Abstract
The co-stimulatory molecule CD40 enhances immunity through several distinct roles in T cell activation and T cell interaction with other immune cells. In a mouse model of immunity to liver stage Plasmodium infection, CD40 was critical for the full maturation of liver dendritic cells, accumulation of CD8+ T cells in the liver, and protective immunity induced by immunization with the P. yoelii fabb/f- genetically attenuated parasite. Using mixed adoptive transfers of polyclonal wild type (WT) and CD40-deficient (CD40−/−) CD8+ T cells into WT and CD40−/− hosts, we evaluated the contributions to CD8+ T cell immunity of CD40 expressed on host tissues including antigen-presenting cells (APC), compared to CD40 expressed on the CD8+ T cells themselves. Most of the effects of CD40 could be accounted for by expression in the T cells’ environment, including the accumulation of large numbers of CD8+ T cells in the livers of immunized mice. Thus, protective immunity generated during immunization with fabb/f- was largely dependent on effective APC licensing via CD40 signaling.
Introduction
Despite the recent success of public health measures, malaria remains widespread in sub-Saharan Africa, Southeast Asia, and South America, and continues to cause morbidity and mortality and impede socioeconomic progress. Climate change threatens to extend the range of Plasmodium-infected Anopheles mosquitoes and the complexity of vector control and swift spread of drug resistance make development of an effective vaccine imperative (1). Live, attenuated Plasmodium vaccines against liver stage infection have shown great promise in the mouse model and are now being optimized in preliminary human clinical trials (1–3). These attenuated strains are invaluable as a model of effective sterilizing immunity and can be used to determine the mechanisms that must be triggered during immunization to generate a protective and long-lasting response that can prevent symptomatic blood stage infection.
Radiation-attenuated sporozoites (RAS) and genetically-attenuated parasites (GAP) elicit strong CD8+ T cell responses that protect immunized mice from infectious challenge (4–6). It is unclear by which cells CD8+ T cells are primed; hepatocytes, liver dendritic cells (DC), and antigen-presenting cells (APC) in the skin-draining lymph node have all been implicated (7–9). Though they are not involved in the effector response, CD4+ T cells are required during immunization to induce protective immunity (6, 10). Recent studies indicate that during immunization with RAS, CD4+ T cells are needed to generate optimal numbers of CD8+ T cells, though they appear not to shape the quality of effector function or memory response (11).
There are several routes by which CD4+ T cells provide help to CD8+ T cells including licensing APC to better prime CD8+ T cells and signaling CD8+ T cells directly via cytokines or surface molecules. Interaction between CD40, a co-stimulatory molecule expressed on APC and CD8+ T cells, and CD40L expressed on CD4+ T cells is a core mechanism of CD4+ T cell help (12, 13). Frequently used to improve responses in anti-pathogen or anti-tumor vaccine studies (14, 15), CD40 stimulation induces APC to secrete inflammatory Th1 cytokines such as IL-12 and IFN-γ, and to upregulate antigen presentation and co-stimulatory molecules, enhancing the cells’ ability to recruit and prime T cells (16). IL-12, IFN-γ, and Th1 responses have been strongly implicated in protection against liver stage Plasmodium infection and other intracellular parasites (17–20). IL-4-secreting CD4+ T cells, a hallmark of Th2 responses, may also be required for protective immunity conferred by RAS, throwing into question whether Th2 or Th1 responses aid immunity against liver stage infection (21, 22).
CD40 signaling also promotes CD8+ T cell activation, proliferation, and can influence the memory program and prevent T cell exhaustion (23, 24). In non-inflammatory model systems, CD40 expressed on the CD8+ T cell is critical for the development of an effective memory response, whereas in viral and bacterial infections it is not required, and CD40 on the APC drives the CD8+ T cell response (25–27). Whether immunity to an intracellular eukaryotic parasite such as the liver stage of Plasmodium relies on CD40 as a route of CD4+ T cell help is unclear. Here we explore the role of CD40 in generating a protective immune response during primary immunization with the late-arresting attenuated strain P. yoelii fabb/f- (28). Rather than using a single T cell specificity to investigate the response to fabb/f- immunization, we chose to examine the total CD8+ T cell response to be able to draw conclusions that would apply to the full range of polyclonal responses to the parasite’s many antigens. An alternative approach to using antigen-specific T cell clones or tetramers would be to examine activated, antigen-specific CD8+ T cells that are CD11ahi CD8αlo (29), however, the high frequency of activated T cells present in both the resting and the immunized liver makes this method difficult to apply to cells collected from the liver (30, 31). We find that without CD40, mice normally protected by P. yoelii fabb/f- immunizations are not able to withstand infectious challenge. Moreover, CD40 signaling is a key requirement for multiple components of the response induced by fabb/f- immunization and CD40 expressed on the CD8+ T cell has a distinct function from that of CD40 expressed on the APC.
Materials and Methods
Ethics Statement
The animal experiments described here were performed according to the regulations of the Institutional Animal Care and Use Committee and the Office of Laboratory Animal Welfare, under the protocol NC-06 approved by the Seattle BioMed Animal Care and Use Committee. Liver perfusions were performed under terminal anesthetization with Avertin (tribromoethanol). Animals were monitored daily and every effort was made to minimize suffering.
Mice
The study used female mice on the C57BL/6 background, between 8–10 weeks of age. WT C57BL/6 and MuMT mice were purchased from the Jackson Laboratory, (Bar Harbor, ME) and housed in the Seattle Biomedical Research Institute vivarium. MHCII−/− and CD40−/− mice originated from Jackson Laboratory, (Bar Harbor, ME) and were bred in-house at Seattle Biomedical Research Institute.
Parasites and immunizations
The fabb/f- parasite has been previously described (28). Parasites were grown in Swiss Webster mice and Anopheles stephensi mosquitoes. Sporozoites were dissected from the salivary glands of mosquitos immediately prior to injection into mice via the tail vein. For experiments in which mice were immunized once and lymphocytes were analyzed 5–7 days later, a dose of 5×104 live fabb/f- sporozoites was used. For the immunization-challenge experiments in Figure 1A and C, mice were immunized twice two weeks apart with 1×104 live fabb/f- sporozoites. Immunized mice and naïve controls were challenged with 100 WT P. yoelii sporozoites one month after the last immunization and were monitored for blood-stage parasitemia by Giemsa-stained blood smear during days 3–14 after challenge. 100 WT P. yoelii sporozoites is a dose we previously found to be infectious to 100% of naïve C57BL/6 mice (32). CD4-depletion was accomplished by injecting mice intraperitoneally with GK1.5 antibody on Days −3, −1, and +1 surrounding each immunization and/or challenge. Successful depletion of >95% of CD4+ T cells was determined by flow cytometric analysis staining for CD4 and CD8 in peripheral blood from each mouse on Day 0.
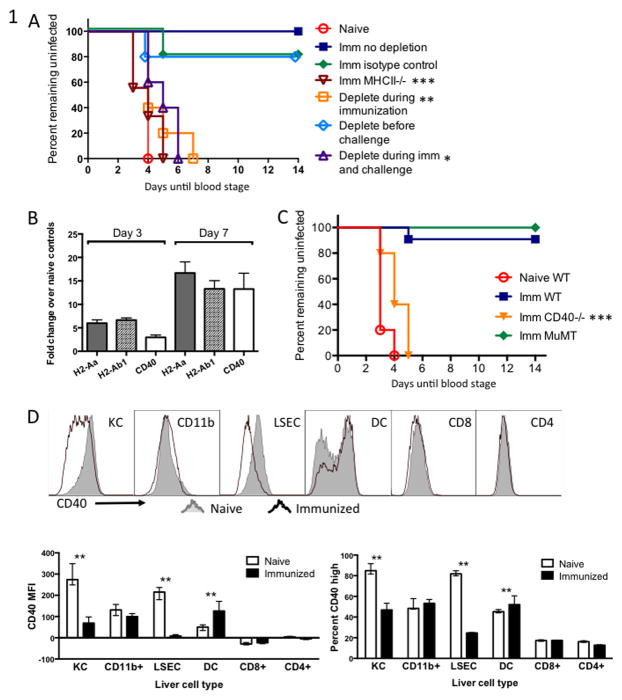
Figure 1. CD4+ T cells and CD40 are needed for protective immunity conferred by fabb/f- immunization.
(A) Groups of WT C57BL/6 mice were immunized twice with 1×104 fabb/f- sporozoites then challenged one month later and monitored for blood-stage parasitemia. Each group was either depleted of CD4+ T cells just prior to each immunization, just prior to challenge, both, or left untreated. (B) Gene expression in the livers of fabb/f- immunized mice measured by q-RT-PCR. WT mice were immunized once with 5×104 fabb/f- sporozoites and 7 days later RNA was isolated from a section of whole liver. Values were normalized to Gapdh and are shown as fold-change over naïve control liver. (C) Groups of WT or gene-deficient C57BL/6 mice were immunized twice with 1×104 fabb/f- sporozoites then challenged one month later and monitored for blood-stage parasitemia. (D) Kupffer cells (KC), CD11b+ infiltrating myeloid cells, liver sinusoidal endothelial cells (LSEC), dendritic cells (DC), CD8+ and CD4+ T cells were isolated from the livers of fabb/f-immunized mice on Day 7 and stained for CD40 surface molecule expression. Representative samples are shown in the top panel and summarized data are shown below. The MFI, right, is based on the entire histogram and percent CD40 high, left, is based on the CD40-bright peak of the dendritic cells. Asterisks represent significant differences between naïve and immunized within one cell type.
Cell isolation for adoptive transfer
For the experiments depicted in Figures 5–6, CD8+ T cells were separated from whole spleen by negative depletion using a MACS column and CD8+ T cell isolation kit (Miltenyi Biotec Inc). The cells were injected into WT and CD40−/− host mice in a 1:1 ratio via the tail vein. Each mouse received 5 × 106 cells total. Host mice and transferred cells expressed different congenic markers to distinguish them by flow cytometry. Host WT mice expressed CD45.1 and CD90.2, transferred WT cells expressed CD45.2 and CD90.1, and CD40−/− mice and transferred cells expressed CD45.2 and CD90.2. (We were unable to differentiate transferred CD40−/− cells from CD40−/− host cells and did not analyze that population, but gave WT and CD40−/− mice the same cell mixture for control purposes).
Figure 5. CD40 expressed on APC drives effector CD8+ T cell differentiation and accumulation in the liver.
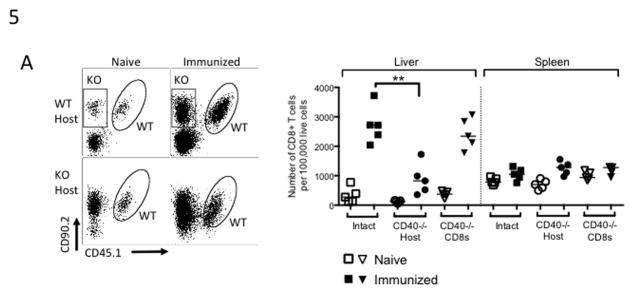
CD8+ T cells were isolated from congenically marked WT or CD40−/− mice and transferred in a 50:50 mix into naïve WT or CD40−/− hosts. Two weeks later the recipients were immunized once with 5×104 fabb/f- sporozoites, then lymphocytes were collected from the liver and spleen on Day 7 after immunization. (A) Left panel: representative plots of CD8+ T cells isolated from livers on Day 7. Oval gates surround transferred WT CD8+ T cells and rectangular gates surround transferred CD40−/− CD8+ T cells (KO). Right panel: aggregate data shown as number of isolated CD8+ T cells per 100,000 live cells. ‘Intact’ are WT CD8+ T cells primed in WT hosts, ‘CD40−/− Host’ are WT CD8+ T cells primed in CD40−/− hosts, and ‘CD40−/− CD8s’ are CD40−/− CD8+ T cells primed in WT hosts.
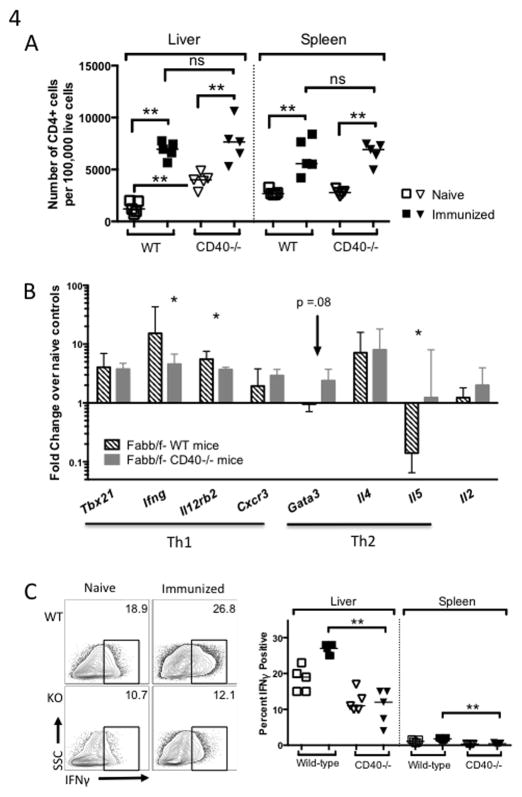
Figure 6. CD40 expressed on CD8+ T cells may limit effector cell differentiation.
Mice were treated as in Figure 5 and cells were collected on Day 5 post-immunization. Expression of genes related to CD8+ T cell differentiation and survival (A), effector activation (B), and exhaustion (C) in CD8+ T cells primed in WT or CD40−/− mice shown as fold change over naïve controls. Upper panels (bar graphs) compare WT CD8+ T cells in WT and CD40−/− hosts (Mann-Whitney test) and lower panels depict paired comparisons of WT and CD40−/− CD8+ T cells primed in WT mice (Wilcoxon matched pairs test).
Cell collection and liver perfusions
Cells were collected from liver, lymph nodes, and spleen using an established method developed in our laboratory (33). While mice were under terminal anesthesia, the liver was exposed and a catheter was inserted into the portal vein. The liver was perfused slowly with 5mL of HBSS containing 5mM Hepes and 5mM EDTA to flush out circulating blood. A small piece of the lower left liver lobe was placed in Trizol for gene expression analysis. The liver was then perfused with 6–8mL of collagenase buffer (HBSS, 5mM Hepes, 5mM CaCl2, .05% type IV Collagenase (Sigma Aldrich)). The gal bladder was removed and the liver dissociated into a single cell suspension in PBS with 5% FBS and spun at low speed to remove hepatocytes. The supernatant was applied to a 20% iodixanol (Optiprep) gradient and spun at 2500rpm for 25 minutes to isolate non-parenchymal cells for flow cytometric and gene expression analysis. Several lymph nodes (the inguinal, coeliac, and portal vein lymph nodes) and the spleen were collected and made into separate lymph node and spleen single cell suspensions for flow cytometric analysis.
Cell staining for flow cytometry and cell sorting
Lymphocytes from the liver, spleen, and lymph nodes were stained and interrogated using panels that included the following antibodies from Biolegend: anti-CD3 (145-2C11), anti-CD8 (53-6.7), anti-CD4 (RM4-5), anti-CD90.2 (53-2.1), anti-CD62L (MEL-14), anti-CD44 (IM7), anti-CD11b (ICRF44) anti-CD122 (5H4), anti-CD86 (GL-1), anti-CD80 (16-10A1), and anti-IA/IE (M5/115.15.2); from BD Biosciences: anti-CD45.1 (A20), anti-IFN-γ (XMG1.2), anti-CD25 (7D4), and anti-CD11c (N418); and from eBioscience: anti-KLRG1 (2F1). Intracellular anti-IFN-γ staining was performed using a Cytofix/Cytoperm kit (BD Biosciences) after cells were stimulated with ionomycin and PMA for 4 hours and treated with Brefeldin A to inhibit protein transport.
To conduct surface marker expression analysis and to sort liver CD8+ and CD4+ T cells and DC into separate populations for gene expression analysis, antibodies from the list above were used in addition to anti-NK1.1 (PK136). Classical DC were identified from high forward- and side-scatter cells that were CD11chi, CD11blo, and MHC class IIhi. The mean number of DC analyzed per mouse was 4,278 cells. CD8+ T cells were identified as CD3+ and CD8+, and adoptively transferred WT and CD40−/− cells were further differentiated by CD45.1 and CD90.2 staining. CD4+ T cells were identified as CD3+, NK1.1- and CD8-, and CD4+. The mean number of CD8+ T cells analyzed per mouse was 6,362 cells, ranging from 675 cells (a low number of WT CD8+ T cells in CD40−/− hosts) to 31,331 cells. The mean number of CD4+ T cells analyzed per mouse was 35,220 cells. Lymphocytes were analyzed using a Beckton-Dickenson LSRII and Beckton-Dickenson FACSDiva software. Liver lymphocytes were sorted using a Beckton-Dickenson FACS Aria II. Data were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR).
Q-RT-PCR Gene expression analysis
Liver tissue or sorted cells were stored in TRIzol (Invitrogen) at −80C. RNA was isolated from TRIzol after treatment with Proteinase K (Qiagen) using chloroform (Sigma-Aldrich) and washed using sodium acetate (Sigma-Aldrich). Complimentary DNA was made using the QuantiTect Reverse Transcription kit (Qiagen) and pre-amplified using TaqMan Real-Time PCR Master Mix and the TaqMan assays for genes of interest (Applied Biosciences). Quantitative real-time PCR was performed on pre-amplified RNA using the same TaqMan assays in a BioMark HD microfluidics system (Fluidigm, South San Francisco, CA). Cycle thresholds where calculated using Fluidigm Real-Time PCR Analysis software. Using Microsoft Excel, gene expression values were calculated using the delta delta CT method and were normalized to the housekeeping gene Hprt. Fold changes in immunized mice were calculated using the median value of strain-specific naïve control mice for each gene.
Statistical analysis
Data were analyzed using Prism6 software (GraphPad). Asterisks represent significant p values obtained by the following statistical tests: Kaplan-Meyer survival curve analysis was used in Figure 1A and C. The Mann-Whitney rank sum test was used for all analyses comparing cells or genes from different host mice (unpaired data). * indicates p < .05, ** p < .01, *** p < .001. Graphs in figures 1–5 depict one representative experiment of 2–3 experiments that each used 4–5 mice per group. The Wilcoxon matched pairs test was used for analyses comparing WT and CD40−/− CD8+ T cells that were primed in the same host mouse (paired data) (Figure 6 lower panels and 7C only), and these graphs show data pooled from 2–3 experiments.
Results
CD4+ T cells and the co-stimulatory molecule CD40 are needed for sterile immunity
Two immunizations of 1 × 104 P. yoelii fabb/f- sporozoites given two weeks apart can protect C57BL/6 mice against an infectious challenge of 100 wild-type (WT) P. yoelii sporozoites one month later [Fig. 1A]. To determine whether CD4+ T cell help was necessary for this protective response, we depleted CD4+ T cells with anti-CD4 antibody during each immunization, just prior to challenge, or both. Immunized, non-depleted mice were protected against blood-stage infection, whereas MHC class II-deficient mice, which completely lack CD4+ T cells, and WT mice that had been CD4-depleted during immunization or during immunization and challenge became infected. In agreement with previous work (6, 10), mice depleted just prior to infectious challenge did not lose protective immunity, indicating that CD4+ T cell help was necessary during the primary response but was not required for the recall response [Fig. 1A].
Because CD40 signaling is a major mechanism of CD4+ T cell help, we investigated whether the molecule was involved in protection conferred by fabb/f- immunization. CD40 was upregulated along with MHC class II molecules in whole liver from mice 7 days after immunization [Fig. 1B]. We immunized CD40-deficient (CD40−/−) mice and observed that they failed to generate protective immunity; they became infected upon challenge. Because both MHCII−/− and CD40−/− mice lack normal antibody responses (34), we also tested protection in B cell-deficient MuMT mice. Immunized MuMT mice were protected against challenge indicating that the lack of protection in MHCII−/− and CD40−/− mice was due to missing CD4+ T cell help and not a failed antibody response [Fig. 1C].
We next examined the change in CD40 expression at the protein level on a variety of immune cells isolated from the liver. Several types of professional and non-traditional antigen presenting cells can be found in the liver, including dendritic cells, liver sinusoidal endothelial cells (LSEC), liver macrophages called Kupffer cells (KC), and CD11b+ myeloid cells that infiltrate the liver upon infection (35). We found that KC and LSEC – both generally recognized as immunosuppressive cell types (36) – downregulated CD40 surface molecule expression upon immunization, whereas DC isolated from the liver upregulated CD40 expression [Fig. 1D]. CD11b+ myeloid cells and CD8+ and CD4+ T cells showed little change in their low-level CD40 expression in response to immunization.
CD40-deficiency results in fewer CD8+ T cells in the liver
Depleting CD4+ T cells during immunization with RAS resulted in a diminished clonal burst of transgenic CD8+ T cells specific for circumsporozoite protein (CS) (11). As CD40 is a main route of CD4+ T cell help, we wished to examine whether CD40 was critical for CD8+ T cell accumulation in the liver. We examined the total CD8+ T cell response in order to capture the entire polyclonal T cell response to immunization. To do this we immunized WT or CD40−/− mice once with fabb/f- sporozoites and analyzed lymphocytes from the liver, spleen, and lymph nodes 7 days later at the peak of the CD8+ T cell response [Fig. 2A]. CD8+ T cells primed in CD40−/− and WT mice expressed similar levels of the activation markers CD44 and CD62L [data not shown]. However, the livers of immunized WT mice contained significantly more CD8+ T cells compared to immunized CD40−/− mice [Fig. 2B]. Because the total number of cells isolated from the murine liver via collagenase perfusion can vary greatly, we chose to analyze T cells as a percentage of total isolated cells in order to be able to make comparisons between organs, individual mice, and experiments performed on different days. This approach is non-standard for lymph node and spleen cells, where the isolation of essentially all lymphocytes is straightforward, but has been adopt widely for cells isolated from tissues where full recovery is impossible, such as the intestine (37). We find that for liver lymphocytes, the percentage is more reproducible than an effort to estimate total numbers of any cell type.
Figure 2. CD8+ T cells primed in CD40−/− mice fail to expand and express markers of exhaustion.
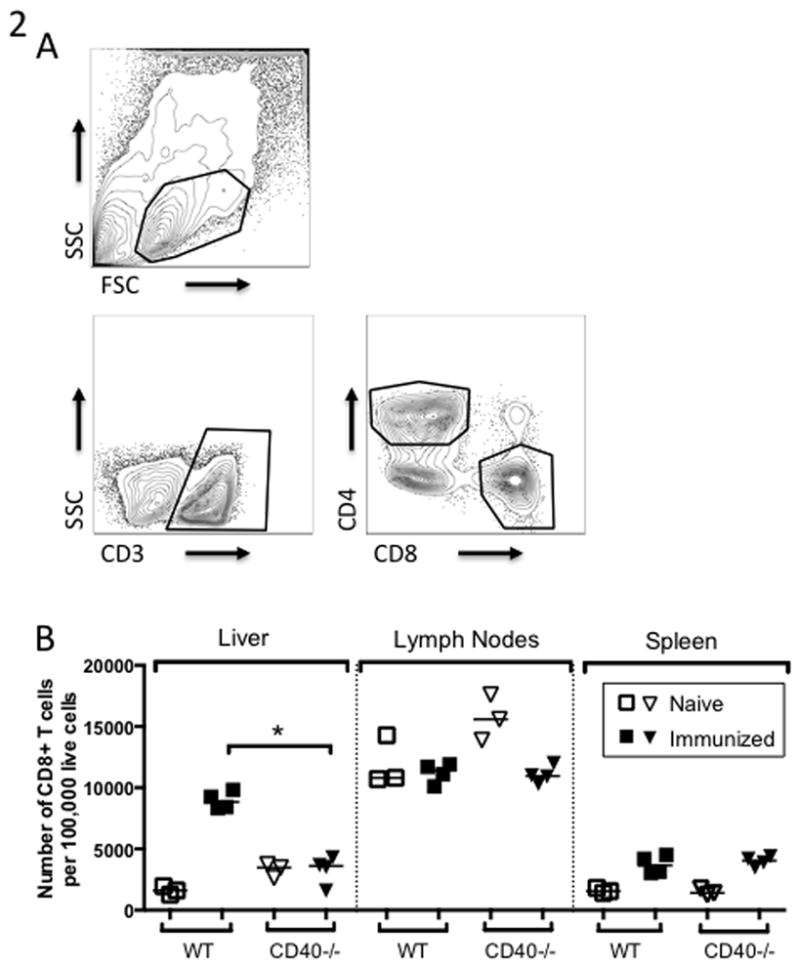
WT or CD40−/− C57BL/6 mice were immunized once with 5×104 fabb/f- sporozoites. Lymphocytes were collected from the liver, lymph nodes, and spleen on Day 7 post-immunization. (A) Strategy for gating CD4+ and CD8+ T cells from non-parenchymal cells isolated from the liver. (B) Number of CD8+ T cells per 100,000 total live cells recovered from liver, lymph nodes and spleen.
CD40-deficient dendritic cells fail to become fully licensed and upregulate a different set of chemokine genes
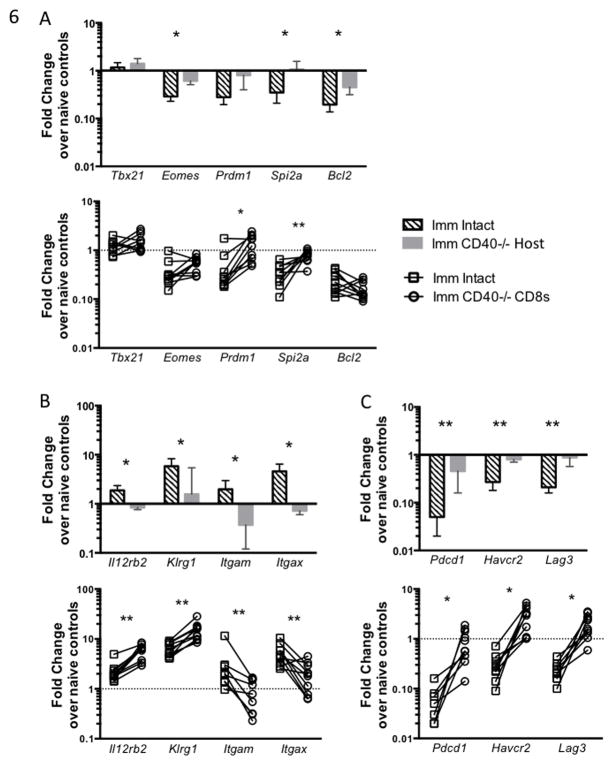
Given the dramatically lower numbers of CD8+ T cells in the liver and lack of protective immunity in CD40−/− mice, we investigated another key outcome of CD40 signaling: APC licensing. We isolated CD11c+ MHCIIhi DC from the livers of immunized mice [Fig. 3A] and measured expression of genes and surface markers associated with APC activation and T cell priming. On Day 5 after immunization, we found that while certain inflammatory genes (Ifng, Nos2) were expressed equally by CD40−/− and WT DC, genes encoding chemokines involved in recruiting inflammatory cells and lymphocytes (Ccl2, Ccl5, Ccl7, Cx3cl1) were induced differently in WT DC and CD40−/− DC [Fig. 3B]. This is striking because CD40 was not simply acting as a rheostat, modulating all T cell functions in parallel. Instead, its effects were specific. Interestingly, it does not appear that lower CD8+ T cell numbers in the livers of CD40−/− mice were due to reduced expression of chemokines that promote T cell recruitment, Ccl5 and Cx3cl1. Additionally, liver DC from WT mice had upregulated surface expression of MHC class II and CD86 at Day 5, but DC from CD40−/− mice expressed lower levels of these molecules [Fig. 3C and D], indicating they had not been licensed to the same extent as WT DC. Expression of CD80 was similar between WT and CD40−/− DC [not shown]. Interestingly, splenic DC did not appear to upregulate these molecules as much as liver DC.
Figure 3. CD40-deficient dendritic cells fail to become fully licensed.
WT or CD40−/− C57BL/6 mice were immunized once with 5×104 fabb/f- sporozoites and dendritic cells were collected from the liver 5 days later. (A) Gating strategy. Liver DC were defined as the CD11blo CD11chi MHCIIhi population within the non-parenchymal cell (NPC) gate which excludes most lymphocytes. (B) Inflammatory and lymphocyte recruitment genes expressed by liver DC as measured by q-RT-PCR. Values are normalized to Hprt and shown as fold change over naïve controls. (C) Representative histograms of MHC class II and (D) CD86 expressed on liver DC, left, and mean fluorescence intensity (MFI) from livers and spleens.
CD40 is necessary for full CD4+ T cell activation and production of IFN-gamma
MHC class II and CD86 expressed on DC are crucial to forming immunological synapses that prime CD4+ T cells (38), thus we investigated whether CD40 deficiency also resulted in suboptimal CD4+ T cell activation during fabb/f- immunization. CD4+ T cells appeared to proliferate similarly in WT and CD40−/− mice after a single immunization with fabb/f- [Fig. 4A], though we did note that CD40−/− mice had significantly more CD4+ T cells prior to immunization, making it hard to definitively conclude that the response was quantitatively similar between groups. CD4+ T cells expressed similar levels of CD44 and CD62L in WT and CD40−/− mice [not shown].
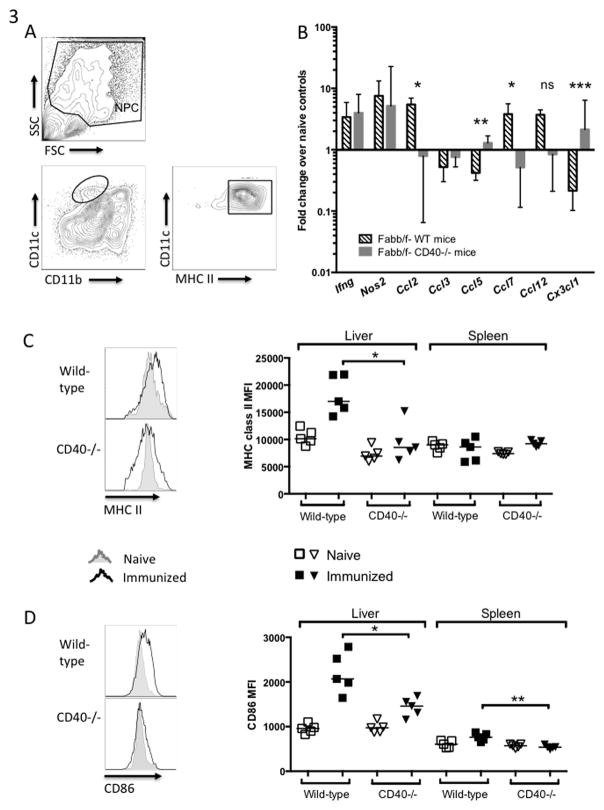
Figure 4. CD4+ T cells primed in CD40−/− mice fail to become fully activated.
WT or CD40−/− mice were immunized once with 5×104 fabb/f- sporozoites and lymphocytes from the liver and spleen were collected on Day 7 (A) or Day 5 (B, C). (A) Number of CD4+ cells collected per 100,000 total live cells. (B) Gene expression of liver CD4+ T cells as measured by q-RT-PCR shown as fold change over naïve controls. (C) Representative plots showing intracellular IFN-γ staining in liver CD4+ T cells and aggregate data from the liver and spleen.
To determine the effect of CD40 deficiency on CD4+ T cell activation and differentiation, we examined expression of several genes in CD4+ T cells isolated from the livers of mice immunized once with fabb/f- sporozoites. CD4+ T cells from CD40−/− mice expressed lower levels of the Th1-associated genes Il12rb2 and Ifng [Fig. 4B]. Additionally, the proportion of IFN-γ+ CD4+ T cells increased upon immunization in WT mice, and there was a significantly lower percentage of IFN-γ+ cells in CD40−/− mice at Day 5 [Fig. 4C]. Taken together, CD4+ T cells appeared to be less activated in CD40−/− hosts by some measures, seeming to lack the proper co-stimulation that would drive them toward Th1 effector cell differentiation.
CD40 expressed on APC drives CD8+ effector cell differentiation and accumulation
CD40 can be expressed both on APC and on CD8+ T cells, providing two possible modes of CD40:CD40L interaction. Because the importance of CD8+ T cell-autonomous CD40 is under considerable debate, we addressed this question in the context of immunity to liver stage Plasmodium infection. To do this, we transferred a 50:50 mixed population of polyclonal WT and CD40-deficient CD8+ T cells into either WT or CD40−/− host mice prior to immunization. This allowed us to examine the effects of APC CD40-deficency by analyzing WT CD8+ T cells in WT and CD40−/− hosts, while also interrogating the effect of CD40 deficiency in CD8+ T cells by comparing WT and CD40−/− CD8+ T cells primed in the same WT host. Upon collecting cells from the liver and spleen at Day 7 post-immunization, we found that CD8+ T cell accumulation in the liver depended on CD40 expressed on APC; the defect in numbers was still present in WT CD8+ T cells primed in CD40−/− hosts but accumulation was normal when CD40−/− CD8+ T cells were primed in WT hosts [Fig. 5A].
We then examined expression of genes associated with effector cell activation and memory precursor differentiation in CD8+ T cells isolated from the livers of mice in the experiment above. WT CD8+ T cells primed in WT hosts appeared to downregulate genes associated with memory precursor effector cells (MPEC) formation, Bcl2, Eomes, and Spi2a compared to those primed in CD40−/− hosts [Fig. 6A, top]. CD40−/− CD8+ T cells primed in WT hosts appeared to upregulate Spi2a, but downregulated the effector-associated gene Prdm1 (Blimp1) [Fig. 6A, bottom]. CD8+ T cells primed by CD40−/− APC expressed lower levels of Il12rb2, Klrg1, and the activation-associated genes Itgam (CD11b) and Itgax (CD11c) at Day 5 post-immunization [Fig. 6B, top]. CD40−/− CD8+ T cells primed in WT hosts also upregulated Itgam and Itgax less than WT CD8+ T cells, but intriguingly, CD40−/− CD8+ T cells upregulated Il12rb2 and Klrg1 even more than WT cells primed in WT hosts [Fig 6B, bottom]. Expression of the IL-12 receptor and IL-12 signaling have been associated with effector cell development but are also needed for survival and recall responses by CD8+ T cells (39, 40). Taken together, these data indicate that activation and differentiation of effector CD8+ T cells relied on CD40 expressed on the APC and not CD8+ T cell-autonomous CD40. Of note, both CD8+ T cells primed in CD40−/− hosts, and those missing CD40−/− themselves, failed to downregulate genes associated with exhaustion, Pdcd1 (PD1), Havcr2 (Tim3), and Lag3, as much as WT CD8+ T cells in intact mice [Fig 6C].
CD40 is required for differentiation of effector cells
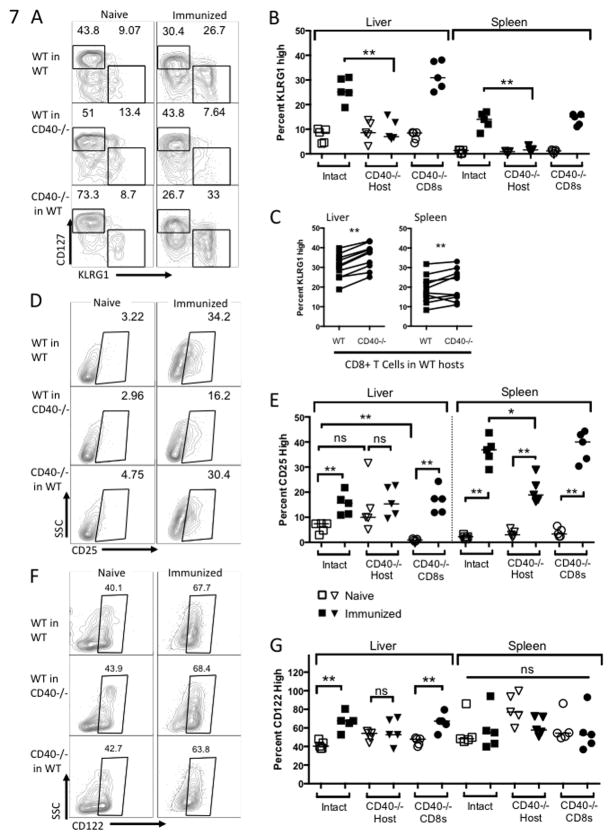
We next investigated expression of cell surface markers associated with differentiation of effector cells and memory precursor effector cells (MPEC), which we defined on the basis of CD127/KLRG1 and CD122 expression. We first observed that significantly more CD8+ T cells primed in WT hosts were expressing KLRG1, a marker expressed by terminally differentiated effector cells, compared to those primed in CD40−/− hosts [Fig. 7A and B]. Surprisingly, more CD40−/− CD8+ T cells expressed KLRG1 than WT CD8+ T cells primed in WT hosts [Fig. 7C]. We also observed that CD25 was upregulated on CD8+ T cells in WT hosts at Day 5 but not on liver CD8+ T cells primed in CD40−/− hosts. In the spleen, CD25 was upregulated in all three groups of mice, but expressed at a higher level on CD8+ T cells primed in WT hosts [Fig. 7D, E].
Figure 7. Host CD40 drives differentiation of effector cells.
Mice were treated as in Figure 5 and cells were collected on Day 5 post-immunization. (A) Representative plots of KLRG1 and CD127 expression on liver CD8+ T cells and (B) aggregate KLRG1 expression data from liver and spleen. Panel (B) compares WT CD8+ T cells in WT and CD40−/− hosts (Mann-Whitney test) and panel (C) compares WT and CD40−/− CD8+ T cells in WT hosts (Wilcoxon matched pairs test). (D) Representative plots of CD25 expression on splenic CD8+ T cells and (E) aggregate data from liver and spleen. (E) Representative plots of CD122 expression on liver CD8+ T cells and (F) aggregate data from liver and spleen.
Given higher proportions of effector cells in WT hosts, we were surprised to see equal or higher proportions of CD127+ and CD122+ memory precursor cells in WT hosts as well. There were no significant differences in levels of CD127 between groups of CD8+ T cells [summary data not shown], which is expressed early in the primary response by cells that will form the memory population (41, 42). Additionally, CD8+ T cells primed in WT hosts upregulated CD122 to a greater extent than those primed in CD40−/− hosts [Fig. 7F, G]. CD122 is a subunit of the IL-15 receptor and its expression on CD8+ T cells during the primary response has been associated with the formation of Tcm as well as long-term survival of those cells in the memory population (43, 44).
Taken together, our observations suggest that CD40 expressed on the APC drives differentiation of effector CD8+ T cells in fabb/f-immunized mice, and that CD40 expressed on the CD8+ T cell itself may serve to limit effector cell differentiation.
Discussion
The role of CD40 stimulation and nature of CD4+ T cell help during immunization with the fabb/f- GAP has not been investigated. We set out to answer three questions: i) Whether CD40 signaling is required for protective immunity, ii) what components of the primary immune response, such as CD4+ T cell lineage or CD8+ T cell differentiation, are regulated by CD40 signaling, and iii) whether any of CD40’s effects are mediated through CD8+ T cell-autonomous CD40 expression. We found that during fabb/f- immunization, CD40 signaling plays key roles in licensing liver DC, generating an effector Th1 response, and driving robust CD8+ T cell activation and differentiation of both effector and memory precursor cells. We observed a clear compartmentalization of CD40 function when expressed on different cell types. While CD8+ T cell accumulation in the liver, CD4+ T cell activation, and DC licensing depended on CD40 expressed on the APC, CD8+ T cell-autonomous CD40 was critical to limit terminal differentiation in CD8+ T cells.
Much insight into the immune response to RAS has been gained though experiments performed with circumsporozoite protein (CS)-specific transgenic CD8+ T cells (9, 45, 46). In the study described here, we chose to examine the polyclonal CD8+ T cell response in its entirety instead of focusing on a single T cell specificity with a transgenic clone or tetramer. The reasons for this are two-fold. First, the late-arresting P. yoelii fabb/f- strain induces a broader T cell repertoire than early-arresting RAS, perhaps due to the increasing variety of antigens expressed later in liver stage development (6). The response of CD8+ T cells to stimuli is often clone-dependent and can vary greatly depending on TCR affinity for a particular epitope and what signals the APC provides in response to that antigen (47, 48), thus the quality of response made by CS-specific T cells may only represent part of the broad CD8+ T cell response to immunization with fabb/f- sporozoites. Other non-CS antigens may be important in generating sterile immunity against liver stage infection. While immunization with CS protein alone can protect mice against infectious challenge (49), a sub-unit vaccine based on CS, called RTS, S, is only partially successful in humans (50) and a response against CS is not required in mice to confer protective immunity (49, 51). As the immunodominant antigen on sporozoites, CS may actually act as a decoy antigen, drawing the immune response away from more protective antigens expressed later in liver stage development (49, 52). An alternative to studying specific T cells would be to examine activated or antigen-experienced CD8+ T cells recognized by their upregulation of CD11a (29), however, we found that the vast majority of CD8+ T cells collected from the liver were activated even in naïve mice, in line with previous studies (30, 31). Secondly, recent experiments describe the importance of innate-like activation of non-antigen-specific CD8+ T cells during various models of infection (53, 54) and our approach allows us to examine both the adaptive and innate T cell responses to GAP immunization. Innate inflammatory responses driven by unrelated antigen, such as RNA from Hepatitis C Virus or the TLR9 ligand CpG, or induced by a primary Plasmodium infection can prevent or reduce liver stage Plasmodium infection in mice 24–48 hours later (20, 55–57) and these innate responses are likely important in generating a long-lived memory T cell response.
CD4+ T cell help is necessary for an effective CD8+ T cell memory response against non-inflammatory antigens, such as tumor cells and certain pathogens that may not carry sufficient danger signals (48). The liver is a tolerogenic environment, tasked with modulating immune responses to harmless food antigen or components of commensal bacteria carried from the gut. Liver pathogens such as Hepatitis C virus appear to exploit this bias, resulting in chronic infection characterized by inflammation but ineffective host defense (58). Better disease outcomes and clearance of HCV are associated with robust CD4+ T cell responses, suggesting that CD4+ T cell help is needed to overcome tolerance in the liver (59). Against liver stage Plasmodium, mice depleted of CD4+ T cells during immunization with RAS failed to experience robust CD8+ T cell expansion and were not protected against challenge (10, 11). In the current study, we found that CD4+ T cell help was also necessary to induce protection during immunization with the fabb/f- GAP. Of note, while we found that sterile immunity conferred by fabb/f- sporozoites against iv challenge did not require a B cell response, antibodies induced by attenuated parasites can provide protection against mosquito bite challenge (60, 61).
While some studies have implicated Th1 cells in protective immunity, several recent studies using the RAS immunization model instead support the concept that CD8+ T cell response requires IL-4 produced by CD4+ T cells and expression of IL-4R on CD8+ T cells, suggesting Th2 cells are important (21, 22). In the current study, we found that CD4+ T cells had upregulated Il4 transcript but not other genes associated with the Th2 lineage. Instead, they were expressing genes and cytokines associated with the Th1 lineage- Tbx21 (Tbet), Il12rb2, Cxcr3, and IFN-γ. This suggests a resolution between the apparently conflicting previous studies, based on the idea that anti-liver stage CD4+ T cell help involves Th1-like cells that nevertheless make IL-4. We found that CD40−/− led to decreased expression of Il12rb2 and IFN-γ, increased expression of the Th2 cytokine Il5, and a trend toward increased expression of Gata3, a Th2 transcription factor, suggesting that it is Th1 responses that are needed for protective immunity. Similar to our findings here, Th1 responses have been associated with protection against several intracellular parasitic infections (62–64), but variations in T helper cell responses may be due to the different mouse and parasite strains used in various studies.
As a principle mechanism of CD4+ T cell help, the requirement for CD40 is similarly contingent upon the type of infection or model system studied. Experiments using bacterial or viral models have produced conflicting results as to whether CD4+ T cells and CD40 are needed at all to prime an effective CD8+ T cell response. In studies that demonstrate it is required, CD40 expressed on the APC was responsible for CD8+ T cell priming and memory formation but CD8+ T cell-autonomous CD40 played no appreciable role (23, 25). However, using a model in which female CD8+ T cells respond to male HY antigen – a system which presumably does not carry as many danger signals as bacterial or viral infection – CD40 was required on both the APC and the CD8+ T cell to generate effective memory responses. Specifically, CD40 expressed on the APC was necessary for clonal expansion while CD40 on the CD8+ T cell itself was required for memory CD8+ T cell formation (26, 27). Whether CD40 plays any role in liver-stage Plasmodium infection has never been examined and thus we were interested in whether the requirement for CD40 mirrored that of bacterial and viral models or that of non-inflammatory antigens.
The best-known effect of CD40 stimulation is licensing APC to better prime T cells. At Day 5 post-immunization we observed that compared to WT mice, DC in CD40−/− mice expressed less cell surface MHC class II and CD86, which are markers of activation and are required to fully prime CD4+ T cells. CD40−/− DC also upregulated a different set of chemokines. While WT DC upregulated the chemokine genes Ccl2 and Ccl7 more than CD40−/− DC, CD40−/− DC upregulated genes for T cell recruitment chemokines Ccl5 and Cx3cl1 more than WT DC. From these data, we surmise that the drastically lower numbers of CD8+ T cells found in the livers of CD40−/− mice are not directly due to a defect in DC recruitment of lymphocytes, but it remains unclear whether reduced CD8+ T cell proliferation or increased cell death results in the lower numbers seen in CD40-deficient hosts. The differences in activation and chemokine expression profile in CD40−/− DC likely result in sub-optimal priming of T cells, leading to the lack of protective immunity we saw in CD40−/− mice immunized with fabb/f-.
Of note, liver APC generally considered to be immunosuppressive – KC and LSEC – downregulated CD40 while liver DC upregulated CD40. Though there are many fewer DC in the liver than KC and LSEC, it appears that enough DC become licensed during fabb/f- immunization in WT mice to prime a protective immune response. We also found it intriguing that fabb/f- immunization induced higher levels of CD86 and MHCII expression by DC in the liver than by DC in the spleen. The majority of CD8+ T cells specific for CS are primed in the lymph node draining the site of mosquito bite inoculation during immunization with RAS (9, 49). However, some evidence suggests that DC in the livers of RAS-immunized mice acquire parasite antigen from dying, infected hepatocytes and confer protection against low-dose challenge when transferred to naïve mice (8, 65). Local T cell priming in the liver may be an important component of immunity conferred by late-arresting vaccine strains such as the fabb/f- GAP. Immunization with fabb/f- sporozoites results in a broader T cell repertoire and more robust protection against infectious challenge than early-arresting RAS (6, 9). Intravenous administration of attenuated parasites, which allows sporozoites easier transport to the liver, provides better protection than intradermal or intramuscular routes (2). Additionally, dead sporozoites provide no protection at all, suggesting that parasite development in the liver is critical to inducing a robust response (66). Whether T cells are primed in the liver during fabb/f- immunization remains unclear, but our observation that liver DC in particular upregulated antigen presentation and co-stimulatory molecules suggests that this question merits further examination.
CD40 stimulation is known to induce IL-12 production in DC and macrophages, and IL-12 signaling in turn drives Th1 and effector cell differentiation and promotes cell survival in both CD4+ and CD8+ T cells (16, 67, 68). We found that both CD4+ and CD8+ cells upregulated Il12rb in response to fabb/f- immunization and that this was abolished in CD40−/− mice, suggesting these cells were unable to receive IL-12 signals. Importantly, IL-12 signaling is required for protection against liver stage Plasmodium infection. IL12rb2-deficient and IL-12-deficient mice are not protected by immunization with attenuated sporozoites. These effects have been partially explained by IL-12 inducing a Th1 response (19, 69), but could also reflect the role of IL-12 signaling in cell survival and programming effective memory CD8+ T cell responses.
In both CD8+ and CD4+ T cells, IL-12 signaling has been linked to better memory responses. IL-12-deficient and IL-12 receptor-deficient cells do not survive well after immunization and they exhibit poor recall responses upon secondary stimulation (40, 67, 70). Increased expression of the Il12rb2 gene in CD4+ and CD8+ T cells in response to immunization with fabb/f- sporozoites could lead to increased cell survival and thus contribute to protective immunity. However, IL-12 signaling appears to be a double-edged sword. Recent work suggests that while a small amount of IL-12 is required to form memory cells, IL-12 is also responsible for driving terminal differentiation of effector CD8+ T cells (71). IL-12 induces transcription factors associated with effector cell differentiation while repressing those associated with memory in a dose-dependent manner (39). IL-12 signaling also leads to upregulation of CD25 (72), the high-affinity subunit of the IL-2 receptor, and IL-2 induces T cell activation and proliferation (73–75). We observed that CD8+ T cells primed in WT hosts upregulated Il12rb2 transcript and expressed more CD25 on their surface than cells primed in CD40−/− hosts. It is likely that lower expression of these molecules resulted in the severe reduction of CD8+ T cells in the liver and reduced KLRG1 expression that we observed in CD40−/− mice.
Unexpectedly, when we investigated whether CD8+ T cell-autonomous CD40 played a role in generating an effective CD8+ T cell response, we found that CD40−/− CD8+ T cells expressed even higher amounts of Il12rb2 transcript than WT cells. Concordantly, CD40−/− CD8+ T cells expressed higher levels of KLRG1 and Prdm1 (which encodes the transcription factor Blimp1) than WT cells, indicating a higher portion of CD40−/− cells had become terminally differentiated effector cells. Interestingly, CD40−/− CD8+ T cells failed to upregulate Itgax, which encodes CD11c, upon immunization despite showing increases in Il12rb2 and KLRG1 expression. CD11c is expressed by multi-functional short-lived effector cells upon immunization with attenuated parasites (76). This may indicate that although CD40−/− CD8+ T cells appear to become effector cells, they are unable to become fully activated. It is unclear whether the same population of CD40−/− CD8+ T cells that upregulated effector-associated genes also accounted for the observed difference in Spi2a expression, which is associated with memory cell formation (77).
Though it is impossible to detect truly “exhausted” cells during the primary response, we found the expression of genes associated with exhaustion in CD40-deficient hosts and CD8+ T cells intriguing. While WT CD8+ T cells in WT hosts downregulated Pdcd1, Lag3, and Havcr1 (which encode the inhibitory receptors PD1, LAG3, and TIM3), WT CD8+ T cells in CD40−/− hosts didn’t downregulate these genes as much, and CD40−/− CD8+ T cells appear to have upregulated Havcr2 and Lag3. CD40−/− CD8+ T cells have been observed to become unresponsive to stimuli over time, characteristic of exhausted cells (27). In line with our results, CD40 expressed on CD8+ T cells has been implicated in the rescue of exhausted T cells in chronic Hepatitis B virus and Toxoplasma gondii infections (24, 78). Though never investigated in liver stage Plasmodium infection, blockade of exhaustion molecules PDL1 and LAG3 speeds clearance of blood-stage infection (79).
Interestingly, we did not observe a correspondingly low proportion of CD127+ or CD122+ memory precursor cells in the WT host mice, even though their CD8+ T cell responses seemed to be largely dominated by effector cells. Instead, WT hosts appeared to prime more effector cells and just as many or more MPEC than CD40−/− hosts.
Large numbers of CD8+ T cells are needed during the primary response to RAS to generate protective immunity (80), likely because a more robust primary CD8+ T cell response means a greater number of memory precursor cells survives to form solid populations of memory cells that can protect against future infection (39, 81). Additionally, recent reports describe the importance of KLRG1+ “long-lived effector cells”, which, despite a lack of prolonged self-renewal, can protect against secondary infection (39, 82). In the current study, we saw a severe reduction in CD8+ T cells in the livers of CD40−/− mice. We also observed reduced expression of genes and surface receptors – such as Il12rb2, CD25, and CD122 – that are indicative of adequate CD4+ T cell help and are required during the primary response for effective CD8+ T cell secondary responses or for the survival of memory cells (43, 44, 73, 83–85). Given these defects in CD40-deficient hosts, it is unsurprising that protective immunity induced by fabb/f- immunization was abrogated in CD40−/− mice.
Taken together, these results suggest that CD40 expressed in the host environment drives APC licensing, CD4+ T cell activation, and robust CD8+ T effector cell responses during fabb/f- immunization. Thus, protective immunity conferred by late-arresting parasite vaccines and mediated by CD8+ T cells relies heavily on APC licensing and strong co-stimulatory signals.
Acknowledgments
Many thanks Sebastian Yuen for technical assistance, Hieu Nguyen for cell sorting, Dat Mai for animal caretaking, and Brandon Sack for manuscript review.
Footnotes
This work was supported by Seattle Biomedical Research Institute, the University of Washington Department of Pathology, and the University of Washington Pathobiology Program “Diseases of Public Health Importance” NIH Training Grant T32AI007509-13.
References
- 1.World Health Organization. World Malaria Report 2012. 2013. pp. 1–24. [Google Scholar]
- 2.Epstein JE, Tewari K, Lyke KE, Sim BKL, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. Live Attenuated Malaria Vaccine Designed to Protect Through Hepatic CD8+ T Cell Immunity. Science. 2011;334:475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 3.Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, Williams J, de la Vega P, Ware L, Komisar J, Polhemus M, Richie TL, Epstein J, Tamminga C, Chuang I, Richie N, O’Neil M, Heppner DG, Healer J, O’Neill M, Smithers H, Finney OC, Mikolajczak SA, Wang R, Cowman A, Ockenhouse C, Krzych U, Kappe SHI. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jobe O, Lumsden J, Mueller AK, Williams J, Silva Rivera H, Kappe SHI, Schwenk RJ, Matuschewski K, Krzych U. Genetically Attenuated Plasmodium berghei Liver Stages Induce Sterile Protracted Protection That Is Mediated by Major Histocompatibility Complex Class I–Dependent Interferon-γ–Producing CD8 +T Cells. J Infect Dis. 2007;196:599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SHI, Harty JT. Superior Antimalarial Immunity after Vaccination with Late Liver Stage-Arresting Genetically Attenuated Parasites. Cell Host and Microbe. 2011;9:451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongfen SE, Torgler R, Romero JF, Rénia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol. 2007;178:7054–7063. doi: 10.4049/jimmunol.178.11.7054. [DOI] [PubMed] [Google Scholar]
- 8.Jobe O, Donofrio G, Sun G, Liepinsh D, Schwenk R, Krzych U. Immunization with Radiation-Attenuated Plasmodium berghei Sporozoites Induces Liver cCD8α+DC that Activate CD8+T Cells against Liver-Stage Malaria. PLoS ONE. 2009;4:e5075. doi: 10.1371/journal.pone.0005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 10.Weiss WR, Sedegah M, Berzofsky JA, Hoffman SL. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 11.Overstreet MG, Chen Y-C, Cockburn IA, Tse S-W, Zavala F. CD4+ T Cells Modulate Expansion and Survival but Not Functional Properties of Effector and Memory CD8+ T Cells Induced by Malaria Sporozoites. PLoS ONE. 2011;6:e15948. doi: 10.1371/journal.pone.0015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 13.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 14.Tang YC, Thoman M, Linton P-J, Deisseroth A. Use of CD40L immunoconjugates to overcome the defective immune response to vaccines for infections and cancer in the aged. Cancer Immunol Immunother. 2009;58:1949–1957. doi: 10.1007/s00262-009-0718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miconnet I, Pantaleo G. A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. Vaccine. 2008;26:4006–4014. doi: 10.1016/j.vaccine.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedegah M, Finkelman F, Hoffman SL. Interleukin 12 induction of interferon gamma-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler NS, Schmidt NW, Harty JT. Differential Effector Pathways Regulate Memory CD8 T Cell Immunity against Plasmodium berghei versus P. yoelii Sporozoites. J Immunol. 2010;184:2528–2538. doi: 10.4049/jimmunol.0903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 20.Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin-12- and Gamma Interferon-Dependent Protection against Malaria Conferred by CpG Oligodeoxynucleotide in Mice. Infect Immun. 2001;69:1643–1649. doi: 10.1128/IAI.69.3.1643-1649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalho LH, Sano G-I, Hafalla JCR, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 22.Morrot A. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202:551–560. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez MGH, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even in the absence of CD4+ T cell help. J Immunol. 2008;180:4382–4390. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]
- 24.Bhadra R, Gigley J, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol. 2011;187:4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois C. A Role for CD40 Expression on CD8+ T Cells in the Generation of CD8+ T Cell Memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 27.Meunier S, Rapetti L, Beziaud L, Pontoux C, Legrand A, Tanchot C. Synergistic CD40 signaling on APCs and CD8 T cells drives efficient CD8 response and memory differentiation. J Leukocyte Biol. 2012;91:859–869. doi: 10.1189/jlb.0611292. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, Aly ASI, Cowman AF, Kappe SHI. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai D, Pham NLL, Harty JT, Badovinac VP. Tracking the Total CD8 T Cell Response to Infection Reveals Substantial Discordance in Magnitude and Kinetics between Inbred and Outbred Hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004;172:5222–5229. doi: 10.4049/jimmunol.172.9.5222. [DOI] [PubMed] [Google Scholar]
- 31.Tu Z, Bozorgzadeh A, Crispe IN, Orloff MS. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007;149:186–193. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JL, Murray S, Vaughan AM, Harupa A, Sack B, Baldwin M, Crispe IN, Kappe SHI. Quantitative Bioluminescent Imaging of Pre-Erythrocytic Malaria Parasite Infection Using Luciferase-Expressing Plasmodium yoelii. PLoS ONE. 2013;8:e60820. doi: 10.1371/journal.pone.0060820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatol. 2011;54:1379–1387. doi: 10.1002/hep.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 35.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D. Preferential Localization of Effector Memory Cells in Nonlymphoid Tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 38.Radziewicz H, Ibegbu CC, Hon H, Bédard N, Bruneau J, Workowski KA, Knechtle SJ, Kirk AD, Larsen CP, Shoukry NH, Grakoui A. Transient CD86 expression on hepatitis C virus-specific CD8+ T cells in acute infection is linked to sufficient IL-2 signaling. J Immunol. 2010;184:2410–2422. doi: 10.4049/jimmunol.0902994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation Directs Memory Precursor and Short-Lived Effector CD8+ T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 41.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busch DH, Kerksiek KM, Pamer EG. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164:4063–4070. doi: 10.4049/jimmunol.164.8.4063. [DOI] [PubMed] [Google Scholar]
- 43.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 Controls both Proliferation and Survival of a Subset of Memory-Phenotype CD8+ T Cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obar JJ, Lefrancois L. Early Signals during CD8+ T Cell Priming Regulate the Generation of Central Memory Cells. J Immunol. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites. PLoS Pathog. 2010;6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 47.Ramsburg EA, Publicover JM, Coppock D, Rose JK. Requirement for CD4 T cell help in maintenance of memory CD8 T cell responses is epitope dependent. J Immunol. 2007;178:6350–6358. doi: 10.4049/jimmunol.178.10.6350. [DOI] [PubMed] [Google Scholar]
- 48.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar KA, Sano G-I, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 50.The RTS, S Clinical Trials Partnership. First Results of Phase 3 Trial of RTS, S/AS01 Malaria Vaccine in African Children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 51.Mauduit M, Tewari R, Depinay N, Kayibanda M, Lallemand E, Chavatte JM, Snounou G, Renia L, Gruner AC. Minimal Role for the Circumsporozoite Protein in the Induction of Sterile Immunity by Vaccination with Live Rodent Malaria Sporozoites. Infect Immun. 2010;78:2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy SC, Kas A, Stone BC, Bevan MJ. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. Proc Nat Acad Sci. 2013;110:6055–6060. doi: 10.1073/pnas.1303834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-Activated Memory CD8 T Cells Control Early Pathogen Loadin an Innate-like, NKG2D-Dependent Manner. CellReports. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, Germain RN. A Spatially-Organized Multicellular Innate Immune Response in Lymph Nodes Limits Systemic Pathogen Spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen A, WXU, Zhou T, Ding Y, Duan J, Huang F. Inhibitory role of toll-like receptors agonists in Plasmodium yoeliiliver stage development. Parasite Immunol. 2009;31:466–473. doi: 10.1111/j.1365-3024.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 56.Liehl P, VZ-LIs, Chan J, Zillinger T, Baptista F, Carapau D, Konert M, Hanson KK, Carret CEL, Lassnig C, Müller M, Kalinke U, Saeed M, Chora AF, Golenbock DT, Strobl B, Encio MP, Coelho LP, Kappe SH, Superti-Furga G, Pichlmair A, Vigário AM, Rice CM, Fitzgerald KA, Barchet W, Mota MM. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2013;20:47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-Mediated Innate Immune Responses against Malaria Parasite Liver Stages. CellReports. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 59.Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226–1234. doi: 10.1136/gutjnl-2011-300620. [DOI] [PubMed] [Google Scholar]
- 60.Sack BK, Miller JL, Vaughan AM, Douglass A, Kaushansky A, Mikolajczak S, Coppi A, Gonzalez-Aseguinolaza G, Tsuji M, Zavala F, Sinnis P, Kappe SHI, Adams JH. Model for In Vivo Assessment of Humoral Protection against Malaria Sporozoite Challenge by Passive Transfer of Monoclonal Antibodies and Immune Serum. Infect Immun. 2014;82:808–817. doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. The CD40/CD40 Ligand Interaction Is Required for Resistance to Toxoplasmic Encephalitis. Infect Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaussabel D, Jacobs F, de Jonge J, de Veerman M, Carlier Y, Thielemans K, Goldman M, Vray B. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect Immun. 1999;67:1929–1934. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 65.Leirião P, Mota MM, Rodriguez A. Apoptotic Plasmodium-infected hepatocytes provide antigens to liver dendritic cells. J Infect Dis. 2005;191:1576–1581. doi: 10.1086/429635. [DOI] [PubMed] [Google Scholar]
- 66.Alger NE, Harant J. Plasmodium berghei: protection against sporozoites by normal mosquito tissue vaccination of mice. 1976;40:269–272. doi: 10.1016/0014-4894(76)90090-4. [DOI] [PubMed] [Google Scholar]
- 67.Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- 68.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 69.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science (New York, NY) 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 70.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T Cell Memory Development Requires IL-12 or Type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel E-M, Gubbels M-J, Ploegh HL, Yap GS. Differential Regulation of Effector- and Central-Memory Responses to Toxoplasma gondii Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells. PLoS Patho g. 2010;6:e1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and Inflammation InduceDistinct Transcriptional Programs that Promote the Differentiation of Effector Cytolytic T Cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged Interleukin-2Ra Expression on Virus-Specific CD8. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8+ memory T cells. Nat Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooney LA, Gupta M, Thomas S, Mikolajczak S, Choi KY, Gibson C, Jang IK, Danziger S, Aitchison J, Gardner MJ, Kappe SHI, Wang R. Short-Lived Effector CD8 T Cells Induced by Genetically Attenuated Malaria Parasite Vaccination Express CD11c. Infect Immun. 2013;81:4171–4181. doi: 10.1128/IAI.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, Ashton-Rickardt PG. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5:919–926. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- 78.Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV. CD40 Activation Rescues Antiviral CD8+ T Cells from PD-1-Mediated Exhaustion. PLoS Pathog. 2013;9:e1003490. doi: 10.1371/journal.ppat.1003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Expl Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8+ T Cells in the Memory Population Mediate Potent Protective Immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. J Exp Med. 2006;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 85.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]