Abstract
Apoptosis of activated hepatic stellate cells (HSCs) has been verified as a potential mechanism to aid in hepatic fibrosis remission. Earlier research suggests that Septin4_i1 may sensitize hepatocellular carcinoma cells to serum starvation-induced apoptosis. Here, we aimed to investigate the effect of Septin4_i1 on HSC apoptosis and explore the associated signaling pathways. We found that Septin4_i1 can induce apoptosis in LX-2 cells and that this is accompanied by an up-regulation in cleaved-caspase-3 and peroxisome proliferator-activated receptor-γ (PPAR-γ) expression and a down-regulation in α-SMA expression. Over-expression of Septin4_i1 reduced phosphorylated Akt and B-cell lymphoma 2 (Bcl-2) expression but had no effect on the expression of p53 and death receptor (DR)-5. The decreased expression of Bcl-2 and the increased expression of cleaved-caspase-3 induced by Sept4_i1 could be reversed by GW501516, a PPAR-β/δ agonist that has been reported by others to enhance Akt signaling. In addition, GW9662, an antagonist of PPAR-γ, could also inhibit apoptosis in LX-2 cells induced by Sept4_i1. In conclusion, our data suggest that Sept4_i1 induces HSC apoptosis by inhibiting Akt and Bcl-2 expression and up-regulating PPAR-γ expression.
Keywords: Hepatic fibrosis, Septin4_i1, Apoptosis, Hepatic stellate cells
Introduction
Hepatic fibrosis results from continuous liver injuries (Friedman 2008). Virus infection, chemical ingestion, and alcoholism, among other factors, often damage hepatocytes and initiate the activation of hepatic stellate cells (HSCs) (Gonzalez et al. 2009). These activated HSCs express α-smooth muscle actin (α-SMA) and secret extracellular matrix (ECM) proteins to initiate hepatic fibrosis. Meanwhile, peroxisome proliferator-activated receptor-γ (PPAR-γ), which is mainly expressed in quiescence HSCs, is down-regulated in activated HSCs (Bataller and Brenner 2001). It has been suggested that apoptosis, phenotype reversal, immune clearance and senescence may all contribute to the clearance of activated HSCs (Kong et al. 2013). In recent years, research has focused on HSC apoptosis, showing that the apoptosis of activated HSCs could lead to the remission of hepatic fibrosis (Yu et al. 2010). Hence, efforts to determine the effective exogenous factors that are involved in the regulation of HSC apoptosis could offer significant benefit for therapies directed against hepatic fibrosis (Sato et al. 2008).
Proteins in the Septin family of GTPases are evolutionarily conserved. These proteins have roles in exocytosis, cytokinesis and other cell processes (Faty et al. 2002; Kinoshita 2006). In particular, Septin4 (Sept4), which resides at chromosome 17q23, is mainly expressed in the liver, brain, and testes (Larisch et al. 2000) and is involved in various physiological processes, including cell apoptosis, cell cycle regulation and fibrogenesis. Previous studies have shown that Sept4 expression levels are altered during the HSC activation (De Minicis et al. 2007). We also previously showed that Sept4 regulates LPS-induced HSCs activation through the TLR4-TGF-β-smad4 pathway (Sun et al. 2013). Iwaisako et al. (2008) observed that the level of Sept4 was down-regulated in mice with liver fibrosis induced by both carbon tetrachloride (CCl4) and bile duct ligation (BDL). These data suggested that Sept4 plays a significant role in hepatic fibrosis. However, these findings have yet to ascertain whether Sept4 has a role in HSC apoptosis and how Sept4 regulates HSC apoptosis. In this study, we sought to determine the function of Sept4_i1 (the first splice variant derived of Sept4) in HSC apoptosis and to explore its potential mechanisms of action.
Materials & Methods
Cell Culture and Materials
LX-2 cells (Xu et al. 2005), an immortalized human HSC line preserved in our laboratory, were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Invitrogen). Cells were cultured in a humidified incubator at 37°C with 5% CO2. GW501516, a potent PPAR-β/δ agonist to enhance Akt signaling (Kostadinova et al. 2012), and GW9662, a PPAR-γ antagonist (both from Santa Cruz Biotechnology; Dallas, TX) were dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 μM. Primary antibodies for caspase-3 and phospho-Akt (Ser473, p-Akt) were purchased from Cell Signaling Technology (Beverly, MA). Primary antibodies for Sept4, total-Akt (T-Akt), p53, B-cell lymphoma 2 (Bcl-2) and PPAR-γ and all secondary antibodies were purchased from Santa Cruz Biotechnology. A primary antibody for glyceraldehyde phosphate dehydrogenase (GAPDH) was provided by Goodhere (China). A terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay (TUNEL) staining kit to measure apoptosis was purchased from Roche (Switzerland). Annexin V-FITC staining kit was also used to observe apoptosis and purchased from Merck/EMD Millipore (Billerica, MA).
Construction and Expression of Recombinant Plasmid pIRES2-EGFP-Sept4_i1
pIRES2-EGFP-Sept4_i1 plasmid was constructed and preserved in our laboratory. In brief, the primers were designed according to the full length sequence of Sept4_i1 (NM_004574.3), as follows: sense primer, 5′- CCCTC GAGGCCACCATGGACCGTTCACTGGGATGGC-3′; antisense primer, 5′- CGGGATCCTTAATAGTTCTCC TTCATCTGTTTTTGTATTTTG -3′. Polymerase chain reaction (PCR) was performed as: 95°C for 2 min, followed by 30 cycles of 95°C for 20 s, 60°C for 20 s and 72°C for 1 min, and then 72°C for 10 min. The obtained PCR products were double-digested with the endonucleases and cloned into pIRES2-EGFP vector (Clontech Laboratories, Inc.; Mountain View, CA). The products were verified with DNA sequencing, and the new recombinant plasmid was named pIRES2-EGFP-Sept4_i1. Plasmids (3–4 μg) were transfected into LX-2 cells for 18 hr using FUGENE 6 (Promega; Fitchburg, WI) according to the manufacturer’s instructions. The cells were then cultured for another 36 hr in DMEM containing 0.25% FBS. The transfection efficiency was visualized by inverted fluorescence microscopy (Leica Microsystems; Wetzlar, Germany). The protein expression of Sept4 was analyzed by western blotting.
Western Blotting
LX-2 cells were transfected with pIRES2-EGFP-Sept4_i1 and treated with appropriate stimuli for the indicated time periods. Culture media was removed and the cells were lysed for protein extraction, quantified according to the method of Bradford (Sangon; China). Equal protein concentrations were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% or 12% gels, and electroblotted onto polyvinylidene fluoride (PVDF) membranes (Merck, EMD Millipore). The membranes were blocked with 5% non-fat milk and then incubated with specific antibodies overnight at 4°C and subsequently with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 hr at room temperature. Membranes were visualized using chemiluminescence (Merck, EMD Millipore).
TUNEL Assay and Annexin V-FITC Staining
Apoptosis was detected using a TUNEL staining kit according to the manufacturer’s instructions (Roche, Switzerland). Briefly, cultured cells were fixed with 4% paraformaldehyde (pH 7.4) for 1 hr at room temperature and then incubated with 0.1% Triton X-100 for 2 min on ice. The cells were then incubated with TUNEL reaction mixture for 60 min at 37°C and counterstained with Hoechst 33258 for 15 min. Apoptotic cells were then visualized by fluorescence microscopy and apoptotic cells were marked as red spots.
The rate of apoptosis was determined using Annexin V-FITC staining. Briefly, the cells were collected and stained with Annexin-V in the dark for 15 min according to the manufacturer’s instructions (Merck, EMD Millipore). The cells were then stained with propidium iodide (PI) and apoptosis was analyzed by flow cytometry. Experiments were performed in triplicate and the data are presented as the mean ± SD. A one-way ANOVA was then used to determine the significant differences using SPSS v15.0 (Chicago, IL). A p-value of <0.05 was considered statistically significant.
Results
Construction of Recombinant Plasmid pIRES2-EGFP-Sept4_i1
Sept4_i1 sequence was amplified and cloned into pIRES2-EGFP vector. The plasmid was confirmed by PCR, restriction enzyme digestion and DNA sequencing (Fig. 1A and data not shown). We show that the sequence obtained was identical to that of Sept4_i1 in GenBank (NM_004574.3). pIRES2-EGFP-Sept4_i1 was then transfected into LX-2 cells and the expression of Sept4_i1 detected by western blotting. As shown in Figure 1B, the expression of Sept4_i1 was increased in the cells transfected with the pIRES2-EGFP-Sept4_i1 plasmid (Sept4_i1) as compared with that in cells transfected with the empty vector.
Figure 1.

pIRES2-EGFP-Sept4_i1 was constructed and Sept4_i1 over-expressed in LX-2 cells. (A) pIRES2-EGFP-Sept4_i1 construct. Lane 1, λ-Hind III DNA Marker. Lane 2, pIRES2-EGFP vector. Lane 3, pIRES2-EGFP-Sept4_i1 plasmid. Lane 4, pIRES2-EGFP-Sept4_i1 plasmid following restriction enzyme digestion. Lane 5, PCR sample of pIRES2-EGFP-Sept4_i1 plasmid. Lane 6, DL2000 plus DNA Marker. (B) Sept4_i1 overexpression in LX-2 cells as compared with untransfected cells and cells expressing the vector only. GAPDH served as a loading control.
Sept4_i1 Induces Apoptosis in LX-2 Cells
To explore the effect of Sept4_i1 in LX-2 cells, the expression levels of α-SMA, caspase-3 and PPAR-γ were detected by western blotting. As shown in Figure 2A, we observed that overexpression of Sept4_i1 up-regulated the expression levels of cleaved-caspase-3 and PPAR-γ and down-regulated the expression level of α-SMA (Fig. 2A). In addition, the over-expression of Sept4_i1 also induced apoptosis in LX-2 cells. As shown in Fig. 2B and Fig. 3, the proportion of apoptotic cells increased in cells transfected with the pIRES2-EGFP-Sept4_i1 plasmid as compared with cells transfected with the control vector. The apoptotic cells also demonstrated morphological changes typical of apoptotic cells, including cell rounding and shrinking. Thus, these results suggest that Sept4_i1 may inhibit the activation of LX-2 cells and induce their apoptosis.
Figure 2.
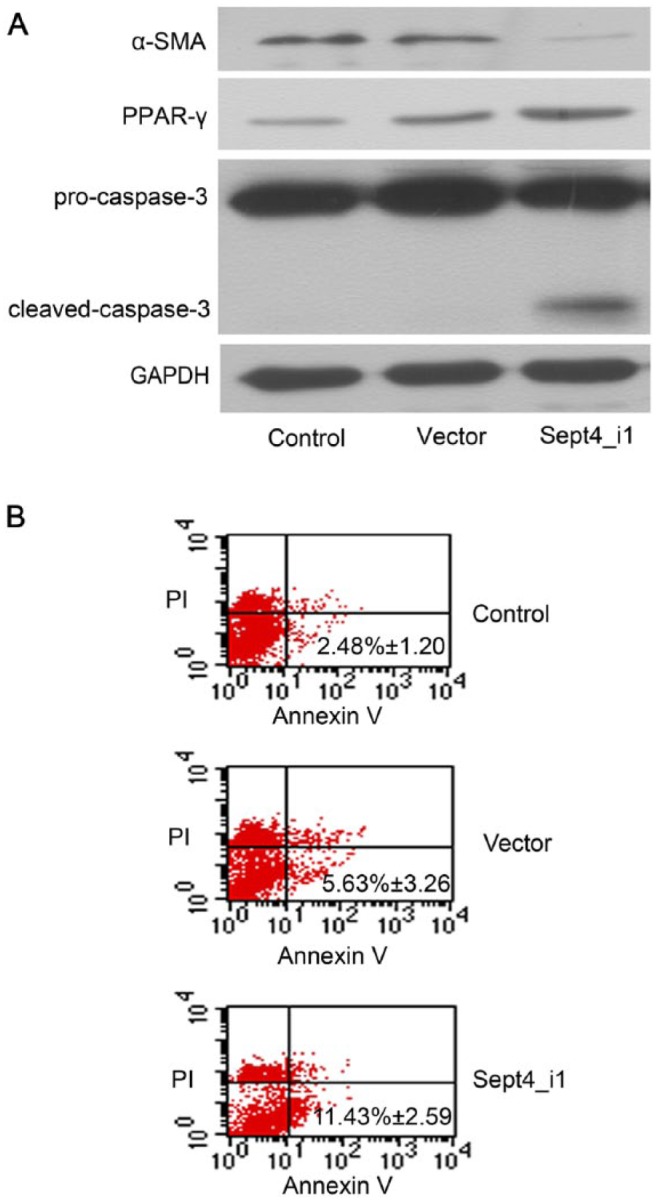
Sept4_i1 induces apoptosis in LX-2 cells. (A) Western blotting shows upregulation in the expression levels PPAR-γ and cleaved-caspase-3 in cells transfected with pIRES2-EGFP-Sept4_i1. The expression of α-SMA was inhibited in transfected LX-2 cells. GAPDH served as a loading control. (B) Sept4_i1 induced apoptosis in LX-2 cells, as detected by Annexin V-FITC staining. The rate of apoptosis of pIRES2-EGFP-Sept4_i1-expressing cells was 11.43% ± 2.59% as compared with 5.63% ± 3.26% in the control (p<0.05). No significant difference existed between the vector only group and the control group (p>0.05).
Figure 3.
Sept4_i1 induces apoptosis in LX-2 cells as detected by TUNEL staining. Cell nuclei were stained by Hoechst 33342. Apoptotic cells are identified by red spotted staining. Sept4_i1 induced apoptosis in LX-2 cells. Scale, 20 µm.
Sept4_i1-induced Apoptosis in LX-2 Cells Depends on Akt/Bcl-2 Expression
Recently, we have found that soluble egg antigens (SEA) from Schistosoma japonicum can facilitate HSC apoptosis by down-regulating p-Akt expression and up-regulating the expression of both p53 and death receptor (DR)-5 (Wang et al. 2014). To identify whether the Sept4_i1-induced apoptosis was associated with the Akt/p53/DR5 pathway, we analyzed the expression changes of p-Akt, p53 and DR5 proteins using western blotting. As shown in Figure 4A, Sept4_i1 overexpression led to a reduction in p-Akt but had no effect on p53 and DR5.
Figure 4.
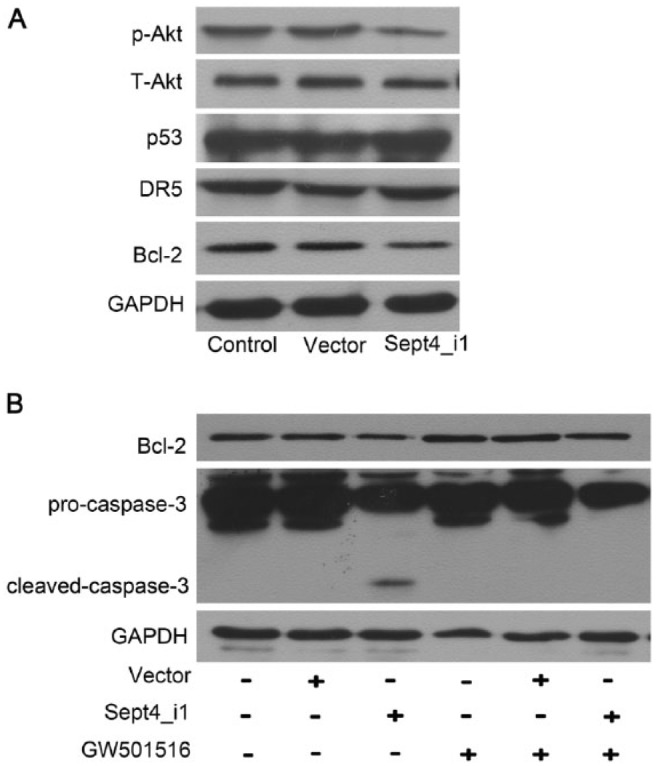
Sept4_i1-induced apoptosis in LX-2 cells is dependent on Akt/Bcl-2 expression. (A) Cells were transfected with pIRES2-EGFP-Sept4_i1 or pIRES2-EGFP vector. The expression levels of Akt, p53, DR5 and Bcl-2 were detected by western blotting. Over-expression of Sept4_i1 reduced the expression levels of p-Akt and Bcl-2. (B) LX-2 cells were transfected with pIRES2-EGFP-Sept4_i1 and treated with the PPAR-β/δ agonist GW501516. Bcl-2 and caspase-3 expressions were analyzed by western blotting. The decreased expression of Bcl-2 and the increased expression of cleaved-caspase-3 induced by Sept4_i1 could all be reversed by supplementation with GW501516.
Previous studies have reported that Bcl-2 family proteins may play important roles in cell apoptosis and may be regulated by the Akt pathway (Li et al. 2013; Liu et al. 2014). Therefore we further sought to observe the relationship between Akt and Bcl-2. We found that Sept4_i1 overexpression reduced the expression of Bcl-2 (Fig. 4A). As shown in Figure 4B, the decreased expression of Bcl-2 induced by Sept4_i1 could be reversed by GW501516, which has been reported by others to enhance Akt signaling (Kostadinova et al. 2012). In parallel, the increased expression of cleaved-caspase-3 expression induced by Sept4_i1 was also inhibited in the cells co-treated with Sept4_i1 and GW501516 (Fig. 4B). These results indicate that Akt/Bcl-2, but not Akt/p53/DR5, may be involved in Sept4_i1-induced apoptosis in LX-2 cells.
Sept4_i1-induced Up-regulation of p-Akt and Bcl-2 in LX-2 Cells Is Associated with PPAR-γ
PPAR-γ is usually expressed in quiescent HSCs and is depleted in activated HSCs (Galli et al. 2000). PPAR-γ is also reported to be involved in apoptosis and suggested to regulate vascular smooth muscle cell proliferation by activating Akt expression (Kumar et al. 2004). In this study, we further explored whether PPAR-γ regulated Akt expression in HSCs. For these experiments, GW9662, a specific PPAR-γ antagonist, was used to inhibit PPAR-γ signaling (Yu et al. 2010). As shown in Figure 5, the expression levels of p-Akt and Bcl-2 were all increased in cells co-treated with Sept4_i1 and GW9662, as compared with cells treated with Sept4_i1 only. The increased expression of cleaved-caspase-3 induced by Sept4_i1 was also inhibited by GW9662. When the cells were treated with GW9662, neither pIRES2-EGFP-Sept4_i1 nor the pIRES2-EGFP vector could modulate the expressions of p-Akt, Bcl-2 and caspase-3 in LX-2 cells. In conclusion, Sept4_i1 induces apoptosis in LX-2 cells through the PPAR-γ/Akt/Bcl-2 pathway.
Figure 5.
Sept4_i1-induced up-regulation of p-Akt and Bcl-2 in LX-2 cells is associated with PPAR-γ. LX-2 cells were treated with pIRES2-EGFP-Sept4_i1 and the PPAR-γ antagonist GW9662 and proteins were extracted and subjected to western blotting. The decreased expression of Bcl-2 and p-Akt and the increased expression of cleaved-caspase-3 induced by Sept4_i1 could all be reversed by GW9662 supplementation.
Discussion
Hepatic fibrosis is a common prognosis for many patients who harbor liver injuries caused by a variety of etiological factors. The activation of HSCs has been identified as the central event in hepatic fibrosis (Anthony et al. 2010; Suarez-Cuenca et al. 2008). Clearance of activated HSCs may become a valid strategy to fibrotic remission and induction of HSC apoptosis has been reported as a potential effective strategy to induce fibrotic reversal (Kong et al. 2013; Yu et al. 2010). Sept4_i1 is a splice variant of Sept4, and composed of 1437 nucleotides. Our previous work has indicated that Sept4_i1 could be regulated in Schistosoma japonicum-induced hepatic fibrosis progression (Duan et al. 2011). Iwaisako et al. (2008) have also reported that Sept4 plays a role in hepatic fibrosis. However, the mechanism of its function in this disease remains unclear. Shen and colleagues found that Sept4_i1 could sensitize hepatocellular carcinoma cells to serum starvation-induced apoptosis (Shen et al. 2012). Thus, in the current study, we attempted to observe whether Sept4_i1 could induce apoptosis in HSCs. We show that Sept4_i1 induces apoptosis in LX-2 cells, indicating that Sept4_i1 may offer an opportunity sensitize HSCs to undergo apoptosis.
The PI3K/Akt pathway participates in many essential intracellular signal transduction pathways, including apoptosis (Scheid and Woodgett 2001). Gabele and colleagues have found that inhibiting the Akt/P70S6K pathway contributes to the down-regulation of collagen and inhibits HSC proliferation (Gabele et al. 2005). Furthermore, Park et al. (2009) have found that suppressing PI3K/Akt signaling can activate FoxO proteins and promote TRAIL-induced cell apoptosis in LX-2 cells. In the present study, we also found a down-regulation in p-Akt levels in LX-2 cells expressing Sept4_i1.
Previously, we reported that SEA from Schistosoma japonicum can induce HSC apoptosis through the Akt/p53/DR5 signaling pathway (Wang et al. 2014). Others have shown that TRAIL also induces apoptosis in human hepatocellular carcinoma cells through the Notch1/Akt/Hdm2/p53 signaling pathway (Wang et al. 2009). However, Sept4_i1-induced apoptosis in LX-2 cells was not associated with Akt/p53/DR5 signaling. Instead, we observed that Bcl-2 was suppressed in Sept4_i1-transfected LX-2 cells. Several studies have shown involvement of the Akt/Bcl-2 pathway in apoptosis in PC12 cells and endothelial cells (Kostadinova et al. 2012; Kumar et al. 2004; Pugazhenthi et al. 2000). Thus, we further used the PPAR-β/δ agonist GW501516 to enhance Akt signaling and observed a change in the expression of Bcl-2 in our Sept4_i1-transfected LX-2 cells. The results showed that GW501516 could up-regulate Bcl-2 expression and inhibit cell apoptosis in Sept4_i1-expressing LX-2 cells. These results suggest that the Akt/Bcl-2 pathway might be involved in Sept4_i1-induced cell apoptosis.
PPARs, including PPAR-α, PPAR-β/δ and PPAR-γ, are a ligand-activated nuclear receptor super-family. PPAR-γ plays a role in tumorigenesis, differentiation, cell cycle regulation and apoptosis (Ouk et al. 2013; Zardi et al. 2013). Yu et al. (2010) showed that PPAR-γ can suppress the activation of HSCs and reverse hepatic fibrosis in mice. They further showed that PPAR-γ can induce apoptosis in HSCs via an extrinsic apoptosis pathway. Zheng and Chen (2004) have also indicated that curcumin-induced apoptosis in HSCs requires PPAR-γ activity. These findings all point to the involvement of PPAR-γ in HSCs apoptosis. In this study, HSC apoptosis was observed in Sept4_i1-transfected LX-2 cells, and was accompanied by the up-regulation of PPAR-γ expression. We further used GW9662 to suppress the expression of PPAR-γ (Yu et al. 2010), and found that GW9662 up-regulated p-Akt and Bcl-2 but down-regulated cleaved-caspase-3 in Sept4_i1-expressing LX-2 cells. From these results, we speculate that PPAR-γ might be involved in Sept4_i1-induced apoptosis in LX-2 cells via the Akt/Bcl-2 pathway. Two previous studies may have presented evidence in support of our hypothesis, with both studies demonstrating that PPAR-γ inhibits Akt phosphorylation and induces cellular apoptosis and migration (Goetze et al. 2002; Hong et al. 2013).
In conclusion, we provide evidence for the mechanism by which Sept4_i1 may induce apoptosis in LX-2 cells. We demonstrate that the up-regulation of PPAR-γ sensitizes LX-2 cells to Sept4_i1-induced apoptosis by suppressing p-Akt and Bcl-2 expression. Sept4_i1 may be a candidate molecule for treatment against hepatic fibrosis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (Grant Numbers 81171589, 81471975, 81401683), the Jiangsu provincial Natural Science Foundation (Grant Number BK20140435), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Anthony B, Allen JT, Li YS, McManus DP. (2010). Hepatic stellate cells and parasite-induced liver fibrosis. Parasit Vectors 3:60-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. (2001). Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21:437-451. [DOI] [PubMed] [Google Scholar]
- De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. (2007). Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132:1937-1946. [DOI] [PubMed] [Google Scholar]
- Duan YN, Qian HY, Qin YW, Zhu DD, He XX, Zhou Q, Yang YN, Bao J, Feng JR, Sun W, Chen JL. (2011). Dynamics of Sept4 expression in fibrotic livers of mice infected with Schistosoma japonicum. Parasitology 138:1003-1010. [DOI] [PubMed] [Google Scholar]
- Faty M, Fink M, Barral Y. (2002). Septins: a ring to part mother and daughter. Curr Genet 41:123-131. [DOI] [PubMed] [Google Scholar]
- Friedman SL. (2008). Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabele E, Reif S, Tsukada S, Bataller R, Yata Y, Morris T, Schrum LW, Brenner DA, Rippe RA. (2005). The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem 280:13374-13382. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb D, Price D, Ceni E, Salzano R, Surrenti C, Casini A. (2000). Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology 31:101-108. [DOI] [PubMed] [Google Scholar]
- Goetze S, Bungenstock A, Czupalla C, Eilers F, Stawowy P, Kintscher U, Spencer-Hansch C, Graf K, Nurnberg B, Law RE, Fleck E, Grafe M. (2002). Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension 40:748-754. [DOI] [PubMed] [Google Scholar]
- Gonzalez SA, Fiel MI, Sauk J, Canchis PW, Liu RC, Chiriboga L, Yee HT, Jacobson IM, Talal AH. (2009). Inverse association between hepatic stellate cell apoptosis and fibrosis in chronic hepatitis C virus infection. J Viral Hepat 16:141-148. [DOI] [PubMed] [Google Scholar]
- Hong S, Xin Y, HaiQin W, GuiLian Z, Ru Z, ShuQin Z, HuQing W, Li Y, Ning B, YongNan L. (2013). The PPARgamma agonist rosiglitazone prevents neuronal loss and attenuates development of spontaneous recurrent seizures through BDNF/TrkB signaling following pilocarpine-induced status epilepticus. Neurochem Int 63:405-412. [DOI] [PubMed] [Google Scholar]
- Iwaisako K, Hatano E, Taura K, Nakajima A, Tada M, Seo S, Tamaki N, Sato F, Ikai I, Uemoto S, Kinoshita M. (2008). Loss of Sept4 exacerbates liver fibrosis through the dysregulation of hepatic stellate cells. J Hepatol 49:768-778. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. (2006). Diversity of septin scaffolds. Curr Opin Cell Biol 18:54-60. [DOI] [PubMed] [Google Scholar]
- Kong D, Zhang F, Zhang Z, Lu Y, Zheng S. (2013). Clearance of activated stellate cells for hepatic fibrosis regression: molecular basis and translational potential. Biomed Pharmacother 67:246-250. [DOI] [PubMed] [Google Scholar]
- Kostadinova R, Montagner A, Gouranton E, Fleury S, Guillou H, Dombrowicz D, Desreumaux P, Wahli W. (2012). GW501516-activated PPARbeta/delta promotes liver fibrosis via p38-JNK MAPK-induced hepatic stellate cell proliferation. Cell Biosci 2:34-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Miller AI, Polverini PJ. (2004). p38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. Journal of Biological Chemistry 279:43352-43360. [DOI] [PubMed] [Google Scholar]
- Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, Book-Melamed N, Timberg R, Duckett CS, Lechleider RJ, Steller H, Orly J, Kim SJ, Roberts AB. (2000). A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2:915-921. [DOI] [PubMed] [Google Scholar]
- Li J, Wu H, Xue G, Wang P, Hou Y. (2013). 17beta-Oestradiol Protects Primary-Cultured Rat Cortical Neurons from Ketamine-Induced Apoptosis by Activating PI3K/Akt/Bcl-2 Signalling. Basic Clin Pharmacol Toxicol [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Zou J, Zhang B, Yuan Z. (2014). Dengue virus subgenomic RNA induces apoptosis through the Bcl-2-mediated PI3k/Akt signaling pathway. Virology 448:15-25. [DOI] [PubMed] [Google Scholar]
- Ouk T, Potey C, Gautier S, Bastide M, Deplanque D, Staels B, Duriez P, Leys D, Bordet R. (2013). PPARs: a potential target for a disease-modifying strategy in stroke. Curr Drug Targets 14:752-767. [DOI] [PubMed] [Google Scholar]
- Park SJ, Sohn HY, Yoon J, Park SI. (2009). Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell Signal 21:1495-1503. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. (2000). Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. Journal of Biological Chemistry 275:10761-10766. [DOI] [PubMed] [Google Scholar]
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. (2008). Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 26:431-442. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. (2001). PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2:760-768. [DOI] [PubMed] [Google Scholar]
- Shen S, Liu M, Wu Y, Saiyin H, Liu G, Yu L. (2012). Involvement of SEPT4_i1 in hepatocellular carcinoma: SEPT4_i1 regulates susceptibility to apoptosis in hepatocellular carcinoma cells. Mol Biol Rep 39:4519-4526. [DOI] [PubMed] [Google Scholar]
- Suarez-Cuenca JA, Chagoya de Sanchez V, Aranda-Fraustro A, Sanchez-Sevilla L, Martinez-Perez L, Hernandez-Munoz R. (2008). Partial hepatectomy-induced regeneration accelerates reversion of liver fibrosis involving participation of hepatic stellate cells. Exp Biol Med (Maywood) 233:827-839. [DOI] [PubMed] [Google Scholar]
- Sun X, Yang Y, Zhu D, Qian H, Duan Y, He X, Gu X, Sun W, Zhu Y. (2013). Expression of Septin4 in human hepatic stellate cells LX-2 stimulated by LPS. Inflammation 36:539-548. [DOI] [PubMed] [Google Scholar]
- Wang C, Qi R, Li N, Wang Z, An H, Zhang Q, Yu Y, Cao X. (2009). Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. Journal of Biological Chemistry 284:16183-16190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang J, Xu F, Zhu D, Duan Y, Chen J, Sun X, He X, Li P, Sun W, Feng J. (2014). Schistosoma japonicum Soluble Egg Antigens Facilitate Hepatic Stellate Cell Apoptosis by Downregulating Akt Expression and Upregulating p53 and DR5 Expression. PLoS Negl Trop Dis 8:e3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. (2005). Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Chu ES, Go MY, Lau RH, Zhao J, Wu CW, Tong L, Poon TC, Sung JJ. (2010). Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol 42:948-957. [DOI] [PubMed] [Google Scholar]
- Zardi EM, Navarini L, Sambataro G, Piccinni P, Sambataro FM, Spina C, Dobrina A. (2013). Hepatic PPARs: their role in liver physiology, fibrosis and treatment. Curr Med Chem 20:3370-3396. [DOI] [PubMed] [Google Scholar]
- Zheng S, Chen A. (2004). Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J 384:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]




