Abstract
Topical microbicides that effectively block interactions between CCR5+ immature Langerhans cells (LC) residing within genital epithelia and R5 human immunodeficiency virus (HIV) may decrease sexual transmission of HIV. Here, we investigated the ability of synthetic RANTES analogues (AOP-, NNY-, and PSC-RANTES) to block R5 HIV infection of human immature LC by using a skin explant model. In initial experiments using activated peripheral blood mononuclear cells, each analogue compound demonstrated marked antiviral activity against two R5 HIV isolates. Next, we found that 20-min preincubation of skin explants with each RANTES analogue blocked R5 HIV infection of LC in a dose-dependent manner (1 to 100 nM) and that PSC-RANTES was the most potent of these compounds. Similarly, preincubation of LC with each analogue was able to block LC-mediated infection of cocultured CD4+ T cells. Competition experiments between primary R5 and X4 HIV isolates showed blocking of R5 HIV by PSC-RANTES and no evidence of increased propagation of X4 HIV, data that are consistent with the specificity of PSC-RANTES for CCR5 and the CCR5+ CXCR4− phenotype of immature LC. Finally, when CCR5 genetic polymorphism data were integrated with results from the in vitro LC infection studies, PSC-RANTES was found to be equally effective in inhibiting R5 HIV in LC isolated from individuals with CCR5 diplotypes known to be associated with low, intermediate, and high cell surface levels of CCR5. In summary, PSC-RANTES is a potent inhibitor of R5 HIV infection in immature LC, suggesting that it may be useful as a topical microbicide to block sexual transmission of HIV.
Sexual transmission across genital mucosa is the most common mode of acquiring human immunodeficiency virus (HIV) (5, 13, 30). It is also well established that most HIV isolates obtained from patients soon after primary infection utilize the coreceptor CCR5 (and not CXCR4) for cellular entry (termed R5 HIV isolates) (40, 44, 45). In addition, genetic studies have shown that individuals with homozygous deletions in the open reading frame (ORF) of CCR5 are largely protected from initial HIV infection despite numerous exposures (4, 12, 16, 35). These observations point to the importance of CCR5 as a critical cofactor involved in sexual transmission of HIV. Whether CCR5 restricts entry of only R5 HIV isolates at the level of the genital mucosa or at sites internally (e.g., in the submucosa or lymph nodes), however, is unknown.
The initial cell types infected following sexual exposure of humans to HIV are also unknown, and transmission is likely to be influenced by a number of factors (e.g., ulceration, presence of other sexually transmitted organisms, menstrual cycle) (21, 26). In female rhesus monkeys, Langerhans cells (LC), resident dendritic cells located with genital mucosal epithelium and other stratified squamous epithelial layers (29), have been shown to be the dominant cell type infected following nontraumatic intravaginal exposure to simian immunodeficiency virus (SIV) (11). It was previously shown that human immature LC express surface CCR5, but not CXCR4, and preferentially bind gp120 envelope protein derived from R5 HIV when compared with envelope protein derived from CXCR4-using X4 HIV (43). It has also been shown that human immature LC are preferentially infected by R5 HIV (14, 28) and that infection is mediated by CD4 and CCR5 (15). These findings suggest that human CCR5+ CXCR4− immature LC act as gatekeepers to allow entry of R5 HIV, but not X4 HIV, at mucosal surfaces.
Based upon this LC gatekeeper hypothesis, we have been engaged in designing and studying agents that could potentially block interactions between CCR5 and R5 HIV at mucosal surfaces. AOP-RANTES {aminooxypentane oxime of [glyoxylyl1]RANTES (amino acids 2 to 68)} was the first synthetically modified chemokine discovered that demonstrated potent antiviral activity against R5 HIV (36). More recently, in an effort to develop even more potent and durable inhibitors of R5 HIV replication, a series of amino-terminally modified RANTES analogues have been designed and tested (10, 18, 22, 25, 32, 38). The aim of the present study was to investigate the ability of three of these RANTES analogues (AOP-RANTES, NNY-RANTES, and PSC-RANTES) to block R5 HIV infection of human immature LC by using an ex vivo skin explant model that mimics early biologic events of HIV transmission (14). Although an excellent model to study HIV infection events in human LC, epidermal tissue explants used in these experiments are devoid of CD4+ T cells. Thus, it is possible that results from this study may not correlate well with actual HIV transmission events if T cells (and not LC) are the first cells infected with HIV following sexual exposure to virus.
There are several additional important caveats in trying to develop a topical microbicide that targets CCR5 to prevent sexual transmission of HIV. Firstly, heterosexual exposure to HIV is likely not limited to R5 HIV isolates but includes a mixture of R5 and X4 HIV strains. Thus, exclusive inhibition of R5 HIV isolates by a RANTES analogue may permit X4 HIV isolates to establish new HIV infections in exposed hosts. Recent studies in vitro have supported this possibility (7, 18, 22, 31, 38). The rare occurrence of X4 HIV infection in highly exposed individuals lacking CCR5 cell surface expression (i.e., those who are homozygous for the CCR5 Δ32 allele) (4, 16, 24), however, suggests that CCR5 entry inhibitors may be suitable and effective microbicides. Secondly, considerable genetic polymorphism in the CCR5 gene (2) has been associated with significant variation in cell surface expression levels of CCR5 and R5 HIV infection levels (15, 20, 32, 33). Thus, the blocking efficacy of RANTES analogues may be variable from individual to individual. Because of these potential problematic issues, we also investigated (i) whether blockade of R5 HIV by RANTES analogues in the presence of X4 HIV increased entry of X4 HIV into LC and (ii) how CCR5 genetic polymorphisms present among the skin donors influenced the antiviral efficacy of RANTES analogues in LC.
MATERIALS AND METHODS
Healthy skin donors.
The Institutional Review Board of the National Cancer Institute approved all aspects of this study, and informed consent was obtained from all healthy individuals volunteering for the suction blister procedure. Volunteers reported no histories of chronic disease (including no histories of HIV risk factors) and no use of medications on a regular basis.
Reagents and viruses.
Phycoerythrin-conjugated mouse anti-human HLA-DR monoclonal antibodies (MAbs) and isotype control antibodies were purchased from Pharmingen (San Diego, Calif.), and fluorescein isothiocyanate-conjugated rat anti-p24 MAb was purchased from Beckman-Coulter (Fullerton, Calif.). RANTES analogues were synthesized and purified as previously described (36, 41). HIVC-92BRO25, a subtype C R5 primary isolate (103.5 infectious units/ml), was propagated in peripheral blood mononuclear cells (PBMC) (see below) and then titers were determined using a 50% tissue culture infective dose method as previously described (38). Purified, pelleted, and titered HIVBa-L (stock at 107.17 50% tissue culture infective doses/ml), an R5 HIV laboratory isolate, was purchased from Advanced Biotechnologies Inc. (Columbia, Md.). HIVCJ1.3, an R5 HIV primary isolate, was a kind gift from David I. Cohen (Queen's College, Flushing, N.Y.); low passage stocks of HIVCJ1.3 were prepared as previously described (14). B2 HIV, a subtype B R5 HIV primary isolate, and B4 HIV, a subtype B X4 HIV primary isolate, were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Rockville, Md.) and propagated in PBMC, and the titers were determined (38). The relative fitness of these subtype B isolates along with a number of other subtype B and C isolates was determined previously (1).
Inhibition of HIV replication in PBMC by RANTES analogues.
After phytohemagglutinin (PHA) stimulation for 48 h, interleukin-2 (IL-2)-treated PBMC were added to 96-well plates (2 × 105 cells/well) containing serially diluted (1:10) drugs: AOP-RANTES, NNY-RANTES, and PSC-RANTES (100 to 0.00001 nM). HIVC-BR025, diluted in RPMI-based complete media (approximately 0.1 multiplicity of infection [MOI]), was then added to wells after a 3-h drug-PBMC incubation. Three days following infection, virus was removed, cells were washed, and media containing the appropriate drug concentration was added back to each well. On days 5, 10, and 15 postinfection, each plate was centrifuged for 5 min, and cell-free supernatant samples (25 μl) were removed and stored at −70°C for subsequent analysis. Virus production was measured in cell-free supernatants using reverse transcriptase assays as previously described (1).
Inhibition of HIV infection in LC by RANTES analogues.
Epithelial tissue explants were prepared from suction blister roofs of healthy volunteers, and LC within these explants were infected as previously described (14, 15). Briefly, 50-μl droplets of RPMI containing known concentrations of RANTES analogues were placed on the inside surface of sterile plastic culture dish covers. Skin explants (i.e., epidermal sheets) were then draped over the droplets with the basal epithelial cell layer down for 20 min at 37°C. Portions (50 μl) of media containing known amounts of HIV were then added to the initial droplet contained beneath the explants and incubated for two additional hours at 37°C. Explants were washed three times with sterile phosphate-buffered saline and then floated in six-well plates containing 4 ml of complete medium for 3 days.
LC that spontaneously emigrated from explants were collected 3 days following HIV exposure and analyzed by flow cytometry as previously described (14, 15). Approximately 3 × 103 to 6 × 103 LC emigrated from a single explant, and cells from three to six explants were used for each condition within a given experiment. Emigrated cells were preincubated in staining buffer for 10 min at room temperature followed by serial incubations with the following: 10 μg of phycoerythrin-conjugated mouse anti-human HLA-DR MAb per ml for 30 min at 4°C, Dead Red (Molecular Probes, Eugene, Oreg.) for 20 min at room temperature, Cytofix/Cytoperm fixing and permeabilization reagents (Pharmingen) for 20 min at 4°C, and 10 μg of fluorescein isothiocyanate-conjugated rat anti-p24 MAb per ml for 30 min at 4°C. Cells were then examined by flow cytometry using a FACScan cytometer (Becton Dickinson, Mountain View, Calif.) equipped with Lysis II software (Becton Dickinson). Dead cells, i.e., Dead Red-positive cells, were excluded from all analyses. For each experiment, as many events as possible were collected by the flow cytometer, which most often resulted in analysis of greater than 5 × 103 Dead Red-negative LC. The percentages of HIV-infected LC (i.e., p24+ LC) reported here were always calculated by first subtracting any background values obtained when uninfected cells were stained with anti-p24 antibodies. Percent inhibition of LC infection by each RANTES analogue was calculated by the following formula: percent inhibition = [(percent HIV p24+ LC from untreated skin explants − percent HIV p24+ LC from skin explants pretreated with RANTES analogue)/(percent HIV p24+ LC from untreated skin explants)] × 100.
Assessment of HIV transmission to CD4+ T cells.
PBMC were isolated from buffy coats by density centrifugation and enriched for CD4+ T cells by negative selection using a commercially prepared MAb cocktail-complement reagent (Lympho-Kwik; One Lambda Inc., Los Angeles, Calif.) according to the manufacturer's guidelines. HIV-exposed explants were floated with the basal epithelial cell sides down in complete media containing 2 × 106 resting allogeneic CD4+ T cells. After 2 days, explants were removed from cultures and discarded. For detection of secreted HIV p24 protein, supernatants were harvested every third day and examined for HIV p24 protein content by enzyme-linked immunosorbent assay (ELISA) (Beckman Coulter, Miami, Fla.) according to the manufacturer's instructions. Of note, no RANTES analogues were added during the entire coculture period.
R5 HIV-X4 HIV competition experiments.
Ten skin explants were preincubated with or without 100 nM PSC-RANTES for 20 min as described above and then incubated with the pair of primary isolates, B2 HIV (an R5 HIV isolate) and B4 HIV (an X4 HIV isolate), for 2 h. As determined by HIV p24 content with an ELISA, approximately equal amounts of each paired R5 HIV and X4 HIV were used to infect LC. Analogue and viruses were then washed from explants, and the skin was then floated in complete media for 3 days. Emigrated LC were collected, DNA was extracted from cells using a QIAGEN QIAamp blood kit (Santa Clara, Calif.), and isolate-specific env fragments were detected and quantified for each HIV isolate in the dual infection using a heteroduplex tracking assay as previously described (1).
CCR5 genotyping.
Genomic DNA was isolated from PBMC (200 μl) of suction blister volunteers by using a QIAamp blood kit. Genotyping by analysis of fragment length polymorphisms (i.e., to detect CCR5 ORFΔ32) was performed as previously described (34, 37). CCR5 promoter polymorphism genotyping and CCR5 compound genotype classification was performed as previously described (15). All genotyping was performed in a blind manner by investigators unaware of the results of the in vitro HIV infection studies in LC.
Statistical analyses.
Statistical analyses were performed using the t test or the Kruskal-Wallis test.
RESULTS
RANTES analogues block R5 HIV replication in activated cultures of PBMC.
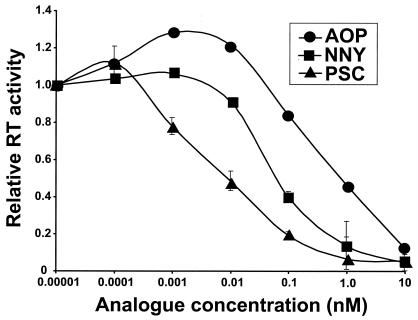
To initially characterize the antiviral activity of RANTES analogues, we first characterized their ability to block R5 HIV replication in PBMC. By using a primary isolate of R5 HIV (HIVC-92BR025), we found that PSC-RANTES was more potent than both AOP- and NNY-RANTES at inhibiting viral replication in PBMC at all doses tested (Fig. 1). Specifically, the 50% inhibitory concentration (IC50) for PSC-RANTES was 8 ± 2.8 pM; for AOP-RANTES, the IC50 was 150 ± 71 pM; and for NNY-RANTES, the IC50 was 98 ± 23 pM.
FIG. 1.
RANTES analogues block R5 HIV replication in activated cultures of PBMC. PHA-IL-2-stimulated PBMC were infected with HIVC-92BRO25, a subtype C R5 primary isolate, in the presence of various doses of either AOP-, NNY-, or PSC-RANTES as described in Materials and Methods. RT, reverse transcriptase.
Preincubation of skin explants with RANTES analogues blocks subsequent R5 HIV infection in LC in a dose-dependent manner.
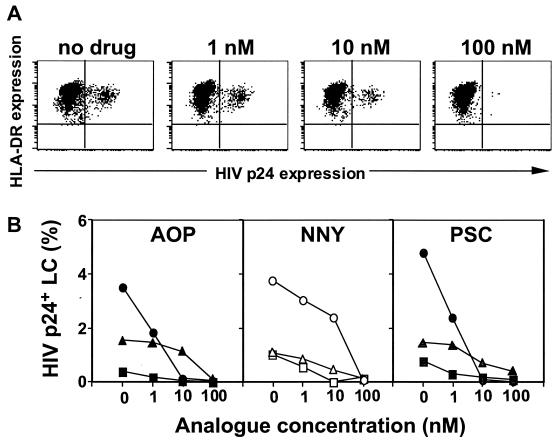
It is known that LC residing within peripheral epithelial tissues demonstrate a relatively immature or resting phenotype when compared to other types of dendritic cells that have migrated to lymph nodes. Recently, we have established an ex vivo tissue explant model whereby immature LC residing within epithelium are exposed to HIV (14). In this study, we tested the ability of RANTES analogues to block R5 HIV infection of LC. Preincubation of skin explants with AOP-RANTES for 20 min blocked subsequent R5 HIV infection of LC in a dose-dependent manner (Fig. 2A). The blocking was confirmed by repeated experiments using skin explants from three additional randomly selected individuals (Fig. 2B). Similarly, preincubation of skin explants with either NNY- or PSC-RANTES blocked subsequent R5 HIV infection of LC in a dose-dependent manner (Fig. 2B). Of note, there was considerable variability in R5 HIV infection levels within LC when using skin explants from different individuals, a finding we have previously demonstrated is attributable to individual differences in the CCR5 genotype (15). Although the blocking efficiency of each RANTES analogue at doses of 1 and 10 nM varied, all three analogues demonstrated complete blocking of R5 HIV replication at doses of 100 nM (Fig. 2B). No cellular toxicity was noted for any of the analogues at the doses used in these experiments (data not shown).
FIG. 2.
Preincubation of skin explants with RANTES analogues blocks subsequent R5 HIV infection in LC in a dose-dependent manner. LC within skin explants were preincubated with the indicated concentrations of RANTES analogues for 20 min, exposed to HIVBa-L for 2 h, cultured for 3 days, and then double stained with anti-p24 and anti-major histocompatibility complex class II MAbs. (A) Representative fluorescence-activated cell sorter analyses from a single experiment using AOP-RANTES. (B) Summary of nine experiments using skin explants from nine individuals with various CCR5 diplotypes. Each line represents data from a different individual skin donor.
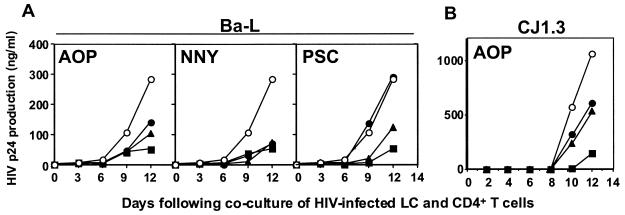
Preincubation of skin explants with RANTES analogues blocks the ability of HIV-exposed LC to subsequently transmit virus to cocultured CD4+ T cells.
Although only relatively few LC are productively infected with HIV using this system, we have shown that these cells transmit relatively high levels of HIV infection in cocultured T cells (14, 15). Thus, we next tested whether preincubation of skin explants with RANTES analogues could block the ability of HIV-exposed LC to subsequently transmit virus to cocultured T cells. Preincubation of skin explants with either AOP-, NNY-, or PSC-RANTES blocked subsequent transmission of HIVBa-L (an R5 HIV laboratory isolate) to cocultured CD4+ T cells in a dose-dependent manner (Fig. 3A). Preincubation of skin explants with AOP-RANTES also blocked subsequent transmission of HIVCJ1.3 (an R5 HIV primary isolate) to CD4+ T cells (Fig. 3B). These data suggest that a C-type lectin-mediated “capture” of virus, which should not be blocked by RANTES analogues, is not a dominant process for LC residing within epithelium, a finding that is consistent with previous published results (15).
FIG. 3.
Preincubation of skin explants with RANTES analogues blocks the ability of HIV-exposed LC to subsequently transmit virus to cocultured CD4+ T cells. LC within skin explants were preincubated with the indicated concentrations of RANTES analogues (open circles, 0 nM; closed circles, 1 nM; closed triangles, 10 nM; closed squares, 100 nM) for 20 min, exposed to the indicated R5 HIV strains for 2 h, and cocultured with allogeneic CD4+ T cells. Culture supernatants were then collected on the indicated days and assessed for p24 content by ELISA. (A) One representative experiment of three using an R5 HIV laboratory isolate (HIVBa-L). (B) One representative experiment of three using an R5 HIV primary isolate (HIVCJ1.3).
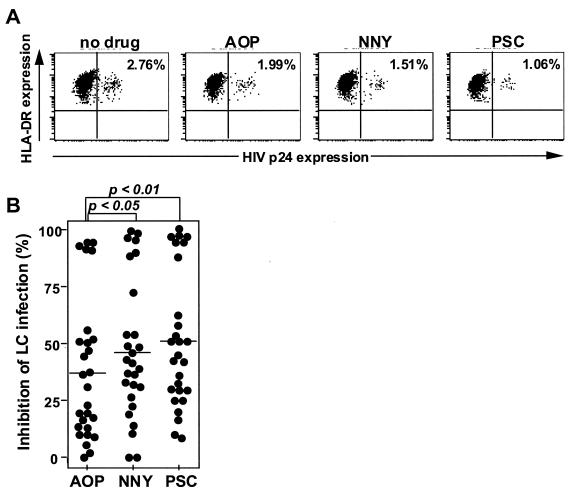
PSC-RANTES is more potent than AOP-RANTES and NNY-RANTES in its ability to block R5 HIV infection of LC.
To elucidate the most potent inhibitor of R5 HIV infection in LC, we directly compared the antiviral activity of the three RANTES analogues. Because of the variability in R5 HIV infection levels in different individual skin donors, these direct-comparison experiments had to be performed using skin from single donors. Since tissue is also a limiting factor for these experiments, only one dose of each analogue could be tested for each direct-comparison experiment. We chose a suboptimal blocking dose (10 nM) in order to observe efficacy differences between the individual analogues. A single representative direct-comparison experiment is shown in Fig. 4A. This type of experiment was performed a total of 27 times, and the percent inhibition for each analogue in each experiment is plotted in Fig. 4B. Both PSC- and NNY-RANTES inhibited R5 HIV infection in LC significantly more than did AOP-RANTES (P < 0.01 and P < 0.05, respectively). PSC-RANTES demonstrated the best blocking ability, although this was not statistically significant when compared to NNY-RANTES. By contrast, we could not demonstrate significant differences among the analogues in their ability to block transmission of HIV to cocultured CD4+ T cells (data not shown). We believe this may relate to the relative lack of sensitivity in this assay when compared to the flow cytometry-based assay that we used to quantify HIV infection levels within LC.
FIG. 4.
PSC-RANTES is more potent than AOP-RANTES and NNY-RANTES in its ability to block R5 HIV infection of LC. LC within skin explants were preincubated with a suboptimal blocking dose (10 nM) of each RANTES analogue for 20 min, exposed to HIVBa-L for 2 h, cultured for 3 days, and then stained with anti-p24 and anti-major histocompatibility complex class II MAbs. (A) Representative fluorescence-activated cell sorter analyses from a single experiment showing comparative blocking ability of each analogue. (B) Summary of 27 separate comparative experiments using LC from 27 skin donors. Percent inhibition of LC infection was calculated as described in Materials and Methods.
PSC-RANTES blocks primary R5 HIV infection of LC without promoting infection by primary X4 HIV.
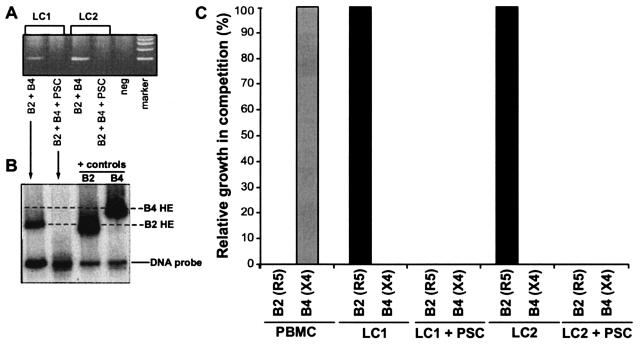
Since it has been reported that high concentrations of RANTES and AOP-RANTES can increase replication of X4 HIV isolates (7, 18, 22, 31, 38), we next studied whether preincubation of skin with PSC-RANTES could lead to enhanced replication of primary X4 HIV in LC. Skin explants were preincubated with 100 nM PSC-RANTES for 20 min and then exposed to a mixture of R5 HIV (the primary isolate B2 HIV) and X4 HIV (the primary isolate B4 HIV). The MOI was approximately 0.01 for both viruses, or the same MOI used for dual infection of skin explants or PBMC in previous studies (1). For these experiments, neither flow cytometry for HIV p24 nor an HIV p24 antigen capture assay could distinguish between these two closely related HIV isolates in the dual infections. Thus, we used a heteroduplex tracking assay designed specifically to detect and quantify single specific HIV isolates present in dual infections (1, 27). Infection by the B2 HIV-B4 HIV mixture could be detected in the skin explants of two donors (Fig. 5A). Heteroduplex tracking assays on the PCR-amplified HIV env DNA isolated from lysed LC revealed that only the R5 isolate, B2 HIV, and not the X4 HIV isolate, B4 HIV, was detected and PCR amplified in these experiments (Fig. 5B), suggesting that X4 HIV could not establish infection in LC. In skin that had been pretreated with PSC-RANTES and then exposed to the B2 HIV-B4 HIV mixture, we could not amplify HIV env DNA from lysed LC (Fig. 5A). This result suggests that 100 nM PSC-RANTES completely blocks infection of LC by R5 HIV and does not promote infection of LC by X4 HIV.
FIG. 5.
PSC-RANTES blocks primary R5 HIV infection of LC without promoting infection of LC by primary X4 HIV. LC within skin explants were preincubated with 100 nM PSC-RANTES for 20 min, simultaneously exposed to relatively equal amounts (MOI of 0.01 for each) of both B2 HIV (an R5 HIV primary isolate) and B4 HIV (an X4 HIV primary isolate) for 2 h, and cultured for 3 days. DNA was then extracted from LC and amplified by PCR using env-specific primers (A) and used in a heteroduplex tracking assay (B) as described in Materials and Methods. (C) Additional competitions between B2 HIV and B4 HIV were performed in PBMC. DNA was extracted after 10 days in these experiments. The percent growth of B2 HIV relative to B4 HIV is plotted for both PBMC and LC experiments.
By contrast to these results in LC, the primary X4 isolate B4 HIV was the only virus detected by the heteroduplex tracking assay in a competition between B4 HIV and B2 HIV in PHA-IL-2-treated PBMC (0.01 MOI of each virus) (Fig. 5C). B4 HIV also competed very well with R5 HIV primary isolates other than B2 HIV in additional PBMC experiments (data not shown). Lastly, unlike what was observed with LC, pretreatment of PBMC with 100 nM PSC-RANTES lead to a fourfold stimulation of B4 HIV replication (data not shown). Taken together, these data suggest that primary R5 HIV isolates have a competitive advantage over primary X4 HIV isolates in establishing infections in LC (but not in PBMC).
PSC-RANTES blocks R5 HIV infection in LC isolated from individuals with a variety of CCR5 diplotypes.
Since R5 HIV infection levels in LC correlate well with the CCR5 diplotype (15), we hypothesized that the ability of RANTES analogues to inhibit R5 HIV infection in LC would also be influenced by the CCR5 diplotype. To test this hypothesis, we examined the ability of PSC-RANTES (at a suboptimal dose of 10 nM) to block R5 HIV infection in LC isolated from 46 different randomly selected skin donors. Because we had previously shown that the A-2459G polymorphic locus in the CCR5 promoter region and the CCR5 ORFΔ32 mutation were the loci most likely to influence R5 HIV infection in LC (15), we examined the genotype of these 46 individuals at these sites. Since the −2459A promoter allele is tightly linked to CCR5Δ32 (15, 20), compound CCR5 genotypes for the two selected CCR5 loci were classified into the following five groups: Awt/Awt (n = 12), Awt/Gwt (n = 13), Gwt/Gwt (n = 9), Awt/AΔ32 (n = 4), and Gwt/AΔ32 (n = 8).
Our findings illustrate that susceptibility of LC to R5 HIV infection varied based upon CCR5 diplotype categories (Table 1). Since we observed no significant differences in infection among the Awt/AΔ32, Gwt/Gwt, and Awt/Gwt diplotype categories with either no treatment (P = 0.969) or 10 nM PSC-RANTES (P = 0.711), further analyses were performed by grouping these three diplotypes into a single category. Differences in LC susceptibility to R5 HIV infection were statistically significant with the highest levels of infection observed for the Awt/Awt diplotype, the lowest levels of infection observed for the Gwt/AΔ32 diplotype, and intermediate levels of infection observed for the combined diplotype category for both no treatment (P < 0.001) and 10 nM PSC-RANTES (P < 0.001) treatment. When analysis of differences between no treatment and 10 nM PSC-RANTES were compared within each diplotype category in paired t tests, 10 nM PSC-RANTES significantly reduced susceptibility to R5 HIV infection for Awt/Awt (P = 0.01), Gwt/Gwt (P = 0.025), Awt/Gwt (P = 0.001), and Gwt/AΔ32 (P = 0.025), but not for Awt/AΔ32 (P = 0.273) (Table 1).
TABLE 1.
PSC-RANTES blocks R5 HIV infection in LC isolated from individuals with a variety of CCR5 diplotypes
| Genotype | n | % HIV p24+ LC
|
Pa | |||
|---|---|---|---|---|---|---|
| No treatment
|
PSC-RANTES (10 nM)
|
|||||
| Mean | SE | Mean | SE | |||
| Awt/Awt | 12 | 3.445 | 0.515 | 2.167 | 0.261 | 0.01 |
| Awt/AΔ32 | 4 | 2.370 | 0.818 | 1.613 | 0.365 | 0.273 |
| Gwt/Gwt | 9 | 2.082 | 0.548 | 1.371 | 0.419 | 0.025 |
| Awt/Gwt | 13 | 1.833 | 0.200 | 1.314 | 0.183 | 0.001 |
| Gwt/AΔ32 | 8 | 0.905 | 0.190 | 0.443 | 0.155 | 0.025 |
Paired t tests were performed to assess the mean difference and statistical significance between no treatment and 10 nM treatment within each diplotype category.
DISCUSSION
Topical microbicides are urgently needed to help prevent sexual transmission of HIV throughout the world (9). In this study, we tested the ability of a set of synthetic RANTES analogues to inhibit CCR5-mediated HIV infection using an ex vivo tissue explant model that mimics certain biologic features of heterosexual HIV transmission (14). PSC-RANTES, when compared to AOP- and NNY-RANTES, was the most potent compound for blocking R5 HIV infection of LC located within skin explants. We found no evidence that PSC-RANTES enhanced infection of LC with X4 HIV, which is a potential concern for microbicides that selectively block R5 HIV infection. Importantly, we also showed that PSC-RANTES inhibited R5 HIV in LC isolated from individuals with a variety of CCR5 diplotypes, suggesting that this drug, and possibly other CCR5 inhibitors, may be widely effective regardless of genetic polymorphism in the CCR5 gene. Thus, these results suggest that a topical microbicide containing PSC-RANTES may be a useful agent in blocking sexual transmission of HIV. Indeed, recent experiments by our group have shown that intravaginal exposure to topical PSC-RANTES for 15 min can completely prevent subsequent intravaginal infection by a CCR5-using SIV isolate in female rhesus macaques (Lederman et al., submitted for publication).
The idea of using RANTES analogues at mucosal sites to block R5 HIV infection is based upon the theory that restricted transmission of R5 HIV (in contrast to X4 HIV) is occurring within mucosal epithelia and not at sites more proximal, such as the submucosal layers or the draining lymph nodes. In the animal studies mentioned above, no PSC-RANTES could be detected within the systemic circulation of topically treated macaques, suggesting that PSC-RANTES was blocking SIV infection locally at or near the site of virus exposure (Lederman et al., submitted). Immature dendritic cells that normally reside in genital mucosal epithelia and other stratified squamous epithelial layers, i.e., LC, are CCR5+ and CXCR4− (43) and have been recently shown to be the major cell type infected in female rhesus macaques following intravaginal exposure to SIV (11). Although we have not yet proven that PSC-RANTES blocks intravaginal SIV infection in macaques by blocking infection of mucosal CCR5+ LC, this is a plausible explanation for our findings.
Although X4 HIV isolates rarely establish infections in recently exposed hosts (16, 24), the fact that high concentrations of RANTES analogues can stimulate replication of primary X4 HIV isolates in long-term PBMC cultures (7, 18, 22, 31, 38) raises a potential concern for the use of PSC-RANTES as a microbicide. This potential problem, however, may not be as critical an issue when CCR5 inhibitors are used as topical microbicides. In the latter setting, exposure to a viral swarm that contains both R5 and X4 HIV isolates would be relatively brief. Genetic variation in gp120, from a CCR5-using virus to a CXCR4-using virus, is unlikely to occur during such short exposure times. Alternatively, topical microbicides containing RANTES analogues may increase or stimulate de novo CXCR4 surface expression on potential initial target cells. Such an increase in CXCR4 expression, however, would have to occur fairly rapidly within tissue for X4 HIV to enter the body, especially if the microbicide was applied minutes prior to HIV exposure. Importantly, we have shown here that primary X4 HIV did not infect LC, either alone or following preincubation of the skin with PSC-RANTES (Fig. 5). Given that activated mature LC express CXCR4 and can be infected with X4 HIV (43, 46), these results indicate that short-term incubation of LC with PSC-RANTES does not induce LC activation. Regardless of these promising ex vivo results in LC, however, the possibility that CCR5 blockade may facilitate transmission of X4 HIV needs to be studied closely if topical microbicides containing PSC-RANTES or other CCR5 inhibitors are ever to be used on humans.
Genetic polymorphism clearly plays a role in influencing both the acquisition of HIV and the course of disease in the years following initial infection (3, 23). Although studies have evaluated the influence of genetic polymorphism on outcomes of highly active antiretroviral therapy treatment of HIV infection (8, 23, 39, 42) and hypersensitivity to treatment (17, 19), an understanding of more global factors influencing efficacy of HIV therapy based on pharmacogenomic approaches is not well developed (6). Regarding CCR5 inhibitors, relatively little is known about how these compounds function in different individuals with varying CCR5 genotypes. Interestingly, Sabbe and colleagues found that internalization of CCR5 on the cell surface of CD4+ T cells induced by exposure to AOP-RANTES was the most rapid in individuals homozygous for the −2459A allele and least rapid in individuals homozygous for the −2459G allele (32). Importantly, we showed here that 10 nM PSC-RANTES significantly inhibited R5 HIV infection in LC isolated from individuals with a variety of CCR5 diplotypes (Table 1). In addition, higher doses of PSC-RANTES (100 nM) blocked R5 HIV infection in LC isolated from individuals with all possible CCR5 genotypes (Fig. 2 and data not shown). These results suggest that CCR5 inhibitors (in this case, PSC-RANTES) would be prophylactically and therapeutically effective, especially at relatively large doses, despite genetic differences in CCR5 among treated individuals.
Acknowledgments
We thank Mark C. Udey, Donald Mosier, and Michael Lederman for helpful discussions and Ana Hancox and Susan Booher for technical assistance.
These studies were supported in part by grant AI51649 from the National Institutes of Health and by a grant from the Swiss National Science Foundation.
REFERENCES
- 1.Ball, S. C., A. Abraha, K. R. Collins, A. J. Marozsan, H. Baird, M. E. Quinones-Mateu, A. Penn-Nicholson, M. Murray, N. Richard, M. Lobritz, P. A. Zimmerman, T. Kawamura, A. Blauvelt, and E. J. Arts. 2003. Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J. Virol. 77:1021-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamshad, M. J., S. Mummidi, E. Gonzalez, S. S. Ahuja, D. M. Dunn, W. S. Watkins, S. Wooding, A. C. Stone, L. B. Jorde, R. B. Weiss, and S. K. Ahuja. 2002. A strong signature of balancing selection in the 5′ cis-regulatory region of CCR5. Proc. Natl. Acad. Sci. USA 99:10539-10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535-551. [DOI] [PubMed] [Google Scholar]
- 4.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 5.Fauci, A. S. 1999. The AIDS epidemic-considerations for the 21st century. N. Engl. J. Med. 341:1046-1050. [DOI] [PubMed] [Google Scholar]
- 6.Flexner, C. W. 2003. Advances in HIV pharmacology: protein binding, pharmacogenomics, and therapeutic drug monitoring. Top. HIV Med. 11:40-44. [PubMed] [Google Scholar]
- 7.Gordon, C. J., M. A. Muesing, A. E. Proudfoot, C. A. Power, J. P. Moore, and A. Trkola. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerin, S., L. Meyer, I. Theodorou, F. Boufassa, M. Magierowska, C. Goujard, C. Rouzioux, P. Debre, and J. F. Delfraissy. 2000. CCR5 delta32 deletion and response to highly active antiretroviral therapy in HIV-1-infected patients. AIDS 14:2788-2790. [DOI] [PubMed] [Google Scholar]
- 9.Harrison, P. F., Z. Rosenberg, and J. Bowcut. 2003. Topical microbicides for disease prevention: status and challenges. Clin. Infect. Dis. 36:1290-1294. [DOI] [PubMed] [Google Scholar]
- 10.Hartley, O., K. Dorgham, D. Perez-Bercoff, F. Cerini, A. Heimann, H. Gaertner, R. E. Offord, G. Pancino, P. Debre, and G. Gorochov. 2003. Human immunodeficiency virus type 1 entry inhibitors selected on living cells from a library of phage chemokines. J. Virol. 77:6637-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 13.Kahn, J. O., and B. D. Walker. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339:33-39. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura, T., S. S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, A. R. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 17.Mallal, S., D. Nolan, C. Witt, G. Masel, A. M. Martin, C. Moore, D. Sayer, A. Castley, C. Mamotte, D. Maxwell, I. James, and F. T. Christiansen. 2002. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359:727-732. [DOI] [PubMed] [Google Scholar]
- 18.Marozsan, A. J., V. S. Torre, M. Johnson, S. C. Ball, J. V. Cross, D. J. Templeton, M. E. Quinones-Mateu, R. E. Offord, and E. J. Arts. 2001. Mechanisms involved in stimulation of human immunodeficiency virus type 1 replication by aminooxypentane RANTES. J. Virol. 75:8624-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McComsey, G., D. J. Tan, M. Lederman, E. Wilson, and L. J. Wong. 2002. Analysis of the mitochondrial DNA genome in the peripheral blood leukocytes of HIV-infected patients with or without lipoatrophy. AIDS 16:513-518. [DOI] [PubMed] [Google Scholar]
- 20.McDermott, D. H., P. A. Zimmerman, F. Guignard, C. A. Kleeberger, S. F. Leitman, and P. M. Murphy. 1998. CCR5 promoter polymorphism and HIV-1 disease progression. Lancet 352:866-870. [DOI] [PubMed] [Google Scholar]
- 21.Miller, C. J., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59-67. [DOI] [PubMed] [Google Scholar]
- 22.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien, S. J., and J. P. Moore. 2000. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol. Rev. 177:99-111. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien, T. R., C. Winkler, M. Dean, J. A. E. Nelson, M. Carrington, N. L. Michael, and G. C. White. 1997. HIV-1 infection in a man homozygous for CCR5Δ32. Lancet 349:1219. [DOI] [PubMed] [Google Scholar]
- 25.Pastore, C., G. R. Picchio, F. Galimi, R. Fish, O. Hartley, R. E. Offord, and D. E. Mosier. 2003. Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES. Antimicrob. Agents Chemother. 47:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 27.Quinones-Mateu, M. E., S. C. Ball, A. J. Marozsan, V. S. Torre, J. L. Albright, G. Vanham, G. van Der Groen, R. L. Colebunders, and E. J. Arts. 2000. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 74:9222-9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani, N., S. Holzmann, C. H. Tripp, F. Koch, and P. Stoitzner. 2003. Langerhans cells-dendritic cells of the epidermis. APMIS 111:725-740. [DOI] [PubMed] [Google Scholar]
- 30.Royce, R. A., A. Sena, W. Cates, and M. S. Cohen. 1997. Sexual transmission of HIV. N. Engl. J. Med. 336:1072-1078. [DOI] [PubMed] [Google Scholar]
- 31.Rusconi, S., S. La Seta Catamancio, P. Citterio, E. Bulgheroni, F. Croce, S. H. Herrmann, R. E. Offord, M. Galli, and M. S. Hirsch. 2000. Combination of CCR5 and CXCR4 inhibitors in therapy of human immunodeficiency virus type 1 infection: in vitro studies of mixed virus infections. J. Virol. 74:9328-9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbe, R., G. R. Picchio, C. Pastore, O. Chaloin, O. Hartley, R. Offord, and D. E. Mosier. 2001. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J. Virol. 75:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salkowitz, J. R., S. E. Bruse, H. Meyerson, H. Valdez, D. E. Mosier, C. V. Harding, P. A. Zimmerman, and M. M. Lederman. 2003. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin. Immunol. 108:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salkowitz, J. R., S. F. Purvis, H. Meyerson, P. Zimmerman, T. R. O'Brien, L. Aledort, M. E. Eyster, M. Hilgartner, C. Kessler, B. A. Konkle, G. C. White II, J. J. Goedert, and M. M. Lederman. 2001. Characterization of high-risk HIV-1 seronegative hemophiliacs. Clin. Immunol. 98:200-211. [DOI] [PubMed] [Google Scholar]
- 35.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 36.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. C. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. W., M. Dean, M. Carrington, C. Winkler, G. A. Huttley, D. A. Lomb, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Kaslow, S. Buchbinder, E. Vittinghoff, D. Vlahov, K. Hoots, M. W. Hilgartner, and S. J. O'Brien. 1997. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277:959-965. [DOI] [PubMed] [Google Scholar]
- 38.Torre, V. S., A. J. Marozsan, J. L. Albright, K. R. Collins, O. Hartley, R. E. Offord, M. E. Quinones-Mateu, and E. J. Arts. 2000. Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs. J. Virol. 74:4868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdez, H., S. F. Purvis, M. M. Lederman, M. Fillingame, and P. A. Zimmerman. 1999. Association of the CCR5delta32 mutation with improved response to antiretroviral therapy. JAMA 282:734. [DOI] [PubMed] [Google Scholar]
- 40.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilken, J., D. Hoover, D. A. Thompson, P. N. Barlow, H. McSparron, L. Picard, A. Wlodawer, J. Lubkowski, and S. B. Kent. 1999. Total chemical synthesis and high-resolution crystal structure of the potent anti-HIV protein AOP-RANTES. Chem. Biol. 6:43-51. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita, T. E., J. P. Phair, A. Munoz, J. B. Margolick, R. Detels, S. J. O'Brien, J. W. Mellors, S. M. Wolinsky, and L. P. Jacobson. 2001. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS 15:735-746. [DOI] [PubMed] [Google Scholar]
- 43.Zaitseva, M., A. Blauvelt, S. Lee, C. K. Lapham, V. Klaus-Kovtun, H. Mostowski, J. Manischewitz, and H. Golding. 1997. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat. Med. 3:1369-1375. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. H. J. Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 67:3345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]
- 46.Zoeteweij, J. P., H. Golding, H. Mostowski, and A. Blauvelt. 1998. Cutting edge: cytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J. Immunol. 161:3219-3223. [PubMed] [Google Scholar]