expressed in most human carcinoma cell lines. These results suggest that TIPE3 may play important roles in carcinogenesis and cell secretion.
Keywords: TNFAIP8L3 (TIPE3), expression, carcinogenesis, secretion
Introduction
TIPE3, tumor necrosis factor-α-induced protein-8 like-3 (also known as TNFAIP8L3), is a newly identified protein of TNFAIP8 family (Sun et al. 2008; Freundt et al. 2008). This family consists of four homologous members—TNFAIP8 (TIPE), TIPE1 (TNFAIP8L1), TIPE2 (TNFAIP8L2), and TIPE3—with a unique structure and unknown function(s) (Zhang et al. 2009). Recently, most studies have focused on TIPE2, which is considered a negative regulator in inflammation and carcinogenesis (Sun et al. 2008; Gus-Brautbar et al. 2012; Cao et al. 2013). TIPE2 deficiency in mice causes fetal inflammatory diseases and its abnormal expression in humans is associated with infectious disease, diabetic nephropathy, stroke and atherosclerosis (Sun et al. 2008; Zhang et al. 2010a, 2012, 2013; Xi et al. 2011; Lou and Liu 2011; Lou et al. 2014). Among these family members, TNFAIP8 is known as an apoptosis regulator and is thought to play an important role in carcinogenesis (Patel et al. 1997; Kumar et al. 2000, 2004; Yoshinaga-Hirabayashi and Yoneda 2001; Zhang et al. 2006; Dong et al. 2010). Hitomi et al. (2008) reported that TIPE1 was a necessary component of both zVAD-fmk caspase inhibitor and TNF-α-induced necroptosis (Galluzzi and Kroemer 2008; Hitomi et al. 2008; Lou and Liu 2011). However, little is known about TIPE3.
Previous studies have demonstrated that TIPE3 is a feedback enhancer of tumorigenesis and phospholipid signaling (Fayngerts et al. 2014). TIPE3 mRNA has been detected in a wide range of murine organs, including the uterus, developing embryos, intestine, lung, brain, bladder, and colon (Fayngerts et al. 2014). However, the expression pattern of TIPE3 protein has not yet been determined and compared between human and mice. Therefore, in this study, we generated polyclonal antibodies that could specifically recognize both human and murine TIPE3 proteins and analyzed the expression of TIPE3 protein in normal human and C57BL/6 murine tissues by immunostaining. Our results suggest that TIPE3 protein is mainly distributed in the cytoplasm and its upregulation may be associated with carcinogenesis.
Materials & Methods
Human Normal Tissues
Human normal tissue microarrays including most of the organs (Table 1) were purchased from Xi’an Alena Biotech CO. (Shanxi, China). We also collected 15 human normal samples from Shandong University Qilu Hospital between March 2012 and July 2012. Standard, 4-µm tissue sections were prepared and stained with hematoxylin and eosin (HE) and examined by light microscopy. All procedures were pre-approved by the Institutional Review Board (IRB) of Shandong University.
Table 1.
TIPE3 Detection in Human and Murine Normal Tissues.
| Tissues | TIPE3 Expression in Human | TIPE3 Expression in Mouse |
|---|---|---|
| Esophagus | − | + |
| Salivary gland | ||
| Duct | N | + |
| Stomach | ||
| Epithelium | + | + |
| Parietal cells | +++ | +++ |
| Small intestine | ||
| Simple columnar epithelium | + | ± |
| Colon | ||
| Simple columnar epithelium | + | N |
| Gland | + | N |
| Adrenal gland | ||
| Chromaffin cells | ++ | ++++ |
| Steroid secreting cells | + | − |
| Pancreas | ||
| Pancreatic acinus | − | − |
| Islet | ++ | ++ |
| Thyroid gland | N | − |
| Central nervous system | ||
| Neuron | ++ | ++ |
| Kidney | ||
| Glomerulus | + | + |
| Proximal convoluted tubule | + | + |
| Distal convoluted tubule | +++ | +++ |
| Urinary bladder | ||
| Transitional epithelium | N | + |
| Trachea | ||
| Epithelium | ++++ | + |
| Lung | ||
| Alveolar cells | + | + |
| Testis | ||
| Spermatogonium | +++ | N |
| Primary spermatocytes | +++ | N |
| Secondary spermatocytes | +++ | N |
| Spermatozoid | +++ | N |
| Ductus deferens | ||
| Epithelium | N | + |
| Epididymis | ||
| Epithelium | N | + |
| Prostate | ||
| Epithelium | + | + |
| Ovary | ||
| Oocytes | N | + |
| Corpus luteum | N | + |
| Oviduct | ||
| Epithelium | N | + |
| Uterus | ||
| Uterine gland | + | N |
| Skin | ||
| Epithelium | +/− | − |
| Skeletal muscle | − | − |
| Cardiac muscle | + | + |
Abbreviation: N, not detected.
Cell Lines
The cell lines used for analysis of TIPE3 expression are indicated in Table 2. Cells were cultured in high-glucose DMEM or RPMI-1640 supplemented with 10% fetal calf serum. Most of these cell lines are from reserves in our laboratory. HEK293 cells were generated by transformation of human embryonic kidney cell with sheared adenovirus 5 DNA (Graham et al. 1977). Hepa1-6 cells were purchased from Cell Resource Center of Shanghai Institutes for Biological Sciences (Shanghai, China). About 107 cells of each cell line were used to extract total RNA.
Table 2.
Detection of TIPE3 mRNA in Cell Lines.
| Cell line | Origin | Species | TIPE3 |
|---|---|---|---|
| HEK-293 | Embryonic kidney | H | ++++ |
| HeLa | Cervix carcinoma | H | + |
| NB4 | Promyelocytic leukemia | H | + |
| U-87 MG | Brain | H | +++ |
| U251 | Astrocytoma | H | + |
| 3AO | Ovarian carcinoma | H | +/− |
| SK-OV-3 | Ovarian adenocarcinoma | H | +/− |
| BEL-7402 | Hepatoma | H | +++ |
| THP-1 | Monocyte | H | +/− |
| K-562 | CML | H | ++ |
| A549 | Lung carcinoma | H | ++ |
| T24 | Transitional cell carcinoma urinary bladder | H | + |
| A2780 | Ovarian carcinoma | H | +/− |
| Hmy2.CIR | B lymphoblast | H | ++++ |
| ECC-1 | Endometrium adenocarcinoma | H | + |
| Tca-8113 | Tongue carcinoma | H | +/− |
| MKN-45 | Gastric | H | +/− |
| MKN-28 | Gastric | H | +/− |
| HGC-27 | Stomach, lymph node metastasis (Undifferentiated carcinoma, can secrete mucin) | H | + |
| SGC-7901 | Gastric adenocarcinoma | H | +/− |
| AGS | Stomach Disease: gastric adenocarcinoma |
H | +/− |
| BGC-823 | Gastric adenocarcinoma | H | + |
| SMMC-7721 | Hepatocellular carcinoma | H | + |
Abbreviation: H, Homo sapiens. +/-, +, ++, +++ and ++++ represent no, faint, moderate, strong and very strong expression of TIPE3.
Antibody Preparation and Specificity Determination
Homologous immunogenic peptides of mouse TIPE3 (mTIPE3, NM 001033535) and human TIPE3 (hTIPE3, NM 207381) were designed and synthesized according to the peptide sequences described in GenBank (www.ncbi.nlm.nih.gov/genbank/). The antigen sequence is as follows: MDSDSGEQSEGEPGC. Specific antibodies were prepared and identified similar to that described for anti-TIPE2 or anti-TIPE1 antibodies (Zhang et al. 2010b; Cui et al. 2011). To determine the specificity of the antibodies, Hepa1-6 cells were transfected with pRK5-mTIPE1 or pRK5-mTIPE3 plasmids, or pRK5 vector as a control using Lipofectamine 2000 according to the manufacturer’s protocol (Life Technologies; Carslbad, CA). EL-4 cells transfected with pRK5-mTIPE were also used as a control. Forty-eight hours later, the cells were lysed and subjected to western blotting, as described previously (Cui et al. 2011). Membranes were incubated with anti-TIPE3 antibodies (1:200) overnight at 4C followed by HRP-conjugated secondary antibody for 30 min at room temperature. The bound peroxidase activity was determined using enhanced chemiluminescence (ECL, F-cheiBIsi.6pro, DNR, Tel Aviv, Israel) using the SuperSignal West Pico kit (Pierce Biotechnology; Rockford, IL).
Animals and Tissues Preparation
All experiments with animals were performed according to the guidelines of the Animal Ethical Committee of Shandong University. Male and female C57BL/6 mice were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Science (Shanghai, China), and were 10 to 12 weeks old at the time of entry into the study. All mice were housed in the Animal Facilities of Shandong University under pathogen-free conditions. Tissues for immunostaining were prepared as previously described (Zhang et al. 2010b).
Immunohistochemistry
Human normal tissue microarrays, murine tissues and human samples were assessed for TIPE3 expression using immunostaining, as described previously (O’Reilly et al. 1997; Zhang et al. 2010b; Baskin et al. 2014) but with minor modifications. After blocking with goat serum (Invitrogen; Carlsbad, CA), the sections were incubated with anti-TIPE3 Ab (1:100) or with the isotype-matched control antibodies (rabbit IgG, 1:200; Invitrogen). Biotinylated goat anti-rabbit IgG mAb (1:200; Invitrogen) was used as the secondary antibody and the reaction developed with Histostain Bulk Kit and DAB kit (Invitrogen). Sections were then mounted in neutral balsam (Invitrogen) and examined with an optical microscope (Olympus BX51, Olympus; Tokyo, Japan). In each case, the intensity (weak, moderate, or strong) and percentage of neoplastic immunoreactive cells (cutoff at 10%) were evaluated. Tumors were scored as follows: -, no appreciable staining or staining in < 10% neoplastic cells; +, tumors with faint appreciable staining in > 10% neoplastic cells; ++, tumors with moderate staining or containing > 10% neoplastic cells with moderate immunostaining; +++, tumors with strong immunostaining in > 10% neoplastic cells or containing > 10% neoplastic cells with strong staining.
Extraction of Total RNA and Quantitative PCR
Total RNA was extracted using TRIZOL, according to the manufacturer’s instructions (Invitrogen). Single-stranded cDNA was synthesized using hexamer primers (Promega; Madison, WI). cDNA was used as templates for the amplification of TIPE3. Primers were designed as follows according to the sequences described by Sun et al(2008): forward for hTIPE3: 5′-TTCAGAGGGGAAAGGGACT-3′, and reverse 5′-AACATCAGGACCTGCGGC-3′. Primers for human β-actin were also designed: forward 5′-ATTGGCAATGAGCGGTTCCG-3′, reverse 5′ -AGGGCAGTGATCTCCTTCTG-3′. All primers were synthesized by Invitrogen. Quantitative PCR (qPCR) was performed using iQSybr Green Supermix (Bio-Rad Laboratories; Hercules, CA) as described previously (Zhang et al. 2010b), with minor modifications. Each sample was run in triplicate. A melting-curve analysis was performed to ensure the specificity of the products. Relative gene expression was analyzed using the 2-△△Ct method and normalized to β-actin levels.
Statistical Analysis
The results are expressed as the mean ± SD. Unpaired t-test was used to determine significance. A value of p<0.05 was considered statistically significant.
Results
TIPE3 is a Cytoplasmic Protein
IHC was performed to determine the expression of TIPE3 protein in murine and human normal organs. Both mTIPE3 and hTIPE3 proteins were mainly detected in the cytoplasm of positively stained cells. TIPE3 protein was detected in a wide range of human and murine organs, as summarized in Table 1.
TIPE3 Expression in Murine Tissues
TIPE3 Expression in Mouse Reproductive System and Brain
In ovary tissue of the adult female mouse, a moderate level of TIPE3 staining was seen in the oocytes of primordial, primary, secondary and mature follicles, but not in the connective tissues between the follicles (Fig. 1A1). Cells of the corpus luteum also expressed a moderate level of TIPE3 protein (Fig. 1A2). Similarly, moderate TIPE3 staining was observed in columnar epithelium of the oviduct (Fig. 1B1). In the reproductive system of the adult male mouse, weak levels of TIPE3 protein staining were observed in the pseudostratified columnar epithelium of the ductus deferens (Fig. 1C1 and 1C2) and in spermatozoa within the epididymal ducts (Fig. 1D1). TIPE3 protein staining was minimal in the epithelium of the epididymis (Fig. 1D1) and prostate gland (Fig. 1F1). In the mouse brain, TIPE3 protein was detectable in the neurons of the grey matter at moderate levels in both the hemicerebrum and cerebellum (Fig. 1E1 and 1E2). TIPE3 protein was not detected in any of the isotype control stainings for follicles (Fig. 1A3), the oviduct (Fig. 1B2), the ductus deferens (Fig. 1C3), the epididymal ducts (Fig. 1D2), the brain (Fig. 1E3) or the prostate gland (Fig. 1F2).
Figure 1.
TIPE3 protein in reproductive system and brain of C57BL/6 mice by IHC. In the ovary, TIPE3 protein was observed in oocytes, follicular cells and granulose lutein cells (A1, 400×; A2, 400×). In the oviduct, TIPE3 protein was observed in the epithelium (B1, 400×). In the ductus deferens, TIPE3 protein was observed in the epithelial cells (C1, 100×; C2 400×). In epididymal tissue, TIPE3 protein was detected in spermatozoa (D1, 400×). In the prostate gland, TIPE3 staining was detected in the epithelium (F1, 400×). In brain, TIPE3 protein was detected in the neurons of the grey matter (E1, 400×; E2 400×). A3, B2, C3, D2, E3 and F2: no staining was detected using the rabbit isotype control antibody (400×) for ovary, oviduct, ductus deferens, epididymal, brain and prostate gland tissues, respectively. Scale (C1), 80 μm; all others, 20 μm.
TIPE3 Expression in Mouse Gastrointestinal System and Cardiac Muscle
In the esophagus, moderate staining was observed in the stratified squamous epithelium (Fig. 2A1 and A2). Similarly, moderate TIPE3 staining was seen in the ductal epithelial cells of the salivary gland (Fig. 2B1). In the stomach, TIPE3 expression was apparent both in the glandular and nonglandular portions of the tissue, with intense staining observed in the parietal cells of the glandular tissue of the stomach (Fig. 2C1 and 2C2) but much lower intensity staining in the epithelium of the nonglandular tissue (Fig. 2D1). In the small intestine, TIPE3 protein was minimal in the simple columnar epithelium and the small intestinal glandular cells, but intense TIPE3 staining was observed in the cells that morphologically resembled the granulocytes in the lamina propria (Fig. 2E1 and 2E2). The expression of TIPE3 protein in the muscular tissue differed according to muscle type. As shown in Fig. 2F1, cardiac muscle tissue was marked by moderate TIPE3 staining. Isotype controls for esophagus, salivary gland, stomach, small intestine, and cardiac muscle were negative (Fig. 2A3, 2B2, 2C3, 2D2, 2E3, 2F2, respectively).
Figure 2.
TIPE3 protein in the gastrointestinal system and cardiac muscle of C57BL/6 mice by IHC. In the esophagus, TIPE3 protein was found in the stratified squamous epithelium (A1, 400×; A2, 400×). In the salivary gland, TIPE3 protein was detected in ducts (B1, 400×). In stomach, TIPE3 protein was observed both in the glandular and nonglandular portions (C1, 100×; C2, 400×; D1, 400×). In the small intestine, TIPE3 protein was detected in the mesenchyme (E1 400×, E2 400×). TIPE3 protein was detected in cardiac muscle (F1, 400×). A3, B2, C3, D2, E3 and F2: no staining was detected with the rabbit isotype antibody (400×) for esophagus, salivary gland, glandular and nonglandular portions of the stomach, small intestine and cardiac muscle. Scale (C1), 80 μm; all others, 20 μm.
TIPE3 Expression in Mouse Urinary, Endocrine and Respiratory Systems
TIPE3 protein was expressed extensively in the renal cortex. The glomerulus and proximal convoluted tubules showed very weak TIPE3 expression, whereas moderate staining was seen in the epithelia of the distal convoluted tubules (Fig. 3A1 and 3A2). Similarly, weak TIPE3 staining was detected in the transitional epithelium of the urinary bladder (Fig. 3B1). TIPE3 protein was not detected with isotype antibody staining for the renal cortex (Fig. 3A3) or the urinary bladder (Fig. 3B2).
Figure 3.
TIPE3 protein in urinary, endocrine and respiratory systems of C57BL/6 mice by IHC. In kidney tissues, TIPE3 was detected in the glomeruli and in the tubular epithelial cells of the proximal convoluted tubules and distal convoluted tubules (A1, 400×; A2, 400×). In the urinary bladder, TIPE3 protein was detected in the transitional epithelium (B1, 400×). In pancreas, TIPE3 protein was observed in the islets, but not in pancreatic acini (C1, 400×; C2, 400×). In the adrenal gland, TIPE3 protein was detected in the medulla (D1, 400×). In the lung, TIPE3 protein was observed in the alveolar cells and the epithelium of the bronchus (E1, 400×; E2 400×). In the trachea, TIPE3 protein was detected in the pseudostratified ciliated columnar epithelium and hyaline cartilage (F1, 400×). A3, B2, C3, D2, E3 and F2: no staining was detected with the rabbit isotype antibody (400×) for, kidney, urinary bladder, pancreas, adrenal gland, lung and trachea, respectively. Scale, 20 μm.
TIPE3 protein showed moderate staining intensity in the islets, but the cells in pancreatic acini, which secrete zymogen granular, were not TIPE3 positive (Fig. 3C1 and 3C2). In the adrenal gland, intense TIPE3 staining was detected in the chromaffin cells of the adrenal medulla, which secrets epinephrine and norepinephrine. In contrast, minimal TIPE3 protein was observed in the steroid-secreting cells of the adrenal cortex (Fig. 3D1). No TIPE3 was detected in the pancreas (Fig. 3C3) or the adrenal gland (Fig. 3D2) with isotype control staining.
In the trachea, weak TIPE3 staining was observed in the chondrocytes of the hyaline cartilage (Fig. 3F1). Similarly, in the lung, weak TIPE3 staining was detected in the pseudostratified ciliated columnar epithelium and the alveolar epithelium (Fig. 3E1 and E2). TIPE3 protein was not detectable with isotype control staining for these tissues.
TIPE3 Expression in Human Tissues
TIPE3 Expression in Human Gastrointestinal and Endocrine Systems and Hemicerebrum
Human TIPE3 protein was expressed widely among the tissues of the digestive tract, with the exception of the esophagus (data not shown). This is contrary to murine TIPE3, which was detected at a moderate level in the esophagus. In the stomach, intense TIPE3 staining was observed in the lamina propria (Fig. 4A1). In the small intestine, moderate TIPE3 staining was detected in the simple columnar epithelial cells of the intestinal villus (Fig. 4B1). Moderate staining was also detected in the simple columnar epithelium of the colon tissues (Fig. 4C1) and in the neurons of grey matter of the hemicerebrum (Fig. 4D1). Isotype control staining showed no cross-reactivity for any of these tissues [stomach (Fig. 4A2), small intestine (Fig. 4B2), colon (Fig. 4C2) and hemicerebrum (Fig. 4D2)].
Figure 4.
TIPE3 protein in human gastrointestinal, hemicerebrum and endocrine systems by IHC. In the stomach, TIPE3 was observed in the mucosa (A1, 400×). In the small intestine, TIPE3 protein was detected in the mesenchyme (B1, 400×). In the colon, TIPE3 was detected in the simple columnar epithelium and glands (C1, 400×). In the central nervous system, TIPE3 was detected in grey matter neurons (D1, 400×). In the adrenal gland, TIPE3 was observed in the medulla (E1, 400×). In the pancreas, TIPE3 was observed in the islets (F1, 400×). A2, B2, C2, D2, E2 and F2: no staining was detected with the rabbit isotype antibody (400×) for stomach, small intestine, colon, hemicerebrum, adrenal gland and pancreas, respectively. Scale, 20 μm.
Consistent with the mouse, a moderate TIPE3 staining was observed in the chromaffin cells in the adrenal medulla, which can secret catecholamine under stress. In contrast, the cells of the adrenal cortex, which secret aldosterone, glucocorticoid or androgens, showed little to no staining for TIPE3 (Fig. 4E1). In the pancreas, intense TIPE3 staining was observed in the endocrine cells of the islets, but no staining was observed in the exocrine cells of pancreatic acini (Fig. 4F1).
TIPE3 Expression in Human Renal, Respiratory and Reproductive Systems
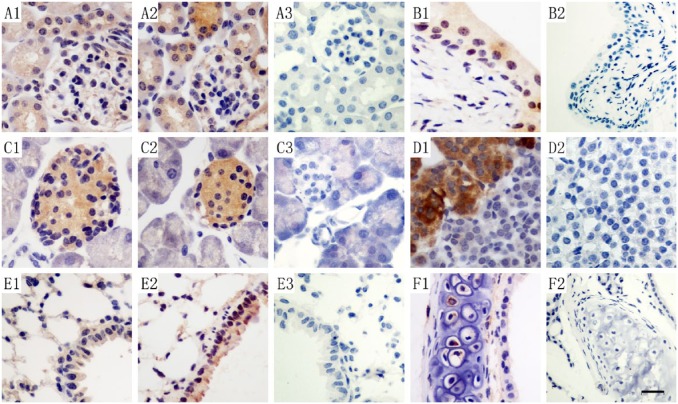
As with the expression pattern of TIPE3 in the mouse kidney, hTIPE3 protein staining was stronger in the distal convoluted tubules than in other parts of the kidney in the human samples (Fig. 5A1). In the respiratory system, intense levels of TIPE3 protein were detected in the pseudostratified ciliated columnar epithelium of the trachea (Fig. 5B1), but weak staining was observed in the alveolar epithelium (Fig. 5C1).
Figure 5.
TIPE3 protein in human kidney, respiratory system, reproductive organs and other organs by IHC. In the kidney, TIPE3 was detected in the glomeruli and in the tubular epithelial cells of the proximal and distal convoluted tubules (A1, 200×). In the trachea, TIPE3 protein was detected in the pseudostratified ciliated columnar epithelium (B1, 400×). In the lung, TIPE3 protein was observed in the alveolar cells (C1, 400×). In the testis, TIPE3 protein was observed in spermatogonia, primary spermatocytes, secondary spermatocytes and spermatozoa (D1, 400×). In the prostate gland, TIPE3 staining was detected in the epithelium (E1, 400×). In the uterus, TIPE3 protein was detected in the uterine glands (F1, 400×). In the skin, TIPE3 protein was detected in the epithelium (G1, 400×). TIPE3 protein expression was low in cardiac muscle and was not detected in skeletal muscle (H1, 400×; I1, 400×). A2, B2, C2, D2, E2, F2, G2, H2, I2, J3 and K3: no staining was detected with the rabbit isotype antibody (400×; A2 200×) for kidney, trachea, lung, testis, prostate, uterus, cardiac muscle and skeletal muscle, respectively. Scale (A1 and A2), 40 μm, others 20 μm.
In testicular tissues, hTIPE3 protein was highly expressed within the spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and spermatozoa. All of the cells in this male reproductive tissues showed strong positive staining (Fig. 5D1). In the prostate gland, hTIPE3 protein was detectable at a weak level in the epithelium (Fig. 5E1). Weak levels of hTIPE3 protein were observed in the uterine tissue samples (Fig. 5F1). There was no positive staining in each tissue with isotype antibodies.
TIPE3 Expression in Human Muscular Tissue and Skin
Consistent with its expression in mice, TIPE3 protein varied with muscle type in human tissues, with weak staining in cardiac muscle and no staining in skeletal muscles (Fig. 5H1 and 5I1). In the skin, very weak TIPE3 staining could be detected in the epidermis (Fig. 5G1). There was no positive staining in each tissue with isotype controls.
TIPE3 Expression in Cultured Cell Lines
The expression levels of TIPE3 in different cultured cell lines are shown in Figure 6, and the results are summarized in Table 2. The highest level of TIPE3 protein was detected in HEK293 cells, which originate from embryonic kidneys (transformed with SV40T antigen and B lymphoblast cell line Hmy2.CIR). TIPE3 was also observed in most human carcinoma cell lines, albeit at different levels. Moderate TIPE3 levels were detected in U-87 MG and U251 cell lines, which are from astrocytoma; in K-562 cells, from chronic myelogenous leukemia; in A549 cells, a lung carcinoma cell line; in HGC-27, an undifferentiated carcinoma cell line from stomach with lymph node metastasis, and in BEL-7402, a hepatoma cell line. Relatively low levels of TIPE3 were detected in HeLa (cervical), SKOV-3 (ovarian adenocarcinoma), 3AO and A2780 cell lines (ovarian epithelial carcinoma), ECC-1 (endometrium adenocarcinoma), T24 (transitional cell carcinoma urinary bladder), and most carcinoma cell lines originating from gastric tissues, such as MKN-28, MKN-45, SGC-7901, AGS, and BGC-823. These data demonstrate that TIPE3 is expressed in most carcinoma cell lines and may play a role in carcinogenesis.
Figure 6.
TIPE3 mRNA in cell lines. TIPE3 mRNA levels were detected in human cell lines by RT-PCR (A and B) and real-time PCR (C and D). The highest TIPE3 mRNA expression was detected in human HMy2.CIR cells and HEK293 cells. Moderate or weak TIPE3 expression was detected in most human carcinoma cell lines. (E) Antibody specificity controls.
Specificity of the TIPE3 Antibodies
As shown in Figure 6E, TIPE3 proteins were detected only in Hepa1-6 cells transfected with mTIPE3 expression vectors (pRK5-mTIPE3). TIPE3 levels were unaffected by the over-expression of other related proteins, TIPE (also known as TNFAIP8) and TIPE1 (a. k. a TNFAIP8) in EL4 cells or Hepa1-6 cells. TIPE3 proteins were not observed in Hepa1-6 cells transfected with vector controls. These results indicate that the antibodies we generated bind specifically to TIPE3 protein.
Discussion
TIPE3 is a newly described member of the tumor necrosis factor-alpha-induced protein 8 (TNFAIP8) family. So far, all of the members of this family are considered to be related to carcinogenesis. TNFAIP8, also known as SSC-S2, GG2-1, and MDC-3.13, is an oncogenic protein and apoptosis regulator (Patel et al. 1997; Kumar et al. 2000, 2004; Yoshinaga-Hirabayashi and Yoneda 2001; Zhang et al. 2006). Similarly, TIPE1 is thought to be essential for TNF-α-induced necroptosis (Galluzzi and Kroemer 2008; Hitomi et al. 2008; Lou and Liu 2011). TIPE2 can regulate cell death by promoting Fas-induced apoptosis (Sun et al. 2008) and is considered as a negative regulator of carcinogenesis as it interferes with Ras activity (Gus-Brautbar et al. 2012).
Previously, we suggested that TIPE3 is an important regulator of tumorigenesis through its activation of phospholipid signaling (Fayngerts et al. 2014). Our findings in this study are consistent with this notion. Using quantitative PCR, we found that TIPE3 mRNA was mainly detected in most human carcinoma cell lines, including U-87 MG, U251, K-562, A549, HGC-27, BEL-7402, and ECC-1; albeit, at different levels. Furthermore, we found a marked upregulation in TIPE3 protein in breast tumor sections (data not shown). These results indicate that, similar to other TIPE proteins, TIPE3 may also be involved in carcinogenesis.
However, a novel finding of this study is the striking parallel in the expression of TIPE3 protein in human and mouse tissues. First, we found that both human and murine TIPE3 proteins were mainly detected in the cytoplasm of the positively stained cells, with little difference in the expression profiling of TIPE3. Second, TIPE3 protein was detectable in a wide range of murine and human organs, such as stomach, lung, prostate, kidney, brain, among others. Third, TIPE3 protein expression was mainly restricted to certain cell types of epithelial origin, especially in the epithelial parts of tissues with glandular structures, such as the digestive tract, breast ductal epithelial cells and the endocrine glands, including the renal proximal tubules, the glucocorticoid-secreting cells of the adrenal medulla, the islets of the pancreas, and the parietal cells of the stomach. These data suggest that TIPE3 may be associated with secretory functions.
TIPE3 protein expression was almost identical across mouse and human organs, which may result from the homologous sequences of TIPE3 between the two species (Sun et al. 2008). Furthermore, to a certain extent, TIPE3 expression was similar to that previously described for TIPE1 and TIPE2 (Zhang et al. 2010b, 2011; Cui et al. 2011); this is likely because of the sequence homology among members of TNFAIP8 family (Sun et al. 2008; Lou and Liu 2011).
Collectively, our data demonstrate that TIPE3 expression is identical in most organs of human and mouse, and that it is mainly restricted to epithelial-derived cells with secretory functions. Furthermore, TIPE3 mRNA was highly expressed in most human carcinoma cell lines. These results suggest that TIPE3 may play an important role in carcinogenesis and secretion.
Acknowledgments
We thank Xiaojing Wang, Yongmei Yang and Haiting Mao for some cell lines. We thank Yunwei Lou for helping to modify the manuscript.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from the National Natural Science Foundation of China (No.81171578, No.81100205).
References
- Baskin DG, Hewitt SM. (2014). Improving the state of the science of immunohistochemistry: the Histochemical Society’s standards of practice. J Histochem Cytochem 62:691-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo C, Zhu F, Wang Q, Wang J, Wang X, Chen Y.H, Zhang L. (2013). Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer 12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Zhang G, Hao C, Wang Y, Lou Y, Zhang W, Wang J, Liu S. (2011). The expression of TIPE1 in murine tissues and human cell lines. Mol Immunol 48:1548-1555. [DOI] [PubMed] [Google Scholar]
- Dong Q, Zhao Y, Liu Y, Wang Y, Zhang P, Jiang G, Dong X, Cui Q, Wang E. (2010). Overexpression of SCC-S2 correlates with lymph node metastasis and poor prognosis in patients with non-small-cell lung cancer. Cancer Sci 101:1562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayngerts S, Wu J, Oxley C, Liu X, Vourekas A, Cathopoulis T, Wang Z, Cui J, Liu S, Sun H, Lemmon M, Zhang L, Shi Y, Chen Y.H. (2014). TIPE3 is the transfer protein of lipid second messengers that promote cancer. Cancer Cell 26:465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt E, Bidere N, Lenardo M. (2008). A different TIPE of immune homeostasis. Cell 133:401-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kroemer G. (2008). Necroptosis: a specialized pathway of programmed necrosis. Cell 135:1161-1163. [DOI] [PubMed] [Google Scholar]
- Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S, Zhang L, Chen Y.H. (2012). The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell 45:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F, Smiley J, Russell W, Nairn R. (1977). Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36:59-74. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Christofferson D, Ng A, Yao J, Degterev A, Xavier R, Yuan J. (2008). Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135:1311-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Whiteside T, Kasid U. (2000). Identification of a novel tumor necrosis factor-alpha-inducible gene, SCC-S2, containing the consensus sequence of a death effector domain of fas-associated death domain-like interleukin-1 betaconverting enzyme-inhibitory protein. J Biol Chem 275:2973-2978. [DOI] [PubMed] [Google Scholar]
- Kumar D, Gokhale P, Broustas C, Chakravarty D, Ahmad I, Kasid U. (2004). Expression of SCC-S2, an antiapoptotic molecule, correlates with enhanced proliferation and tumorigenicity of MDA-MB 435 cells. Oncogene 23:612-616. [DOI] [PubMed] [Google Scholar]
- Lou Y, Liu S. (2011). The TIPE (TNFAIP8) family in inflammation, immunity, and cancer. Mol Immunol 48:1548-1555. [DOI] [PubMed] [Google Scholar]
- Lou Y, Zhang G, Geng M, Zhang W, Cui J, Liu S. (2014). TIPE2 Negatively Regulates Inflammation by Switching Arginine Metabolism from Nitric Oxide Synthase to Arginase. PLoS One 9:e96508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly L, Gu D, Sarvetnick N, Edlund H, Phillips J, Fulford T, Cooke A. (1997). a-Cell neogenesis in an animal model of IDDM. Diabetes 46:599-606. [DOI] [PubMed] [Google Scholar]
- Patel S, Wang F, Whiteside T, Kasid U. (1997). Identification of seven differentially displayed transcripts in human primary and matched metastatic head and neck squamous cell carcinoma cell lines: implications in metastasis and/or radiation response. Oral Oncol 33:197-203. [DOI] [PubMed] [Google Scholar]
- Sun H, Gong S, Carmody R, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen Y.H. (2008). TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y, Qu Z, Cui J, Zhang G, Liang X, Ma C, Gao C, Chen Y.H, Liu S. (2011). Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol 48:1203-1208. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Hirabayashi T, Yoneda Y. (2001). Expression of SCC in ovarian granulose cells and cultured cells, induced rapid structural changes in mitochondria. Ital J Anat Embryol 106:51-57. [PubMed] [Google Scholar]
- Zhang C, Chakravarty D, Sakabe I, Mewani R, Boudreau H, Kumar D, Ahmad I, Kasid U. (2006). Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther 13:947-955. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen Y.H, Shi Y. (2009). Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol 16:89-90. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang Y, Wei X, Zhen J, Wang Z, Li M, Miao W, Ding H, Du P, Zhang W, He M, Yi F. (2010a). Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. Biochim Biophys Acta 1802:1078-1086. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, Qu Z, Guo C, Chen Y, Zhang Y, Liu S. (2010b). Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol 47:2435-2442. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma C, Chen Y.H, Zhang L. (2011). The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol 48:1209-1215. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wei X, Liu L, Liu S, Wang Z, Zhang B, Fan B, Yang F, Huang S, Jiang F, Chen Y.H, Yi F. (2012). TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem 287:32546-32555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Zhang W, Lou Y, Xi W, Cui J, Geng M, Zhu F, Chen Y.H, Liu S. (2013). TIPE2 deficiency accelerates neointima formation by downregulating smooth muscle cell differentiation. Cell Cycle 12:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]