Summary
Objective
Using a hypothesis driven approach, subcortical and cortical regions implicated in anxiety disorders in the general population were examined in children with recent-onset epilepsy with versus without anxiety compared to controls. This study reports frequency of anxiety disorders while examining familial, clinical and demographic variables associated with anxiety in children with epilepsy.
Method
Participants included 88 children with epilepsy aged 8–18 years; 25 with a current anxiety disorder and 63 children with epilepsy no current anxiety disorder. 49 controls without anxiety disorders were included. T1 volumetric MRI scans were collected; subcortical volumes and cortical thickness were computed using the FreeSurfer image analysis suite. Analyses focused on adjusted measures of subcortical volumes and cortical thickness.
Results
Relative to controls, larger left amygdala volumes were found in the Epilepsy Anxiety group compared to the Epilepsy No Anxiety group (p = 0.027). In the hippocampus there were no significant differences between groups. Examination of cortical thickness demonstrated that the Epilepsy Anxiety group showed thinning in left medial orbitofrontal (p=0.001), right lateral orbitofrontal (p=0.017), and right frontal pole (p=0.009). There were no differences between groups in age, sex, IQ, age of onset, medications, or duration of epilepsy. There were more family members with a history of anxiety disorders in the Epilepsy Anxiety group compared to the Epilepsy No Anxiety group (p=0.005).
Significance
Anxiety is a common psychiatric comorbidity in children with recent-onset epilepsy with volumetric enlargement of the amygdala and thinner cortex in orbital and other regions of prefrontal cortex, suggesting structural abnormalities in brain regions that are part of the dysfunctional networks reported in individuals with anxiety disorders in the general population. These findings are evident early in the course of epilepsy, are not related to chronicity of seizures, and may be linked to a family history of anxiety and depressive disorders.
Keywords: Pediatric Epilepsy, Anxiety Disorders, Amygdala
Introduction
While anxiety disorders are known to be common in children with epilepsy,1–5 there is little understanding of the distribution of the specific types of anxiety disorders or their neurobiological substrate.
Additionally, the contributions of familial factors and clinical epilepsy characteristics that may impact the expression of anxiety have not been closely examined. It is important to better understand the nature of risk factors associated with anxiety disorders in childhood epilepsy particularly since general population studies have demonstrated that anxiety disorders appear first in childhood and adolescence and persist over time. In fact, 75% of adults with anxiety disorders report having had anxiety disorders in youth.6 Notably in epilepsy, adults have high rates of anxiety, depression, and suicide, indicating the potential for long-term impact of anxiety initially presenting in childhood.7–9
A spectrum of disorders is subsumed under the diagnosis of anxiety disorders (e.g., generalized anxiety, social phobia, separation anxiety, and specific phobia). In the general population, it is increasingly understood that anxiety disorders occur at certain points in childhood. For example, specific phobias appear first at age 6, social phobia at age 8, and panic disorder often not until late adolescence or early adulthood.10 In terms of frequency in adolescence, the anxiety disorder with the highest lifetime prevalence rate is specific phobia (19%) with social phobia (9%) and separation anxiety (8%) coming in at a close second and third.10 Although anxiety disorders in childhood epilepsy are common, ranging from 13% to 48% across several studies,1–5 there has been very little characterization of the distribution of specific anxiety disorders represented in children with epilepsy, especially using contemporary diagnostic procedures. It is important to identify anxiety disorders that are common in childhood epilepsy in order to specify best practices for screening and treatment options. The first aim of this study is to characterize the most common types of anxiety disorders based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)11 present in children at or near the diagnosis of epilepsy.
The etiology underlying anxiety disorders in epilepsy has been controversial ranging from psychosocial to biological to familial (aggregation). The psychosocial view holds that seizures are unpredictable, frightening and thus a potentially significant factor precipitating anxiety.12 The biological view has been understudied, and there are a limited number of imaging studies of anxiety in children with epilepsy. In children with chronic focal seizures, Daley et al.13 reported children with depression and/or an anxiety disorder had significantly larger left amygdala volumes. Importantly, in the general literature including the pediatric, adult, and animal literature, the amygdala has been implicated in playing a critical role in the development of anxiety disorders.14; 15 The amygdala mediates increases in arousal and begins, primarily via central nucleus outputs, the cascade of neural, endocrine, and behavioral components of a fear response. Additionally, connections between prefrontal cortices and the amygdala play a role in the alert and fear response system. The hippocampus is thought to process contextual information about the feared stimulus or situation, while anterior orbitofrontal cortex has been shown to be involved in processing and regulating ongoing emotion, as well as determining an executive response based on the environmental requirements.16 When this system goes awry, contextual specificity of a threatening stimulus and/or appropriate regulation to the perceived threat is lost causing a misinterpretation of danger and an inappropriate or exaggerated fear response.17; 18 As a result of aberrant communication between these cortical and subcortical regions, the system interprets and responds as if danger were present, and this over response in the system has been implicated in anxiogenesis.18 Notably, the brain regions implicated in the fear response system have not been examined in children with epilepsy with anxiety disorders. The second aim of this study is to examine the critical neuroanatomical regions reviewed above in this distributed fear system in children with recent-onset epilepsy.
Third, a growing body of literature suggests that familial or genetic factors may be related to cognitive and behavioral comorbidities of childhood epilepsy.19–21 In the general population, parental psychiatric history plays an important role in the expression of psychopathology in offspring.22 A number of family aggregation studies have implicated a close relationship between depressive and anxiety disorders in parents and subsequent expression of anxiety disorders in offspring,23; 24 but this has not been examined in pediatric epilepsy. The third aim of this study is to provide preliminary information regarding the link between first-degree relatives with depression and/or anxiety and anxiety in children with epilepsy.
Fourth, there are number of epilepsy-related factors that have been linked to mood disorders and anxiety in epilepsy. For example, antiepilepsy medications (AEDs) have been associated with depression, irritability, and anxiety.25–27 More frequent seizures and a longer duration of epilepsy have been inconsistently associated with an increased risk for depression.28 The final aim of this study is to examine the epilepsy variables that may be associated with anxiety including age of onset, duration, number of AEDs, seizure severity and epilepsy syndrome.
In summary, to begin to elucidate factors involved with the expression of anxiety disorders in children with recent-onset epilepsy, the purpose of this study is to investigate the following: 1) to characterize the different types of anxiety disorders present in children with epilepsy; 2) to utilize a hypothesis-driven network approach to examine anterior prefrontal cortex and subcortical (amygdala and hippocampus) brain regions implicated in the general anxiety literature to play a critical role in the development of an anxiety disorder; 3) to examine the role of family history of anxiety and depression; and 4) to identify clinical epilepsy variables associated with anxiety disorders in epilepsy.
Participants
Study participants were aged 8–18 years including 49 healthy controls (27 females [55%]; M age = 13.22 [3.10]); and 88 children with recent-onset epilepsy (Table 1). All children had a full scale IQ above 70. Twenty-five children with epilepsy met the DSM-IV11 criteria for a current anxiety disorder. The remaining 63 children with epilepsy did not meet criteria for any diagnosis of anxiety. Inclusion criteria were a diagnosis of epilepsy within the past 12 months, no other developmental disabilities or neurological disorders, normal neurological examinations and normal clinical imaging results. A board-certified pediatric neurologist (blinded to interview data) confirmed that all participants had focal or generalized seizures and provided independent confirmation of specific types of epilepsy (i.e., Rolandic epilepsy, juvenile myoclonic epilepsy, childhood absence epilepsy, etc.). Control participants were age- and gender-matched first-degree cousins of epilepsy participants who presented with no history of seizures, current anxiety disorder, any initial precipitating injuries (e.g., febrile convulsions), other developmental or neurological disease, or loss of consciousness greater than 5 minutes. Five control participants were diagnosed with a current anxiety disorder during the clinical interview and were not included in subsequent analyses. Children and parents participated in a psychiatric diagnostic interview; children underwent IQ testing and MRI.
Table 1.
Demographic and Clinical Variables
| Epilepsy − Anxiety (n = 63) | Epilepsy + Anxiety (n = 25) | |
|---|---|---|
| Age (years) | 12.94 (3.55) | 12.10 (2.96) |
| Sex (n; %) | 30F (47.6%)/33M (52.4%) | 15F (60.0%)/10M (40%) |
| Duration (mos.) | 7.97 (3.57) | 8.48 (3.44) |
| Age of Onset (yrs) | 12.15 (3.58) | 11.23 (2.94) |
| Seizure Typea (n; %) | ||
| Focal | 26 (41%) | 17 (68%) |
| Generalized | 36 (57%) | 8 (32%) |
| Unknown | 1 (2%) | |
| Seizure Severity Scale M(SD) | 20.05 (6.26) | 18.84 (6.01) |
| Anti-epileptic drugs (n; %) | ||
| No Meds | 6 (9.5%) | 6 (24.0%) |
| Monotherapy | 57 (90.5%) | 17 (68.0%) |
| Polytherapy | 0 (0.0%) | 2 (8.0%) |
| Carbamazepine | 6 (9.5%) | 2 (8.0%) |
| Ethosuximide | 5 (7.9%) | 1 (4.0%) |
| Gabapentin | 1 (1.6%) | 0 (0.0%) |
| Lamotrigine | 5 (7.9%) | 3 (12.0%) |
| Levetiracetam | 7 (11.1%) | 4 (16.0%) |
| Oxcarbazepine | 12 (19.0%) | 6 (24.0%) |
| Topiramate | 1 (1.6%) | 0 (0.0%) |
| Valproic acid | 20 (31.7%) | 3 (12.0%) |
Groups differ at p<0.05
First-degree cousins were used as controls rather than siblings or peer groups because: (i) first-degree cousins are more genetically distant from participants with epilepsy and less pre-disposed than siblings to shared genetic factors that may contribute to anomalies in brain structure and cognition; (ii) a greater number of first-degree cousins were available than siblings in the age range and (iii) the family link was anticipated to facilitate participant recruitment and retention over time compared to peer controls.
Standard protocol approvals, registrations, and consents
Research approval was obtained from the Health Sciences Institutional Review Board at the University of Wisconsin School of Medicine and Public Health. Written informed consent was obtained from parents or legal guardians of participating youth, written informed consent was obtained from participants age 18, and written informed assent was obtained from participants age 8–17. All pertinent medical records were obtained after signed release of information was obtained.
Procedures
Psychiatric diagnostic interview
Each child and parent participated in an independent semi-structured interview using the Kiddie–Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS) by a trained research assistant or three PhD psychologists (including JEJ and DCJ). A consensus diagnosis was reached when parent and child interviews were conducted by different interviewers. Additionally, interviews were recorded and randomly selected for review to insure inter-rater reliability. Post-traumatic stress disorder (PTSD) and obsessive compulsive disorder (OCD) were not included as anxiety disorders as recent classification systems (DSM-V and International Classification of Disease [ICD-10]) currently place both of these disorders in separate categories due to research indicating that these disorders have core clinical features that are distinct from other anxiety disorders.30–32 There were 3 children with OCD and 1 child with PTSD, and these children were excluded in the analyses. Additionally, due to the highly comorbid nature of anxiety disorders, children with other comorbid diagnoses (i.e. ADHD and depression) were not excluded from this study.10
Family history of anxiety and depression in first degree relatives (parents and siblings) was based on self-report by the parent who participated in the KSADS interview. Family members were included only if they received treatment for the disorders. No determination of the different types of anxiety or depression was made.
Measures of Cognition and Seizure Severity
Full-scale IQ (FSIQ) was obtained using the 4 subtest Wechsler Abbreviated Scale of Intelligence (WASI).33 The children’s Liverpool Seizure Severity Scale is a parent completed questionnaire, developed to measure seizure severity in children with epilepsy.34 Higher scores reflect greater seizure severity.
Imaging Protocol
Images were obtained on a 1.5 T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, U.S.A.). Sequences acquired for each participant included (1) T1-weighted, three-dimensional spoiled gradient recall (SPGR) acquired with the following parameters: TE = 5 ms, TR = 24 ms, flip angle = 40 degrees, NEX = 1, slice thickness = 1.5 mm, slices = 124, plane = coronal, FOV = 200 mm, matrix = 256 × 256; (2) proton density (PD); and (3) T2-weighted images acquired with the following parameters: TE = 36 ms (for PD) or 96 ms (for T2), TR = 3,000 ms, NEX = 1, slice thickness = 3.0 mm, slices = 64, slice plane = coronal, FOV = 200 mm, matrix = 256 × 256. Images were transferred to a Mac OSX computer for processing with the FreeSurfer image analysis suite, a set of software tools for the study of cortical and subcortical anatomy which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). A brief technical presentation of these procedures is presented in Dabbs et al.35
Data Reduction and Analyses
Demographic and clinical characteristics were assessed using t- and chi-square tests. To control for effects of age, sex, and intracranial volume (ICV), these variables were regressed on left and right amygdala and hippocampal subcortical brain volumes to produce standardized age/sex/ICV-residualized volumes for each participant. Similarly, cortical thickness residual values were computed controlling for age, sex, and global cortical thickness for the cortical regions of interest (ROI). Finally, standardized mean control group residuals for each subcortical volume and cortical thickness ROI were subtracted from the respective residual value for each participant with epilepsy. Each difference score thus represents deviation from the control mean. Hypothesized regions of interest implicated in the fear response system included the amygdala and hippocampus, and volume differences between patient groups (anxious vs. nonanxious) were assessed using t-tests. Cortical thickness values in left and right prefrontal regions of interest were compared across the epilepsy groups in a similar fashion.
To control for Type I error due to multiple statistical comparisons we used 2 approaches: one theoretical, the other analytical. From a theoretical perspective, we limited our analysis to regions of interest we hypothesized, based on existing studies, would be associated with anxiety. Limiting our analyses to bilateral amygdala and hippocampal volumes and cortical thickness in bilateral anterior prefrontal cortex (5×2 = 10 ROI’s total), all of which have been associated with anxiety and/or negative affect in general, focused our investigation and decreased the possibility of family-wise error. Second, from an analytical perspective, we cut the number of pairwise comparisons from 30 to 10 by examining how children with epilepsy both with and without anxiety differ in subcortical volumes/cortical thickness relative to controls.
Results
Distribution of Anxiety Disorders
A number of anxiety disorders were represented among children with recent-onset epilepsy (Figure 1), including specific phobias (11 out of 25, 44%), separation anxiety (8 out of 25, 32%), social phobia (6 out of 25, 24%), generalized anxiety disorder (5 out of 25, 20%), and anxiety disorder NOS (3 out of 25, 12%). These numbers do not equal 100%, as 8 children had more than 1 anxiety disorder. Six children from the Epilepsy + Anxiety group were identified with a comorbid depressive disorder; 4 were identified with comorbid ADHD. Removing these participants from the analyses did not statistically change our volumetric and cortical thickness findings across the two epilepsy groups; these participants were included in all analyses. Participating parents often reported symptoms of anxiety prior to the epilepsy diagnosis, but in most cases, children were not formally diagnosed and were untreated for the anxiety disorder, making it difficult to determine the exact age of onset.
Figure 1.
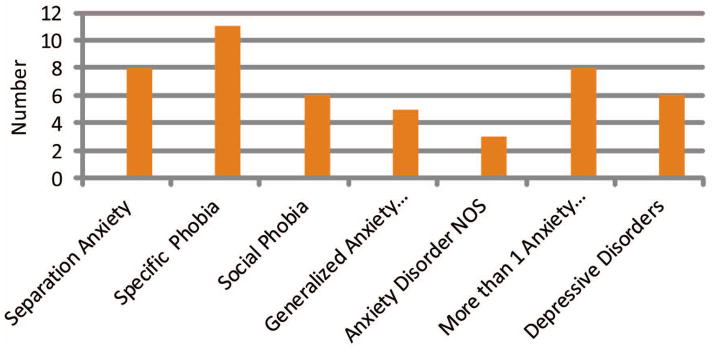
Number of Anxiety and Depressive Disorders
Amygdala and Hippocampus
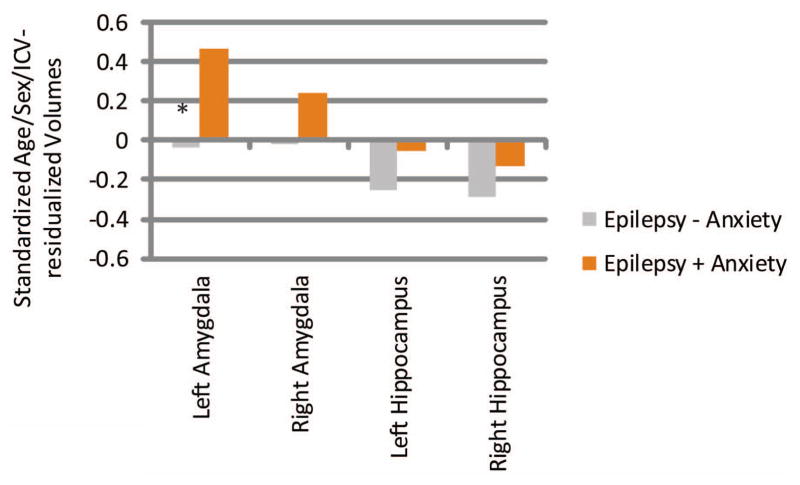
Left and right amygdala hippocampus volumes, after controlling for age, sex, and intracranial volume were as follows (standardized residual units): left amygdala (Epilepsy + Anxiety M = 0.41, SD 1.28=; Epilepsy − Anxiety M = −0.11, SD = .81; Control group M = −0.07, SD = 1.00); right amygdala (Epilepsy + Anxiety M = 0.21, SD = 1.18; Epilepsy − Anxiety M = −0.05, SD = 0.89; Control group M = −0.04, SD = 1.01); left hippocampus (Epilepsy + Anxiety M = 0.07, SD = 1.11; Epilepsy − Anxiety M = −0.13, SD = 0.89; Control group M =0.12, SD = 1.05); right hippocampus (Epilepsy + Anxiety M = 0.03, SD = 1.56; Epilepsy − Anxiety M = −0.13, SD = 0.72; Control group M = 0.16, SD = 0.91). See Supplemental File 1 for means and SD’s.
Left and right age/sex/ICV-residualized subcortical volume difference scores (patient – control) are presented in Figure 2. Significantly larger left amygdala volumes were seen in the Epilepsy + Anxiety group (M = 0.47, SD = 1.28) than in the Epilepsy − Anxiety group (M = −0.04, SD = 0.81), t(86) = 2.25, p = 0.027. Similar but non-significant differences for right amygdala volumes were found between groups (Epilepsy + Anxiety group M = 0.24, SD = 1.18; Epilepsy − Anxiety group M = −0.02, SD = 0.89), p = 0.262. Neither left nor right age/sex/ICV-residualized hippocampal volumes reached significance in group comparisons.
Figure 2.
Number of Anxiety and Depressive Disorders
Standardized Age/Sex/ICV-residualized Volumes
Frontal Lobe Cortical Thickness
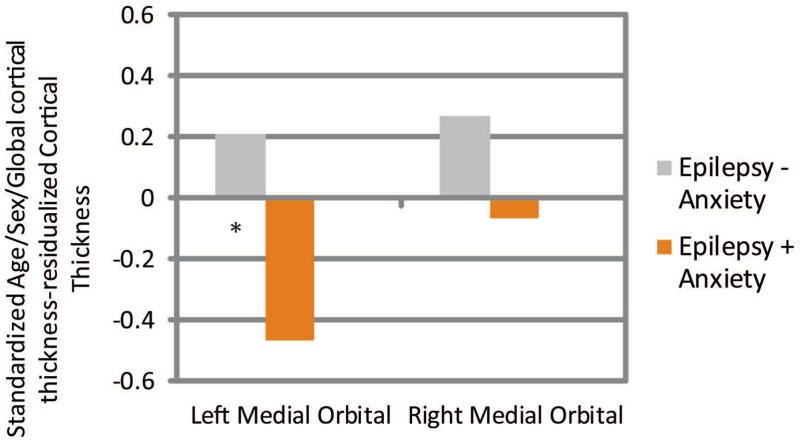
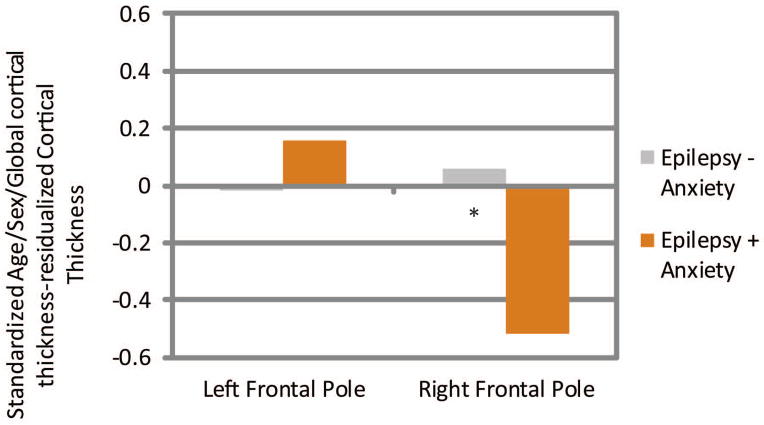
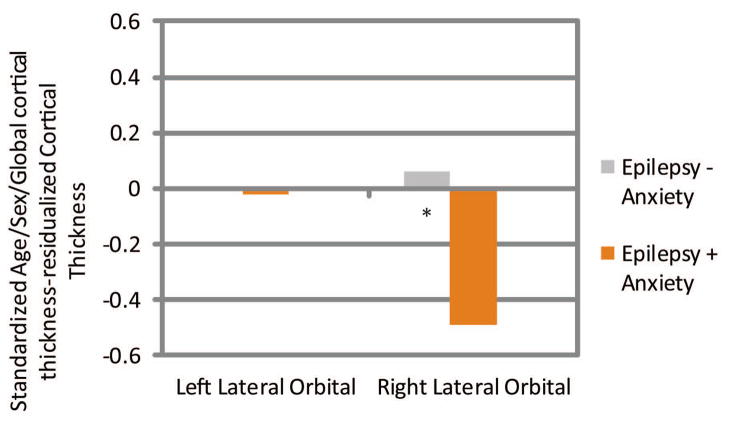
Age/sex/global cortical thickness-corrected cortical thickness difference scores in left and right medial orbital, lateral orbital, and frontal pole ROI’s were then compared across groups. Children with epilepsy and anxiety showed significantly thinner cortex in the left medial orbitofrontal (Epilepsy + Anxiety M = −0.47, SD = 0.83; Epilepsy − Anxiety M = 0.21, SD = 0.86, t(86) = 3.36, p = 0.001), right lateral orbitofrontal (Epilepsy + Anxiety M = −0.49, SD = 1.13; Epilepsy − Anxiety M = 0.06, SD = 0.88, t(86) = 2.44, p = 0.017), and right frontal pole (Epilepsy + Anxiety M = −0.52, SD = 1.01; Epilepsy − Anxiety M = 0.06, SD = 0.88, t(86) = 2.67, p = 0.009) (see Figures 3–5). See Supplemental Files 1 and 2 for means and SD’s, and an uncorrected vertex map showing areas of decreased cortical thickness in children with epilepsy and anxiety.
Figure 3.
Medial Orbital Prefrontal Cortex
Standardized Age/Sex/ICV-residualized Cortical Thickness
Figure 5.
Frontal Pole
Standardized Age/Sex/ICV-residualized Cortical Thickness
Familial History of Anxiety and Depression
In terms of family history, Epilepsy + Anxiety children (71%) were significantly more likely to have a first-degree family member with a history of anxiety or depression compared to the Epilepsy − Anxiety group (37%) (p=0.005) and controls (25%) (p<0.001). There was no difference in family history of anxiety or depression when comparing the Epilepsy − Anxiety group with controls. (p=0.177).
Demographic and Clinical Characteristics
As shown in Table 1, participants across all 3 groups (healthy controls, Epilepsy − Anxiety, Epilepsy + Anxiety) did not differ in age or proportion of females to males. Comparing clinical characteristics across Epilepsy − Anxiety and Epilepsy + Anxiety children, the groups did not differ in duration of epilepsy or age of seizure onset. Number of AEDs did not differ between groups when comparing monotherapy vs. polytherapy; the number of each medication is shown in Table 1. Epilepsy syndrome differed across groups, with 68% of Epilepsy + Anxiety children carrying a diagnosis of focal seizures vs. 32% with generalized seizures; in the Epilepsy − Anxiety group, more children with generalized seizures (57%) than focal seizures (43%) were present, p ≤ 0.05. Additionally, there were no differences in parent reported seizure severity in the Epilepsy + Anxiety and Epilepsy − Anxiety groups.
Discussion
The four primary findings from this study include the distribution of specific anxiety disorders in children with epilepsy, the neuroanatomical correlates of anxiety in children with epilepsy, the contribution of family history and the role of clinical epilepsy variables in anxiety.
Distribution of anxiety disorders
Anxiety disorders in children with recent-onset epilepsy were common with 28% of the epilepsy sample meeting DSM-IV criteria for an anxiety disorder. The distribution of specific anxiety disorders yielded elevated rates across a diversity of diagnoses including specific phobias (44%), separation anxiety (32%), social phobia (24%), generalized anxiety disorder (20%), and anxiety disorders NOS (12%). The children with epilepsy in this sample had higher rates of specific phobia (44% vs. 15.8%), separation anxiety (32% vs. 1.6%), social phobia (24% vs. 8.2%), and generalized anxiety disorder (20% vs. 1.1%) when compared to the rates reported in the National Comorbidity Survey- Adolescent Supplement (NCS-A).10 This finding indicates that rates of anxiety disorders are likely higher in children with epilepsy when compared to youth in the general population; a similar finding is reported in adults with epilepsy and depression. It is important to begin to characterize the specific types of anxiety disorders that are common in children with epilepsy as very little is known about the prevalence of specific anxiety disorders common in epilepsy. There are no controlled trials for the treatment of anxiety in pediatric epilepsy, and current treatment recommendations are from the general population and include a combination of SSRIs and cognitive behavioral therapy. Additionally, the general population studies continue to indicate that anxiety disorders commonly appear in childhood and adolescence and persist over time.6 As is well documented, adults with epilepsy have high rates of anxiety, depression, and suicide, indicating the negative impact of anxiety initially presenting in childhood.7–9
Neuroanatomical correlates of anxiety
The relationship between anxiety and neurobiology was examined in a hypothesis driven manner, focusing on regions demonstrated to be related to anxiety in the general literature. First, children with epilepsy and anxiety were found to have significantly larger left amygdala volumes compared to children with epilepsy without anxiety when compared to controls after controlling for age, sex and brain size. There has been only one structural MR study of children with epilepsy with affective and anxiety disorders. Daley et al.13 reported a similar finding with larger left amygdala volumes in children with chronic epilepsy with complex focal seizures; thus confirming that left amygdala findings appear to be reliable in pediatric epilepsy with anxiety and affective disorders.
In the general literature, amygdala volume differences have been reported in children with anxiety disorders, but the lateralized patterns of enlargement have been variable. Compared to controls, children with generalized anxiety disorder were found to have significantly larger right amygdala and total amygdala volumes.36 Among medication naive children with depression and comorbid anxiety, larger ratio differences were noted between the amygdala and hippocampal volumes indicating larger amygdala volumes on both the left and right with an increase in anxiety symptoms.37 Similarly, in the current study, there was a trend for larger right amygdala volumes in children with epilepsy and anxiety.
In this sample, children with recent-onset epilepsy and anxiety had significantly thinner cortex in the left medial and right lateral orbital frontal cortex and right frontal pole, which is considered a part of the anterior prefrontal cortex. The prefrontal cortex has been implicated in playing a significant role in the anxiety system,38; 39 particularly as it inhibits or modulates input from the amygdala resulting in an extinction response. This decrease in cortical thickness finding may indicate that the prefrontal cortex is not developing in a similar fashion as compared to healthy controls and children with epilepsy without anxiety. In healthy adults, decreased cortical thickness in the ventromedial prefrontal cortex was found to be correlated with increased activity in the amygdala when presented with emotional tasks.40 It was hypothesized that decreased thickness might indicate fewer neurons available to produce an inhibitory response.
In summary, this study replicated the MR findings of increased left amygdala volumes reported by Daley et al. 13 and identified thinner anterior prefrontal cortex in the sample of children with recent-onset epilepsy and anxiety, providing support for the idea of a biological vulnerability for anxiety in epilepsy that is similar to results reported in the general literature.
Clinical and family history as predictors of anxiety
Children with recent-onset epilepsy and anxiety were more likely to have focal epilepsy compared to children with epilepsy without anxiety. However, cortical and subcortical significant findings were not associated with syndrome differences (focal vs. generalized), suggesting that these neurobiological differences are associated with anxiety rather than epilepsy syndrome. Additionally, there were no significant differences in number of AEDs, seizure severity, age of onset or duration of epilepsy between the groups with and without anxiety.
McLaughlin et al.22 recently examined the role of parental psychiatric history in the expression of psychopathology in offspring and reported that any psychiatric disorder increased the risk of a psychiatric disorder identified in offspring. When examining anxiety and depression specifically, a number of family aggregation studies have implicated a close relationship between the two disorders in parents and subsequent expression of anxiety disorders in offspring.23; 24 In this study, children with recent-onset epilepsy and anxiety were more likely to have a first-degree relative with a history of anxiety or depression compared to children with epilepsy without anxiety and healthy control children. Notably, Daley et al.13 reported no relationship between family history of mood disorders and anxiety and larger left amygdala findings.
Limitations
There are several limitations of this study. First, there is no comparison group of control children with anxiety because rates of current anxiety diagnoses were low. As a result, our only comparisons can occur with children without anxiety or epilepsy (healthy controls) and children with epilepsy without anxiety. Unaffected siblings of children with some epilepsies have been shown to exhibit cognitive differences compared to healthy controls; therefore, first degree cousins were selected as controls. It is possible that cousins may exhibit some shared genetic psychiatric, cognitive, or imaging characteristics; however, this issue remains unexplored in epilepsy populations. The number of children with epilepsy and anxiety is modest; however, the two comparison groups are of reasonable sample size. Additionally, children with epilepsy and anxiety were over represented in terms of seizure syndrome as a significant proportion of these children had focal epilepsy. However, given this sample was similar to the Daley et al.13 sample, it provided partial replication of those study findings. Finally, children with diverse anxiety disorders were analyzed together in this investigation, and similar to the general population, some of the children had other Axis I disorders (i.e. ADHD and depression) since anxiety disorders are highly comorbid with other psychiatric disorders.10 It remains to be determined whether anatomic findings would vary as a function of specific anxiety disorders and with or without other comorbid disorders, and this is an issue worth pursuing in the future with larger sample sizes.
Conclusion
Anxiety is a common psychiatric comorbidity in children with recent-onset epilepsy which has an underlying neurobiological substrate consisting of volumetric enlargement of the amygdala and thinner cortex in orbital and other regions of prefrontal cortex, suggesting the presence of abnormalities in brain structures that are involved in the dysfunctional networks found in individuals with anxiety disorders in the general population. These anatomical findings are evident early in the course of epilepsy, are not related to chronicity of seizures, and are linked to a family history of anxiety and depressive disorders.
Supplementary Material
Supplemental Figure 1. Vertex analysis of cortical thickness
Areas of thinner cortex identified by a whole brain vertex-wise analysis. Regions of thinner cortex in anxious compared to non-anxious epilepsy participants, p < 0.05, controlling for age and gender. Uncorrected for multiple comparisons.
Figure 4.
Lateral Orbital Prefrontal Cortex
Standardized Age/Sex/ICV-residualized Cortical Thickness
Acknowledgments
This study was supported by NIH (NINDS 3ROI 44351), which provided funding for study design, data collection, and data analysis. The funder was not involved in manuscript preparation or publication decisions. JEJ wrote the first draft of this manuscript. No authors received any honorarium, grant, or other form of payment for producing this manuscript.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures of Conflicts of Interest:
DCJ, KLC, and KD report no disclosures.
JEJ, DAH, and MS receive research support from NIH (NINDS 3RO1 44351 [coinvestigators]).
DAH receives research support from CURE (Citizens United for Research on Epilepsy).
CES has received compensation as a consultant for Questcor Pharmaceuticals (2011), serves on the Scientific Board of The Charlie Foundation, serves as an Associate Editor of Epilepsia and Co-editor-in-Chief for Basic Science of Epilepsy Currents, has received royalties from publication of the books: (1) Epilepsy and the Ketogenic Diet, and (2) Epilepsy: Mechanisms, Models and Translational Perspectives, and received support from NIH (NINDS RO1 44351 [coinvestigator]).
BPH serves as an Associate Editor for Epileosy & Behaviorand receives research support from the NIH (NINDS 3RO1 NS44351 [PI], RO1 AG027161 [coinvestigator], P50AG3314 [coinvestigator], 1RO1NS064034 [coinvestigator], RO1AG031790 [coinvestigator]), and Citizens United for Epilepsy Research (Co-PI).
References
- 1.Alwash RH, Hussein MJ, Matloub FF. Symptoms of anxiety and depression among adolescents with seizures in Irbid, Northern Jordan. Seizure. 2000;9:412–416. doi: 10.1053/seiz.2000.0427. [DOI] [PubMed] [Google Scholar]
- 2.Ott D, Caplan R, Guthrie D, et al. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–914. doi: 10.1097/00004583-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Adewuya AO, Ola BA. Prevalence of and risk factors for anxiety and depressive disorders in Nigerian adolescents with epilepsy. Epilepsy Behav. 2005;6:342–347. doi: 10.1016/j.yebeh.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Caplan R, Siddarth P, Gurbani S, et al. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46:720–730. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones JE, Watson R, Sheth R, et al. Psychiatric comorbidity in children with new onset epilepsy. Developmental Medicine & Child Neurology. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim-Cohen J, Caspi A, Moffitt TE, et al. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 7.Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52 (Suppl 1):21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- 8.Hecimovic H, Salpekar J, Kanner AM, et al. Suicidality and epilepsy: a neuropsychobiological perspective. Epilepsy Behav. 2011;22:77–84. doi: 10.1016/j.yebeh.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Brandt C, Schoendienst M, Trentowska M, et al. Prevalence of anxiety disorders in patients with refractory focal epilepsy--a prospective clinic based survey. Epilepsy Behav. 2010;17:259–263. doi: 10.1016/j.yebeh.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Avenevoli S, McLaughlin KA, et al. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychological Medicine. 2012;42:1997–2010. doi: 10.1017/S0033291712000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: 1994. [Google Scholar]
- 12.Hamid H, Ettinger AB, Mula M. Anxiety symptoms in epilepsy: salient issues for future research. Epilepsy Behav. 2011;22:63–68. doi: 10.1016/j.yebeh.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Daley M, Siddarth P, Levitt J, et al. Amygdala volume and psychopathology in childhood complex partial seizures. Epilepsy Behav. 2008;13:212–217. doi: 10.1016/j.yebeh.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 17.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- 18.Ferrari MC, Busatto GF, McGuire PK, et al. Structural magnetic resonance imaging in anxiety disorders: an update of research findings. Rev Bras Psiquiatr. 2008;30:251–264. doi: 10.1590/s1516-44462008000300013. [DOI] [PubMed] [Google Scholar]
- 19.Hesdorffer DC, Caplan R, Berg AT. Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia. 2012;53:301–307. doi: 10.1111/j.1528-1167.2011.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levav M, Mirsky AF, Herault J, et al. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- 21.Smith AB, Kavros PM, Clarke T, et al. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin KA, Gadermann AM, Hwang I, et al. Parent psychopathology and offspring mental disorders: results from the WHO World Mental Health Surveys. Br J Psychiatry. 2012;200:290–299. doi: 10.1192/bjp.bp.111.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieb R, Isensee B, Hofler M, et al. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- 24.Hettema JM, Prescott CA, Myers JM, et al. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 25.Caplan R. Psychopathology in pediatric epilepsy: role of antiepileptic drugs. Front Neurol. 2012;3:163. doi: 10.3389/fneur.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddy CM, Rickards HE, Cavanna AE. Behavioral adverse effects of antiepileptic drugs in epilepsy. J Clin Psychopharmacol. 2012;32:362–375. doi: 10.1097/JCP.0b013e318253a186. [DOI] [PubMed] [Google Scholar]
- 27.Mula M, Monaco F. Antiepileptic drugs and psychopathology of epilepsy: an update. Epileptic Disord. 2009;11:1–9. doi: 10.1684/epd.2009.0238. [DOI] [PubMed] [Google Scholar]
- 28.Plioplys S, Dunn DW, Caplan R. 10-year research update review: psychiatric problems in children with epilepsy. J Am Acad Child Adolesc Psychiatry. 2007;46:1389–1402. doi: 10.1097/chi.0b013e31815597fc. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Brewin CR, Lanius RA, Novac A, et al. Reformulating PTSD for DSM-V: life after Criterion A. J Trauma Stress. 2009;22:366–373. doi: 10.1002/jts.20443. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: 2013. [Google Scholar]
- 32.World Health Organization. ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; Geneva: 1992. [Google Scholar]
- 33.Wechsler D. Wechsler Abbreviated Intelligence Scale. The Psychological Corporation; San Antonio, T.X: 1999. [Google Scholar]
- 34.Baker GA, Jacoby A, Smith DF, et al. Development of a novel scale to assess life fulfillment as part of the further refinement of a quality-of-life model for epilepsy. Epilepsia. 1994;35:591–596. doi: 10.1111/j.1528-1157.1994.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 35.Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009 doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bellis MD, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 37.MacMillan S, Szeszko PR, Moore GJ, et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 38.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 40.Foland-Ross LC, Altshuler LL, Bookheimer SY, et al. Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. J Neurosci. 2010;30:16673–16678. doi: 10.1523/JNEUROSCI.4578-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Vertex analysis of cortical thickness
Areas of thinner cortex identified by a whole brain vertex-wise analysis. Regions of thinner cortex in anxious compared to non-anxious epilepsy participants, p < 0.05, controlling for age and gender. Uncorrected for multiple comparisons.