Abstract
The study of T cell immunity at barrier surfaces has largely focused on T cells bearing the αβ T cell receptor. However, T cells that express the γδ T cell receptor are disproportionately represented in peripheral tissues of mice and humans, suggesting they too may play an important role responding to external stimuli. Here we report that in a murine model of cutaneous infection with Vaccinia Virus, dermal γδ T cell numbers increased ten-fold in the infected ear and resulted in a novel γδ T cell population not found in naïve skin. Circulating γδ T cells were specifically recruited to the site of inflammation and differentially contributed to dermal populations based on their CD27 expression. Recruited γδ T cells, the majority of which were CD27+, were Granzyme B+ and made up about half of the dermal population at the peak of the response. In contrast, recruited and resident γδ T cell populations that made IL-17 were CD27−. Using a double chimera model that can discriminate between the resident dermal and recruited γδ T cell populations, we demonstrated their divergent functions and contributions to early stages of tissue inflammation. Specifically, the loss of the perinatal thymus-derived resident dermal population resulted in decreased cellularity and collateral damage in the tissue during viral infection. These findings have important implications for our understanding of immune coordination at barrier surfaces and the contribution of innate-like lymphocytes on the front lines of immune defense.
INTRODUCTION
The skin is host to a network of T lymphocytes, some of which take up permanent residence within the tissue, having been recruited there throughout life, while others are only transiently present (1, 2). Following antigen-specific activation in central lymphoid organs, TCRαβ effector cells traffic to peripheral sites, including the skin, and some become resident memory T cells (TRM). These cells are recruited into the tissue as a result of local infection or inflammatory signals and remain there to protect against future external insults (3–8). In contrast to TCRαβ TRM cells, TCRγδ T cells have been shown to home to tissues and acquire specific functions as a result of developmental programming and their response via TCR recognition of foreign antigens in poorly defined (9–11). This hardwiring, which drives them particularly to barrier tissues such as the intestine, reproductive tract and skin, seems ideally suited to ensure that γδ T cells participate in innate host defense.
Within the murine skin, dendritic epidermal T cells (DETC) are resident in the epidermis. They seed the skin during fetal development, express an invariant Vγ5 TCR, have dendritic morphology and are relatively immobile (12). They have been shown to participate in keratinocyte maintenance, to respond to wounding, and to assist in the healing process (13–15). A different subset of TCRγδ T cells are resident in the dermis. Dermal γδ T cells comprise up to half of the dermal T cells in mice (16) and 2–9% in humans (17). This populations has been shown to participate in host defense against bacterial infection of the skin (16, 18) as well as to contribute to psoriasis in the mouse (19, 20) and humans (21). It is now appreciated that in mice, dermal γδ T cells exit the thymus and seed the dermis near the time of birth (11, 22). Characterization of dermal γδ T cells by several groups has revealed their memory phenotype and functions related to that of CD4+ TH17 cells, being capable of producing IL-17 upon stimulation in vitro. Dermal γδ T cells express CCR6, CXCR6, CD103, CD44, IL-23R and IL-7R (21–23). However, it is unclear whether IL-17-producing dermal γδ T cell populations observed during infection or inflammation are derived from the resident population seeded there at birth or if unique populations are recruited from the circulation.
A recent study identified a population of γδ T cells that is enriched in skin draining lymph nodes (dLN) of mice with properties similar to those of dermal γδ T cells (22). This discovery demonstrated a potential link between dermal γδ T cells and those found in the circulation. While several models of systemic viral infection (24, 25) have elicited a γδ T cell response in the spleen and LN, these studies did not address questions concerning tissue trafficking or provide a relevant context for natural modes of infection. Importantly, studies with human PBMC have found that γδ T cells in the circulation are impacted in patients with inflammatory bowel disease (26), melanoma (27) and psoriasis (28), suggesting that local inflammation can impact global γδ T cell homeostasis. These findings point to the need to understand the trafficking patterns of γδ T cells during steady state conditions and in the context of disease.
Emerging data from studies of cells in central lymphoid organs suggest that CD27 delineates γδ T cell functional subsets, leading to a paradigm shift for how γδ T cells are classified (11, 29). Expression of CD27 by mouse γδ T cells is tightly correlated with their ability to produce IFNγ, where failure to express CD27 correlates with IL-17 production (30). Importantly, analogous γδ T cell populations have been found in human PBMC (31–33), making this a relevant axis from which to orient experiments in model organisms.
In the current study, we analyzed the γδ T cell response in mouse skin to local infection with Vaccinia Virus (VV). We sought to dissect the contribution of resident versus immigrant γδ T cells following infection and to determine whether γδ T cells recruited from the circulation give rise to long-term residents in the skin after the cutaneous infection has cleared. Many previous reports have studied the γδ T cell response in TCRα−/− or TCRβ−/− mice which lack TCRαβ T cells (24, 25) or performed transfers of γδ T cells isolated from TCRβ−/− mice (34) to investigate the anti-viral response. In such scenarios the γδ T cell makeup and response is likely abnormal (35). Analyzing γδ T cells in a wild-type setting allowed us to better appreciate their role in a skin infection. Our experiments revealed that CD27+ and CD27− γδ T cells are specifically recruited from the circulation to the site of infection, but surprisingly do not become resident in the tissue, even when encountering a relatively empty niche. The immigrant γδ T cells have functional attributes distinct from the resident dermal γδ T cells, the latter being primarily responsible for enhancing the inflammatory response in the tissue.
METHODS
Mice
C57BL/6, B6.SJL-Ptprca Pepcb/BoyJ and TCRδ−/− mice were obtained from the Jackson Laboratory and TCRδ-H2BeGFP (TCRδ-GFP) mice were obtained from I. Prinz (Hannover Medical School) and have been described previously (36). Mice were housed in specific pathogen-free conditions in the animal facilities at the University of Washington and unless otherwise specified were used at 6–10 weeks of age. All experiments were done in accordance with the Institutional Animal Care and Use Committee guidelines of the University of Washington.
Infections
2×106 plaque forming units (PFU) of recombinant Vaccinia Virus expressing full-length chicken ovalbumin (VV-OVA) was used for epicutaneous infection by skin scarification, as described previously (37). Mice were anesthetized with isoflurane and 5 µl of diluted virus was applied to the dorsal side of the ear. The skin area was then gently scratched 20 times with a 28-gauge needle. For flow cytometry experiments, both ears of mice were infected and combined to comprise one sample and the numbers were divided by two to obtain the number of cells/ear.
Adoptive transfer and sorting
C57BL/6 or CD45.2+ TCRδ-GFP mice received 7×105 γδ T cells enriched from spleen and skin dLN of CD45.1+ TCRδ-GFP mice by i.v. injection one day before infection. Enrichment of γδ T cells was performed by incubating single cell suspensions with biotin-labeled anti-TCRβ and anti-CD19 mAb for 30 minutes on ice, followed by passage through the Stem Cell Biotin negative selection kit. Mice were infected on one ear and the infected (or uninfected) ears from two mice were combined for each sample. In some experiments, the enriched population was further purified as follows: cells were stained with fluorochrome-conjugated anti-CD27 and sorted on a FACS Aria for the GFP+TCRβ−CD19−CD27+ or GFP+TCRβ−CD19−CD27− populations, of which >97% were TCRγδ+. 6×105 CD27+ or 2.7×105 CD27− sorted γδ T cells from B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were transferred into separate C57BL/6 (CD45.2) hosts. Mice that received sorted cells were infected on both ears and ears from a single mouse were combined for a sample.
Flow Cytometry
Single cell suspensions were prepared from spleens and LN by mechanical disruption. Dorsal and ventral halves of ears were separated, minced and then enzymatically digested for 2 hrs at 37 °C in Liberase TM (Roche). Both ears from each mouse were combined for processing to obtain adequate cell numbers. All tissues were passaged through a 100-µm nylon sieve (BD Bioscience). In some experiments, to avoid the Liberase digestion (which cleaved CD27), dorsal and ventral halves of the ears were separated and floated dermal side down in complete media overnight at 37°C. Supernatant, into which cells had migrated, was collected the following day and stained. Cells were stained for 30 min on ice with the appropriate mixture of monoclonal antibodies and washed with PBS containing 1% BSA. The following conjugated mAb were obtained from BD Pharmingen, eBioscience or BioLegend: anti- TCRβ (H57–597), CCR6 (140706), CD27 (LG.3A10), CD103 (2E7), Vγ5 (BioLegend Vγ3 clone 536), CD44 (IM7), CD45.1 (A20), CD45.2 (104). In the ear, γδ T cells from TCRδ-GFP were identified as GFP+Vγ5− (dermal) or GFP+Vγ5+ (DETC), according to the nomenclature described by Heilig & Tonegawa (38). For the analysis of intracellular IFNγ, IL-17 and Granzyme B, single cell suspensions were incubated in the presence or absence of 50 ng/mL PMA and 500 ng/mL Ionomycin for 4 hours at 37°C in the presence of Brefeldin A. Cells were stained for surface markers and then processed using the BD PharMingen Cytofix/Cytoperm kit. Samples were analyzed on a FACSCanto II (BD) using Flowjo software (Tree Star).
In situ proliferation
Infected mice were injected i.p. with 2mg in 200µl 50-Bromo-2-deoxyuridine (BrdU) 1 hour before being euthanized. Single cell suspensions were obtained from each tissue and stained using the BD Biosciences APC BrdU Kit. Cells were fixed with PFA before Cytofix/Cytoperm to ensure retention of TCRδ-GFP signal.
Bone marrow chimeras
Chimeras were made as previously described (22). Briefly, perinatal thymocytes (pThy) were harvested 0–48 h after birth and 5×106 cells were transferred i.v. to congenic recipients that had been lethally irradiated with a dose of 1000 rad in a cesium irradiator. The next day, 1–2×106 congenic bone marrow (BM) cells were transferred. These mice are referred to as “BMpThy chimeras” while controls without pThy are referred to as “BM chimeras”. Chimeras were analyzed or infected at least 8 weeks after reconstitution.
Virus titration
The viral load in organs was determined by plaque assays on 143B cells with dilutions of a 40% tissue homogenate added to confluent wells. Titers reported are log10 PFU per whole LN or ear.
Histology
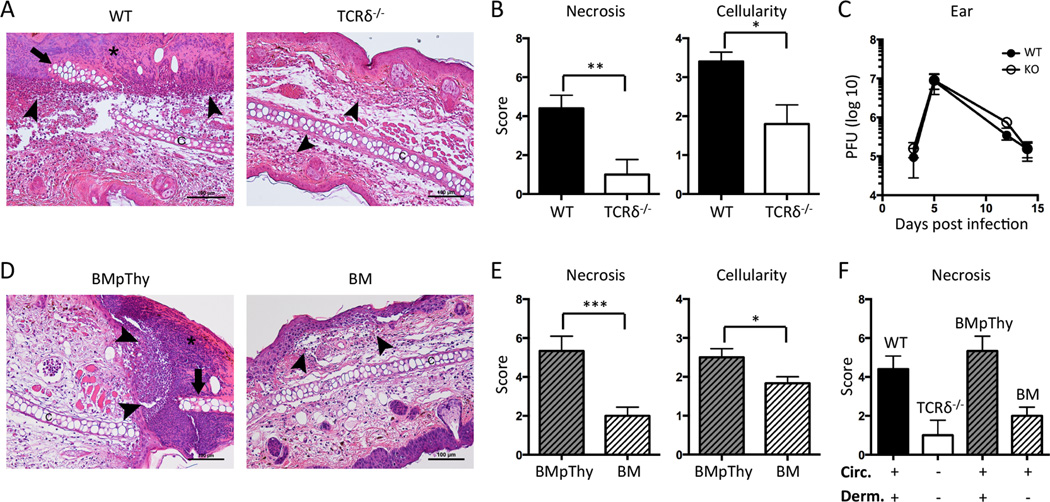
Ears from infected mice were fixed in 10% buffered formalin for 3 days before being embedded vertically in paraffin. Sections were hematoxylin and eosin stained and the severity of inflammation and necrosis was determined on a scale of 0–4 (4 being the most severe). Scoring of all samples was blinded and uninfected mice were used as controls.
Statistical analysis
Prism software (GraphPad) was used for all statistical analysis. The two-tailed, unpaired student t-test was used for comparisons of γδ T cell frequencies and histological scores.
RESULTS
γδ T cells accumulate at the site of infection with the emergence of a novel dermal population
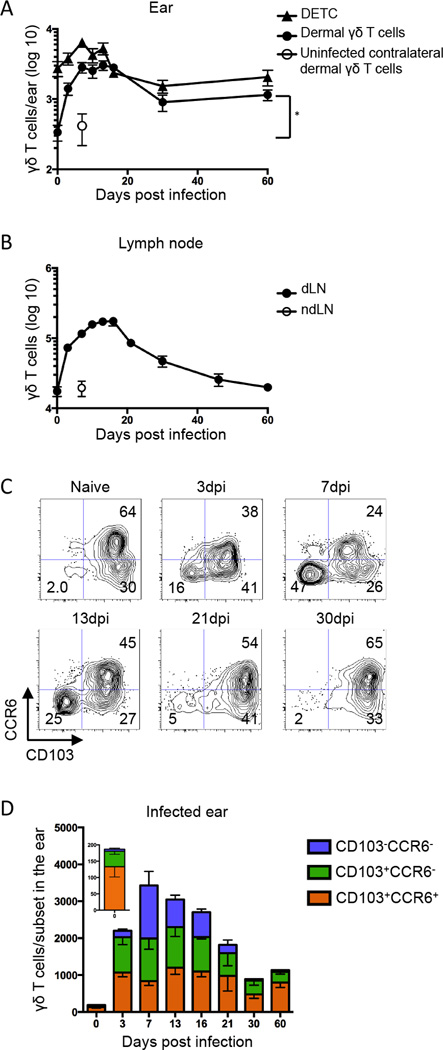
Although it is known that γδ T cells reside at barrier surfaces in mice and humans (16, 17), few studies have addressed the role they play during infection of the skin, particularly in response to viral infection. To address this, we used a model of Vaccinia Virus (VV) scarification, in which virus localizes primarily to the site of inoculation (39). Following infection of the ear pinna, we observed the number of dermal γδ T cells increased approximately ten-fold in the infected ear and dLN (Figure 1A & B). The response kinetics were slightly unusual, as the numbers in the skin and dLN remained elevated for a protracted period of time. Furthermore, the number of dermal γδ T cells at late time points was significantly higher than in naive ears, suggesting that infection could result in prolonged alterations in the dynamics of the dermal γδ T cell population. The number of DETC also increased about three-fold following infection and returned to the number found in control ears at late time points (Figure 1A).
Figure 1.
Following skin scarification with Vaccinia virus (VV), γδ T cells accumulate in draining lymph node and dermis. TCRδ-GFP mice were infected with VV on the ear by scarification. γδ T cells were gated as TCRβ−GFP+ in the LN and as TCRβ−GFP+Vγ5+ (Dendritic Epidermal T Cells, DETC) or TCRβ−GFP+Vγ5− (dermal γδ T cells) in the ear. A) Number of DETC and dermal γδ T cells in the ear following infection. Open symbol represents the number of dermal γδ T cells in the contralateral uninfected ear at 7dpi. B) Number of γδ T cells in the dLN following infection. Open symbol represents the number of γδ T cells in the contralateral non-draining LN (ndLN) at 7dpi. C) Representative flow plots show CCR6 and CD103 profile of dermal γδ T cells following infection. Numbers denote percent of total dermal γδ T cell population within the indicated quadrant. D) Cell numbers within the indicated dermal γδ T cell subsets over the course of infection per ear (insert, day 0). Error bars signify SEM. Data are compiled from >5 (A, B) or >3 (C, D) independent experiments, n=3–13 mice per group. Statistics performed with the two-tailed, unpaired t-test, *p<0.001.
Dermal γδ T cells have been reported to express CCR6 and CD103 (22). We confirmed that in resting skin, all dermal γδ T cells are CD103+ and over half are CCR6+ (Figure 1C & D). Skin scarification with VV resulted in dramatic changes in the dermal γδ T cell population. By three days post infection, a novel subset appeared in the dermis that was CCR6 and CD103 double negative (DN). This novel dermal γδ T cell subset accounted for almost half of the γδ T cell population at the peak of the response, then disappeared entirely from the dermis by 30 days post infection (Figure 1C & D). We also assessed TCR Vγ chain expression using commercially available antibodies on the three dermal populations and found they were all similarly heterogeneous (data not shown).
Circulating γδ T cells are rapidly recruited to the VV infected ear
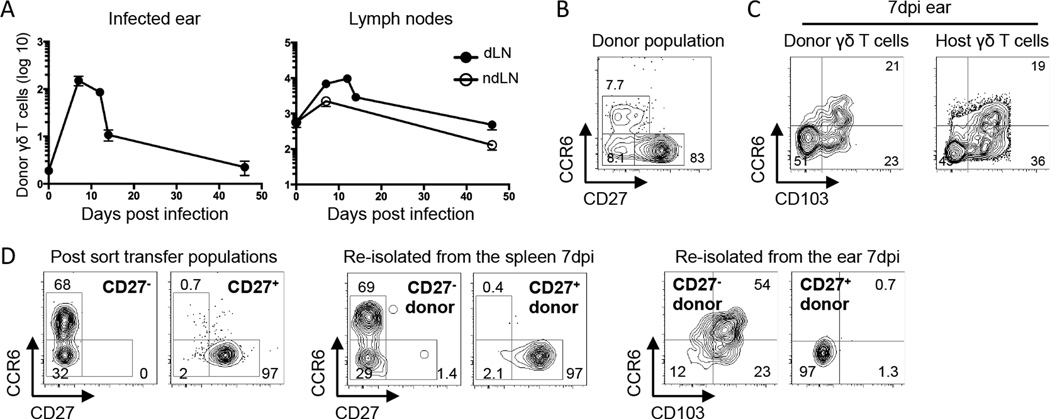
We performed an adoptive transfer to investigate the origin of the dermal γδ T cell populations following infection and to determine the contribution of circulating γδ T cells to the expanded dermal population. Accordingly, 7×105 γδ T cells isolated from the spleen and skin dLN of naïve TCRδ-GFP mice were transferred intravenously into normal C57BL/6 recipients before VV scarification. Infection of the recipients showed that donor γδ T cells were specifically recruited to the skin of the infected ear and their numbers peaked 7 days post infection (Figure 2A). While these adoptively transferred, circulating cells were capable of entering the dermis during active infection, very few remained permanently integrated into the dermis at a late time point.
Figure 2.
Circulating γδ T cells migrate specifically to VV infected skin and contribute to all three dermal subsets. C57BL/6 mice (CD45.2) or CD45.2+ TCRδ-GFP mice received 7×105 γδ T cells isolated from spleen and skin dLN of CD45.1+ TCRδ-GFP mice. The following day, mice were infected with VV on the ear. A) Graphs show numbers of transferred TCRδ-GFP+ cells recovered from the LN or ear, shown as +/− SEM. B) Representative flow plot shows the CCR6 and CD27 profile of the donor γδ T cell population prior to transfer. C) Representative flow plots show CCR6 and CD103 profile of donor and recipient dermal cells at 7dpi. D) γδ T cells from spleen and skin dLN of TCRδ-GFP mice were sorted based on CD27 expression and 6×105 CD27+ or 2.7×105 CD27− γδ T cells were transferred into separate C57BL/6 hosts. Recipients were infected with VV on the ear the following day. Flow plots show the phenotype of CD27− and CD27+ at the time of transfer and 7dpi in the spleen and ear. Numbers denote the percentage of cells within the indicated quadrant. Data are representative of at least two independent experiments, n=3–7 mice per group.
In the peripheral lymphoid organs and the circulation, it has been established that γδ T cells can be functionally segregated based on CD27 expression (11, 29). We found that CD27 and CCR6 staining were mutually exclusive in peripheral lymphoid organs and identified three γδ T cell populations with distinct surface and transcription factor staining patterns (Figure 2B & Supplemental Figure 1A). In line with previous reports that CD27− γδ T cells produce IL-17 upon stimulation, we found that baseline expression of the transcription factor RORγt was higher in CD27− than in CD27+ γδ T cells. Conversely, the transcription factor Tbet was highest in the CD27+ γδ T cells. CCR6+CD27− γδ T cells expressed the highest levels of CD44 and CD103, while the small number of CD27−CCR6− cells and the CD27+ cell subset had lower levels of CD44 and only half expressed CD103.
Transfer experiments revealed that the donor γδ T cells contributed to all three dermal γδ T cell populations defined by CD103 and CCR6 staining (Figure 2C), but it was not clear how these populations related to those in the circulation. Our initial attempts to stain for CD27 on γδ T cells isolated from the ear were negative. Further analysis revealed that CD27 was cleaved during the digestion process used to isolate cells from the ear. Floating ears (dorsal and ventral sides separated) overnight in complete media without enzymatic digestion released some γδ T cells from the tissue and permitted analysis of CD27 expression. This technique revealed that although CD27 was not found on dermal γδ T cells in naïve animals, it was expressed on some dermal γδ T cells following infection, all of which belonged to the CD103−CCR6− DN population (Supplemental Figure 1B). This suggested that the CD103−CCR6− DN subset appearing in infected ears was an immigrant population of circulating CD27+ γδ T cells. Because yields and subset composition from the floating technique were variable, we opted to use CD103 and CCR6 for further analyses of skin samples.
To ask whether CD27+ or CD27− γδ T cells from the circulating pool differentially contributed to the three dermal γδ T cell subsets, we sorted and transferred them into separate hosts before ear scarification with VV. The CD27− donor γδ T cells contributed to all three dermal populations defined by CD103 and CCR6, although few were DN and most expressed CD103 (Figure 2D). In contrast, immigrant γδ T cells from the CD27+ donor population were exclusively CD103−CCR6− DN, even though 50% of cells in the donor population expressed CD103 (Supplemental Figure 1A). On a per cell basis, the CD27− γδ T cells were recruited more efficiently to the infected skin (data not shown). These experiments additionally demonstrate that in vivo in the presence of inflammatory signals, CD27 expression remains stable in the LN and spleen as a marker of distinct γδ T cell lineages. Based on these experiments and the fact that we observed very limited proliferation within the tissue (Supplemental Figure 2), it appears that the majority of γδ T cell accumulation is due to recruitment.
Adult BM- and perinatal thymus-derived γδ T cells occupy discrete niches
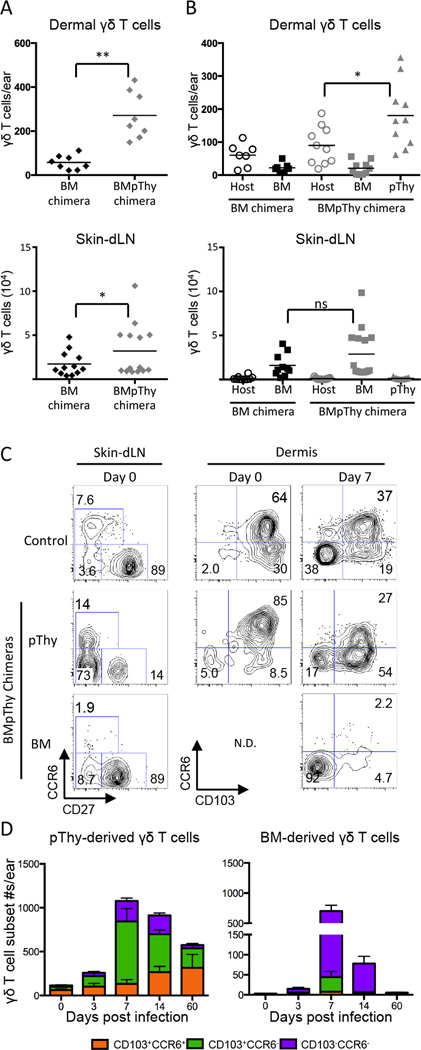
To tease apart the distinct roles of the resident dermal and circulating γδ T cell populations, we generated mice with and without resident dermal γδ T cells (22). We found that γδ T cells in the circulation and dermis were sensitive to whole body irradiation and that donor BM failed to reconstitute the dermal (Figure 3A) or the circulating CCR6+ γδ T cell populations (Supplemental Figure 3). The majority of the γδ T cells in the spleen and LN derived from the BM were CD27+ with a minority of CD27−CCR6− γδ T cells. Although small numbers of host γδ T cells remained in the dermis following irradiation (Figure 3B), their phenotype was abnormal (data not shown) and their numbers were significantly lower than in unirradiated mice.
Figure 3.
Contribution of adult BM-derived and perinatal thymus-derived γδ T cells to the dermal response. CD45.2+ TCRδ-GFP mice were irradiated and reconstituted with BM (BM only) or with BM plus pThy (BMpThy). A) Graphs show the total number of γδ T cells > 8 weeks following reconstitution in the dermis and ski dLN. Geometric mean is shown. B) Contribution of BM-derived and pThy-derived γδ T cells in the dermis and LN. Geometric mean is shown. C) and D) BMpThy chimeras were infected with VV on the ear > 8 weeks following reconstitution. C) CCR6 and CD27 profiles of γδ T cells in the skin dLN and CCR6 and CD103 profiles of γδ T cells in the dermis at day 0 and 7dpi (N.D. = not done). Numbers denote the percentage of that population within the indicated quadrant. D) The number of BM- and pThy-derived γδ T cells contributing to subsets in the dermis following infection. Error bars signify SEM. Statistics performed with the two-tailed, unpaired student t-test, *p<0.02, **p<0.0001, NS = not significant. Data show results from >3 (A, B) or >2 independent experiments (C, D), n=3–15 mice per group.
γδ T cell populations lost due to irradiation were replaced by transferring perinatal thymocytes (pThy) into irradiated hosts along with congenically marked BM cells to generate mice referred to here as BMpThy chimeras. This resulted in the restoration of both dermal γδ T cells (Figure 3A & B) and the circulating CCR6+ population by pThy (Figure 3C). Total γδ T cell numbers in the dermis returned to normal levels and the phenotype of the pThy-derived dermal γδ T cells resembled that of unirradiated mice. Although pThy-derived γδ T cells contributed to all three circulating populations defined by CD27 and CCR6 staining, CD27+ cells were a relatively minor population, allowing us to generally discriminate between the resident dermal and recruited γδ T cell populations.
Using BMpThy chimeras, we confirmed that, following VV infection, adult BM-derived γδ T cells (largely CD27+ in the circulation) were transiently recruited from the circulation and contributed primarily to the CCR6−CD103− DN dermal subset (Figure 3D). In contrast, perinatal thymocyte-derived γδ T cells contributed mainly to the two CD103+ subsets in the dermis and their expansion during infection. As early as day 3 following infection, pThy-derived γδ T cells had expanded in the dermis and their numbers remained elevated for a protracted period of time. In contrast, the number of the BM-derived γδ T cells peaked at 7 days post infection and then declined dramatically. Importantly, at a late time point the BM-derived γδ T cells were absent from the ear while the pThy-derived γδ T cells plateaued at a level similar to wild type (WT) mice following infection.
Adult BM- and pThy-derived γδ T cells are functionally distinct
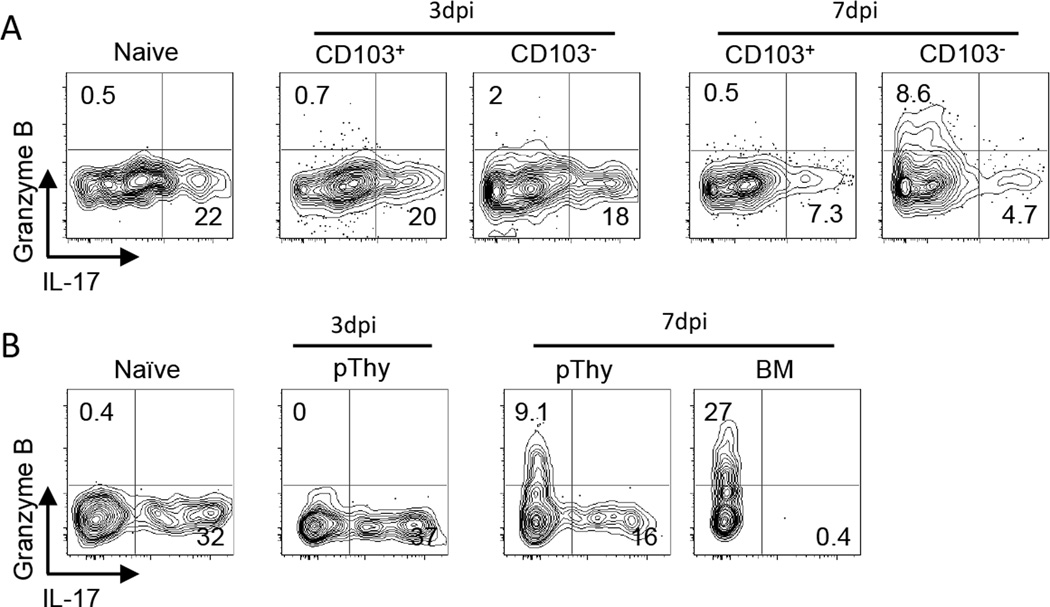
Several groups have established that dermal γδ T cells are fated to produce IL-17 (16, 21, 40). We found that a fraction of dermal γδ T cells in uninfected ears produced this cytokine following in vitro stimulation with PMA/Ionomycin. On day 3 after VV scarification, CD103+ γδ T cells in the dermis were capable of producing IL-17 at a level similar to that of naïve tissue (Figure 4A). Although at any given time point the majority of IL-17 production was by CD103+ γδ T cells (Supplemental Figure 4A), it was surprising that CD103− γδ T cells also produced IL-17 in the dermis. Using the float method in order to preserve CD27 expression, we found that all the IL-17 production was by CD27− γδ T cells (Supplemental Figure 4B), which we predict are derived from the circulating CCR6−CD27− population (Figure 2C). Notably, there were no Granzyme B+ γδ T cells in the naïve dermis at day 3 post infection, but by day 7 (which is the peak for the immigrant population), the CD103− γδ T cells produced Granzyme B. None of the γδ T cells in the dermis stained positive for IFNγ at any time following VV scarification (Supplemental Figure 4C).
Figure 4.
Adult BM-derived and pThy-derived γδ T cells in the dermis are functionally distinct. A) TCRδ-GFP mice and B) BMpThy chimeras were infected with VV on the ear and sacrificed on days 3 and 7. Ears were digested in Brefeldin A and cells were re-stimulated with PMA/Ionomycin for 4 hours at 37°C followed by intracellular staining for cytokines and Granzyme B. Representative flow plots are shown and gates are based on unstimulated naïve samples. Numbers indicate the percentage of that population in each quadrant. Data are representative of at >2 independent experiments.
We repeated these intracellular staining experiments with BMpThy chimeras to determine whether the BM- and pThy-derived populations had distinct functions (Figure 4B). At day 3 post infection, there were too few BM-derived cells to be analyzed, but the pThy-derived γδ T cells readily produced IL-17. By day 7 post infection, a clear distinction between the two populations could be seen, demonstrating that in this infection model, the function of adult BM-derived γδ T cells from the circulation was to make Granzyme B while the dermal pThy-derived γδ T cells gave rise to a population capable of making either IL-17 or, to a lesser extent, Granzyme B.
Resident dermal γδ T cells enhance the early immune response to infection
To determine whether γδ T cells contributed to inflammation and resolution of infection, we assessed tissue pathology and viral titers in wild type (WT) and TCRδ−/− mice, which lack all γδ T cells. By 3 days post infection, WT ears had evidence of both epidermal and cartilage necrosis, often with entire sections of the epidermis replaced by serocellular crusts (Figure 5A). The dermis of these mice predominantly contained accumulation of intact and degenerate neutrophils along with some macrophages and a fewer lymphocytes. In contrast, there was less infiltration of neutrophils and macrophages in the ears of TCRδ−/− mice and the cartilage and epidermis remained largely intact. Comparing the histological analysis for necrosis (combined for epidermis and cartilage) and cellularity, WT mice scored significantly higher than TCRδ−/− mice (Figure 5B). By day 7 post infection both groups had similar pathology (data not shown) and there was no difference in viral load between the ears of WT and TCRδ−/− mice over the course of infection either by PFU assay (Figure 5C) or by qPCR (data not shown).
Figure 5.
Dermal γδ T cells accelerate early collateral damage and contribute to increased tissue cellularity during VV infection. Mice were infected with VV on the ear. A) Representative histological sections of ears from C57BL/6 WT and TCRδ−/− mice 3dpi at 20× magnification. B) Graphs show scores for necrosis (epidermis and cartilage scored separately and then combined) and cellularity. Mean +/− SEM is shown. C) WT and TCRδ−/−mice were sacrificed at the indicated time points following infection and ears were taken for plaque assay. Error bars indicate SEM. D) Representative histological sections and E) scores for BM chimeras and BMpThy chimeras 3dpi. Mean +/− SEM is shown. F) Comparison of necrosis scores for WT, TCRδ−/−, BMpThy chimeras and BM chimeras in relation to the presence (+) or absence (−) of γδ T cell populations (Circ = circulating γδ T cells, Derm = dermal γδ T cells). Arrows indicate necrotic cartilage, arrowheads indicate neutrophilic infiltrates and asterisks indicate serocellular crusts. Statistics performed with the two-tailed, unpaired student t-test, n=5–6 mice per group. *p<0.05, **p<0.01, ***p<0.005 Data are representative of >1 independent experiments, n=3–6 mice per group (C) or >2 (A, B, D, E) independent experiments, n=5–6 mice per group.
We next compared histologic changes of BMpThy chimeras and BM chimeras to determine whether the differences between WT and TCRδ−/− mice could be assigned to the presence or absence of resident dermal γδ T cells. By and large, the BMpThy chimeras recreated the picture seen in WT mice with high scores for necrosis and cellularity. However, the BM chimeras had mild neutrophil infiltration, little to no cartilage necrosis and rare epidermal necrosis (Figure 5D & E). It was striking to see that replacement of dermal γδ T cells in BMpThy mice recapitulated results obtained with the WT mice and similarly, that BM mice mirrored γδ T cell null mice (Figure 5F). Thus, the loss of the dermal CD103+ γδ T cell population appears sufficient to reduce ear inflammation and damage.
DISCUSSION
Scarification of the skin with VV was used as a vaccine to eradicate small pox infection and the virus is now appreciated as an important vector for vaccine development and potentially cancer treatment (41). We have employed VV scarification of mouse ears to interrogate the distinct roles of recruited and resident dermal γδ T cells and to better understand their diversity. In this study, we describe for the first time the expansion, recruitment and contraction of γδ T cells in the dermis following localized VV infection. About half of the expanded dermal γδ T cells at day 7 were comprised of a unique population of CD103−CCR6− γδ T cells that rapidly appeared in the dermis in response to infection. This novel population was derived primarily from circulating CD27+ γδ T cells. A portion of the immigrant γδ T cells were phenotypically similar to the CD103+ IL-17 producing dermal-resident γδ T cell population and derived from circulating CD27− γδ T cells, although some were also found in the CD103− fraction. These immigrant populations were functionally distinct and played a discrete role from the resident dermal population, which was ultimately responsible for driving increased cellularity and tissue damage at an early time-point.
The number of dermal γδ T cells increased approximately 10-fold following VV infection and our data suggests that the majority of this increase can be ascribed to immigration rather than expansion of the resident population. Thus 7×105 adoptively transferred γδ T cells made up 5% of the total γδ T cell population in the spleen and LNs before infection (data not shown) and this donor:host γδ T cells ratio was maintained in the dermis at day 7 following infection. Furthermore, the CD103/CCR6 profile of the adoptively transferred immigrants resembled that of the host γδ T cells in the dermis day 7 post-infection (Figure 2C), suggesting that not only is the entire CD103− population recruited, but many of the CD103+ γδ T cells in the dermis may be as well. This in combination with the fact that we found very little in situ proliferation (Supplemental Figure 2) leads us to conclude that the accumulation of γδ T cells in the dermis is primarily due to recruitment.
The ability of immigrant cells to diversify into all the dermal subsets lead us to ask what the contribution was of the individual populations in the circulation. Emerging evidence confirms that CD27 delineates functional γδ T cell subsets in both mice (11, 29, 30) and humans (31–33). Transfer of CD27+ and CD27− circulating γδ T cells into separate hosts followed by VV scarification demonstrated their unique contribution to CD103− or CD103+ dermal populations, respectively. These experiments also confirmed the independent maintenance of these two populations even in the presence of inflammation. The stability of these two subsets and the constancy of the CCR6+ and CCR6− ratio within the CD27− population offer an important tool for studying γδ T cells during inflammation. Although other groups have compared CD27+ and CCR6+ γδ T cells (11, 29), the circulating CCR6−CD27− subset has received less attention. This latter population is uniformly RORγt+ and distinguished from the CCR6+ population by lower levels of CD103 and CD44 (Supplemental Figure 1A). Future studies will be needed to explore the nuances between these populations.
Although there is some disagreement concerning the radiosensitivity of γδ T cells and their reconstitution in an irradiated adult mouse (11, 16, 22), our chimera experiments revealed that, unlike the DETC population, γδ T cells in the circulation and dermis are radiosensitive. Our results confirm that transfer of perinatal thymocytes into an irradiated host generates bonafide CCR6+CD27− γδ T cells in the circulation and residents in the dermis. Differential kinetics and functional profiles of the BM (immigrant) and pThy (resident) γδ T cell populations in the BMpThy chimeras allowed us to ask whether they had distinct roles following infection. Determining the role of dermal γδ T cells by comparing WT and TCRδ−/− mice is complicated by the absence of normal DETC in the null mice (14), cells that are known to play a role in keratinocyte growth and survival, perhaps impacting tissue damage or recovery. Whole body irradiation allowed us to specifically deplete the resident dermal γδ T cells, creating mice that lack this population while retaining healthy BM-derived circulating γδ T cell populations. Comparing mice specifically with or without dermal γδ T cells revealed that the dermal resident population was responsible for the increased inflammation observed in infected WT animals (Figure 5D & E).
The ability of pThy-derived dermal γδ T cells to recruit inflammatory cells, such as neutrophils, is likely due to their production of IL-17 (16, 20, 42) and recruitment of neutrophils by γδ T cells directly results in increased tissue damage (43). We demonstrated increased necrosis and cellularity of infected ears 3 days after VV infection, indicating that the resident IL-17+ γδ T cells are early responders to viral infection. In contrast, the immigrating population of CD103−CCR6− DN γδ T cells, which appeared concurrently, only upregulated Granzyme B expression at a later time-point. Although classically associated with cytotoxicity, which has been shown for γδ T cells (44), Granzyme B has direct antiviral effects within cells and can cleave structural proteins when released into the extracellular matrix (45, 46). Importantly for our study, extracellular Granzyme B release has been shown to have remodeling activity (47), potentially facilitating migration of cells into the tissue and impacting wound repair. Considering the absence of γδ T cells had no effect on viral load (Figure 5C) and the number of γδ T cells within the dermis remained elevated for an extended period of time, these Granzyme-producing cells may play a non-traditional role during inflammation and in the recovery process after viral clearance.
Little is known about whether γδ T cells can form ‘memory’. The majority of studies have examined at total γδ T cell population re-expansion (48, 49) or relied on CD44 expression (33), which is now appreciated to be regulated differently in γδ T cells than classical αβ T cells (50). When we transferred congenically marked γδ T cells, none of the immigrant γδ T cells remained in the dermis as residents after infection was cleared (Figure 2B). This was true for the CD27+ circulating γδ T cells characterized as CD103−CCR6− DN cells in the dermis and for the CD27− circulating cells that give rise to all three populations in the dermis at day 7 post infection. Similarly in the BMpThy chimeras, BM-derived circulating γδ T cells that entered during the infection did not remain there long term (Figure 3D). This indicates that inflammation is not sufficient to drive the long-term skin residence of γδ T cells that enter the skin from the circulation. In contrast, it has been observed after oral Listeria monocytogenes infection that a population of γδ T cells with memory-like function develop and persist in intestinal tissues (51). However, generating resident dermal γδ T cells requires a neonatal population (or their precursors), derived in our case from the transfer of perinatal thymocytes into irradiated hosts.
The presence of pThy-derived γδ T cells in the dermis was required to increase cellularity near the site of infection and to amplify the overall potency of the immune response as demonstrated by increased tissue damage in WT and BMpThy mice. While the complete absence of γδ T cells did not translate to increased viral load in this or related systems (52, 53), other models have shown that mice lacking γδ T cells are more susceptible to bacterial infection (54) and have a decreased capacity for wound healing (13). Together, the data indicate that γδ T cells are a dynamic population with a range of functions, but that they might not respond equally to all inflammatory cues. Importantly, since there is no DETC counterpart in human skin (55), it has become increasingly important to isolate the relevance of γδ T cells in the dermis in models relevant to human disease. Our study employing the adoptive transfer of circulating γδ T cells into normal hosts and the creation of chimeras specifically lacking a dermal resident population will be useful for interrogating γδ T cell subsets and furthering our understanding of the dermal immune network.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the University of Washington Histology and Imaging Core for preparation of histological sections, Dr. E. Gray for critical discussion and technical advice, and Dr. P. Fink for review of the manuscript. We also thank Dr. J. Turner (U of Chicago) for the TCRδ-H2BeGFP mice originally derived by I. Prinz (MH-Hannover).
REFERENCES
- 1.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 2.Tong PL, Roediger B, Kolesnikoff N, Biro M, Tay SS, Jain R, Shaw LE, Grimbaldeston MA, Weninger W. The Skin Immune Atlas: Three-dimensional analysis of cutaneous leukocyte subsets by multiphoton microscopy. J. Invest. Dermatol. 2014 doi: 10.1038/jid.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, Mueller SN. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. U.S.A. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 2013;3:238–245. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 5.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 7.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata K. Close link between development and function of γδ T cells. Microbiol. Immunol. 2012;56:217–227. doi: 10.1111/j.1348-0421.2012.00435.x. [DOI] [PubMed] [Google Scholar]
- 10.Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, Coutinho A. Localization of γδ T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E, Chennupati V, Föhse L, Naumann R, Weiss S, Krueger A, Förster R, Prinz I. Development of Interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu. Rev. Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 13.Havran WL, Jameson JM. Epidermal T cells and wound healing. J. Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Infect. Dis. 172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 15.Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell. Mol. Life. Sci. 2011;68:2399–2408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCRδ repertoire in normal human skin is restricted and distinct from the TCRδ repertoire in the peripheral blood. J. Invest. Dermatol. 2001;116:275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 18.Maher BM, Mulcahy ME, Murphy AG, Wilk M, O'Keeffe KM, Geoghegan JA, Lavelle EC, McLoughlin RM. Nlrp-3-driven Interleukin 17 production by γδ T cells controls infection outcomes during Staphylococcus aureus surgical site infection. Infect. Immun. 81:4478–4489. doi: 10.1128/IAI.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byamba D, Kim DY, Kim D-S, Kim T-G, Jee H, Kim SH, Park T-Y, Yang S-H, Lee S-K, Lee M-G. Skin-penetrating methotrexate alleviates imiquimod-induced psoriasiform dermatitis via decreasing IL-17-producing γδ T cells. Exp. Dermatol. 2014;23:492–496. doi: 10.1111/exd.12448. [DOI] [PubMed] [Google Scholar]
- 20.Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG. Deficiency in IL-17-committed Vγ4+ γδ T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang H-G, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray EE, Suzuki K, Cyster JG. Cutting Edge: Identification of a motile IL-17-producing γδ T cell population in the dermis. J. Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas JD, Gonzalez FHM, Schmitz S, Chennupati V, Föhse L, Kremmer E, Förster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFNγ producing γδ effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 24.Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. Interferon γ-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by γδ T cells. J. Immunol. 2001;166:6784–6794. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 26.Mann ER, McCarthy NE, Peake STC, Milestone AN, Al-Hassi HO, Bernardo D, Tee CT, Landy J, Pitcher MC, Cochrane SA, Hart AL, Stagg AJ, Knight SC. Skin- and gut-homing molecules on human circulating γδ T cells and their dysregulation in inflammatory bowel disease. Immunology. 2012;170:122–130. doi: 10.1111/j.1365-2249.2012.04649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M. Reduced number and impaired function of circulating γδ T cells in patients with cutaneous primary melanoma. J. Invest. Dermatol. 2003;120:829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- 28.Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J. Infect. Dis. 187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmolka N, Serre K, Grosso AR, Rei M, Pennington DJ, Gomes AQ, Silva-Santos B. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat. Immunol. 2013;14:1093–1100. doi: 10.1038/ni.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.deBarros A, Chaves-Ferreira M, d'Orey F, Ribot JC, Silva-Santos B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur. J. Immunol. 2010;41:195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 32.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17–producing human Vγ9Vδ2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 33.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J. Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 35.Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, Tigelaar RE, Owen MJ, Hayday AC. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat. Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 36.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of γδ T cell development in the adult thymus. Nat. Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nat. Immunol. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 39.Tscharke DC, Smith GL. A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J. Gen. Virol. 80:2751–2755. doi: 10.1099/0022-1317-80-10-2751. [DOI] [PubMed] [Google Scholar]
- 40.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Veradi PH, Titong A, Hagen CJ. A vaccinia virus renaissance: new vaccine and immunotherpeutic uses after smallpox eradication. Hu. Vac. Immuno. 2012;8:961–970. doi: 10.4161/hv.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 43.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of γδ T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J. Leukoc. Biol. 2004;76:545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 44.Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, Lam K-T, Peiris JSM, Lau Y-L, Tu W. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian Influenza viruses. J. Infect. Dis. 2009;200:858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero V, Andrade F. Non-apoptotic functions of granzymes. Tissue Antigens. 2008;71:409–416. doi: 10.1111/j.1399-0039.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 46.Andrade F. Non-cytotoxic antiviral activities of granzymes in the context of the immune antiviral state. Immun. Rev. 2010;235:128–146. doi: 10.1111/j.0105-2896.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 47.Buzza MS, Zamurs L, Sun J, Bird CH, Smith AI, Trapani JA, Froelich CJ, Nice EC, Bird PI. Extracellular Matrix Remodeling by Human Granzyme B via Cleavage of Vitronectin, Fibronectin, and Laminin. J. Biol. Chem. 280:23549–23558. doi: 10.1074/jbc.M412001200. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao L, Huang D, Wei H, Wang RC, Chen CY, Shen L, Zhang W, Jin J, Chen ZW. Expansion, reexpansion, and recall-like expansion of Vγ2Vδ2 T cells in smallpox vaccination and monkeypox virus infection. J. Virol. 2009;83:11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, Hayday AC. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 2013;15:80–87. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheridan BS, Romagnoli PA, Pham Q-M, Fu H-H, Alonzo F, III, Schubert W-D, Freitag NE, LeFrancois L. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J-O, Cha H-R, Kim E-D, Kweon M-N. Pathological effect of IL-17A-producing TCRγδ+ T cells in mouse genital mucosa against HSV-2 infection. Immunol. Lett. 2012;147:34–40. doi: 10.1016/j.imlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy MK, Zhu L, Procario MC, Weinberg JB. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology. 2014;456–457:259–267. doi: 10.1016/j.virol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elbe A. T-Cell receptor αβ and γδ T cells in rat and human skin — are they equivalent? Semin. Immunol. 1996;8:341–349. doi: 10.1006/smim.1996.0045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.