Abstract
Background
Standards for detection of adenomas during screening colonoscopy are widely used to measure examination quality. No such standards exist for sessile serrated adenomas.
Objective
To measure both the adenoma detection rate (ADR) and sessile serrated adenoma detection rate (SSADR) during screening colonoscopy before and after quality improvement/financial incentive measures.
Design
Retrospective determination of baseline ADR/SSADR by the endoscopist, followed by prospective collection of data after informing physicians of baseline detection rates.
Setting
Tertiary cancer center with large cancer screening program.
Patients
A total of 2833 average-risk colorectal cancer screening patients ages 50 to 75 undergoing initial colonoscopy.
Interventions/data collection
Electronic medical record for indication and demographics, endoscopy report and pathology report.
Main outcome measurements
Detection rates of adenomas and sessile serrated adenomas by gender.
Results
Overall ADR in males and females was 50.6% and 36.6%, respectively. Overall detection of advanced adenomas in males and females was 12.4% and 6.5%, respectively. Overall SSADR in males and females was 10.1% and 7.1%, respectively. In 108 patients (3.8% of entire group) SSAs were the only premalignant lesions found. Detection rates of both types of premalignant polyps improved over time but did not reach statistical significance.
Limitations
Single-center experience with limited sample size and small group of endoscopists.
Conclusions
ADRs far in excess of current standards are achievable. Cecal withdrawal time is associated with ADR. Prevalence of SSA rivals that of advanced adenomas and is greater than current medical literature suggests. The combination of monitoring and financial incentives did not result in statistically significant improvement in ADR.
INTRODUCTION
Current standards for adenoma detection rates (ADRs) of 25% in men and 15% in women during screening colonoscopy were established based on studies published in an era before the availability of high definition colonoscopes, electronic chromoendoscopy (eg, narrowing-band imaging) and widespread utilization of split bowel preps. [1–7] Recent reports from multiple practice settings demonstrate ADRs significantly above the standards suggesting that the bar has been set too low.[8, 9] However, such reports are not universal as other studies report ADRs barely exceeding the current standards.[10–12] The limited ability of colonoscopy as frequently performed to detect many adenomas was brought out by the tandem colonoscopy studies.[13] Missed adenomas would provide an explanation for the incomplete protection from colon cancer provided by colonoscopy and the failure to approach the cancer reduction rates projected in the National Polyp Study.[14, 15]
The shortfall in colonoscopy’s ability to reduce colon cancer rates is particularly striking in the right colon.[16, 17] This has been attributed in part to flat lesions, such as, sessile serrated adenomas (SSA), whose cancer risks have only recently received widespread attention.[18–20] Although SSAs are well described in the pathology literature, their natural history is poorly defined. Their prevalence is unclear but is estimated to be less than 2%.[21] Despite the growing significance of SSAs, no standards exist to inform the endoscopist if their detection rate is adequate. The implicit assumption is that an endoscopist with adequate ADR will have a suitable detection rate for SSAs.
Our aim was to assess the detection rates for both adenomas and sessile serrated adenomas in screening average risk patients to determine if their detection rates were correlated. As these data were collected as part of a quality improvement effort, an additional objective was to determine the impact of informing endoscopists about their individual detection rates on future performance.
METHODS
Patients
Data was collected from all first time screening colonoscopies in average risk individuals 45–75 years of age from July 2010 through May 2013. The Endoscopy Center at MD Anderson Cancer Center is open access with patients referred for screening colonoscopy from their primary oncologic service or through Cancer Prevention Center. The latter patients were frequently evaluated by gastroenterology mid-level providers before their procedures. Screening examinations were identified by manual review of all colonoscopy reports performed during the study period by three of the authors (ST, MS, and WR). If a colonoscopy report was identified as a potential screening examination, clinic notes preceding the procedure date were reviewed to verify that the colonoscopy was the first for the patient and that no symptoms or conditions were prompting the examination. Patients were excluded if they (1) were suspected of having a colon cancer syndrome based on family or personal history of cancer, (2) had multiple first-degree relatives with colon cancer history or 1 first degree relative <45 years of age at time of diagnosis of colon cancer, (3) personal history of Crohn’s disease or ulcerative colitis, (4) had prior colonoscopy or (5) had prior colon resection. Demographic, clinical, and endoscopic data were entered into a secure database. Initially the data collection was part of a QA/QI project and IRB approval was not required. However, as data collection was proving labor intensive, a broader initiative was undertaken to develop a means to automate the data collection as much as possible. So, IRB approval was obtained from the University of Texas MD Anderson Cancer Center with the current study forming the baseline data set to serve as a standard for a subsequent electronic neoplastic polyp detection rate monitoring system based on Natural Language processing. A waiver of consent was obtained from the IRB.
Procedure
Colonoscopy was performed following a standard split dose preparation regimen established in 2009.[22] Quality measures being actively monitored at time of study were cecal withdrawal time and cecal intubation rates. For the purposes of this study cecal withdrawal time was calculated only on examinations with no polyps. All procedures were performed with Olympus Series 180 colonoscopes (Center Valley, Pennsylvania) that have narrow-band imaging capability. Use of narrow-band imaging and distal cap attachment was left to the individual endoscopist’s discretion. Procedural reports were generated using a standard template using Endoworks software (Olympus America Center Valley, Pennsylvania). Incomplete examinations due to bowel prep or technical difficulty were included. A total of 13 gastroenterologists performed 90 or more screening colonoscopies during the study period and are included in the analysis.
Pathology
Specimens were reviewed by a group of nine academic gastrointestinal pathologists who were assigned to read specimens on a schedule that varied month by month. For the purposes of this study pathology results were taken from original pathological reports, no review of earlier results was done. Polyp submission method, either as individual polyps in each container or batched, was left to the individual endoscopists. If multiple polyps were submitted in 1 container but a number not specified, it was assumed to have 2 polyps. Endoscopists were performing procedures on a set weekly schedule. Polyp size was determined from the pathology report. Select cases are routinely reviewed at a weekly pathology conference. Polyps not retrieved after removal were considered to be non-neoplastic.
Incentive and Quality Improvement Program
In February 2012 all physicians were given their individual ADRs and SSA detection rates. In addition, they were given data on the performance of the group overall, as well as, de-identified individual data of all group members. At the same time a clinical performance incentive program for fiscal year 2012 (ending August 31, 2012) was revealed with bonus payments dependent in part on individual ADR. Half the incentive was based on individual productivity but to be eligible to participate a normalized ADR of 30% was needed on screening examinations. Another quarter of the incentive was based on achieving an ADR of 35% or higher. No incentive program was offered the following fiscal year. Endoscopists did not receive updated ADR reports and data collection continued until May 2013.
Statistical methods
Descriptive analysis was performed using means and standard deviation for continuous measures. Correlations were determined by the method of Pearson. Mean adenoma per procedure (MAP) was calculated by dividing total number of adenomas removed by total number of screening examinations performed by individual endoscopist. The variable gender mix of the various endoscopist’s practices was taken into account by calculating separate male and female ADRs, as well as, a normalized ADR assuming a population that was gender balanced (half male and half female). Confidence intervals were calculated for normalized ADR as recommended by Do et al.[23] Comparisons of detection rates for significance were by the Fisher exact test. Logistic regression was performed to assess predictors of ADR and SSA detection using Stata software, version 13.1 (Stata Corp, College Station, TX). Covariates included in the analysis were patient factors: age, gender, race, body mass index (BMI) and physician factor: years of practice post training.
RESULTS
Demographics
A total of 2833 patients underwent first time colonoscopy during the study period. This represents approximately 18% of colonoscopy volume in our laboratory over the time period and most of the patients were women (n=1830; 64.6%). whites made up 68% of the group, followed by African-Americans (12.7%), Hispanics (11.2%) and Asians (8.1%). The mean age of the study group was 55 years with an average BMI of 29. Family history of colon cancer in 1st degree or 2nd degree relatives was present in 283 patients, 10% of the total.
Adenoma detection rate
Adenomas were found in 1189 patients for an overall ADR of 41% (men, 50.6%; women, 36.6%). Assuming a gender balanced population, the normalized ADR was 43.6%. The ADR range in men was 29.3–66.1%, whereas in women the range was even larger 14.5–57.4% (Table 1). The male ADR median was 53.1% with interquartile range (IQR) 64.8–36.5%. Female median ADR was 38% with IQR 43–25.7%. The median normalized ADR was 46.9% with an IQR of 51.1–31.2%. Multivariate analysis was performed to determine factors associated with ADR. The expected factors such as age, male gender and obesity were significant with odds ratio (OR) 1.04 [1.02–1.05], 1.71 [1.46–2.01], and 1.31 [1.07–1.59] respectively. Race was not significant. Years since fellowship had a weak but statistically significant inverse relationship with ADR, OR=0.98 [0.97–0.99]. The average BMI of each endoscopist’s patient group was not correlated with their ADR, R=0.15.
Table 1.
Individual and group ADRs for both genders and normalized gender-balanced rate with confidence interval (CI).
| Doc | # Exams | Males | M-ADR | Females | F-ADR | N-ADR (CI) |
|---|---|---|---|---|---|---|
| A | 238 | 85 | 64.7% | 153 | 43.8% | 54.2% (6.3%) |
| B | 148 | 40 | 65.0% | 108 | 38.0% | 51.5% (8.0%) |
| C | 167 | 62 | 66.1% | 105 | 44.8% | 55.4% (7.5%) |
| D | 297 | 107 | 65.4% | 190 | 57.4% | 61.4% (5.5%) |
| E | 325 | 113 | 51.3% | 212 | 29.7% | 40.5% (5.3%) |
| F | 234 | 87 | 51.7% | 147 | 42.2% | 47.0% (6.4%) |
| G | 171 | 56 | 53.6% | 115 | 28.7% | 41.1% (7.4%) |
| H | 239 | 81 | 53.1% | 158 | 43.7% | 48.4% (6.3%) |
| I | 94 | 41 | 34.2% | 53 | 22.6% | 28.4% (9.1%) |
| J | 457 | 152 | 34.9% | 305 | 31.8% | 33.3% (4.3%) |
| K | 269 | 102 | 38.2% | 167 | 22.2% | 30.2% (5.5%) |
| L | 96 | 41 | 29.3% | 55 | 14.6% | 21.9% (8.3%) |
| M | 98 | 36 | 61.1% | 62 | 40.3% | 50.7% (9.9%) |
| Total | 2833 | 1003 | 50.6% | 1830 | 36.6% | 43.6% (1.8%) |
The overall advanced adenoma detection rate was 8.6% (men, 12.4% and women, 6.5%). The correlation between ADR and advanced adenoma detection was 0.61. Mean adenoma per procedure (MAP) for the overall study group was 0.81 (men, 1.13 and women, 0.63) (Table 2). The correlation between normalized ADR and normalized MAP was 0.93.
Table 2.
Individual and group mean adenoma per procedure (MAP) and advanced adenoma (AA) detection rates by gender.
| Doc | Total Adenomas |
Male MAP |
Female MAP |
Pts w AA |
%M-AA | %F-AA |
|---|---|---|---|---|---|---|
| A | 302 | 1.89 | 0.92 | 20 | 11.76% | 9.15% |
| B | 138 | 1.35 | 0.78 | 8 | 12.50% | 7.65% |
| C | 148 | 1.42 | 0.63 | 21 | 19.35% | 13.96% |
| D | 342 | 1.48 | 0.97 | 36 | 14.02% | 12.54% |
| E | 235 | 1.06 | 0.54 | 22 | 11.71% | 8.00% |
| F | 229 | 1.39 | 0.73 | 28 | 16.09% | 12.81% |
| G | 102 | 0.76 | 0.47 | 13 | 14.29% | 9.32% |
| H | 208 | 1.23 | 0.68 | 19 | 13.58% | 9.51% |
| I | 44 | 0.44 | 0.51 | 6 | 4.88% | 6.21% |
| J | 287 | 0.83 | 0.53 | 29 | 6.58% | 6.41% |
| K | 133 | 0.69 | 0.40 | 24 | 12.74% | 9.67% |
| L | 30 | 0.46 | 0.20 | 8 | 12.19% | 8.82% |
| M | 100 | 1.28 | 0.87 | 9 | 16.67% | 10.75% |
| Total | 2298 | 1.13 | 0.63 | 243 | 12.36% | 9.43% |
Serrated adenoma detection rate
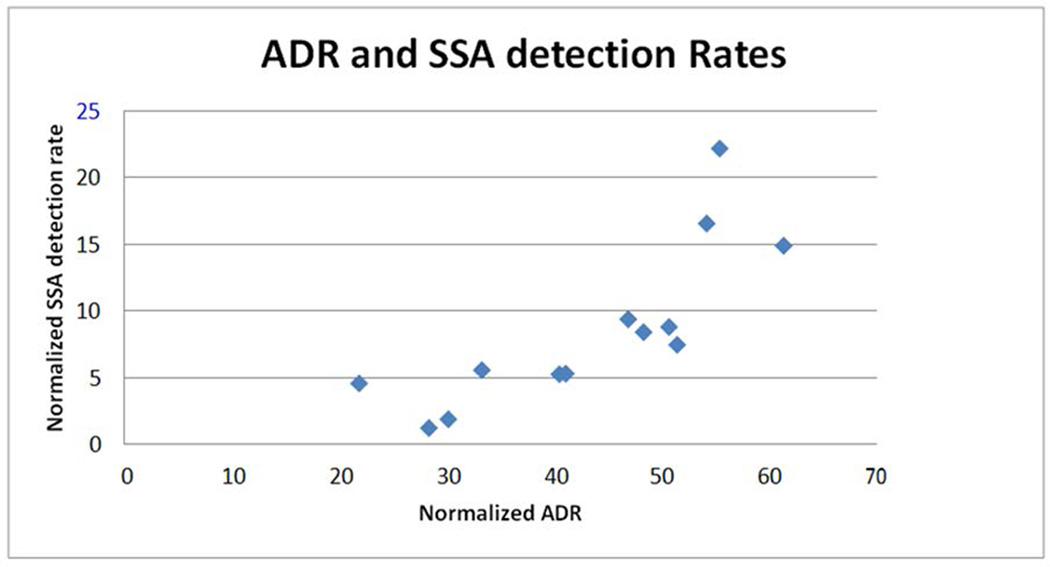
The overall sessile serrated adenoma detection rate was 8.2% (men, 10.1% and women, 7.1%). The range of normalized sessile serrated detection rates was 1.2% to 22.1% (Table 3). The median SSA detection rate was 7.39% with an IQR of 12.12–4.68%. The correlation between ADR and SSA detection rates was 0.78. Yet the disparity between SSA detection rates is greater than for ADRs (Figure 1). Three endoscopists had SSA detection rates higher than 14% and account for almost half of the SSA lesions detected (112 of 231) yet performed only 24.7% (702) of total screening colonoscopies. They also have the highest ADRs with an average normalized rate of 56.7%. Four endoscopists had normalized ADRs in the 47–51%, more than twice the current standard and above the average for the group, with an average normalized SSA detection rate of 8.1% slightly lower than the overall group’s rate. Excluding the three endoscopist with highest SSA detection rates lowers the group’s SSA detection rate to 5.6%.
Table 3.
Individual detection rates of SSA by gender and a normalized value
| Doc | Total SSA | %M-SSA | %F-SSA | Normal SSA |
|---|---|---|---|---|
| A | 37 | 20.00% | 13.07% | 16.54% |
| B | 11 | 7.50% | 7.41% | 7.46% |
| C | 34 | 29.03% | 15.24% | 22.14% |
| D | 41 | 18.69% | 11.05% | 14.87% |
| E | 17 | 5.31% | 5.19% | 5.25% |
| F | 22 | 9.20% | 9.52% | 9.36% |
| G | 8 | 5.36% | 4.35% | 4.86% |
| H | 18 | 11.11% | 5.70% | 8.41% |
| I | 1 | 2.44% | 0.00% | 1.22% |
| J | 26 | 5.26% | 5.90% | 5.58% |
| K | 5 | 1.96% | 1.80% | 1.88% |
| L | 4 | 7.32% | 1.81% | 4.57% |
| M | 7 | 8.33% | 6.45% | 7.39% |
| Total | 231 | 10.07% | 7.10% | 8.58% |
FIGURE 1.
Adenoma detection rate (ADR) compared with sessile serrated adenoma (SSA) detection rate for each endoscopist.
A multivariate analysis was performed to evaluate factors associated with SSA detection. Patients with sessile serrated adenomas are more likely to be male and less likely to be African American or Hispanic as compared to Whites. There was no difference by age or BMI. The endoscopists’ years of experience after fellowship was inversely associated with SSA detection but the effect size was small (OR 0.98; 95% CI, 0.97 – 1.00). A slight majority of SSA patients also had adenomas. Comparing patients with both types of neoplastic polyp to SSA patients without adenomas shows that patients with both types are more likely to be male, older and heavier. However, it should be noted that for 108 patients, 3.8% of the total group, SSAs were the only neoplastic lesion found.
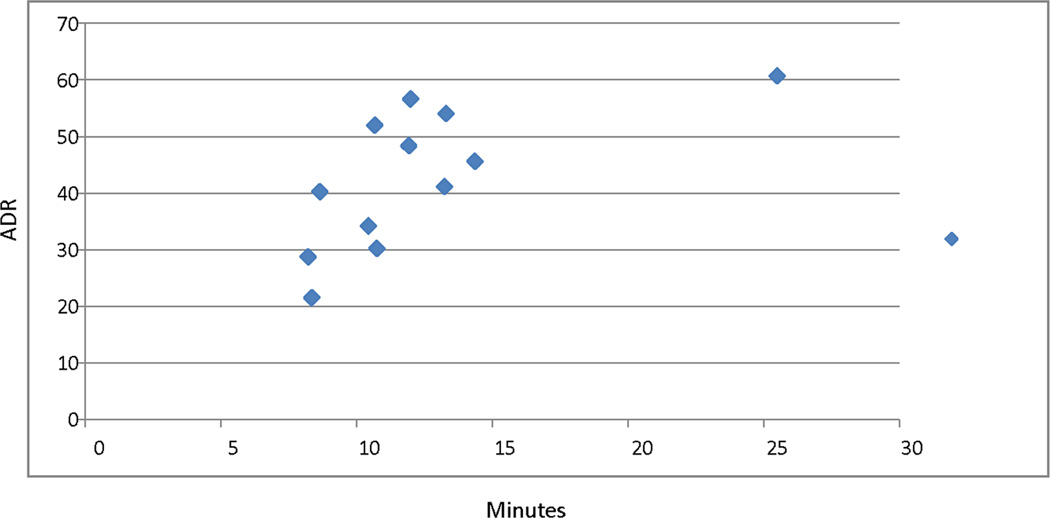
Correlation between average cecal withdrawal time and ADR is 0.69 with a trend toward increasing ADR with increasing time for withdrawal (Figure 2). For SSA detection rate the correlation with cecal withdrawal time is lower at 0.53. Beyond 13–14 minutes the increment in ADR appears to diminish. Correlation between ADR and obesity was assessed for both males and females. For males r=−0.02 and for females r=0.20.
FIGURE 2.
Adenoma detection rate (ADR) by average cecal withdrawal time during normal screening examinations in minutes.
To evaluate if our patient population differed significantly from those in other screening programs we separated our patients into those with a non-colon cancer history and those without any cancer diagnosis. Table 4 shows that ADRs were well above standards in both males and females without cancer. Those patients with a non-colon cancer history had slightly higher ADRs but were also older with the age difference being statistically significant for both genders (p<.0001) whereas ADR differences did not reach statistical significance. The SSA detection rate in males was comparable between those with or without a cancer history. However, in females those without a cancer history had a statistically higher rate of SSA detection.
Table 4.
Demographic and polyp detection rates for those patients with no history of cancer and those with a non-colon cancer history
| No Cancer History | Non-colon Cancer History | ||
|---|---|---|---|
| N | 1465 | 1368 | |
| Male Average Age | 54.07 (5.28) | 58.56 (7.00) | p<.0001 |
| Male ADR | 48.5%(2.1%) | 53.4% (2.4%) | p=.13 |
| Male SSA-DR | 11.17% (2.6%) | 8.7%(2.6%) | p=.20 |
| Female Average Age | 53.98 (5.30) | 55.77 (6.37) | p<.0001 |
| Female ADR | 34.8% (1.6%) | 38.4% (1.6%) | p=.12 |
| Female SSA-DR | 8.7% (1.8%) | 5.5%(1.5%) | p=.01 |
SSA-DR = SSA detection rate confidence interval in parenthesis
Both overall ADR and sessile serrated adenoma detection rates increased over time for both genders following the distribution of both individual and group ADR data (Table 5). However, although the increases were encouraging, they did not reach statistical significance.
Table 5.
Polyp detection rates before and after informing endoscopist of their individual rates as well as the group rates.
| Jul 2010–Jan 2012 | Feb 2012–May 2013 | ||
|---|---|---|---|
| Male ADR | 49.2% | 53.1% | p=.24 |
| Female ADR | 35.2% | 38.5% | p=.16 |
| Male SSA detection | 9.8% | 10.6% | p=.66 |
| Female SSA detection | 6.6% | 7.9% | p=.27 |
DISCUSSION
Our data shows that it is possible to achieve ADRs more than double the current standard in both men and women in our screening population. The benefits of ADRs well above the standards have been recently demonstrated by Corley et al who found reduction in interval cancer occurrence associated with higher ADR.[24] Adherence to the current standards has the potential for over half of the patients with adenomas to leave the endoscopy unit with their adenomas having been missed with inappropriate follow-up instructions with an attendant elevated risk for interval cancer development.
In this study, the overall SSA detection rate was 8.2% comparable to the rate of advanced adenoma detection. Endoscopist using the estimated prevalence of 2% as their goal for SSA detection can be missing 75% or more of the patients with these lesions.
The variability in SSA detection rates far exceeded that in ADR (18:1 vs. 3:1). Normalized ADR greater than 40% did not necessarily translate into above average SSA detection. However, the very high ADRs (54–61%) seen in those with SSA detection rates of 10% or more argues that those adept at SSA detection are also excellent in adenoma detection, a correlation seen with flat polyp detection as well [25]. This suggests that training directed toward detection of SSA that tend to be subtle and flat would lead to enhancement of ADR.
Improving neoplastic polyp detection rates remains a challenge.[26] Our unit introduced universal split prep regimen and narrow-band imaging in late 2009. Although data for increased ADRs with split preps is consistent, the utility of narrow-band imaging in promoting adenoma detection is unclear as reports are conflicting.[27–30]. Clearly taking more time on withdrawal is essential but not sufficient. This benefit appeared to have a plateau effect beyond 10 minutes in the study by Lee et al and 13 min in our data.[31] However, time alone is not enough as previous attempts to mandate a withdrawal time length did not improve ADR and little correlation with ADR was seen with cecal withdrawal times within a limited range, 6–11min.[32, 33] However, our data and that of Lee TJW et al, 2013 would argue that the ideal withdrawal time is 10 minutes or more.[31] In addition to time, the quality of colonoscopic examination is critical to improve ADR. High quality technique focused on cleaning and diligent inspection during withdrawal as assessed by video recording has been shown to correlate with higher ADR.[34]
Informing endoscopists of their performance has had mixed effects on subsequent performance.[35–37] In general, interventions to enhance ADRs have had little to no effect [26]. This includes tying productivity bonuses to ADR as mirrored in our experience. Still the time trends in our group were favorable for ADR and SSA detection in both sexes, although differences did not reach statistical significance and were quite modest for SSA detection in males. Some may view demands for enhanced productivity and higher ADR standards as opposing goals, but our data argues that the 2 aims can be compatible.
Focusing exclusively on non-sessile serrated adenoma detection misses the significant number of patients with SSA particularly those without a concomitant adenomas, 3.8% of the total study group.[38] Training endoscopists to find flat subtle lesions like sessile serrated adenomas may be the most fruitful path to take to improve overall detection of neoplastic lesions. The four individuals with higher than average SSA detection rates had high average normalized ADR of 54.2%. Putting the focus on detection of a relatively new lesion is possibly less threatening to endoscopists particularly those who argue that they have been finding adenomas for decades. We have initiated a training program focused on detection of SSAs to reduce our variation in detection rates using the best practices of our high detectors. This will consist of video demonstration, as well as, didactic lectures.
Strengths of this paper include the fact that it reflects the work of a dedicated group of gastroenterologist using a comprehensive approach to the preparation and evaluation of patients for screening colonoscopies. In addition, pathologic diagnoses are provided by an experienced group of gastrointestinal pathologists so that we are not relying on classifications like “proximal serrated lesion” to determine if a lesion is neoplastic. In addition, lesion size was based on pathological assessment and not endoscopic estimates that can inflate advanced adenoma detection rates.[39] Limitations are that it reflects the work of a small group in a single center and was collected in part retrospectively. Concerns that our patient population was somehow at higher risk for adenomas should be tempered with the analysis presented showing that even in patients without a cancer history our collective detection rates are far in excess of the current standards. In all likelihood the differences seen between cancer and non-cancer patients are due to the cancer history group being slightly older as significant changes in ADR are seen within 5 years as patient’s age. [40, 41]. Nevertheless, we cannot totally rule out that our screening population has a higher adenoma prevalence than is typical. Additional limitations are the small sample size, lack of data on bowel prep scores, and lack of reliable data on onset of use of caps by those endoscopists using the device. Nevertheless, improving ADR performance can be straight-forward for many practices. Greater utilization of split preps with educational efforts to enhance patient compliance is an important initial step to improving ADRs.[42] Spending a few additional minutes on withdrawal from the cecum could lead to further enhancement of both ADR and SSA detection.[43] Reviewing published reports on endoscopic appearance of SSAs will aid better recognition of these lesions as well.
In our screening population adenoma detection rates twice the current standard are achievable. If comparable adenoma prevalence rates exist in the general population undergoing screening, the current ADR standards create the potential for many high risk patients to be missed. Colonoscopy is not as effective a cancer prevention tool when used by poorly trained individuals nor in those who perform hasty examinations despite their training.[44] The fact that endoscopist miss adenomas, the lesion that has been the focus of decades of effort, makes it understandable that reported sessile serrated adenoma detection rates are so low. Our 8% SSA rate is 4 times that found in a recent review.[21] If other groups report comparable results for adenoma and SSA detection, the GI societies need to consider updating the ADR standards and to develop new standards for the detection of sessile serrated adenomas. The recent report by Corley et al on ADR and interval cancer rates adds impetus to the notion that pushing ADRs beyond the current standards is beneficial to patients [24]. Once detected, neoplastic lesions need to be removed completely, a particular problem with SSAs. [45]. Finally, effective programs to train endoscopist to detect SSA lesions must be created.
TAKE HOME MESSAGE.
Adenoma detection rates (ADRs) twice as high as current standards are readily achieved. Sessile serrated adenoma detection rates are comparable to those for advanced adenomas.
High ADRs does not assure above average detection rates for sessile serrated adenomas. Quality improvement initiatives can enhance detection rates of both types of polyps.
Acknowledgement
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number K07CA160753 to M.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHORS CONTRIBUTIONS
WA Ross: Conception and design of study, data analysis, drafting of manuscript and final approval of manuscript
S Thirumurthi: Data analysis, critical revision and final approval of manuscript
PM Lynch: Data analysis, critical revision and final approval of manuscript
A Rashid: Data analysis, critical revision and final approval of manuscript
M Pande: Data analysis, critical revision and final approval of manuscipt
MA Shafi: Data analysis and final approval of manuscript
JH Lee: Data analysis, critical revision and final approval of manuscript
GS Raju: Conception of study, data analysis, critical revision and final approval of manuscript
References
- 1.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME Colorectal Cancer Study G. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. The New England journal of medicine. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. The American journal of gastroenterology. 1990;85:969–974. [PubMed] [Google Scholar]
- 3.Kadakia SC, Wrobleski CS, Kadakia AS, Meier NJ. Prevalence of proximal colonic polyps in average-risk asymptomatic patients with negative fecal occult blood tests and flexible sigmoidoscopy. Gastrointestinal endoscopy. 1996;44:112–117. doi: 10.1016/s0016-5107(96)70126-8. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. The New England journal of medicine. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK. Quality in colonoscopy: cecal intubation first, then what? The American journal of gastroenterology. 2006;101:732–734. doi: 10.1111/j.1572-0241.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Lehman GA, Ulbright TM, Smith JJ, Pound DC, Hawes RH, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. The American journal of gastroenterology. 1993;88:825–831. [PubMed] [Google Scholar]
- 7.Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. The New England journal of medicine. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 8.Coe SG, Wallace MB. Assessment of adenoma detection rate benchmarks in women versus men. Gastrointestinal endoscopy. 2013;77:631–635. doi: 10.1016/j.gie.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE. Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the new hampshire colonoscopy registry. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1308–1312. doi: 10.1016/j.cgh.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhangu A, Bowley DM, Horner R, Baranowski E, Raman S, Karandikar S. Volume and accreditation, but not specialty, affect quality standards in colonoscopy. The British journal of surgery. 2012;99:1436–1444. doi: 10.1002/bjs.8866. [DOI] [PubMed] [Google Scholar]
- 12.Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460–1467. e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. The American journal of gastroenterology. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 14.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. The New England journal of medicine. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 15.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Annals of internal medicine. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 17.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Annals of internal medicine. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 18.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. The New England journal of medicine. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA : the journal of the American Medical Association. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 20.Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:760–767. doi: 10.1016/j.cgh.2012.12.004. quiz e54–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. The American journal of gastroenterology. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. quiz 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raju GS, Vadyala V, Slack R, Krishna SG, Ross WA, Lynch PM, et al. Adenoma detection in patients undergoing a comprehensive colonoscopy screening. Cancer medicine. 2013;2:391–402. doi: 10.1002/cam4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do A, Weinberg J, Kakkar A, Jacobson BC. Reliability of adenoma detection rate is based on procedural volume. Gastrointestinal endoscopy. 2013;77:376–380. doi: 10.1016/j.gie.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England journal of medicine. 2014;370:1298–1306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltenbach T, McGill SK, Kalidindi V, Friedland S, Soetikno R. Proficiency in the diagnosis of nonpolypoid colorectal neoplasm yields high adenoma detection rates. Digestive diseases and sciences. 2012;57:764–770. doi: 10.1007/s10620-011-1921-6. [DOI] [PubMed] [Google Scholar]
- 26.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointestinal endoscopy. 2011;74:656–665. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, et al. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2013 doi: 10.1136/gutjnl-2013-304578. [DOI] [PubMed] [Google Scholar]
- 28.Gurudu SR, Ramirez FC, Harrison ME, Leighton JA, Crowell MD. Increased adenoma detection rate with system-wide implementation of a split-dose preparation for colonoscopy. Gastrointestinal endoscopy. 2012;76:603–608. e1. doi: 10.1016/j.gie.2012.04.456. [DOI] [PubMed] [Google Scholar]
- 29.Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406–1412. doi: 10.1136/gut.2007.137984. [DOI] [PubMed] [Google Scholar]
- 30.Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointestinal endoscopy. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJW, Blanks RG, Rees CJ, Wright KC, Nickerson C, Moss SM, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy. 2013;45:20–26. doi: 10.1055/s-0032-1325803. [DOI] [PubMed] [Google Scholar]
- 32.Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2013;62:236–241. doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney MS, Cury MS, Neeman N, Ngo LH, Lewis JM, Chuttani R, et al. Effect of Institution-Wide Policy of Colonoscopy Withdrawal Time ≥7 Minutes on Polyp Detection. Gastroenterology. 2008;135:1892–1898. doi: 10.1053/j.gastro.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointestinal endoscopy. 2011;74:128–134. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Coe SG, Crook JE, Diehl NN, Wallace MB. An endoscopic quality improvement program improves detection of colorectal adenomas. The American journal of gastroenterology. 2013;108:219–226. doi: 10.1038/ajg.2012.417. quiz 27. [DOI] [PubMed] [Google Scholar]
- 36.Kahi CJ, Ballard D, Shah AS, Mears R, Johnson CS. Impact of a quarterly report card on colonoscopy quality measures. Gastrointestinal endoscopy. 2013;77:925–931. doi: 10.1016/j.gie.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:1335–1340. doi: 10.1016/j.cgh.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Lu F-I, van Niekerk DW, Owen D, Tha SPL, Turbin DA, Webber DL. Longitudinal Outcome Study of Sessile Serrated Adenomas of the Colorectum: An Increased Risk for Subsequent Right-sided Colorectal Carcinoma. The American Journal of Surgical Pathology. 2010;34:927–34. doi: 10.1097/PAS.0b013e3181e4f256. [DOI] [PubMed] [Google Scholar]
- 39.Eichenseer PJ, Dhanekula R, Jakate S, Mobarhan S, Melson JE. Endoscopic mis-sizing of polyps changes colorectal cancer surveillance recommendations. Diseases of the colon and rectum. 2013;56:315–321. doi: 10.1097/DCR.0b013e31826dd138. [DOI] [PubMed] [Google Scholar]
- 40.Diamond SJ, Enestvedt BK, Jiang Z, Holub JL, Gupta M, Lieberman DA, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointestinal endoscopy. 2011;74:135–140. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenspan M, Rajan KB, Baig A, Beck T, Mobarhan S, Melson J. Advanced Adenoma Detection Rate Is Independent of Nonadvanced Adenoma Detection Rate. The American journal of gastroenterology. 2013;108:1286–1292. doi: 10.1038/ajg.2013.149. [DOI] [PubMed] [Google Scholar]
- 42.Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:1225–1231. doi: 10.1016/j.cgh.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 43.Butterly L, Robinson CM, Anderson JC, Weiss JE, Goodrich M, Onega TL, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. The American journal of gastroenterology. 2014;109:417–426. doi: 10.1038/ajg.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan C, Rex DK, Zullo A, Cooper GS. Loss of efficacy and cost-effectiveness when screening colonoscopy is performed by nongastroenterologists. Cancer. 2012;118:4404–4411. doi: 10.1002/cncr.27664. [DOI] [PubMed] [Google Scholar]
- 45.Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, et al. Incomplete Polyp Resection During Colonoscopy—Results of the Complete Adenoma Resection (CARE) Study. Gastroenterology. 2013;144:74.e1–80.e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]