Abstract
Objective
Functional connectivity (FC) among language regions is decreased in adults with epilepsy compared to controls, but less is known about FC in children with epilepsy. We sought to determine if language FC is reduced in pediatric epilepsy, and examined clinical factors that associate with language FC in this population.
Methods
We assessed FC during an age-adjusted language task in children with left-hemisphere focal epilepsy (n=19) compared to controls (n=19). Time series data were extracted for three left ROIs and their right homologues: inferior frontal gyrus (IFG), middle frontal gyrus (MFG), and Wernicke's area (WA) using SPM8. Associations between FC and factors such as cognitive performance, language dominance, and epilepsy duration were assessed.
Results
Children with epilepsy showed decreased interhemispheric connectivity compared to controls, particularly between core left language regions (IFG, WA) and their right hemisphere homologues, as well as decreased intrahemispheric right frontal FC. Increased intrahemispheric FC between left IFG and left WA was a positive predictor of language skills overall, and naming ability in particular. FC of language areas was not affected by language dominance, as the effects remained when only examining study participants with left language dominance. Overall FC did not differ according to duration of epilepsy or age of onset.
Significance
FC during a language task is reduced in children, similar to findings in adults. In specific, children with left focal epilepsy demonstrated decreased interhemispheric FC in temporal and frontal language connections and decreased intrahemispheric right frontal FC. These differences were present near the onset of epilepsy. Greater FC between left language centers is related to better language ability. Our results highlight that connectivity of language areas has a developmental pattern and is related to cognitive ability.
Keywords: Functional neuroimaging, Seizures, Neuropsychological Assessment
Cognition requires coordinated processing between distributed brain regions, which can be quantified through functional connectivity (FC).1 FC is the correlation of signal changes between brain regions across time during resting state or active task scans. Adults with epilepsy demonstrate reduced FC within the language network despite equivalent hemodynamic response patterns during fMRI language tasks.2–4 Decreased FC in adults is also associated with worse language performance,2,5 indicating a relationship between cognitive ability and connectivity. Less is known about FC of language areas in pediatric epilepsy populations. Numerous pediatric epilepsy studies of FC have been conducted during resting state6,7 but only one study has focused on FC during a cognitive task.8 In a study of working memory, children with frontal lobe epilepsy demonstrated decreases in connectivity compared to controls, and decreased FC within the frontal lobe of patients was associated with cognitive impairment.8 FC during language tasks, however, has not been investigated in children with focal epilepsy, despite the presence of language difficulties in this age group.9
We address a gap in the understanding of language FC in pediatric focal epilepsy by determining if 1) language FC is reduced in children with epilepsy, 2) FC is associated with cognitive skills, and 3) FC is associated with clinical factors, such as language dominance, age of onset, duration of epilepsy, seizure frequency, and number of anti-epileptic medications (AEDs). Based on adult studies, we predicted that children with epilepsy would show reduced FC overall compared to controls. We also hypothesized that increased language FC would correlate with better neuropsychological performance, particularly language. Atypical language patterns are known to occur in epilepsy10 and reduced FC may be influenced by atypical language dominance. One adult study controlled for language dominance by including only controls and patients with left language dominance and did not find reduced left hemisphere functional connectivity in individuals with epilepsy.11 Thus, we hypothesized that group differences in FC would diminish when examining only left-language dominant individuals because FC differences may be biased by epilepsy patients with atypical language dominance. Finally, we hypothesized that patients with indicators of more severe epilepsy (i.e., longer duration, earlier age of onset, higher seizure frequency, more AEDs), would have lower FC.12,13
Methods
Participants
Thirty-eight native English-speaking right-handed children between the ages of six and 12 participated in this study. Nineteen were patients with left-hemisphere focal epilepsy (13 male, 6 female; M age=10.24 years, SD=2.05 years) and 19 were typically developing (TD) controls (10 male and 9 female; M age=10.09 years, SD=1.73 years). Another 33 patients were excluded due to 1) epileptogenic focus other than the left hemisphere, 2) left-handedness, 3) questionable task accuracy, 4) technical problem with raw imaging data, and/or 5) movement. Regarding movement, we excluded subjects with mean or max x, y, or z motion exceeding >3 mm (see below for further detail). Following epilepsy imaging guidelines,14 1.5T structural MRI high resolution epilepsy protocol was normal in 18 patients; one patient had mesial temporal sclerosis (MTS). All patients had a left hemispheric seizure focus based on interictal or ictal EEG, and clinical characteristics (see Table 1). Precise seizure focus localization was determined for 15 of 19 patients using ictal EEG (n=6), interictal EEG (n=8), and clinical seizure characteristics alone (n=1). Four additional patients had seizures lateralized to the left hemisphere, with undetermined location of seizure focus (interictal EEG: n=2; clinical seizure characteristics alone: n=2). Seizure frequency varied from no seizures in the past year to daily seizures. The patients were on various AEDs, with seven patients on more than one medication. TD controls were drawn from a larger sample15 and selected to match the epilepsy sample based on age (±11 months). When several TD controls had similar ages to a patient, intellectual functioning was then used to match the subjects. TD controls with atypical language patterns were excluded from the analyses. All TD controls had normal neurologic examinations and normal structural T1 3T MRI. Handedness was measured by child-friendly items from the Edinburgh Handedness Inventory (EHI).16
Table 1. Seizure Characteristics, Neuropsychological Assessment, and FMRI Task Performance.
Mean and standard deviations of FMRI Task Performance, WASI, CELF-IV-CL, and EOWPVT scores for Patients and TD Controls.
| Group | ||
|---|---|---|
|
| ||
| Seizure Characteristics | Patients | |
| Localization (left focus) | 6 temporal, 4 frontal, 1 fronto-temporal, 1 parietal, 2 temporal-parietal, 1 occipital | |
|
| ||
| Seizure Duration (mean) | 3.4 years (±3.2; range=0.3-10.4 years) | |
|
| ||
| Age of Onset | 6.9 years (±2.6; range=1.0-11.0 years) | |
|
| ||
| Seizure Frequency* | Daily: n=5, weekly: n=2, monthly: n=5, none in past 6 months: n=2, none in past year: n=4 | |
|
| ||
| AEDs* | Carbamazepine: n=3, clonazepam: n=1, diazepam: n=2, lacosamide: n=1, lamotrigine: n=3, levetiracitam: n=3; lorazepam: n=1, oxcarbazepine: n=9, phenobarbital: n=1, phenytoin: n=1, topiramate: n=1, valproate: n=3, zonisimide: n=2 | |
|
| ||
| Measure | Patients | Controls |
|
| ||
| Intellectual Assessment | ||
|
| ||
| FSIQ | 106 (±19) (n=18) | 110 (±11) |
| VIQ | 107 (±19) (n=18) | 111 (±14) |
| PIQ | 103 (±16) (n=18) | 106 (±11) |
|
| ||
| Language Measures | ||
|
| ||
| CELF-IV-CL | 103 (±14) (n=17) | 108 (±11) |
| EOWPVT | 107 (±16) (n=18) | 112 (±16) |
|
| ||
| fMRI Task Performance | ||
|
| ||
| Overall Accuracy | 71% (±25%) (n=17) | 80% (±16) (n=18) |
Seizure frequency and AED information was not available for 1 patient.
Controls had no history of developmental, learning, neurological, or psychiatric disorders and IQ>70. Epilepsy patients also had IQ>70 and no significant developmental, psychiatric, or additional neurological history (beyond epilepsy) with the exception of learning disorders or attention disorders (e.g., ADHD). Three patients were diagnosed with ADHD and one was diagnosed with a learning disorder and ADHD. Five additional patients had learning difficulties without a formal diagnosis, as indicated by special education services or grade retention. All participants were recruited from the community through flyers, a widely distributed hospital newsletter, and a pre-recorded “on-hold” message for the hospital.
The study was approved by Children's National Medical Center Institutional Review Board, with informed consent provided by the parents, and assent was provided by all children prior to any study procedure.
Neuropsychological testing and Task Performance
Neuropsychological evaluation of intellectual and language functioning was performed in a separate session. Data were unavailable for one patient (see Table 1). Intellectual functioning was assessed by the Wechsler Abbreviated Scale of Intelligence (WASI).17 Language functioning was assessed with the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-IV)18 and the Expressive One-Word Picture Vocabulary Test (EOWPVT).19 Dependent variables included the CELF-IV Core Language (CL) score, which measures expressive and receptive language, and the EOWPVT total score, which measures naming vocabulary. Task performance was evaluated by the overall accuracy for the task (true positives divided by the sum of the number of correct answers possible and the number of commissions, multiplied by 100).
MRI
Details of acquisition methods and experimental paradigm were previously described,15 but are summarized here. Whole-brain functional MRI was collected on a 3.0 Tesla Siemens Magnetom Trio equipped with a standard CP head coil. Images were collected parallel to the anterior commissure–posterior commissure plane.20 We used a block design composed of five epoch cycles; each cycle consisted of an experimental condition that alternated with a control condition and each hemicycle lasted 30 seconds. Total scan time was five minutes. Individual stimuli were presented every three seconds for a total of 10 per block. Tasks were presented via E-prime software version 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA). Visual stimuli were presented through a rear projection screen; auditory stimuli were digitized and presented via pneumatic earphones. Responses were performed via fiber-optic push button response recorded by PC in E-prime.
Experimental Paradigm
Auditory description decision task
For the experimental condition, the participant hears an auditory clue (5-6 word sentences) that describes and names an object (e.g., “A long yellow fruit is a banana”) and pushes a button when the correct object is described. Seventy percent of items are correct targets and 30% are foils. The task is designed to provide in-scanner monitoring of performance by requiring a semantic decision identified by button press response. Task performance was assessed by accuracy data (i.e., number of correct button responses). The control condition consists of reverse speech with tone identification where the participant pushes a button when the tone follows reverse speech (70% have tones, 30% are foils). The task was adjusted by age-level with different versions of the tasks developed based on word frequency normative data derived from children's reading materials21. The auditory description decision task is known to activate frontal and temporal language areas.15
Image Preprocessing and FC Preprocessing
Preprocessing and group analyses were performed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK) using MATLAB (Version 8.1 Mathworks, Inc., Sherborn, MA). Each subject's anatomical scan was coregistered to the SPM8 white matter template and then segmented. The mean EPI image was then coregistered to the segmented gray matter and the transformation was applied to all of the EPI images. Functional images were spatially normalized to the MNI standard anatomical space, spatially smoothed using an 8mm full width at half maximum Gaussian kernel, and temporally filtered (high-pass filter: 128s). For the group analyses, individual t-maps were generated with movement parameters as covariates of no interest.
Given the potential sensitivity of FC analyses to motion,22 participants were excluded for excessive movement resulting in >3 mm for both mean or max movement (Controls: mean x=0.038 ± 0.34, mean y=0.068 ± 0.057, mean z=0.12 ± 0.043, max x=0.19 ± 0.21, max y=0.30 ± 0.18, max z=0.68 ± 0.59; Patients: mean x=0.047 ± 0.040, mean y= 0.083 ± 0.085, mean z=0.17 ± 0.14, max x=0.34 ± 0.69, max y=0.32 ± 0.23, max z=0.92 ± 0.67). Movement parameters were not different between groups (epilepsy vs. TD controls) (p's>0.05). The three left regions of interest (ROIs) were defined by significant clusters of functional activation from whole brain activation (thresholded at FWE, p=0.05) for a larger group map of pediatric TD controls within the anatomical boundaries of Wernicke's Area (WA), inferior frontal gyrus (IFG; Broca's area), and middle frontal gyrus (MFG), similar to masks used in a previous study.15 We entered the center coordinates for each of the three left ROIs into an fMRI database (http://beta.neurosynth.org) to confirm that >18 studies of language also found activation within approximately six mm of our center coordinates for each ROI. This demonstrated that our ROIs were not unduly influenced by our sample and confirmed that these are common areas for language activation. Right homologues were defined as mirror images of left ROIs. Thus, six ROIs were used. Activation time series data were extracted for each subject from each ROI with MarsBar.23 Individual linear correlation coefficients were computed between pairs of time course signals extracted from the ROIs, partialling out the following parameters to control for motion and physiological noise: framewise displacement (FD), regressor of volume index that exceeds 0.5mm FD,24 and signal from ventricle and white matter regions of interest.
Determination of Language Dominance
To determine language dominance, we calculated a lateralization index (LI) for each subject's IFG and WA ROIs and categorized language based on a commonly-used cut-off value of 0.20.25 Using the LI Toolbox bootstrap method (LI-Toolbox for SPM8),26 ROIs were individually categorized as left-lateralized if LI ≥ 0.20, bilateral if LI <|0.20|, or right if LI ≤ -0.20.20 The participants categorized as left language dominant were considered to have typical language dominance, and those with right, bilateral, or crossed patterns were considered to be atypical (see Table 2). Crossed dominance patterns occurred when participants had discrepant LIs between the two ROIs (e.g., left IFG, right WA), but participants with bilateral activation in one region and unilateral activation in the other were considered dominant on the side of unilateral region activation.15
Table 2. Language Dominance Patterns and Overall FC by Language Pattern.
Patterns of language dominance: typical (left) and atypical (right, bilateral, crossed) for Patients and TD Controls.
| Rates | ||
|---|---|---|
|
|
||
| Language Dominance | Patients (n=19) | Controls (n=19) |
| Typical | ||
| Left | 15 | 19 |
|
| ||
| Atypical | ||
| Right | 2 | 0 |
| Crossed | 2 | 0 |
| Bilateral | 0 | 0 |
| TOTAL | 4 | 0 |
Data analyses
The primary analysis examined FC differences between patients and controls (across inter- and intrahemispheric connections of IFG, MFG and WA; see Figure 1a for all paired ROI combinations). Time course correlation coefficients were transformed to Fisher's z-scores and submitted into a mixed model ANOVA with group (TD controls vs. epilepsy) as a between-group factor and connection as a within-subject factor (inter- and intrahemispheric connections of IFG, MFG and WA). To ensure that connectivity results were not a matter of activation differences, we also compared activation between the two groups (TD controls vs. epilepsy) at a threshold of p=0.05, FWE.
Figure 1. FC between paired language regions for each group (Patients vs. TD Controls).
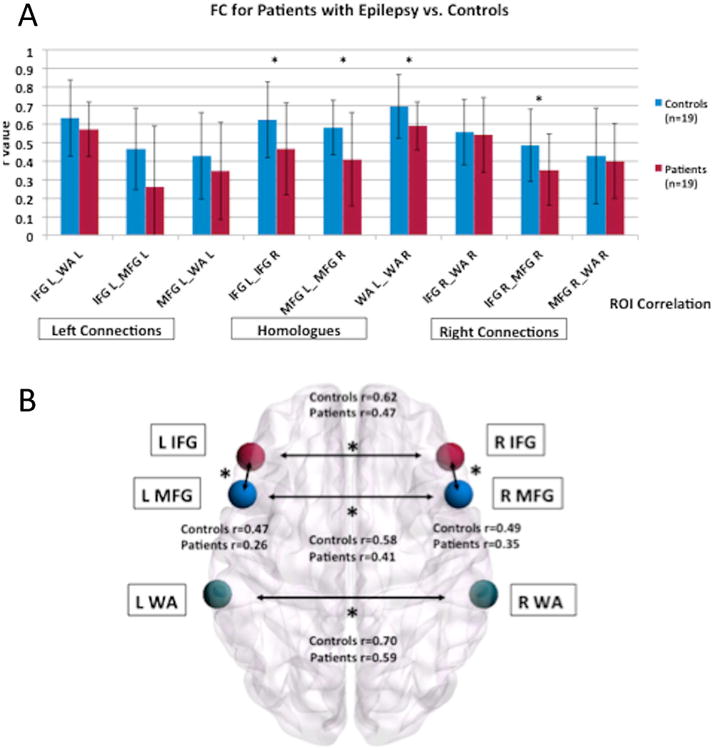
a) Graph of the FC values for each paired language region for each group. b) Visual depiction of the ROIS and the significantly different connections between Patients and TD Controls. The brain networks were visualized with BrainNet Viewer (http://nitrc.org/projects/bnv/).41 * indicates language connections with a significant difference between groups.
We performed additional analyses to examine the effects of neuropsychological performance, epilepsy characteristics (age of onset, epilepsy duration, seizure frequency, number of AEDs), and language dominance on language connectivity. Group differences (epilepsy and TD controls) in neuropsychological performance were assessed by one-way ANOVA. To examine the relationship between language FC and neuropsychological performance, hierarchical multiple linear regression models were used to predict naming vocabulary (EOWPVT scores) and overall language ability (CELF-IV-CL scores) from FC for IFG and WA connections. Regarding predictor variables, for both models the main intrahemispheric language connection (left IFG and left WA) implicated in adult FC studies was entered into the model first, followed by the two interhemispheric connections (left and right IFG, left and right WA).
The relationship between 1) seizure duration and 2) age of onset and FC for ROI pairs was examined using Pearson r correlation. Regarding seizure frequency, we divided the pediatric epilepsy group into a group with more frequent seizures (total: n=7 (daily: n=5, weekly: n=2)) and those with fewer seizures (total: n=11(monthly: n=5; none in past six months: n=2; none in past year: n=4)). For AEDs, we divided the epilepsy group into a group taking one AED or less (n=11) and those taking more than one AED (n=7). For the seizure frequency and AED analyses, we used a mixed model ANOVA with group (more vs. less frequent or 0-1 AED vs. >1 AED) as a between-group factor and connection as a within-subject factor (inter- and intrahemispheric connections of IFG, MFG and WA). We examined a cumulative total of four tests of clinical variables (age of onset, seizure duration, seizure frequency, and number of AEDs); therefore, all p-values were adjusted for multiple comparisons using the Bonferroni procedure (p=0.013 for these analyses).
To investigate the effect of language dominance, we ran the mixed model ANOVA with group (TD controls vs. epilepsy) as a between-group factor and connection as a within-subject factor for only the left dominant language participants.
Results
FMRI Activation during the Language Task
The activation maps during the auditory description decision task revealed activation of a fronto-temporal network (p=0.05, FWE; k>20 voxels). We did not find any significant differences between patients and controls used in this study. Task performance (overall accuracy) was not different between groups (see Table 1).
Functional Connectivity: Group Differences in Paired ROI Time Course Correlations
A main effect of group (F(1,36)=6.80, p=0.01, Cohen's d=0.47) demonstrated that FC was stronger in TD controls than in patients (Figure 1A). A main effect of ROI (F(8,36)=10.31, p<0.001) demonstrated that FC strength differed between language regions, with the strongest connection between left and right WA (See Figure 1). There was no two-way (group × connection) interaction, suggesting that both groups show a similar FC profile among core language regions. Exploratory analyses revealed that patients had lower interhemispheric connections of core homologous temporal, inferior frontal, and middle frontal language regions compared to controls: left and right WA (p=0.02), left and right IFG (p=0.04), and left and right MFG (p=0.02) (Figure 1B and 2). Patients also had a lower left and right intrahemispheric connection than controls for IFG and MFG (left IFG and MFG: p=0.04; right IFG and MFG: p=0.03).
Figure 2. Color-coded matrices representing FC for the 9 main language connections.
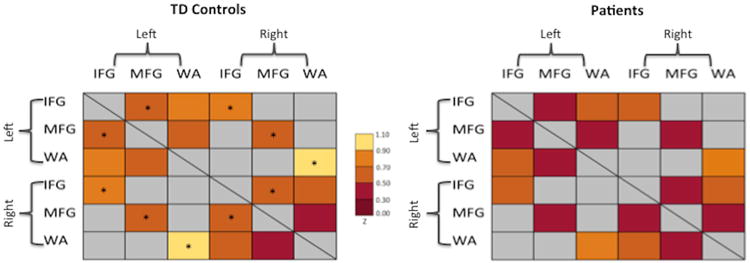
Matrices for TD Controls are displayed on the left panel and Patients with epilepsy on the right. The colors range from dark red (z=0.0-0.30) to yellow (z=0.90-1.10). * indicates language connections with a significant difference between groups. Connections that were not examined in the current study are identified by a gray color in the matrices.
Relationship Between FC and Neuropsychological Performance
The mean score for all neuropsychological measures was within the average to high average range for all participants (see Table 1). Intellectual functioning (FSIQ, VIQ, PIQ) and language skills (CELF-IV, EOWPVT) were not different between groups likely due to matching and inclusion criteria. Hierarchical linear regression analyses revealed that the left intrahemispheric connection (left WA and left IFG) was a significant positive predictor of naming vocabulary (EOWPVT scores), accounting for 12% of the variance, F(1,35)=4.97, p=0.03 (Table 3; Figure 3A). When the two interhemispheric connections (left and right IFG, left and right WA) were added (step 2), the model approached significance, F(3,33)=2.53, p=0.07, and accounted for an additional 6% of the variance. All models presented passed tests for multicollinearity.
Table 3. Hierarchical Regression Analyses Predicting Language Ability.
Results of the hierarchical multiple linear regression models used to predict naming vocabulary (EOWPVT scores) and overall language ability (CELF-IV-CL scores) from FC for IFG and WA connections (IFG L-WA L, IFG L-IFG R, WA L-WA R).
| EOWPVT | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Predictor | b | SE B | β | t | ΔF | ΔR2 | |
| Step 1 | 4.97* | 0.12 | |||||
|
| |||||||
| IFG L-WA L | 21.76 | 9.76 | 0.35 | 2.23* | |||
|
| |||||||
| Step 2 | 1.28 | 0.06 | |||||
|
| |||||||
| IFG L-WA L | 15.60 | 10.79 | 0.25 | 1.45 | |||
| IFG L-IFG R | 10.55 | 8.54 | 0.23 | 1.24 | |||
| WA L-WA R | 4.09 | 9.49 | 0.07 | 0.43 | |||
|
| |||||||
| * p <.05 | |||||||
|
| |||||||
| CELF-IV-CL | |||||||
|
| |||||||
| Predictor | b | SE B | β | t | ΔF | ΔR2 | |
|
| |||||||
| Step 1 | 4.83* | 0.12 | |||||
|
| |||||||
| IFG L-WA L | 16.49 | 7.51 | 0.35 | 2.20* | |||
|
| |||||||
| Step 2 | 0.41 | 0.02 | |||||
|
| |||||||
| IFG L-WA L | 13.36 | 8,51 | 0.29 | 1.57 | |||
| IFG L-IFG R | 5.76 | 6.77 | 0.17 | 0.85 | |||
| WA L-WA R | -4.51 | 7.57 | -0.11 | -0.60 | |||
p < .05
Figure 3. Correlations between FC and neuropsychological variables.
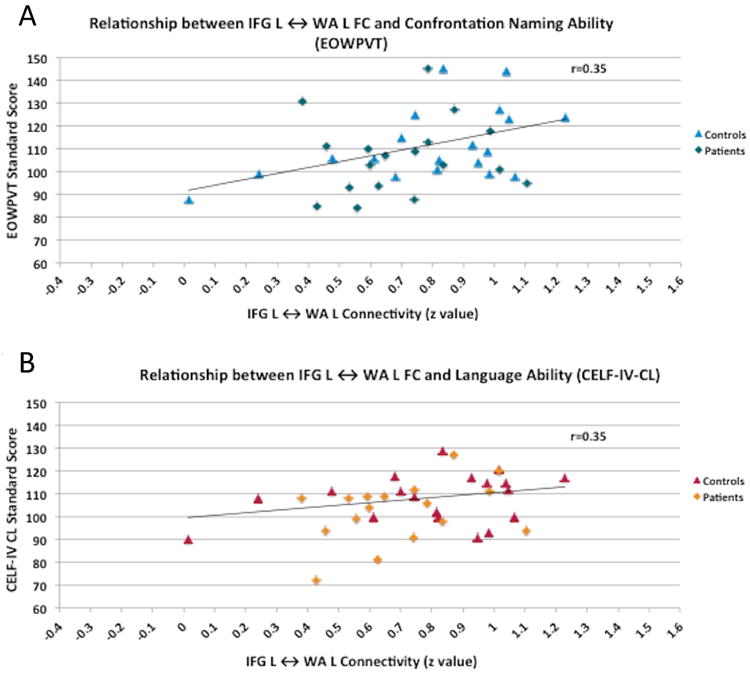
Correlation between FC for IFG L ↔ WA L and naming vocabulary (EOWPVT) and overall language ability (CELF-IV-CL).
The left intrahemispheric connection (left WA and left IFG) was also a significant positive predictor of overall language ability (CELF-IV-CL) and accounted for 12% of the variance, F(1,34)=4.83, p=0.04 (Table 3; Figure 3B). However, the model was not significant when the two interhemispheric connections (left and right IFG, left and right WA) were added (step 2) suggesting that left intrahemispheric connection alone was a strong predictor.
Effect of Epilepsy Characteristics on FC of Language Regions
We also examined the relationship between language FC and 1) age of onset, 2) seizure duration, 3) seizure frequency, and 4) number of AEDs. Neither age of onset nor seizure duration was correlated with FC of the language regions. One connection, right MFG to right WA, demonstrated a negative correlation with age of onset (r=-0.47, p=0.05), but this did not survive Bonferroni correction. Using mixed model ANOVA, we also did not find language FC differences based on seizure frequency (more vs. less frequent) or numbers of AEDs (0-1 AED vs. >1 AEDs).
Effect of Language Dominance on FC of Language Regions
The majority of study participants had typical language dominance (left WA, left IFG), as expected, but four (21%) patients with epilepsy demonstrated atypical language dominance, including right-lateralized (right WA, right IFG) and crossed patterns (left IFG, right WA or right IFG, left WA) (see Table 2). For the left language dominant participants, TD controls demonstrated stronger connectivity than patients (F(1,32)=5.17, p=0.03, Cohen's d=0.46), similar to the results from the overall analysis (with atypical and typical language patterns included).
Discussion
In the present study, children with left focal epilepsy, and predominantly normal MRIs, demonstrated reduced FC of language areas compared to TD controls. The pediatric epilepsy group showed a decrease specifically in the homologues of IFG and WA and the right intrahemispheric IFG-MFG connection. Gross pathology, movement, and IQ did not differ between groups, removing these characteristics as potential contributing factors. The FC of language areas was related to language ability, such that increased connectivity between left language areas (IFG and WA) correlated with better performance on language measures. Contrary to our hypothesis, FC did not differ according to the epilepsy characteristics we examined.
We found reduced interhemispheric frontal and temporal connectivity (left and right WA; left and right IFG) in children with epilepsy. Reduced synchrony between frontal language regions (left and right IFG) has also been identified in studies of adults with epilepsy.2 In contrast to adult epilepsy studies,3,4 however, we did not find differences in left intrahemispheric FC. Different stages of language development for children compared to adults may account for these discrepant findings. Evidence suggests that maturation of language connectivity is protracted, and there is a shift from strong interhemispheric connectivity during infancy/childhood to strong intrahemispheric connectivity in adulthood.27,28 Two-day-old infants show strong FC between hemispheres during an auditory language task.29 Typically developing children (ages 5-7) demonstrate strong functional interhemispheric connectivity, particularly in the superior temporal regions, while adults display strong intrahemispheric connectivity (between left frontal and temporal language areas) during a language task.28 Overall, the evidence suggests that intrahemispheric, fronto-temporal functional connections are strengthened later in life,28–30 when language skills are more advanced.31 The protracted development of FC parallels structural studies using diffusion tensor imaging where fronto-temporal connections mature slower than other language region connections.29,32–34
Similar to these findings, in the current study of children (ages 6-12) the interhemispheric, homologous language connections were strong in both the TD control and epilepsy groups, despite reduced FC in these connections for children with epilepsy compared to controls. The strongest connectivity was between left and right WA, particularly for the control group. The previously mentioned body of research, in combination with our findings, identifies interhemispheric connectivity as an important stage in the typical development of language. We postulate that greater interhemispheric FC in children may be an index of available plasticity for the establishment of language dominance, and this plasticity diminishes through childhood.29,32–34 In atypical development, such as preterm children, reduced interhemispheric structural connectivity of the temporal lobe predicts language impairment.35 Thus, our hypothesis is that reduced interhemispheric connectivity at an early age may represent reduced capacity for the brain to compensate for neural disruption to the language networks. Epilepsy—or any source of atypical development, such as factors related to preterm birth—may disrupt or alter the FC developmental process for some children, which then may be a predictor of outcome.
We expand previous work by finding that children who had stronger left intrahemispheric language FC had better language ability. Increased left WA and left IFG FC was a positive predictor of naming vocabulary in particular. Adult studies have also shown that stronger cognitive ability is related to increased intrahemispheric FC, such that adults with left focus epilepsy exhibited a positive correlation between verbal IQ and left intrahemispheric FC.2 These results suggest that the shift from inter- to intrahemispheric connections across development may facilitate, or be a marker of, increased cognitive efficiency and language ability. Epilepsy may disrupt and/or delay the ongoing functional specialization of network connections that underlie the development of cognitive skills, such as language. As noted above, adult epilepsy studies found a group difference (controls > patients) in left intrahemispheric connectivity2–4 and we did not for our pediatric sample. A possible explanation for these discrepant findings may relate to cognitive ability, since our pediatric groups (TD controls and epilepsy) had comparable language skills.
Overall differences in connectivity in children with epilepsy did not depend on duration of epilepsy or age of onset. Previous FC and electrocorticographical studies of both childhood and adult onset epilepsy suggest that a prolonged duration of epilepsy is related to decreased network synchrony in adulthood.12,13 However, our findings are supported by other studies that show that seizure activity does not necessarily account for differences in cognitive ability; for example, lower cognitive skills are evident at onset of epilepsy.36 Our study differs from prior results in several important ways. First, we studied children, whereas others studied adults. Second, our patient population had a shorter duration and range of epilepsy (0.3-10.4 years) than the adult series (0–50 years),12 which may suggest a timeframe for when ongoing epilepsy interferes with FC. This has important implications for timing of interventions because our findings and others8 suggest that decreased FC is associated with lower cognitive skills.
A higher incidence of atypical language patterns is known to occur in patients with epilepsy, up to 30% compared to 5% in TD populations.10 The majority of adult studies of language FC in epilepsy include patients with epilepsy that have typical and atypical language representation. Based on one adult study of controls and patients with left language dominance,11 we hypothesized that differences in FC between controls and patients would relate to these known differences in language dominance. Our findings did not support the hypothesis that language dominance influences changes in FC between language areas overall. However, definitive conclusions regarding the relationship of language dominance and connectivity are limited because the sample size of patients with atypical language representation was small (n=4).
Limitations
In this study we used large ROIs, which has the advantage of capturing variability in patient language localization,10,37 but the disadvantage of averaging time courses across large numbers of voxels. Another disadvantage may be that large ROIs averages variability from within smaller functional ROIs (e.g., BA 44, 45, 47) and may have limited our ability to identify relationships between performance and more localized patterns of synchrony. Whole-brain, voxel-wise analyses may allow for further characterization of FC and the relationship between network synchrony and cognitive performance. The use of whole-brain, rather than ROI, analyses may provide additional insights by examining the effect, or association, of epilepsy on other cognitive networks and their interplay with language systems.38 The differences between our pediatric study and the adult studies may be related to methodological differences, such as larger and/or different ROIs for our FC analysis. It is also possible that these differences were due to differences in the experimental tasks: for example tasks that produce frontal versus those that yield temporal and frontal language area activation. Future investigations should also look at intrinsic connectivity via resting-state study designs.39
The laterality and location of the seizure focus was based on available clinical and elctrophysiological data. The assurance of clinical features and interictal and ictal EEG in lateralizing and, more importantly, localizing the seizure focus are well known and are a limitation to this study. We recognize the migratory nature of epileptiform discharges in some patients who have normal MRI; thus a limitation of this study is the cross sectional design which prevents serial EEG to provide additional confirmation of the focus. While we addressed several possible influences on FC including IQ, task performance, MRI abnormalities, age of onset, duration, seizure frequency, and number of AEDs there remain limitations to these analyses and other factors could be examined, such as type of AED. Assessing the impact of such are challenging particularly within modest sample sizes given the heterogeneity of response, even when children are taking the same dose of the same AED. Further, one would expect that potential medication effects would be global rather than affecting subsets of the distributed language network, as found in the current study. Similarly, the majority of epilepsy patients in our sample had normal structural MRIs; however, microscopic anatomical abnormalities cannot be excluded. Furthermore, our sample reflects general epilepsy populations where 30-50% have comorbid learning problems40 and these patients were not excluded; however, by matching on IQ when possible, our patients did not differ on the neuropsychological measures we used in this study. Future studies with larger samples are needed to examine the relationship between these comorbidities and language processing, as well as other cognitive systems. Future studies with larger samples will also be able to examine fully the effect of atypical language dominance on connectivity. As atypical language dominance is a minority pattern, large samples are required to accumulate a large enough sample for statistical analysis. Language dominance might be expected to affect intrahemispheric connections but not connections between homologous regions.
Conclusions
The results of the current study contribute to our understanding of the functional connectivity of language regions during development through two important findings. First, interhemispheric FC is decreased in children with left lateralized focal epilepsy compared to controls, particularly in the primary frontal and temporal language connections, and these differences are present near the onset of epilepsy. Second, our findings extend the theory that connectivity has a developmental pattern and is related to cognitive ability. Greater intrahemispheric FC between left language centers is related to better language ability in general, and naming vocabulary in particular.
We confirm that we have read Epilepsia's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
This work was supported by The National Institutes of Neurological Disorders and Stroke, National Institutes of Health [R01 NS44280 to W.D.G., M01RR020359 K23NS065121-01A2 to M.B., 5K23MH086111 to B.E.Y.], the National Center for Research Resources, the Intellectual and Developmental Disabilities Research Center, and Children's National Medical Center Grant [HD040677-07]. Finally, the authors would like to acknowledge the valuable contributions of Virginia Terwilliger and the participation of patients and typically developing children.
Footnotes
Disclosure of Conflicts of Interest: Dr. Sepeta has received research support from Epilepsy Foundation of America (EFA). Dr. Yerys received funding from Frederick and Elizabeth Singer Foundation (PI: Gaillard and Kenworthy) and is funded by NIMH grants 5K23MH086111 and 1R21MH092615, as well as Institutional Discretionary Funds - The Children's Hospital of Philadelphia. Dr. Vaidya is funded by NIMH grant MH084961. Dr. Gaillard's income derives from clinical revenue generated for CNMC clinical care. Federal support provided by NINDS 1P30HD40677-01, 2K12NS052159-06A1 NIMH RO1 MH084961, 1R21MH092615, NSF 095998, CDC 1UO1DP003255, DOD/USAMRAA W81XWH-11-2-0198, and PICORE 527. Foundation support from Epilepsy Foundation of America, American Epilepsy Society, Infantile Epilepsy Research Foundation (Lundbeck), and CURE. The department conducts industry supported trials form which no salary support is derived includes: Ovation Pharmaceuticals, King Pharmaceuticals, PRA International/Eisai. Stock (held with spouse): Johnson and Johnson; Lily, Glaxo-Smith-Kline, Pfizer, Siemens and General Electric. Editorial board of Epilepsia and Epilepsy Research. Dr. Berl is funded by NINDS grant K23NS065121-01A2. The remaining authors have no conflicts of interest.
References
- 1.Friston KJ, Frith CD, Liddle PF, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 2.Pravatà E, Sestieri C, Mantini D, et al. Functional Connectivity MR Imaging of the Language Network in Patients with Drug-Resistant Epilepsy. AJNR Am J Neuroradiol. 2011;32:532–40. doi: 10.3174/ajnr.A2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlooswijk MCG, Jansen JFA, Majoie HJM, et al. Functional connectivity and language impairment in cryptogenic localization-related epilepsy. Neurology. 2010;75:395–402. doi: 10.1212/WNL.0b013e3181ebdd3e. [DOI] [PubMed] [Google Scholar]
- 4.Waites AB, Briellmann RS, Saling MM, et al. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 5.Friston KJ, Frith CD, Liddle PF, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 6.Besseling RMH, Jansen JFA, Overvliet GM, et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. NeuroImage: Clinical. 2013;2:239–46. doi: 10.1016/j.nicl.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MH, Smyser CD, Shimony JS. Resting-State fMRI: A Review of Methods and Clinical Applications. [cited 2014 Jan 21];AJNR Am J Neuroradiol. 2012 doi: 10.3174/ajnr.A3263. Internet. Available from: http://www.ajnr.org/content/early/2012/08/30/ajnr.A3263. [DOI] [PMC free article] [PubMed]
- 8.Braakman HMH, Vaessen MJ, Jansen JFA, et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia. 2012 doi: 10.1111/epi.12044. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson GM. High incidence of language disorder in children with focal epilepsies. Developmental Medicine & Child Neurology. 2002;44:533–7. doi: 10.1017/s0012162201002511. [DOI] [PubMed] [Google Scholar]
- 10.Mbwana J, Berl MM, Ritzl EK, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132:347–56. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protzner AB, McAndrews MP. Network alterations supporting word retrieval in patients with medial temporal lobe epilepsy. J Cogn Neurosci. 2011;23:2605–19. doi: 10.1162/jocn.2010.21599. [DOI] [PubMed] [Google Scholar]
- 12.Van Dellen E, Douw L, Baayen JC, et al. Long-term effects of temporal lobe epilepsy on local neural networks: a graph theoretical analysis of corticography recordings. PLoS ONE. 2009;4:e8081. doi: 10.1371/journal.pone.0008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Li Q, Lai Y, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32:438–49. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard WD, Chiron C, Helen Cross J, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50:2147–53. doi: 10.1111/j.1528-1167.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- 15.Berl MM, Mayo J, Parks EN, et al. Regional Differences in the Developmental Trajectory of Lateralization of the Language Network. Human Brain Mapping. 2012 doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corportation; 1999. [Google Scholar]
- 18.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals, Fourth Edition. Toronto, Canada: The Psychological Corportation/A Harcourt Assessment company; 2003. [Google Scholar]
- 19.Gardner MF. Expressive One-Word Picture Vocabulary test. Norato, CA: Academic Therapy Publications; 1990. [Google Scholar]
- 20.Gaillard WD, Berl MM, Moore EN, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–71. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- 21.Carroll JB, Davies P, Richman B. The American Heritage word frequency book. Houghton Mifflin Boston; 1971. [cited 2013 Mar 22]. Internet. Available from: http://www.getcited.org/pub/101366297. [Google Scholar]
- 22.Müller RA, Shih P, Keehn B, et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brett M, Anton JL, Valabregue R, et al. Region of interest analysis using an SPM toolbox. Sendai, Japan: 2002. abstract. [Google Scholar]
- 24.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seghier ML. Laterality index in functional MRI: methodological issues. Magnetic resonance imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–30. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Bitan T, Lifshitz A, Breznitz Z, et al. Bidirectional Connectivity between Hemispheres Occurs at Multiple Levels in Language Processing But Depends on Sex. The Journal of Neuroscience. 2010;30:11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS ONE. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perani D, Saccuman MC, Scifo P, et al. Neural language networks at birth. [cited 2013 Feb 6];PNAS. 2011 doi: 10.1073/pnas.1102991108. Internet. Available from: http://www.pnas.org/content/early/2011/08/31/1102991108. [DOI] [PMC free article] [PubMed]
- 30.Bitan T, Burman DD, Lu D, et al. Weaker top-down modulation from the left inferior frontal gyrus in children. NeuroImage. 2006;33:991–8. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nippold MA, Hesketh LJ, Duthie JK, et al. Conversational Versus Expository Discourse: A Study of Syntactic Development in Children, Adolescents, and Adults. J Speech Lang Hear Res. 2005;48:1048–64. doi: 10.1044/1092-4388(2005/073). [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Evans A, Hermoye L, et al. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage. 2007;38:239–47. doi: 10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubois J, Dehaene-Lambertz G, Perrin M, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 35.Northam GB, Liégeois F, Tournier JD, et al. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain. 2012;135:3781–98. doi: 10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermann BP, Seidenberg M, Bell B. Do seizures damage the brain. Elsevier; 2002. [cited 2011 May 9]. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects; pp. 429–38. Internet. Available from: http://www.sciencedirect.com/science/article/B7CV6-4B2Y99Y-3Y/2/ce5dbb7c54c7d1ecd180d1afd25cf20a. [DOI] [PubMed] [Google Scholar]
- 37.Thivard L, Hombrouck J, du Montcel ST, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. NeuroImage. 2005;24:841–51. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Bettus G, Guedj E, Joyeux F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–91. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaidya CJ, Gordon EM. Phenotypic Variability in Resting-State Functional Connectivity: Current Status. Brain Connectivity. 2013;3:99–120. doi: 10.1089/brain.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fastenau PS, Shen J, Dunn DW, et al. Academic Underachievement Among Children With Epilepsy: Proportion Exceeding Psychometric Criteria for Learning Disability and Associated Risk Factors. J Learn Disabil. 2008;41:195–207. doi: 10.1177/0022219408317548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia M, Wang J, He Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]


