Abstract
The Herpes Simplex Virus type 1 virion tegument phosphoprotein 11/12 (HSV-1 VP11/12) is a major antigen targeted by CD8+ T cells from HSV-seropositive individuals. However, whether and which VP11/12-epitope-specific CD8+ T cells play a role in the “natural” protection seen in seropositive healthy asymptomatic (ASYMP) individuals (who have never had clinical herpes disease) remain to be determined. In this study, we used multiple prediction computer-assisted algorithms to identify 10 potential HLA-A*02:01-restricted CD8+ T cell epitopes from the 716 amino acids sequence of VP11/12. Three out of ten epitopes exhibited high to moderate binding affinity to HLA-A*02:01 molecules. In ten sequentially studied HLA-A*02:01 positive and HSV-1-seropositive ASYMP individuals, the most frequent, robust and polyfunctional effector CD8+ T-cell responses, as assessed by a combination of tetramer frequency, granzyme B, granzyme K, perforin, CD107a/b cytotoxic degranulation, IFN-γ and multiplex cytokines assays, were predominantly directed against three epitopes: VP11/1266–74, VP11/12220–228 and VP11/12702–710. Interestingly, ASYMP individuals had significantly higher proportion of CD45RAlowCCR7lowCD44highCD62LlowCD27lowCD28lowCD8+ effector memory T cells (TEM) specific to the three epitopes, compared to symptomatic (SYMP) individuals (with a history of numerous episodes of recurrent ocular herpetic disease). Moreover, immunization of HLA-A*02:01 transgenic mice with the three ASYMP CD8+ TEM cell epitopes induced robust and polyfunctional epitope-specific CD8+ TEM cells that were associated with a strong protective immunity against ocular herpes infection and disease. Our findings outline phenotypic and functional features of protective HSV-specific CD8+ T cells that should guide the development of an effective T-cell-based herpes vaccine.
INTRODUCTION
Herpes Simplex Virus type 1 (HSV-1) infection is widespread in human populations (1–5). A staggering 1 billion individuals worldwide currently carry the virus that causes a wide range of diseases throughout their life (1–5). Complications range from mild, such as cold sores and genital lesion, to serious, such as permanent brain damage from encephalitis in adults and neonates and blinding corneal inflammation (5, 6). HSV infections are prevalent and permanent, as the virus establishes latency in the neurons of sensory ganglia after a primary infection (7–10). The majority of HSV-seropositive individuals are asymptomatic (ASYMP) (7–10). They do not experience any recurrent herpetic disease (e.g., cold sore, ocular or genital herpes) even though the virus spontaneously reactivates from latency and sheds multiple times each year in their body fluids (i.e., tears, saliva, nasal and vaginal secretions) (2, 3, 11, 12). In contrast, a small proportion of HSV-seropositive individuals are symptomatic (SYMP) and experience endless recurrences of herpetic disease, usually multiple times a year (13, 14), often requiring continuous antiviral therapy (i.e., acyclovir and derivatives). Notably, in some HSV-1-seropositive SYMP individuals, sporadic reactivation of the virus from latency and corneal re-infection can cause blinding recurrent herpetic stromal keratitis (rHSK), a T-cell mediated immunopathological lesion of the cornea (4, 5, 15). Therapeutic manipulation of the immune system (immunotherapy) is an attractive strategy to impact symptomatic disease, HSV-1 shedding, and ultimately, HSV-1 transmission in the community (7–10). For this to occur, one must first identify the HSV-1 antigens/epitopes involved in the apparent protection seen in seropositive ASYMP individuals, who appear to immunologically contain infection and disease.
Among the 84+ HSV-1 encoded protein antigens (Ags), perhaps the least characterized immunologically are the tegument proteins, which are located between the capsid and the envelope (7–10). We recently focused on identifying protective T cell epitopes from HSV-1 and HSV-2 tegument proteins because: (i) vaccine clinical trials which have concentrated during the last 2 decades on HSV envelope glycoproteins (mainly gB and gD) have failed to produce protective immunity against herpes infection and disease (16, 17); (ii) tegument proteins are injected into the cytoplasm of host cells during viral entry, resulting in an immediate processing by antigen presenting cells (APC) and T cells activation shortly after infection (7–10); (iii) the abundant virion phosphoprotein 11 and 12 (or VP11/12) tegument protein, encoded by the UL46 gene, is an excellent target for proteolysis, an event that precedes T-cell epitope presentation in association with HLA molecules (18); (iv) using a proteome-wide approach, covering all 84+ HSV-1 encoded proteins, we and others have found HSV tegument proteins in general, and the abundant VP11/12 tegument in particular, to be major targets of CD8+ T cells from HSV-seropositive healthy ASYMP individuals (who have never had clinical herpes) (7–10, 19–22). However, the repertoire of HSV-1 VP11/12 epitopes that induce protective CD8+ T cells in ASYMP individuals and the nature VP11/12 epitope-specific CD8+ T cells involved in protective immunity remain to be determined. This information is necessary for a successful design of an effective T cell-based therapeutic vaccine strategy.
In the present study, we identify three novel protective ASYMP epitopes from VP11/12 tegument protein that are strongly recognized by CD8+ T cells from HSV-seropositive healthy ASYMP individuals (VP11/1266–74, VP11/12220–228 and VP11/12702–710). A significantly higher proportion of HSV-1 VP11/12- epitope-specific effector memory CD8+ T cells (TEM cells) were detected in ASYMP individuals compared to SYMP patients. Frequent, robust and polyfunctional effector CD8+ T-cell responses were directed predominantly against these three epitopes in ASYMP individuals. Moreover, immunization of HLA Tg mice with the three VP11/12 CD8+ T cell epitopes induced strong protective immunity against ocular herpes infection and disease, associated with strong and poly-functional VP11/12 epitope-specific CD8+ TEM cell responses. Altogether, our findings identify previously unknown protective epitopes from the VP11/12 tegument protein and delineate quantitative and qualitative features of a protective HSV-specific CD8+ T cell response that should be taken into consideration during herpes vaccine development.
MATERIALS AND METHODS
Human study population
During the last decade (i.e., January 2003 to September 2014), we have recruited and screened 693 individuals for HSV-1- and HSV-2-seropositivity at Gavin Herbert Eye Institute and UCI Institute for Clinical and Translational Science (ICTS). (Table I). Four hundred eighty-eight individuals were White, 205 were non-White (African, Asian, Hispanic and others), 355 were females, and 338 were males. Among these, a cohort of 261 immuno-competent individuals, with an age range of 18–65 (median 32), who are seropositive for HSV-1 and seronegative for HSV-2, were enrolled in the present study. All patients were negative for HIV, HBV and had no history of immunodeficiency. 607 patients were HSV-1, HSV-2 or HSV-1/HSV-2 seropositive, among which 575 patients were healthy and ASYMP (individuals who, in the absence of therapy, have never had any recurrent herpes disease, ocular, genital or elsewhere, based on their self-report and physician examination. Even a single episode of any herpetic disease in their entire life excludes the individual from this group). The remaining 32 patients were defined as HSV-seropositive SYMP having suffered frequent and severe recurrent genital, ocular and/or oro-facial lesions, with two patients having had clinically well-documented repetitive herpes stromal keratitis (HSK) (one patient with ~20 episodes over 20 years that necessitated several corneal transplantations).
Table I.
Cohorts of HLA-A*02:01 positive, HSV seropositive Symptomatic and Asymptomatic individuals enrolled in the study.
| Subject-level Characteristic | All Subjects (n= 693) |
|---|---|
| Gender [no.(%)]: | |
| Female | 355 (52%) |
| Male | 338 (48%) |
| Race [no.(%)]: | |
| White | 488 (71%) |
| Nonwhite | 205 (29%) |
| Age [median (range) yr.]: | 32 (18–65 yr.) |
| HSV status [no. (%)]: | |
| HSV-1-positive | 261 (38%) |
| HSV-2-positive | 323 (46%) |
| HSV-1- & 2-positive | 23 (3%) |
| HSV-negative | 86 (12%) |
| HLA [no.(%)] | |
| HLA-A*02:01-positive | 330 (52%) |
| HLA-A*02:01-negative | 257 (48%) |
| Herpes Disease Status [no.(%)] | |
| ASYMPTOMATIC (ASYMP) | 575 (94%) |
| SYMPTOMATIC (SYMP) | 32 (6%) |
Signs of recurrent disease in SYMP patients were defined as herpetic lid lesions, herpetic conjunctivitis, dendritic or geographic keratitis, stromal keratitis, and iritis consistent with HSK, with one or more episodes per year for the past 2 years. However, at the time of blood collection, SYMP patients had no recurrent disease (other than corneal scarring) and had no recurrences during the previous 30 days. They have no ocular disease other than HSK; have no history of recurrent genital herpes; are HSV-1 seropositive and HSV-2 seronegative. Because the spectrum of recurrent ocular herpetic disease is wide, our emphasis is mainly on the number of recurrent episodes not on the severity of the recurrent disease. No attempt was made to assign specific T cell epitopes to specific severity of recurrent lesions. Patients are also excluded if they: (1) were pregnant or breastfeeding; or (2) were on acyclovir or other related anti-viral drugs or any immunosuppressive drugs at the time of blood sample collection. SYMP and ASYMP groups were matched for age, gender, serological status, and race. Eighty-six healthy control individuals were seronegative for both HSV-1 and HSV-2 and had no history of ocular herpes, genital lesions or oro-facial herpes disease. All subjects were enrolled at the University of California, Irvine under approved Institutional Review Board-approved protocols (IRB# 2003-3111 and IRB#2009-6963). A written informed consent was received from all participants prior to inclusion in the study.
Bioinformatics analyses
HSV-1 VP11/12 open reading frames utilized in this study were from strain 17 (NCBI accession number P 10230). The 716 aa sequence (Fig. S1) of the VP11/12 protein was screened for HLA-A2.1-restricted epitopes using different computational algorithms as previously described: BIMAS software (Bioinformatics and Molecular Analysis Section, NIH (Washington, DC); http://bimas.dcrt.nih.gov/molbio/hla_bind/) and the SYFPEITHI algorithm (http://www.syfpeithi.de/) (1, 2). Potential cleavage sites for human proteasome were also employed in this screening and were identified using NetChop 3.0 (http://www.cbs.dtu.dk/services/NetChop/) and the MHC Pathway database (http://www.mhc-pathway.net) (1, 2).
Peptide synthesis
To determine potential vaccine candidates, ten HLA-A*0201 binding peptides of herpes simplex virus type 1 (strain 17) VP11/12 with high estimated half-time of dissociation (T1/2) were synthesized by Magenex (San Diego, CA) on a 9050 Pep Synthesizer Instrument using solid-phase peptide synthesis and standard 9-fluorenylmethoxy carbonyl technology (PE Applied Biosystems, Foster City, Calif.). Stock solutions were made at 1 mg/ml in PBS. All peptides were aliquoted and were stored at −20°C until assayed.
Cell Lines
The T2 (174 × CEM.T2) mutant hybrid cell line derived from the T-lymphoblast cell line CEM was obtained from the ATCC. T2 cells lack the functional transporter associated with antigen processing (TAP) heterodimer and fail to express normal amounts of HLA-A*0201 on the cell surface. HLA-A*0201 surface expression can be stabilized with exogenous loading of peptide that are able to bind to the MHC class I molecule. The T2 cell line was maintained in IMDM (ATCC) supplemented with 10% heat-inactivated fetal calf serum (FCS) and 100 U of penicillin/mL, 100 U of streptomycin/ml (Sigma-Aldrich).
Stabilization of HLA-A*0201 on class-I-HLA-transfected B × T hybrid cell lines
To determine whether synthetic peptides could stabilize HLA-A*0201 molecules expression on the T2 cell surface, peptide-inducing HLA-A*0201 up-regulation on T2 cells was examined according to a protocol described previously (23, 24). T2 cells (3 × 105/well) were incubated with different concentrations of individual VP11/12 peptide (as indicated in Fig. 1) in 48-well plates for 18 hours at 26°C. Cells were then incubated at 37°C for 3 hours in the presence of 0.7 ul/ml of BD-Golgi stop, to block cell surface expression of newly synthesized HLA-A*0201 molecules, and human β-2 microglobulin (1ug/ml). The cells were washed with FACS buffer (1% BSA and 0.1% sodium azide in phosphate-buffered saline) and stained with anti-HLA-A2.1 specific monoclonal antibody (clone BB7.2) (BD-Pharmingen, USA) at 4°C for 30 min. After incubation, the cells were washed with FACS buffer, fixed with 1% paraformaldehyde in phosphate-buffered saline, and analyzed by flow cytometry using a LSRII (Becton Dickinson). The acquired data were analyzed with FlowJo software (BD Biosciences, San Jose, CA). Expression was measured by LSRII flow cytometer and mean fluorescence intensity (MFI) was recorded. Percent MFI increase was calculated as follows: Percent MFI increase = (MFI with the given peptide − MFI without peptide)/(MFI without peptide) × 100. Each experiment was performed 3 times, and means ± SD values were calculated.
Figure 1. Stabilization of HLA-A*0201 molecules by VP11/12 peptides on the surface of T2 cells.
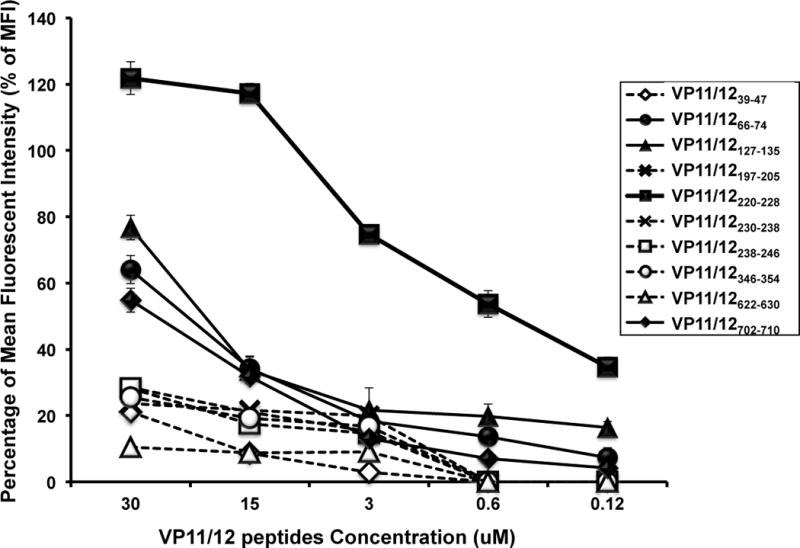
T2 cells (3 × 105) were incubated with serial dilutions of the indicated VP11/12 peptide, as described in Materials & Methods. Cells were then stained with FITC conjugated anti-HLA-A2 mAb (BB7.2). The graph represents the percent of mean fluorescence intensity (MFI) reflecting an increase in the expression of HLA-A2 molecules on the surface of T2 cells triggered by various concentrations of VP11/12 peptides. The percent of MFI increase was calculated as follows: Percent of MFI increase = (MFI with the given peptide − MFI without peptide)/(MFI without peptide) × 100. Solid lines represent peptides that bind with high to moderate affinity to HLA-A02:01 molecules, as determined by stabilization of high levels of HLA-A*02:01 molecules on the surface of T2 cells when incubated with the indicated molarity of VP11/12 peptide. Broken lines represent low affinity peptide binders as determined by low levels of HLA-A*02:01 molecules stabilized on the surface of T2 cells. Error bars show standard deviation (SD) obtained from 3 independent experiments.
HSV specific serotyping through Enzyme-Linked Immunosorbent Assay
The sera collected from random donors were tested for anti-HSV antibodies. ELISA was performed on sterile 96-well flat-bottomed microplates. The plates were coated with the HSV-1 antigen in coating buffer overnight at 4°C. The next day plates were washed with PBS–1% Tween 20 (PBST) five times. Nonspecific binding was blocked by incubating them with a 5% solution of skimmed milk in PBS (200 ul/well) at 4°C for 1 hr. at room temperature (RT). The microplates were washed three times with PBS–Tween and incubated with various sera at 37°C for 2 hrs. Following five washes, biotinylated rabbit anti-human IgG, diluted 1:20,000 with PBST, was used as the secondary antibody and incubated at 37°C for 2 h. After five washes streptavidin was added at a 1:5000 dilution and incubated for 30 min at RT. After five additional washes, the color was developed by adding 100 ul of TMB substrate. The mixture was incubated for 5–15 min at room temperature in the absence of light. The reaction was terminated by adding 1 M H2SO4. The absorbance was measured at 450 nm.
Peripheral blood mononuclear cell (PBMC) isolation
100 mL of an individual’s blood were drawn into yellow-top Vacutainer® Tubes (Becton Dickinson, USA). The PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, USA). The cells were washed in PBS and re-suspended in complete culture medium consisting of RPMI-1640 medium containing 10 FBS (Bio-Products, Woodland, CA, USA) supplemented with 1× penicillin/L-glutamine/streptomycin, 1× sodium pyruvate, 1× non-essential amino acids, and 50 μM of 2-mercaptoethanol (Life Technologies, Rockville, MD, USA). Aliquots of freshly isolated PBMC were also cryopreserved in 90% FCS and 10% DMSO in liquid nitrogen for future testing.
HLA-A2 typing
The HLA-A2 status was confirmed by PBMC staining with 2 μl of anti-HLA-A2 mAb, (clone BB7.2) (BD-Pharmingen, USA), at 4°C for 30 min. The cells were washed and analyzed by flow cytometry using a LSRII (Becton Dickinson). The acquired data were analyzed with Flowjo software (BD Biosciences, San Jose, CA).
Flow cytometry analysis
PBMC were analyzed by flow cytometry after staining with fluorochrome-conjugated human specific monoclonal antibodies (mAbs). Anti-human antibodies were: CD3 (clone SK7) PE-Cy7, CD44 (clone G44-26) A700, CD8 (clone SK1) APC-Cy7, CCR7 (clone 150503) Alexa Flou 700, CD28 (clone CD28.2) APC, CD45RO PE-Cy5, CD45RA FITC, CD62L APC, IFN-γ Alexa Fluor 647, T-bet (clone O4-46) Alexa Fluor 488 (BD Pharmingen);, Granzyme B (clone GB11) A647, CD107a (clone H4A3) FITC, CD107b (clone H4B4) FITC (BioLegend); CD27 (clone 0323) Alexa Fluor 700, Granzyme K (clone G3H69) PerCP eFlour 710, Perforin (clone d69) FITC, Eomes (clone WD1928) eFluor 660 (eBioscience). Draining lymph nodes (DLN) and splenocytes were analyzed by flow cytometry after staining with fluorochrome-conjugated mouse specific monoclonal antibodies (mAbs). Anti-mouse antibodies were: CD8 (clone 53-6.7) PE-Cy7, CD44 (clone IM7) APC-Cy7, CD62L (MEL-14) A700, CD107a (clone 1D4B) FITC, CD107b (clone Ha1/29) FITC, IFN-γ (clone XMG1.2) A700 (BD Pharmingen); Granzyme B (clone GB11) A647, (BioLegend).
Surface staining mAbs against various cell markers were added to a total of 1 × 106 PBMC in phosphate-buffered saline (PBS) containing 1% FBS and 0.1% sodium azide (FACS buffer) for 45 minutes at 4°C. After washing with FACS buffer, cells were permeabilized for 20 min on ice using the Cytofix/Cytoperm Kit (BD Biosciences) and then washed twice with Perm/Wash Buffer (BD Bioscience). Intracellular cytokine staining mAbs were then added to the cells and incubated for 45 min on ice in the dark. Cells were washed again with Perm/Wash and FACS Buffer and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For each sample, 200,000 total events were acquired on BD LSRII Fortessa. Ab capture beads (BD Biosciences) were used as individual compensation tubes for each fluorophore in the experiment. To define positive and negative populations, we employed fluorescence minus controls for each fluorophore used in this study, when initially developing staining protocols. In addition, we further optimized gating by examining known negative cell populations for background level expression. The gating strategy was similar to that used in our previous work (2). Briefly, we gated on single cells, dump− cells, viable cells (Aqua Blue), lymphocytes, CD3+ cells, and CD8+ cells before the final gating on functional cells. For epitope-specific cells, VP11/12 tetramer specific CD8+ T cells were gated for different markers used in co-staining. Data analysis was performed using FlowJo version 9.7.5 (TreeStar, Ashland, OR). Statistical analyses were done using GraphPad Prism version 5 (La Jolla, CA).
mRNA isolation and real-time RT-PCR analysis
Total RNA was isolated from fresh PBMCs using the Direct-zol RNA MiniPrep kit (Zymo Research) and reverse transcribed with High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer’s instructions. Quantitative real-time PCR was carried out on a Rotor-Gene 3000 (Corbett Research) using the LightCycler 480 SYBR Green 1 Master (Roche). The data were normalized to GAPDH for transcription factor analysis. Relative mRNA expression levels were calculated using the formula mentioned below and data are expressed as fold increase.
Tetramer/VP11/12 peptide staining
Fresh PBMCs were analyzed for the frequency of CD8+ T cells recognizing the VP11/12 peptide/ tetramer complexes, as we previously described (7–10). The cells were incubated with VP11/12 peptide/tetramer complex for 30–45 min at 37°C. The cell preparations were then washed with FACS buffer and stained with FITC-conjugated anti-human CD8 mAb (BD Pharmingen). The cells were washed and fixed with 1% paraformaldehyde in PBS. The cells were then acquired on a BD LSR II and data were analyzed using FlowJo version 9.5.6 (Tree Star).
CD107 cytotoxicity assay
To detect VP11/12-specific cytolytic CD8+ T cells in PBMC and cornea cells, intracellular CD107a/b cytotoxicity assay was performed as described by Betts et al. with a few modifications (25, 26). Briefly, 1×106 PBMCs from patients, in addition to spleen cells, DLN cells and TG cells from mice infected with HSV, were transferred into 96-well V bottom FACS plates (BD) in R10 medium and stimulated with 10 different VP11/12 peptides (10μg/mL) in the presence of anti-CD107a-FITC and CD107b-FITC (BD Pharmingen) and BD-Golgi stop (10ug/ml) for 5–6 hours at 37°C. ConA (10μg/mL) (Sigma) and no peptide were used as positive and negative controls, respectively. At the end of the incubation period the cells were harvested into separate tubes and washed once with FACS buffer and then stained with PE-conjugated anti-human CD8 antibody for 30 min. Cells were fixed, permeabilized, and stained with additional antibodies against IFN-γ using FIX/PERM and PERM/Wash solution (BD).
HLA-A*0201 transgenic mice
HLA-A*02:01 transgenic mice, previously described (27–29), were kindly provided by Dr. Francois Lemonier (Pasteur Institute, Paris) and were bred at UCI. These mice represent the F1 generation resulting from a cross between HLA-A*02:01/Kb transgenic mice (expressing a chimeric gene consisting of the 1 and 2 domains of HLA-A*02:01 and the 3 domain of H-2Kb) created on the BALB/c genetic background. The genotype of the HLA transgenic mice used in this study was confirmed as HLA-A*02:01, the most common A*02 subtype, supporting that the immunogenic peptide epitopes reported here are likely presented by the HLA-A*02:01 molecule. All animal studies were conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care and according to Institutional Animal Care and Use Committee-approved animal protocols (IACUC # 202-2372).
Virus production
HSV-1 (strain McKrae) was grown and titrated on RS (rabbit skin) cells. UV-inactivated HSV-1 was generated as we previously described (3). HSV inactivation was confirmed by the inability to produce plaques when tested on RS cells.
Ocular challenge of “humanized” HLA transgenic mice with HSV-1
Two groups of age-matched female HLA-A*02:01 transgenic mice were challenged with 2×105 PFU of strain McKrae as eye drops. Control mice were inoculated using mock samples of virus. Following ocular challenge, mice were monitored for ocular herpes infection and disease. Some of them remained asymptomatic (exhibited no symptoms or disease) while some of them developed symptoms and were considered symptomatic.
Immunization of HLA-A*02:01 transgenic mice with VP11/12 immuno-dominant peptide epitopes
Three groups of age-matched female HLA-A*02:01 transgenic mice (n = 10 each) were immunized subcutaneously with the immuno-dominant CD8+ T cell human epitopes (VP11/1266–74, VP11/12220–228 and VP11/12702–710) delivered with the CD4+ T cell VP11/12129–143 epitope emulsified in CpG1826 adjuvant (GROUP 1), with the immuno-dominant CD8+ T cell human epitopes (VP11/1266–74, VP11/12220–228 and VP11/12702–710) delivered with the CD4+ T cell PADRE epitope emulsified in CpG1826 adjuvant (GROUP 2), or with the CpG1826 adjuvant alone (MOCK) on days 0 and day 21. All immunizations were carried out with 100 μM of each peptide.
Monitoring of ocular herpes infection and disease
Animals were examined for signs of ocular disease by slit lamp. Clinical assessments were made immediately before inoculation and on days 1, 3, 5, 7, 10, 14 and 21 thereafter. The examination was performed by investigators blinded to the treatment regimen of the mice and scored according to a standard 0–4 scale: 0, no disease; 1, 25%; 2, 50%; 3, 75%; and 4, 100% staining, as previously described (42). To quantify replication and clearance of HSV-1 from the eyes, mice were swabbed daily with moist, type 1 calcium alginate swabs. Swabs were placed in 1.0 ml titration media (Media 199, 2% penicillin/streptomycin, 2% newborn calf serum) and frozen at −80°C until titrated on RS cell monolayers, as described previously (42). Mice were also examined for survival in a window of 30 d after challenge, as we previously described (42).
Statistical analyses
Data for each assay were compared by analysis of variance (ANOVA) and Student’s t test using GraphPad Prism version 5 (La Jolla, CA). Differences between the groups were identified by ANOVA and multiple comparison procedures, as we previously described (30). Data are expressed as the mean ± SD. Results were considered statistically significant at p < 0.05.
RESULTS
1. In silico prediction of potential HLA-A*02:01-restricted T cell epitopes from the HSV-1 VP11/12 protein
The amino acid sequence of HSV-1 VP11/12 tegument protein (strain 17) was screened for potential HLA-A*02:01-binding regions using BIMAS, SYFPEITHI and MAPPP predictive computational algorithms (1). The HLA-A*02:01 haplotype is prevalent in over 50% of the world’s population irrespective of gender and ethnicity (29). Based on these analyses, ten potential peptide epitopes with high predicted affinity to HLA-A*0201 molecules were selected (Table II). All 10 VP11/12 peptide epitopes shared the HLA-A*0201-binding motifs: leucine or valine at the second position and a leucine, valine, methionine or alanine at the ninth position. Based on the above computational algorithms, these VP11/12 peptides bear putative antigenic and immunogenic HLA-A*0201-binding epitopes and thus are more likely to be less constrained than other parts of the VP11/12 molecule, resulting in increased accessibility to proteolysis, an event that precedes T-cell epitope presentation in association with HLA molecules (28, 31–35).
Table II.
Schematic representation showing the relative location within HSV-1 VP11/12 of the potential CD8+ T cell epitopes studied.
| Peptide | Sequence | MW | No. (aa) | BIMASa | SYFPEITHIb | MAPPPc | MHCPredd | HLA-A*0201 (IC50 nM) |
|---|---|---|---|---|---|---|---|---|
| VP11/1239–47 | CLLPTPEGL | 942.1 | 9 | 38.730 | 25 | ND | 0.4 | ND |
| VP11/1266–74 | FLTCTDRSV | 1041.1 | 9 | 63.988 | 22 | 0.9727 | 0.15 | 83 |
| VP11/12127–135 | ILTQYWKYL | 1227.4 | 9 | 339.555 | 21 | 0.9576 | 0.49 | ND |
| VP11/12197–205 | RIQQYMFFM | 1263.5 | 9 | 50.119 | 13 | 1.0000 | 0.27 | 81 |
| VP11/12220–228 | RLNELLAYV | 1090.2 | 9 | 3607.314 | 30 | 0.8284 | 0.23 | 709 |
| VP11/12230–238 | VLYRWASWM | 1211.4 | 9 | 148.643 | 16 | 0.7441 | 0.27 | ND |
| VP11/12238–246 | MLWTTDKHV | 1130.3 | 9 | 981.379 | 20 | 0.7346 | 0.21 | 1293 |
| VP11/12346–354 | TLTGYGVWA | 967.0 | 9 | 27.324 | 18 | 0.5100 | ND | ND |
| VP11/12622–630 | RVYEEIPWM | 1222.4 | 9 | 72.146 | 16 | ND | 0.37 | ND |
| VP11/12702–710 | ALSALLTKL | 929.1 | 9 | 49.134 | 29 | 0.7905 | 0.55 | 313 |
The sequence of the HSV-1 tegument protein (VP11/12) was submitted to screening of potential HLA-A*0201 epitopes using several computer algorithms. The ten peptides were selected on the basis of the HLA-A*0201 binding motif sequence from HSV-1 VP11/12. The numbers in the four right columns show predicted IC50 as calculated by (i) BIMAS: http://www-bimas.cit.nih.gov/molbio/hla_bind: (ii) SYFPEITHI: http://www.syfpeithi.de; (iii) MAPPP: http://www.mpiib-berlin.mpg.de/MAPPP and (iv) MHCPred: http://www.mhc-pathway.net. The sequences of synthesized peptides are based on the HSV-1 (Strain 17). ND = Not determined.
2. Three VP11/12 epitopes bind and stabilize HLA-A*02:01 molecules on the surface of target cells
Peptides corresponding to the ten highest probable VP11/12 epitope peptides were synthesized and experimentally tested for binding affinity to soluble HLA-A*0201 molecules (Table II, far right column). The VP11/1266–74 and VP11/12197–205 peptides showed highest affinity binding to HLA-A*0201 molecules (KD affinity values of 81 nM and 83 nM, respectively). Peptides VP11/12702–710 and VP11/12220–228 showed medium binding affinity to soluble HLA-A*0201 molecules (KD of 313 nM and 709 nM, respectively). None of the remaining six peptides bind with significant affinity to HLA-A*0201 molecules.
Cell surface expression of HLA-A*02:01 molecules depends primarily on peptide transport into the ER/Golgi by the transporter associated with antigen transport (TAP). Class-I-HLA-transfected B × T hybrid cell lines (T2 lines) are deficient in TAP but still express low amounts of MHC class I on the surface of the cells (1). We next performed a stabilization assay of HLA-A*02:01 molecules on T2 cells. The T2 cell binding assay is based upon the ability of peptides to stabilize the MHC class I complex on the surface of the T2 cell line. Each of the ten VP11/12 peptides were tested at a concentration of 0.12 μM, 0.6 μM, 3 μM, 15 μM and 30μM (Fig. 1). Out of 10 VP11/12 peptides, VP11/12220–228 showed the highest binding affinity to HLA-A*02:01 molecules. VP11/12220–228 significantly increased levels of HLA-A*02:01 molecules on the surface of the T2 cells in a dose-dependent manner (p < 0.001). From the remaining nine peptides, VP11/12127–135, VP11/1266–74 and VP11/12702–710 were medium binders to HLA-A*02:01 molecules (Fig. 1) Despite many attempts, the remaining six peptides produced no significant stabilization of HLA-A*02:01 molecules on the surface of T2 cells (Fig. 1). It is of note that the VP11/12197–205 peptide that appeared to bind to soluble HLA-A*02:01 did not significantly increase the levels of HLA-A*02:01 molecules on the surface of the T2 cells. Inversely, the VP11/12127–135 peptide that appeared to increase the levels of HLA-A*02:01 molecules on the surface of the T2 cells did not bind to soluble HLA-A*02:01 molecules.
Altogether, the above results suggest that, three out of ten potential VP11/12 peptides, VP11/1266–74, VP11/12220–228 and VP11/12702–710, bind with high affinity to soluble HLA-A*02:01 molecules and significantly increase the levels of HLA-A*02:01 molecules on the surface of the T2 cells. These three peptides might therefore lead to efficient functional presentation to HLA-A*02:01 molecules and stimulation of CD8+ T cells.
3. Frequent VP11/12220–228 and VP11/12702–710-specific CD8+ T cells detected in HLA-A*02:01 positive HSV-seropositive Asymptomatic individuals
The characteristics of the symptomatic (SYMP) and asymptomatic (ASYMP) study populations used in the present study, with respect to age, gender, HLA-A*02:01 frequency distribution, HSV-1/HSV-2 seropositivity and status of ocular herpetic disease are presented in Table I and detailed in the Materials and Methods. Since HSV-1 is the main cause of ocular herpes, only individuals who are HSV-1 seropositive and HSV-2 seronegative were enrolled in the present study. These HSV-1 seropositive individuals were segregated into two groups: (i) 10 HLA-A*02:01 positive HSV-1-infected ASYMP individuals, who have never had detectable levels of any clinical herpes disease; and (ii) 10 HLA-A*02:01 positive HSV-1-infected SYMP individuals with a history of numerous episodes of well-documented recurrent clinical herpes diseases, such as herpetic lid lesions, herpetic conjunctivitis, dendritic or geographic keratitis, stromal keratitis, and iritis consistent with rHSK, with 1 or more episodes per year for the past 2 years. None of the SYMP patients were on acyclovir or other anti-viral or anti-inflammatory drug treatments at the time of blood sample collections.
We compared the frequency of CD8+ T cells specific to each of the ten VP11/12 peptide epitopes, using HLA-A*02:01 specific tetramers/anti-CD8 mAbs, in the peripheral blood of 10 HLA-A*02:01 positive, HSV-1 seropositive ASYMP and 10 HLA-A*02:01 positive, HSV-1 seropositive SYMP individuals, as described above (Fig. 1). All 10 peptides were tested here because lack of binding may not always translate into lack of frequency or function (1, 2). The low frequencies of peripheral blood mononuclear cells- (PBMCs-) derived HSV-specific CD8+ T cells complicate a direct ex vivo detection with tetramers using a typical number of PBMC (~106 cells). Additionally, a prior expansion of CD8+ T cells by HSV-1 or peptide stimulation in an in vitro culture would hamper a reliable determination of the frequency, phenotype and function of epitope-specific CD8+ T cells. We therefore opted to measure the frequencies of VP11/12 epitopes-specific CD8+ T cells ex vivo using 10 × the number of PBMCs (~10 × 106) per tetramer/CD8 mAbs panel. The representative dot plots shown in Fig. 2A indicates similar frequencies of CD8+ T cells specific to subdominant VP11/1266–74 epitope and higher frequencies of CD8+ T cells specific to two immuno-dominant VP11/12220–228 and VP11/12702–710 epitopes detected in one ASYMP individual (top row) compared to one SYMP individual (lower row). Fig. 2B shows median frequencies detected in 10 SYMP and 10 ASYMP individuals. The highest and most significant frequencies of tetramer(+) CD8+ T-cells that were consistently detected in both SYMP and ASYMP individuals, were recorded against VP11/12220–228 and VP11/12702–710 epitopes (7.1% to 14.7%). Significantly higher frequencies of CD8+ T cells specific to both VP11/12220–228 and VP11/12702–710 epitopes were detected in ASYMP vs. SYMP individual (P = 0.03 and P = 0.02, respectively). Similarly, low frequencies of CD8+ T cells were detected in both SYMP and ASYMP individuals against VP11/1266–74 epitope (P = 0.4). Despite repeated attempts, with and without in vitro expansions, the remaining seven VP11/12 peptide/HLA-A*02:01 tetramers consistently detected a low and non-significant frequency of CD8+ T cells in HLA-A*02:01 positive HSV-seropositive SYMP and ASYMP individuals (data not shown).
Figure 2. Frequencies of CD8+ T cells specific to VP11/12220–228 and VP11/12702–710 epitopes detected in HLA-A*02:01 positive HSV-seropositive ASYMP individuals compared to SYMP individuals.
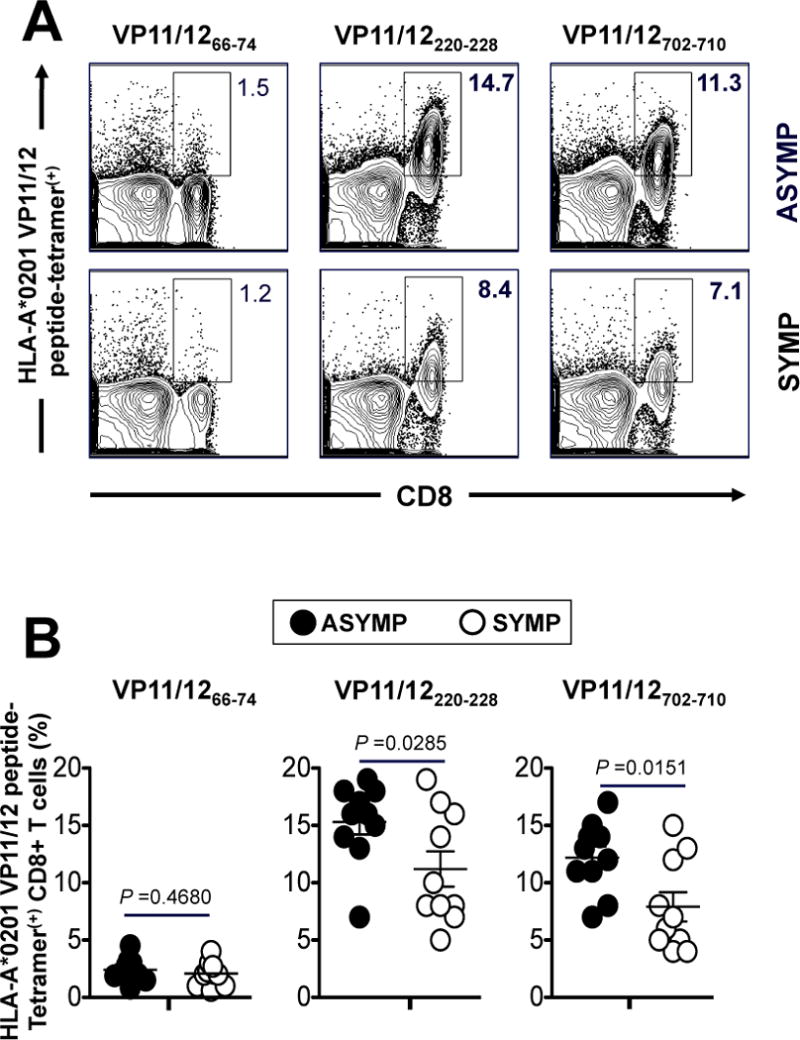
PBMCs (~10 × 106) derived from ten HLA-A*02:01 positive HSV-1 seropositive ASYMP individuals and from ten HLA-A*02:01 positive HSV-1 seropositive SYMP individuals were analyzed ex vivo by FACS for the frequency of CD8+ T cells specific to three VP11/12 epitopes using HLA-A*0201 VP11/12 peptide-tetramer complexes representing each of the three high to medium peptide binders (VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes), as shown in Fig. 1. (A) Representative FACS data of the frequencies of CD8+ T cells, specific to VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes, detected in PBMCs from one HLA-A*02:01 positive HSV-1 seropositive ASYMP individual (top panels) and one HLA-A*02:01 positive HSV-1 seropositive SYMP individual (lower panels). (B) Average frequencies of PBMC-derived CD8+ T cells, specific to VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes, detected from ten HLA-A*02:01 positive HSV-1 seropositive ASYMP individuals compared to ten HLA-A*02:01 positive HSV-seropositive SYMP individuals. The results are representative of 2 independent experiments in each individual. The indicated P values, calculated using one-way ANOVA Test, show statistical significance between SYMP and ASYMP individuals.
Thus, although CD8+ T-cells from ASYMP and SYMP both frequently recognized the VP11/12220–228 and VP11/12702–710 epitopes, there was a significantly higher frequency of recognition by CD8+ T-cells from ASYMP individuals.
4. Asymptomatic individuals have a higher frequency of VP11/12 epitope-specific CD8+ TEM cells while Symptomatic individuals have a higher frequency of VP11/12 epitope-specific CD8+ TCM cells
HSV-specific memory CD8+ T cells are categorized into two major phenotypically distinct TEM and TCM sub-populations (1, 4, 11, 36). We next investigated whether there is a difference in the frequencies of CD8+ TEM and TCM sub-populations specific to HSV-1 VP11/12 epitopes in SYMP vs. ASYMP individuals. Since the frequency of total CD8+ T cells specific to VP11/1266–74 epitope was low, we focused on CD8+ T cells specific to the immunodominant VP11/12220–228 and VP11/12702–710 epitopes, using specific tetramers, as described above. ASYMP individuals had significantly higher percentages of CD44highCC62LlowCD8+ TEM cells specific to VP11/12220–228 (Figs. 3A, and 3B) and VP11/12702–710 (3C and 3D) epitopes compared to SYMP individuals (p < 0.05, ANOVA test). In contrast, significantly higher percentages of VP11/12220–228 and VP11/12702–710 epitope-specific CD8+CD44highCC62Lhigh TCM cells were consistently detected in SYMP individuals compared to ASYMP individuals (p < 0.01, ANOVA test).
Figure 3. Frequent VP11/12-epitope specific CD44highCD62LlowCD8+ TEM cells detected in ASYMP individuals compared to SYMP individuals.
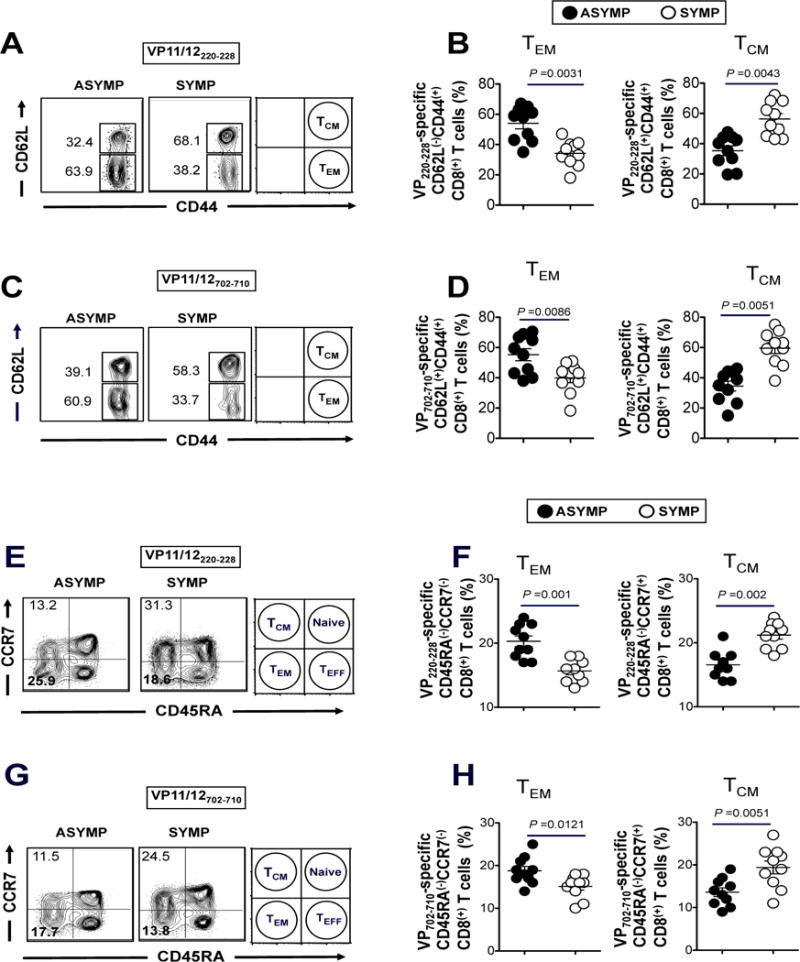
The phenotype of CD8+ T cells specific to VP11/12 peptide/Tetramer complexes representing each of the two immunodominant VP11/12220–228 and VP11/12702–710 epitopes were analyzed in term of TEM and TCM cells. (A) Representative FACS data of the frequencies of CD44highCD62LlowCD8+ TEM cells and CD44highCD62LhighCD8+ TCM cells specific to VP11/12220–228 peptide/Tetramer complexes from one HLA-A*02:01 positive HSV-1 seropositive ASYMP individual and from one HLA-A*02:01 positive HSV-1 seropositive SYMP individual. (B) Average frequencies of blood-derived CD8+ T cells specific to VP11/12220–228 peptide/Tetramer complexes with either TEM or TCM phenotype analyzed from 10 ASYMP and 10 SYMP individuals. (C) Representative data of the frequencies of CD44highCD62LlowCD8+ TEM and CD44highCD62LhighCD8+ TCM cells specific to VP11/12702–710 epitope from one ASYMP individual and one SYMP individual. (D) Average frequencies of blood-derived CD8+ T cells specific to VP11/12702–710 epitope with either TEM or TCM phenotype analyzed from 10 ASYMP and 10 SYMP individuals. Representative FACS data of the frequencies of CD45RAlowCCR7lowCD8+ TEM cells and CD45RAlowCCR7highCD8+ TCM cells, specific to (E) VP11/12220–228 epitope and (F) VP11/12702–710 epitope, analyzed in one ASYMP individual and one SYMP individual. Average frequencies of PBMC-derived CD8+ T cells with either TEM or TCM phenotype, specific to (G) VP11/12220–228 epitope and (H) VP11/12702–710 epitope, analyzed from 10 ASYMP and 10 SYMP individuals. The results are representative of 2 independent experiments in each individual. The indicated P values, calculated using one-way ANOVA Test, show statistical significance between SYMP and ASYMP individuals.
Using different markers of CD8+ TEM and TCM sub-populations, we similarly found that ASYMP individuals had significantly higher percentages of CD45RAlowCCR7lowCD8+ TEM cells specific to VP11/12220–228 (Figs. 3E and 3F) and VP11/12702–710 (Figs. 3G and 3H) epitopes, while SYMP individuals had significantly higher percentages of CD45RAlowCCR7highCD8+ TCM cells (p < 0.01, ANOVA test).
Altogether, the phenotypic properties of HSV-1 VP11/12 epitope-specific memory CD8+ T cells revealed a clear dichotomy in HSV-specific memory CD8+ T cell sub-populations, with ASYMP individuals featuring higher frequencies of the “experienced” HSV-specific CD8+ TEM cells. Thus, the results suggest that at a second pathogen encounter (e.g. following HSV-1 reactivation from latency or re-infection), ASYMP individuals, but not SYMP individuals, would better contain herpes infection and disease by mounting faster and stronger protective HSV-specific CD8+ TEM cell responses.
5. HSV-1 VP11/12 epitope-specific effector CD8+ T cells from Asymptomatic individuals are multi-functional
We compared the function of HSV-VP11/12 epitope-specific effector CD8+ T cells from SYMP vs. ASYMP individuals.
We first compared the level of granzyme B (GzmB), granzyme K (GzmK) and perforin (PFN) expressed on gated VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD8+ T cells from SYMP and ASYMP individuals (Fig. 4A). We found high levels of GzmB, GzmK and PFN expressed on VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD8+ T cells from ASYMP individuals compared to VP11/12 epitope-specific CD8+ T cells from SYMP individuals. This suggests a positive correlation of strong HSV-specific CD8+ T cell cytotoxic responses with protection against ocular herpetic disease (Fig. 4A).
Figure 4. Asymptomatic individuals had a significantly higher proportion of polyfunctional HSV-1 VP11/12-epitope-specific CD8+ T cells.
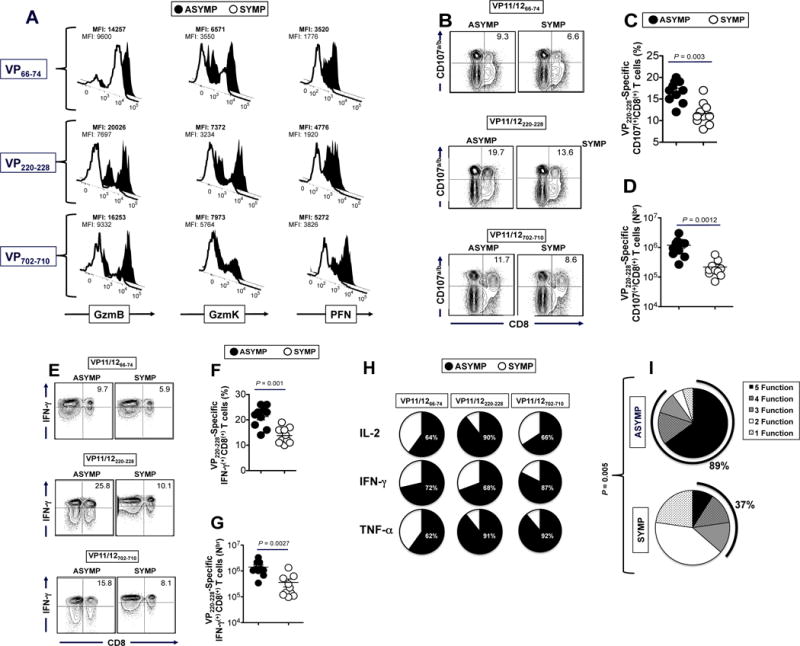
(A) VP11/12 epitope-primed CD8+ T cells from ASYMP individuals express high level of lytic granules compared to CD8+ T cells from SYMP individuals. FACS was used to determine the level of expression of GzmB, GzmK and PFN on tetramer gated CD8+ T cells specific to VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes, as described in Materials & Methods. Samples were acquired on BD LSR II and data analysis was performed using FlowJo. The numbers on the top of each histogram represent mean fluorescent intensity (MFI) depicting the level of expression of each cytotoxic molecule. Numbers in bold represent mean fluorescent intensity (MFI) of ASYMP individual. (B) Representative FACS data of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD107a/bhighCD8+ T cells from one ASYMP vs. one SYMP individual. Average percentage (C) and average absolute number (D) of VP11/12220–228 epitope-specific CD107a/bhighCD8+ T cells from 10 ASYMP and 10 SYMP individuals. (E) Representative FACS data of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific IFN-γhighCD8+ T cells from one ASYMP vs. one SYMP individual. Average percentage (F) and average absolute number (G) of VP11/12220–228 epitope-specific IFN-γhighCD8+ T cells from 10 ASYMP and 10 SYMP individuals. (H) Summary pie charts showing the average amount of each cytokine produced by CD8+ T-cells from ASYMP patients (n = 10, black) and SYMP patients (n = 8, white), as detected by Luminex assay. The average frequencies of cytokine-producing CD8+ T cells from SYMP and ASYMP individuals are shown inside each pie chart. (I) Each pie chart represents the overall mean of CD8+ T cell functions from 10 HLA-A*02:01-positive ASYMP and 10 SYMP individuals in responses to stimulation with either SYMP or ASYMP VP11/12 peptides. Each sector of the pie chart represents the number of CD8+ T cell functions produced.
We next examined the ability of each of the 3 VP11/12 epitope peptides to induce the expression of CD107a/b following in vitro stimulation of CD8+ T cells from SYMP vs. ASYMP individuals. CD107a and CD107b are lysosomal-associated membrane glycoproteins that surround the core of the lytic granules in cytotoxic T lymphocytes (CTL) (1, 25). Upon TCR engagement and epitope stimulation, CD107a/b are exposed on the cell membrane of CD8+ CTL. Thus, the level of CD107a/b expression on the surface of CTL is used as a direct assay for the human epitope-specific CTL response (25). To assess whether VP11/12 epitope-specific CD8+ T cells display CTL activity, fresh PBMC-derived CD8+ T cell lines were generated from HLA-A*02:01-positive ASYMP and HLA-A*02:01-positive SYMP individuals following in vitro stimulation with individual VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope peptides. The cytotoxicity of each of the three CD8+ T cell lines was measured against autologous target monocyte-derived DCs infected with HSV-1 by detecting the level of CD107a/b expression by FACS on gated CD8+ T cells. As shown in Figs. 4B to 4D, a higher percentage of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD8+ T cells from ASYMP individuals expressed significant levels of CD107a/b. In contrast, fewer VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD8+ T cells from SYMP patients expressed CD107a/b. As expected, no significant percentage of CD8+ T cells up-regulated CD107a/b after incubation with mock infected or target cells (data not shown). The results indicate that VP11/12-specific CD8+ T cells from ASYMP individuals: (i) have higher cytotoxic activity compared to CD8+ T cells from SYMP patients; (ii) have cytotoxic activity against HSV-1 infected cells; and (iii) are able to specifically recognize endogenously processed VP11/12 epitopes from HSV-1 infected target cells. The results also indicate that VP11/1266–74, VP11/12220–228 and VP11/12702–710 are functional cytotoxic epitopes.
We next determined the ability of VP11/12 epitopes to stimulate the production of IFN-γ by CD8+ T cells from SYMP vs. ASYMP individuals. Freshly isolated CD8+ T cells from SYMP and ASYMP individuals were stimulated in vitro for 6 hrs. with individual VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope peptides, as described in Materials and Methods. The percentage and number of IFN-γ+CD8+ T cells were compared from SYMP and ASYMP individuals by intracellular FACS staining. Significantly higher percentages of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific IFN-γ-producing CD8+ T cells were detected in ASYMP individuals, as compared to SYMP individuals (p < 0.01, Figs. 4E to 4G).
The levels of IL-2, IFN-γ and TNF-α effector cytokines produced by CD8+ T cells from ASYMP vs. SYMP individuals following in vitro re-stimulation with VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes were also compared by the Luminex micro-beads system. As shown in Fig. 4H, CD8+ T cells from ASYMP patients produced high levels of IL-2, IFN-γ and TNF-α consistent with differentiated T cells (the black filed portion of the pie charts). In contrast, CD8+ T cells from SYMP individuals produced less effector cytokines consistent with less differentiated T cells (the white portion of the pie charts).
The percentage of ASYMP and SYMP individuals that showed positive significant results for one or several CD8+ T cell functions following in vitro re-stimulation with each VP11/12 peptide are summarized in Fig. 4I. Overall, 89% of ASYMP individuals had HSV-specific CD8+ T cells with 3 to 5 functions, indicating their ability to display concurrent polyfunctional activities: (i) production of high levels of GzmB, GzmK and Perforin; (ii) production of high levels of CD107a/b lytic granules (cytotoxic activity), (iii) expression of IFN-γ (FACS); (iv) production of high levels of IL-2, IFN-γ and TNF-α effector cytokines (Luminex); and (v) low levels of expression of T-cell activation markers CD27 and CD28 (FACS). In contrast, only 37% of SYMP individuals had HSV-specific CD8+ T cells with 3 functions while the majority had just one or two functions, suggesting that CD8+ T cells from SYMP patients tended to have two or less functions (P < 0.005).
To further study the differences in HSV-specific CD8+ T cell function from SYMP vs. ASYMP individuals we studied the expression of CD27 and CD28 on VP11/12 epitope-specific CD8+ T cells. Cell surface expression of CD27 and CD28 co-stimulatory molecules is often associated with continuous antigenic stimulation (37, 38). Increased frequencies of VP11/12220–228-specific CD8+CD27+CD28+ T cells were detected from SYMP individuals compared to ASYMP individuals (Figs. 5A and 5B). We also analyzed the expression of PD-1, a marker of T cell exhaustion (39). As shown in Figs. 5C and 5D, SYMP patients exhibited higher frequencies of VP11/12220–228-specific PD1+CD8+ T cells compared to ASYMP individuals, suggesting functional exhaustion of HSV-specific effector CD8+ T cells from SYMP patients.
Figure 5. Asymptomatic individuals had a significantly higher proportion of differentiated and functional VP11/12-epitope-specific CD27highCD28highPD-1lowCD8+ T cells.
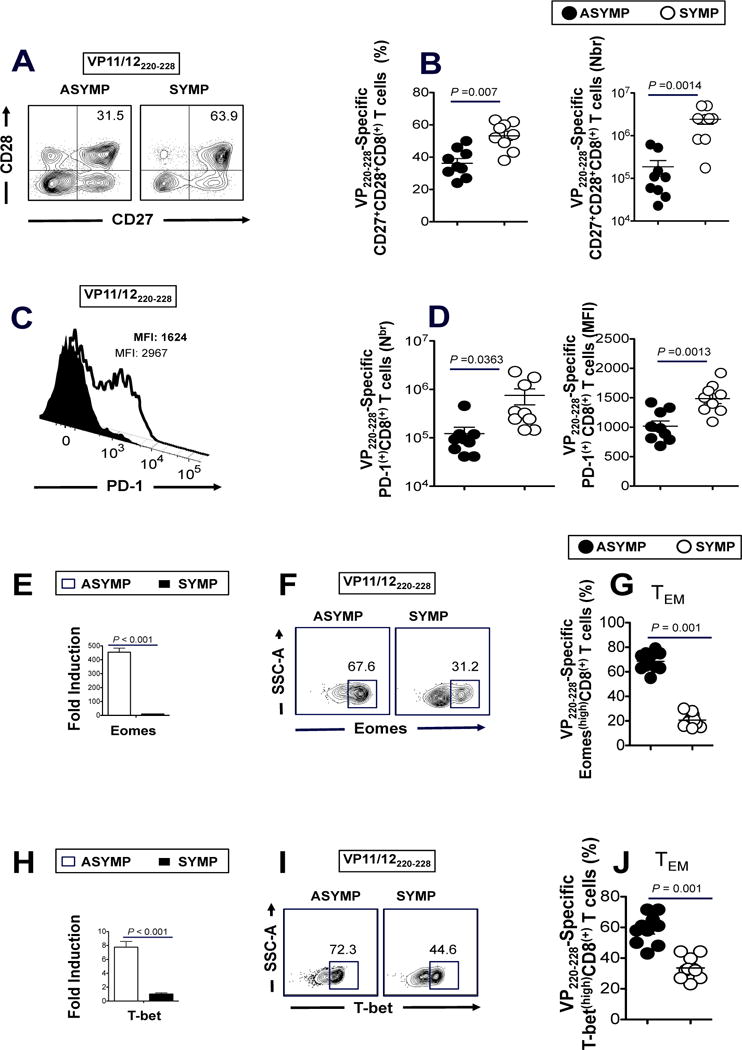
Representative (A) and average frequencies (B) of VP220–228 epitope-specific CD8+ T cells from 10 ASYMP and vs. 10 SYMP individuals that are CD27highCD28high. Representative (C) and average frequencies (D) of VP220–228 epitope-specific CD8+ T cells from 10 ASYMP and vs. 10 SYMP individuals that are PD-1high. (E and H) The expression patterns of T-bet and Eomes transcription factors was analyzed, at RNA level using RT-PCR, from total CD8+ T cells derived from either SYMP (open square) or ASYMP individuals (closed square). Representative FACS data of the percentage of Eomes (F) and T-bet (I) positive HSV-1 VP11/12220–228 epitope-specific CD8+ T cells, derived from one SYMP individual (right) and one ASYMP individual (left). Average frequencies of the expression patterns of Eomes (G) and T-bet (J) transcription factors analyzed by FACS at the protein level from gated VP11/12220–228-specific CD8+ TEM cells derived from 10 SYMP (open circles) and 10 ASYMP individuals (closed circles). The results are representative of 3 independent experiments in each individual. The indicated P values, calculated using one-way ANOVA Test, show statistical significance between SYMP and ASYMP individuals.
To determine the differentiation status of HSV-specific CD8+ T cells from SYMP vs. ASYMP individuals we compared the expression patterns of eomesodermin (Eomes) and T-bet transcription factors ex vivo on CD8+ T cells from SYMP and ASYMP individuals at both mRNA (using RT-PCR) and protein level (using FACS). Eomes and T-bet function as a molecular switch between TCM and TEM cell differentiation by driving differentiation of CD8+ TEM cells at the expense of TCM cells (40–44). As shown in Figs. 5E to 5G, a significantly higher proportion of VP11/12220–228-specific CD8+ T cells from ASYMP individuals expressed Eomes at the RNA level (using RT-PCR) Fig. 5E. In addition, a higher proportion of VP11/12220–228-specific CD8+ T cells from ASYMP individuals were Eomeshigh (P < 0.001, Figs. 5F to 5G). Similar results were obtained when analyzing the expression of T-bet in VP11/12 CD8+ T cells from SYMP vs. ASYMP individuals (Figs. 5H to 5J).
Altogether, the results indicate that VP11/12-specific CD8+ T cell responses associated with control of herpes disease differ in ASYMP vs SYMP individuals: (i) a stepwise loss of CD28 and CD27 together with an increase in IFN-γ and cytolytic activity by VP11/12-epitope specific CD8+ T cells from ASYMP individuals are both characteristics of less-differentiated effector T cells; (ii) high expression of PD-1 exhaustion marker on HSV-1 VP11/12-specific effector CD8+ T cells from SYMP individuals, suggest a total or partial dysfunctionality; (iii) higher expression of Eomes and T-bet by CD8+ T cells T cells from ASYMP individuals, which may favor effector to memory CD8+ T cell transition (E>>>M) and the formation of a higher percentage of CD8+ TEM cells as seen in ASYMP individuals (45); and (iv) ASYMP individuals mostly multi-functional CD8+ T cells with simultaneous expression of 3 to 5 functions, whereas SYMP individuals had a majority of CD8+ T-cells with just one or two simultaneous functions. You said this above!
6. Immunization with three VP11/12 CD8+ TEM epitopes that are highly recognized in Asymptomatic individuals elicited a strong protective immunity in the “humanized” HLA-A*02:01 transgenic mouse model of ocular herpes
We next set up a pre-clinical vaccine trial, using our established susceptible “humanized” HLA-A*02:01 transgenic mouse model (HLA Tg mice on BALB/c genetic background) (1), to evaluate whether immunization with ASYMP immunodominant CD8+ TEM epitopes (i.e. VP11/1266–74, VP11/12220–228 and VP11/12702–710) will confer protection against ocular herpes infection and disease.
Groups of susceptible HLA Tg mice (n = 10 mice per group) were immunized subcutaneously twice, 21 days apart with a mixture of VP11/1266–74, VP11/12220–228 and VP11/12702–710 peptide epitopes together with an immunodominant CD4+ T cell epitope recently identified from VP11/12 tegument protein (VP11/12129–143, Group 1) or with a mixture of VP11/1266–74, VP11/12220–228 and VP11/12702–710 peptide epitopes together with a promiscuous CD4+ T cell epitope (PADRE, Group 2). The CD8+ and CD4+ T cell epitope peptides were emulsified in CpG1826 adjuvant and delivered subcutaneously as a mixture. As negative control, mock-immunized mice received CpG1826 adjuvant alone (Mock, Group 3). Two weeks after the second and final immunization, animals from all groups received an ocular HSV-1 challenge (2 × 105 pfu, McKrae strain) without scarification. The mice were then assessed for up to 30 days post-challenge for ocular herpes pathology, ocular viral titers and survival.
As shown in Figs. 6A and 6B, on day 10 post infection, the pathology clinical scores observed in the immunized groups were significantly lower compared to those recorded in the mock-immunized group (p = 0.001, for all). Furthermore, significantly less virus was detected on day 7 post-infection (the peak of viral replication) in the eye swabs of the vaccinated groups compared to mock-immunized group (p = 0.01, Fig. 6C). Most animals in the vaccinated groups survived infection (80% and 60%) compared to 0% survival in the mock-immunized group (p < 0.005) (Fig. 6D).
Figure 6. Protective immunity against ocular herpes infection and disease induced by immunodominant VP11/12 CD8+ TEM epitopes in “humanized” HLA transgenic mice.
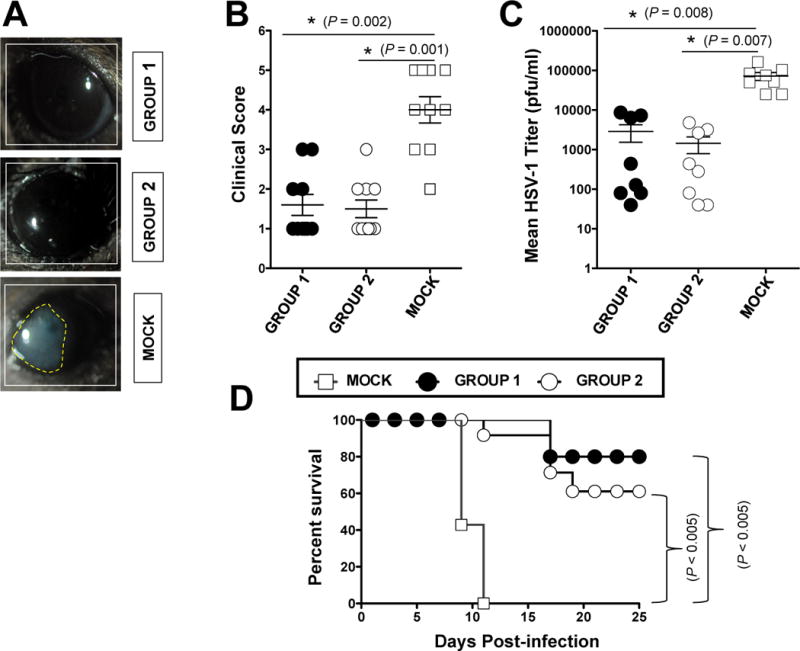
Three groups of age-matched male HLA Tg mice (n = 10 each) were immunized subcutaneously, on days 0 and 21, with a mixture of three human CD8+ TEM cell epitope peptides (VP11/66–74, VP11/12220–228 and VP11/12702–710) delivered either with a novel CD4+ T cell epitope (VP11/12129–143) emulsified in CpG1826 adjuvant (Group 1) or with the promiscuous CD4+ T-cell PADRE epitope peptide emulsified in CpG1826 adjuvant (Group 2). CpG1826 adjuvant alone was used as control (Group 3, mock-vaccinated). Two weeks after the final immunization, all animals were challenged ocularly with 2 × 105 pfu of HSV-1 (strain McKrae). The eye disease was detected and scored two weeks after immunization as described in Material & Methods (A and B). Virus titrations were determined from eye swabs on day 7 post-infection, as described in Material & Methods (C). Survival was determined in a window of 30 days post-challenge, as described in Material & Methods (D). The results are representative of 2 independent experiments. The indicated P values, calculated using one-way ANOVA Test, show statistical significance between protected (ASYMP) and non-protected (SYMP) mice.
Altogether, these results indicate that immunization with immunodominant HSV-1 VP11/1266–74, VP11/12220–228 and VP11/12702–710 CD8+ TEM peptide epitopes decreased ocular herpes disease, decreased virus replication, and protected against lethal ocular herpes in susceptible HLA Tg mice.
7. Frequent VP11/12-specific CD8+ TEM cells are detected in the cornea of HSV-1 infected “ASYMP” HLA transgenic mice
We next determined the phenotype and function of CD8+ T cells from the corneas of protected (“ASYMP”) vs. non-protected (“SYMP”) HLA Tg mice that were vaccinated and HSV-1 challenged, as above. For simplicity only the extremes of ASYMP and SYMP mice were used for phenotypic and functional characterization of cornea resident CD8+ T-cells (Fig. 7). ASYMP mice were mice from group 1 in the experiment shown in Fig. 6B that had a clinical score of 1.0. SYMP mice were mice from the mock group in the experiment shown in Fig. 6B that had a clinical score of 5 or 4. As shown in Figs 7A and 7B, protected (“ASYMP”) mice had a significantly higher percentage of cornea-resident VP11/12220–228 epitope-specific CD44highCD62LlowCD8+ TEM cells, as compared to non-protected (“SYMP”) mice (49.6 +/− 3% vs. 25.1 +/− 2%, p < 0.05). In contrast, a significantly higher percentage of cornea-resident VP11/12220–228 epitope-specific CD44highCD62LhighCD8+ TCM cells were detected in “SYMP” mice, as compared to “ASYMP” mice (p < 0.01, Fig. 7A and 7B). Similar results were obtained for CD8+ T cells specific to VP11/12702–710 epitope (Fig. 7C and 7D). Similar results were obtained for CD8+ T cells specific to the sub-dominant VP11/1266–74 epitope (data not shown). These results indicate that “ASYMP” cornea-resident VP11/12 epitope-specific CD8+ T cells with TEM phenotype are associated with protection from ocular herpes disease.
Figure 7. The corneas of protected HSV-1 infected “ASYMP” HLA transgenic mice contain frequent VP11/12-specific polyfunctional CD8+ TEM cells.
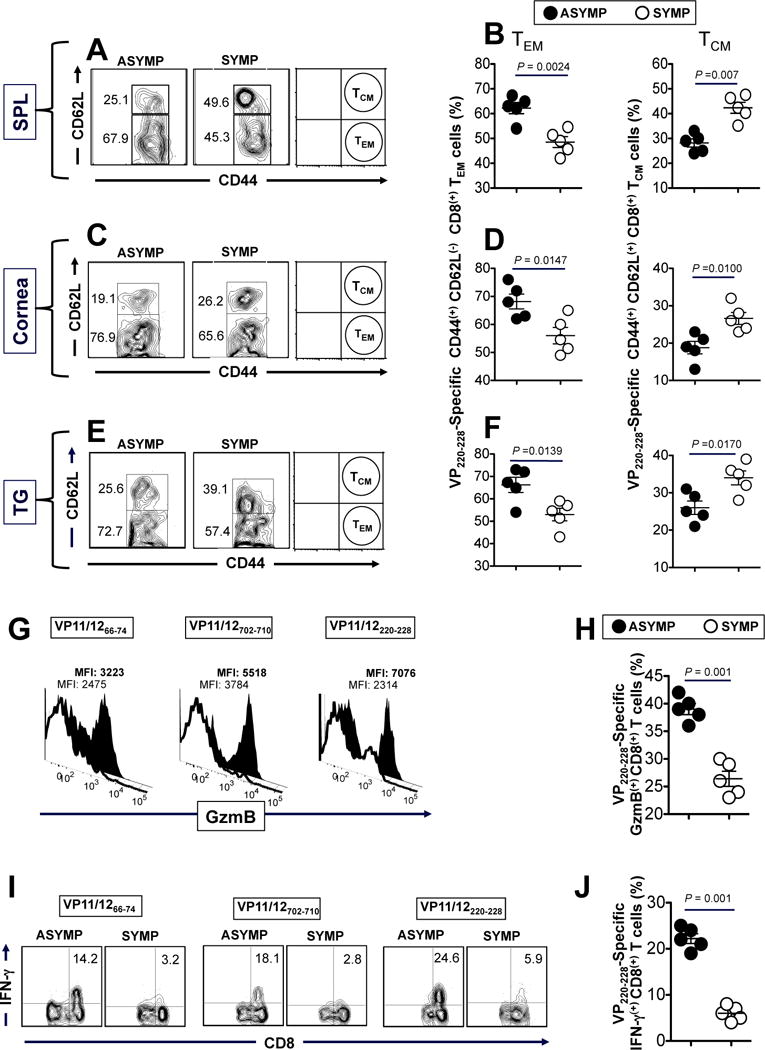
(A) Representative FACS data of the frequencies of CD44highCD62LlowCD8+ TEM and CD44highCD62LlowCD8+ TCM cells specific to VP11/12220–228 peptide/Tetramer complexes detected the corneas of one protected “ASYMP” vs. one non-protected “SYMP” HLA Tg mice. The spleen, cornea, and trigeminal ganglia were assayed for CD8+ T cell responses on day 9 post-infection. (B) Average frequencies of VP11/12220–228 epitope-specific TCM and TEM CD8+ cells detected in the corneas of five protected ASYMP and vs. five non-protected SYMP HLA Tg mice. ASYMP mice had a clinical score of 1.0 in Fig. 6B. SYMP mice had a score of 5 or 4 in Fig. 6B). Representative (C) and average frequencies (D) of VP11/1266–74 epitope-specific TCM and TEM CD8+ cells detected in the cornea five protected ASYMP and vs. five non-protected SYMP HLA Tg mice. (E) Representative data of level of expression of GzmB on cornea-derived CD8+ T cells specific to VP11/66–74, VP11/12220–228 and VP11/12702–710 detected from protected “ASYMP” mice (black histogram) and non-protected “SYMP” mice (white histogram). (F) Average frequencies of GzmB(+)CD8+ T cells specific to VP11/66–74, VP11/12220–228 and VP11/12702–710 detected from the corneas of protected “ASYMP” mice (black circles) and non-protected “SYMP” mice (white circles). (G) Representative data of the % IFN-γ(+)CD8+ T cells specific to VP11/66–74, VP11/12220–228 and VP11/12702–710 detected from the corneas of protected “ASYMP” mice (right) and non-protected “SYMP” mice (left). (H) Average frequencies of IFN-γ(+)CD8+ T cells specific to VP11/66–74, VP11/12220–228 and VP11/12702–710 detected from the corneas of protected “ASYMP” mice (black circles) and non-protected “SYMP” mice (white circles). 1×106 cells for each assay and the results are representative of 2 independent experiments. The indicated P values, calculated using one-way ANOVA Test, show statistical significance between SYMP and ASYMP mice.
To further study the differences in HSV-specific CD8+ T cell function from “SYMP” vs. “ASYMP” mice, we compared the level of expression of GzmB, as determined by mean fluorescent intensity (MFI), as well as the percentage of GzmB+CD8+ T cells, on gated VP11/12 epitope-specific CD8+ T cells. GzmB was also significantly higher in “ASYMP” VP11/12 epitope-specific CD8+ T cells compared to “SYMP” VP11/12 epitope-specific CD8+ T cells (p < 0.005, Fig. 7E). In addition, a higher percentage of VP11/12 epitope-specific CD8+ T cells expressing GzmB were also detected in “ASYMP” corneas compared to “SYMP” corneas, suggesting more protective effector VP11/12-specific CD8+ T cells in “ASYMP” corneas (p < 0.01, Fig. 7F).
We next determined the ability of CD8+ T cells from “SYMP” vs. “ASYMP” mice to produce IFN-γ following recall with the immunizing VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope peptides. CD8+ T cells from “SYMP” cornea and “ASYMP” cornea were stimulated in vitro for 6 hrs. with individual immunodominant (VP11/12220–228 and VP11/12702–710) and subdominant (VP11/1266–74) epitope peptides, as described in Materials and Methods. The percentages of IFN-γ+CD8+ T cells were compared from SYMP and ASYMP mice by intracellular FACS staining. Significantly higher percentages (Figs. 7G and 7H) of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitope-specific CD8+ T cells producing IFN-γ were detected in protected “ASYMP” corneas, as compared to non-protected “SYMP” corneas (p < 0.001).
Altogether, the phenotypic and functional properties of HSV-1 VP11/12 epitope-specific CD8+ T cells in HLA Tg mice revealed that: (i) the “ASYMP” mice, but not “SYMP” mice, had frequent protective HSV-1 VP11/12-specific CD8+ TEM cells; (ii) HSV-1 VP11/12-specific CD8+ T cells from “ASYMP” mice displayed more concurrent polyfunctional activities than CD8+ T cells from SYMP mice; (iii) HSV-1 VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes may represent strong T cell epitope candidates for inclusion in immunotherapeutic and immune-prophylactic herpes vaccines. Of note, the sequences of VP11/1266–74, VP11/12220–228 and VP11/12702–710 epitopes are highly conserved between HSV1 strains (100%) and HSV-2 strains (77% to 100%) (Table S1). However, no significant homology exists between the amino acid sequences of these three epitopes and the VP11/12 amino acid sequences of VZV (44%) and CMV (33%) strains (Table S1). The results also confirm that HLA Tg mice are a useful animal model for understanding the mechanisms by which human epitope-specific CD8+ T cells mediate control of herpes infection and disease, as we previously suggested (13).
DISCUSSION
The HSV-1 derived tegument virion phosphoproteins (VPs), which are located between the capsid and the outer viral envelope proteins, contain a large number of possible protective CD8+ T cell epitopes (9), however only a handful of epitopes have been identified so far. In this study we identified three protective “ASYMP” human CD8+ TEM cells epitopes from the abundant tegument VP11/12 protein: VP11/1266–74, VP11/12220–228 and VP11/12702–710. These new VP11/12 epitopes displayed high-affinity binding to purified HLA-A*0201 molecules, stabilized HLA-A*0201 molecules on target cells, recalled CD8+ T cells in HSV-1 seropositive ASYMP individuals, and in “ASYMP” HLA-A*0201 transgenic mice ocularly infected with HSV-1. In addition, we found that two major VP11/12-epitope specific CD8+ T cell subpopulations, which have remarkably different phenotype and function, one of which was mostly in SYMP and the other mostly in ASYMP individuals. Notably, ASYMP individuals had a significantly higher proportion of differentiated polyfunctional VP11/12-epitope specific CD8+ TEM cells. In contrast, SYMP patients had significantly higher frequencies of less-differentiated mono-functional VP11/12-epitope specific CD8+ TCM cells. To the best of our knowledge, this is the first report of (i) protective CD8+ T cells epitopes from the HSV-1 VP11/12 tegument protein and (ii) phenotypic and functional differences of HSV-1 VP11/12-specific effector and memory CD8+ T cells from ocular herpes SYMP and ASYMP individuals. Our findings provide a framework for the rational design of a herpes vaccine based on tegument proteins that elicit long-term CD8+ T cell protective immunity.
CD8+ T cells recognize their target Ags as 8- to 10-aa-long peptides, which are derived from intracellular, processed viral Ags and presented by HLA class I molecules (e.g. HLA-A*0201 molecules) expressed at the surface of infected cells (46, 47). Despite the HSV-1 84+ open reading frames encoding hundreds of potential HLA-compatible CD8+ T-cell epitopes, only a handful of epitopes/protein drive robust CD8+ T cells in seropositive individuals (48–51). Tegument proteins are delivered to the cytoplasm of host cells during viral entry, resulting in an immediate processing by antigen presenting cells (APC) shortly after infection (7–10), They hence constitute an immediate target Ag to induce host T cells (7, 8). Considering the recent failures of clinical vaccine trials using HSV envelope glycoproteins (mainly gB and gD) (16, 17); and because tegument proteins are major targets of CD8+ T cells from herpes seropositive individuals (7–10, 19–22), it is surprising how only few reports exist identifying protective T cell epitopes on tegument proteins and characterizing the phenotype and function of HSV-1 tegument proteins derived epitope-specific “protective” CD8+ T cells from HSV-seropositive healthy ASYMP individuals, who appear to have acquired a “natural” protective immunity. This information is necessary for the successful design of an effective T cell-based therapeutic vaccine strategy. Rather than just using a few in vitro predictive assays to map CD8+ T cell epitopes from HSV-1 VP11/12 protein, in the present study we have several computational algorithms followed by both in vitro and in vivo functional immunological assays. Only peptides that conform to given sequence motifs preferentially bind with high affinity to HLA class I molecules (1), and the amino acid residues at specific positions are conserved for peptides that bind HLA-A*0201 molecules with high affinity (1, 4). Potential HLA-A*0201-binding peptides can be selected based on the presence of leucine (L), isoleucine (I) or methionine (M) as the dominant residue at position 2 and valine (V), or a residue with an aliphatic hydrocarbon side chain at the C-terminus (1). Following identification of antigenic VP11/12 peptides in HLA-A*0201 positive HSV1 seropositive humans it is important to address whether each peptide can be correctly processed from the native antigen and subsequently displayed on the surface of an APC. Of the 10 antigenic VP11/12 peptides studied in this report, only three (VP11/1266–74, VP11/12220–228 and VP11/12702–710) were generated from native VP11/12 protein by natural processing. This observation illustrated the importance of experimentally evaluating whether peptides generated by computer algorithms and various HLA-peptides binding assays are able to functionally stimulate CD8+ T cells in vitro and in vivo.
Many immunological assays have been developed to validate computationally-predicted human CD8+ T-cell epitopes, including frequency of epitope-specific CD8+ TEM vs. TCM cells by tetramer assays, cell membrane HLA stabilization assays, CD8+ T-cell cytokine analysis, IFN-γ intracellular assay GzmB, GzmK Perforin, CD107a/b degranulation cytotoxic assays, CD27/CD28 activation markers, and PD-1 exhaustion marker (52–54). When used individually, each screen is not sufficient to conclusively identify functional CD8+ T-cell epitopes (52, 54–56). However, combinations of these screens can provide strong evidence of functional epitopes (2, 54, 57). Using these multiple screens we identified three high affinities VP11/12 epitopes that were naturally processed and able to recall frequent functional human CD8+ TEM cells in HSV-1 seropositive ASYMP individuals. Of interest is that the VP11/12702–710 peptide that appeared to bind with only low affinity to HLA-A*02:01 molecules in vitro still induced strong IFN-γ-producing cytotoxic CD8+ TEM cell responses. Although the reason for this is not completely understood, this result highlights the importance of screening potential peptide epitopes using multiple immunological assays.
Epitope-based vaccine approaches offer several potential advantages over conventional protein- or DNA-based vaccine approaches in terms of purity, lot-to-lot consistency, cost of production, and a high specificity in eliciting immune responses (1, 58). In addition, peptide-epitope technology has proven an extremely useful strategy to avoid potentially harmful immune responses (1, 58). Among other advantages, the epitope-based vaccines avoid the potential for “pathogenic” epitopes to inadvertently drive unwanted pathogenic responses (59). Such pathogenic responses might contribute to exacerbation of disease as we recently found for an HLA-A*02:01-restricted CD8+ T cell epitope from the HSV-1 gK protein (60). Since our approach to epitope identification in this study was tailored specifically for HLA-A*02:01-restricted epitopes, the results reported here do not imply that these are the only human CD8+ T-cell epitopes present in VP11/12. However, this study describes a model system that could be applied to any HLA class I haplotype and to any other herpes tegument, structural or regulatory proteins to identify other SYMP vs. ASYMP epitopes.
The choice of animal model is crucial in herpes vaccine development (reviewed in (67)). A major goal of this study was to examine the protective efficacy of human HLA-restricted epitopes. Thus, the ideal animal model should mount an HLA-restricted immune responses specific to human epitopes, while at the same time mimicking as many aspects of herpes infection and disease as possible (68). Normal mice (e.g. BALB/c or C57BL/6) have been the standard choice for most immunologists and results from these mice have yielded tremendous insights into the role of H2-restricted mouse T cells in protection against herpes infection and disease (4, 23, 35, 68–71). However, normal mice do not mount specific “humanized” T cell responses to HLA-restricted epitopes (reviewed in (67)). In contrast, similar to humans, HLA Tg mice can develop “humanized” T cell responses to HLA-restricted epitopes (74).
A question of practical importance is the translation of the current pre-clinical findings for the development of an epitope-based clinical vaccine for a genetically heterogeneous human population. (15, 27, 58, 61–63). A universal herpes vaccine should induce protective ASYMP CD8+ T-cell responses in the context of many different HLA alleles. This report focused on HLA-A*0201, because of its high prevalence in most populations worldwide, regardless of race and ethnicity (64–67). The high degree of HLA polymorphism that might be a hindrance to epitope-based vaccines can be dealt with by the inclusion of multiple HLA-restricted epitopes that are recognized in the context of diverse related HLA alleles, and by designing peptide-based vaccines with higher CD8+ T cell epitope densities. A combination of nine HLA supertypes has been defined to provide an almost perfect coverage (greater than 99%) of the entire repertoire of HLA molecules (68). Since a mixture of multiple ASYMP epitopes is likely to produce polyclonal CD8+ T-cell lines (one T-cell clone for each epitope) and more effector T-cells than a single epitope (69), we used multiple CD8+ T-cell epitopes to induce a broader T-cell response (15, 69). To further increase efficacy, a multi-epitope-based peptide vaccine should include several CD8+ T-cell epitopes from several different glycoproteins and tegument proteins each chosen to represent the HLA supertypes known to provide recognition in a large proportion of the global population, regardless of race and ethnicity.
Focusing the immune response toward selected ASYMP epitopes could be of value in the case of herpes infections, where T cells directed against the ASYMP epitopes might have been inactivated and T cells specific for SYMP epitopes might have escaped T-cell tolerance. In addition, monitoring immunogenicity following vaccination is easily done due to the fact that the epitopes used in the vaccine also serve as the required diagnostic reagents needed to follow T-cell responses in the vaccines. The efficacy of peptide epitopes immunization on different T cell memory populations has been recently reported (70). Most notably, peptide immunotherapy appeared to induce strong and long-lived tissue-resident TEM cells, but not circulating TCM cells (70). The present study supports our recently advanced “SYMP/ASYMP concept” by showing that immunization with immunodominant VP11/12 epitopes that are mainly recognized by CD8+ TEM cells from “naturally” protected ASYMP individuals also protected HLA Tg mice against ocular herpes infection and disease.
Humans are not immunologically “naïve” and often develop T cell responses that cross-react between different viruses including members of the same (or different) family (71–73). However, this is unlikely for the three VP11/12 epitopes discovered in this study because: (i) alignment of their sequences with published sequences of VP11/12 proteins from other human herpesviruses (e.g. VZV and CMV), did not reveal significant homologies that might be translated to cross-reactive T cell responses (Table S1); (ii) the three HLA-A*02:01-restricted immunodominant VP11/12 epitopes reported here as recognized by CD8+ T cells from HLA-A*02:01 positive HSV-1 seropositive individuals are also recognized with the same magnitude and breath by CD8+ T-cells induced in “pathogen-free” HLA-A*02:01 Tg mice following HSV-1 ocular infection. These results suggest that CD8+ T cells detected in humans are likely induced by HSV-1 VP11/12 epitopes, rather than cross-reactive epitopes from other viruses within the α, β, γ herpes family. However, one cannot definitely exclude a cross-reactivity of these HSV-1 VP11/12 epitopes with epitopes from other non-herpes pathogens outside the herpes family.
To gain more insight into the nature of HSV-specific CD8+ T cell sub-populations that are predominant in SYMP vs. ASYMP individuals, we used several immunological assays to determine the population size, the phenotype and the function of CD8+ T cells obtained from peripheral blood. Although we are aware that information gained from peripheral blood T cells may not be completely reflective of tissue-resident T cells, because of the obvious ethical and practical considerations of obtaining tissue-resident CD8+ T cells (i.e. from the cornea or from trigeminal ganglia, the site of acute and latent infections, respectively) our investigations were limited to human PBMC-derived CD8+ T cells. Under the above circumstances, HSV-specific CD8+ T cells from ASYMP patients had a greater frequency of poly-functional VP11/12 epitope-specific CD8+ TEM cells, while HSV-specific CD8+ T cells from SYMP individuals had more mono-functional VP11/12 epitope-specific CD8+ TCM cells. These results suggest that compared to SYMP individuals, at a second pathogen encounter (e.g. following HSV-1 reactivation from latency), ASYMP individuals, would mount faster and stronger protective HSV-specific CD8+ TEM cell responses allowing a better clearance of herpes infection and disease.
We also found a low expression of Eomes associated with high expression of PD-1 in VP11/12-specific CD8+ T cells from SYMP individuals. In contrast, high expression of T-bet associated with low expression of PD-1 was recorded in VP11/12-specific CD8+ T cells from ASYMP individuals. This is consistent with differentiated and functional CD8+ T cells in ASYMP individuals. It is likely that the observed decrease in T-bet expression in SYMP individuals contributes to a restrain in terminal differentiation of secondary effector and memory CD8+ T cells as recently reported in another system (74). On one hand, T-bet and Eomes are key transcription factors involved in the long-term renewal of memory CD8+ T cells to their characteristic effector potency (40–44). T-bet drives differentiation of CD8+ TEM cells at the expense of TCM cells (43). T-bet is also overexpressed in CD8+ T cells that differentiated in the absence of CD4+ T cell help, a condition that is associated with defective central-memory formation (43). Eomes enables CD8+ T cells to compete for the memory cell niche (41). Additionally, CD8+ T cells deficient in T-bet and Eomes fail to differentiate into functional killers, which is required for defense against viral pathogens (42). Instead, virus-specific CD8+ T cells lacking both T-bet and Eomes differentiate into an interleukin-17-secreting autoimmune and inflammatory T cell lineage (42). The above observations together with our results are reminiscent of the CD8+ T cell fate implicated in SYMP herpes disease. On the other hand, T cell exhaustion has a major role in the failure to control persistent infections, likely including latent HSV-1 and HSV-2 infections. High expression of inhibitory receptors, including PD-1, and the inability to sustain functional T cell responses contribute to T cell exhaustion. We found a low expression of T-bet associated with high expression of PD-1 in VP11/12-specific CD8+ T cells from SYMP individuals. In contrast, high expression of T-bet associated with low expression of PD-1 was recorded in VP11/12-specific CD8+ T cells from ASYMP individuals. Our results suggest a persistent antigenic stimulation in SYMP individuals, and are in agreement with a recent report showing persistent antigenic stimulation caused down-regulation of T-bet and resulted in more severe exhaustion of CD8+ T cells (40). To our knowledge this is the first study to show differential expression of transcription factors in HSV-specific human memory CD8+ T cells among HLA-A*02:01 positive, HSV-1 seropositive SYMP and ASYMP individuals. It is likely that the concerted action of T-bet, Eomes and other transfection factors results in the development of fully differentiated CD8+ TEM cells in ASYMP individuals that migrate to inflamed tissues (i.e. eyes and sensory ganglia) following antigen recognition and secrete IFN-γ and/or release cytotoxic granules that contain GzmB and perforin (75, 76).
The underlying protective and non-protective immune mechanisms of TEM and TCM cells in ocular herpes remain to be fully elucidated. A recent study suggests that CD8+ T cells are required to eliminate virus more efficiently from the cornea but play a minimal role in immunopathology (77). However, the TEM and TCM cells phenotype of these cells was not reported and the translation of these findings to the human situation, where “SYMP” CD8+ TCM cells and “ASYMP” CD8+ TEM cells maybe in play, still remains to be determined. Based on our present findings, we propose a mechanism build on a novel “symptomatic/asymptomatic concept”, in which the corneal lesions are not a direct consequence of damage caused by the virus or by auto-reactive or bystander T cells, but rather from the balance between non-protective TCM cell responses specific to SYMP HSV-1 epitopes and protective TEM cell responses specific to non-overlapping ASYMP HSV-1 epitopes. Both direct and indirect mechanisms might be involved in CD8+ TEM cell mediated protection and in CD8+ TCM cells mediated susceptibility to recurrent herpetic disease. The polyfunctionality of CD8+ TEM cells segregated with immunologic control of herpes infection and disease, in both ASYMP individuals and in “ASYMP” HLA Tg mice. Thus, “polyfunctionality” of HSV-specific CD8+ TEM cells may be an important factor accounting for the immunologic control of herpes. Epitope-specific CD8+ TEM cell lines from ASYMP individuals appeared to recognize naturally processed epitopes on HSV-1 infected cells and had strong virus inhibitory cytotoxic activity. A formal demonstration that ASYMP CD8+ TEM cells, with these functions intact, cause immunologic control of recurrent herpes infection and disease would require passive-transfer studies in humans or in experimental animals that develop spontaneous recurrent disease (11).
In conclusion, the present study provides several new insights about the specificity, the phenotype and function of HSV-1 VP11/12 epitope-specific CD8+ T cell sub-population that segregate with immunologic control of ocular herpes infection and disease in both ASYMP individuals and in “humanized” HLA-A*02:01 transgenic mice. We report three, previously unknown, HLA-A*02:01-restricted ASYMP epitopes derived from HSV-1 VP11/12 that tend to recall polyfunctional CD8+ TEM cell responses in HLA-A*02:01 positive HSV seropositive ASYMP humans and in “humanized” HLA-A*02:01 transgenic mice protected from ocular herpes infection and disease. Understanding the detailed mechanisms by which HLA-A*02:01-restricted ASYMP CD8+ T cells are associated with protection against disease will reveal underlying principles of immune control of herpes, which is critical for the rational design of a T cell based vaccine strategy. Overall these findings demonstrate quantitative and qualitative features of an effective HSV-specific CD8+ T cell response that should be considered in the testing of the next generation of herpes vaccines.
Supplementary Material
Acknowledgments
The authors would like to thank Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the Tetramers used in this study. We also thank Barbara Bodenhoefer (RN) from UC Irvine’s Institute for Clinical and Translational Science (ICTS) for helping with blood drawing from HSV-1 seropositive symptomatic and asymptomatic individuals.
This work is supported by Public Health Service research grants EY14900, EY019896, EY024618, EY013191, 1R56AI098985, and 1R56AI093133, from the NIH, Discovery Eye Foundation, The Discovery Center for Eye Research and a Research to Prevent Blindness Challenge grant.
Abbreviations
- HSV
Herpes Simplex Virus
- ASYMP
Asymptomatic
- SYMP
Symptomatic
- VP
Virion phosphoprotein
- HLA
Human Leukocyte Antigen
- IFN-γ
Interferon Gamma
- GzmB
Granzyme B
- GzmK
Granzyme K
- PFN
Perforin
- PBMCs
Peripheral Blood Mononuclear Cells
- TCM
Central Memory CD8+ T Cell
- TEM
Effector Memory CD8+ T Cell
- Tg
Transgenic
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
References
- 1.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol. 2013;191:5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008;180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol. 2012;189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol. 2014;75:715–729. doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. The Challenges and Opportunities for the Development of a T-Cell Epitope-Based Herpes Simplex Vaccine. Vaccine. 2014;34:715–729. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chentoufi AA, BenMohamed L, Van De Perre P, Ashkar AA. Immunity to ocular and genital herpes simplex viruses infections. Clin Dev Immunol. 2012;2012:732546. doi: 10.1155/2012/732546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long D, Skoberne M, Gierahn TM, Larson S, Price JA, Clemens V, Baccari AE, Cohane KP, Garvie D, Siber GR, Flechtner JB. Identification of novel virus-specific antigens by CD4(+) and CD8(+) T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology. 2014;464–465:296–311. doi: 10.1016/j.virol.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. Journal of virology. 2006;80:5509–5515. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. Journal of virology. 2012;86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, Molina DM, Jasinskas A, Nakajima-Sasaki R, Felgner J, Hermanson G, BenMohamed L, Felgner PL, Davies DH. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by proteome-wide antibody profiling. Journal of virology. 2012;86:4328–4339. doi: 10.1128/JVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. Journal of virology. 2008;82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. Journal of virology. 2005;79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 17.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MA, Bucks MA, O’Regan KJ, Courtney RJ. The HSV-1 tegument protein pUL46 associates with cellular membranes and viral capsids. Virology. 2008;376:279–289. doi: 10.1016/j.virol.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Muller WJ, Dong L, Vilalta A, Byrd B, Wilhelm KM, McClurkan CL, Margalith M, Liu C, Kaslow D, Sidney J, Sette A, Koelle DM. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol. 2009;90:1153–1163. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, Benmohamed L. Future of an “Asymptomatic” T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virol. 2012;7:371–378. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A. 2003;100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 23.Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, Reiter Y, Phillips J, Linehan WM, Kasten-Sportes C, Pastan I, Berzofsky JA. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 24.Stuber G, Leder GH, Storkus WT, Lotze MT, Modrow S, Szekely L, Wolf H, Klein E, Karre K, Klein G. Identification of wild-type and mutant p53 peptides binding to HLA-A2 assessed by a peptide loading-deficient cell line assay and a novel major histocompatibility complex class I peptide binding assay. Eur J Immunol. 1994;24:765–768. doi: 10.1002/eji.1830240341. [DOI] [PubMed] [Google Scholar]
- 25.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 26.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 27.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002;106:113–121. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BenMohamed L, Krishnan R, Longmate J, Auge C, Low L, Primus J, Diamond DJ. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum Immunol. 2000;61:764–779. doi: 10.1016/s0198-8859(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 29.Boucherma R, Kridane-Miledi H, Bouziat R, Rasmussen M, Gatard T, Langa-Vives F, Lemercier B, Lim A, Berard M, Benmohamed L, Buus S, Rooke R, Lemonnier FA. HLA-A*01:03, HLA-A*24:02, HLA-B*08:01, HLA-B*27:05, HLA-B*35:01, HLA-B*44:02, and HLA-C*07:01 monochain transgenic/H-2 class I null mice: novel versatile preclinical models of human T cell responses. J Immunol. 2013;191:583–593. doi: 10.4049/jimmunol.1300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009;2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BenMohamed L, Thomas A, Bossus M, Brahimi K, Wubben J, Gras-Masse H, Druilhe P. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine. 2000;18:2843–2855. doi: 10.1016/s0264-410x(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 32.Daubersies P, Thomas AW, Millet P, Brahimi K, Langermans JA, Ollomo B, BenMohamed L, Slierendregt B, Eling W, Van Belkum A, Dubreuil G, Meis JF, Guerin-Marchand C, Cayphas S, Cohen J, Gras-Masse H, Druilhe P. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nature medicine. 2000;6:1258–1263. doi: 10.1038/81366. [DOI] [PubMed] [Google Scholar]
- 33.Bottius E, BenMohamed L, Brahimi K, Gras H, Lepers JP, Raharimalala L, Aikawa M, Meis J, Slierendregt B, Tartar A, Thomas A, Druilhe P. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J Immunol. 1996;156:2874–2884. [PubMed] [Google Scholar]
- 34.McKinney DM, Skvoretz R, Livingston BD, Wilson CC, Anders M, Chesnut RW, Sette A, Essex M, Novitsky V, Newman MJ. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J Immunol. 2004;173:1941–1950. doi: 10.4049/jimmunol.173.3.1941. [DOI] [PubMed] [Google Scholar]
- 35.Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, Vissers DC, ten Bosch GJ, Kester MG, Sijts A, Wouter Drijfhout J, Ossendorp F, Offringa R, Melief CJ. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med. 2001;193:73–88. doi: 10.1084/jem.193.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 37.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–7918. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Channappanavar R, Twardy BS, Suvas S. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One. 2012;7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 45.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 46.Bernardin F, Kong D, Peddada L, Baxter-Lowe LA, Delwart E. Human immunodeficiency virus mutations during the first month of infection are preferentially found in known cytotoxic T-lymphocyte epitopes. Journal of virology. 2005;79:11523–11528. doi: 10.1128/JVI.79.17.11523-11528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang QJ, Huang XL, Rappocciolo G, Jenkins FJ, Hildebrand WH, Fan Z, Thomas EK, Rinaldo CR., Jr Identification of an HLA A*0201-restricted CD8(+) T-cell epitope for the glycoprotein B homolog of human herpesvirus 8. Blood. 2002;99:3360–3366. doi: 10.1182/blood.v99.9.3360. [DOI] [PubMed] [Google Scholar]
- 48.Pelte C, Cherepnev G, Wang Y, Schoenemann C, Volk HD, Kern F. Random screening of proteins for HLA-A*0201-binding nine-amino acid peptides is not sufficient for identifying CD8 T cell epitopes recognized in the context of HLA-A*0201. J Immunol. 2004;172:6783–6789. doi: 10.4049/jimmunol.172.11.6783. [DOI] [PubMed] [Google Scholar]
- 49.Stock AT, Jones CM, Heath WR, Carbone FR. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol Cell Biol. 2006;84:543–550. doi: 10.1111/j.1440-1711.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 50.Storkus WJ, Zarour HM. Melanoma antigens recognised by CD8+ and CD4+ T cells. Forum (Genova) 2000;10:256–270. [PubMed] [Google Scholar]
- 51.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. Journal of virology. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertz T, Yanover C. Identifying HLA supertypes by learning distance functions. Bioinformatics. 2007;23:e148–155. doi: 10.1093/Bioinformatics/btl324. [DOI] [PubMed] [Google Scholar]
- 53.Botten J, Alexander J, Pasquetto V, Sidney J, Barrowman P, Ting J, Peters B, Southwood S, Stewart B, Rodriguez-Carreno MP, Mothe B, Whitton JL, Sette A, Buchmeier MJ. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. Journal of virology. 2006;80:8351–8361. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr Protoc Immunol. 2013 doi: 10.1002/0471142735.im1803s100. Chapter 18: Unit 18 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harndahl M, Rasmussen M, Roder G, Dalgaard Pedersen I, Sorensen M, Nielsen M, Buus S. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur J Immunol. 2012;42:1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- 56.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilchuk P, Spencer CT, Conant SB, Hill T, Gray JJ, Niu X, Zheng M, Erickson JJ, Boyd KL, McAfee KJ, Oseroff C, Hadrup SR, Bennink JR, Hildebrand W, Edwards KM, Crowe JE, Jr, Williams JV, Buus S, Sette A, Schumacher TN, Link AJ, Joyce S. Discovering naturally processed antigenic determinants that confer protective T cell immunity. J Clin Invest. 2013;123:1976–1987. doi: 10.1172/JCI67388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines–yesterday, today, and tomorrow. Lancet Infect Dis. 2002;2:425–431. doi: 10.1016/s1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 59.Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, Morishige N, Wahlert AJ, Brown DJ, Jester JV, Wechsler SL, BenMohamed L. Functional Foxp3+ CD4+CD25(Bright+) “Natural” Regulatory T Cells are Abundant in Rabbit Conjunctiva and Suppress Virus-Specific CD4+ and CD8+ Effector T Cells During Ocular Herpes Infection. J Virol. 2007;81:6911–6919. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Investigative ophthalmology & visual science. 2009;50:2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 61.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol. 2002;32:2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide Epitopes Extended by Ne-Palmitoyl Lysine Moiety Increases Uptake and Maturation of Dendritic Cell Through a Toll-Like Receptor 2 Pathway and Triggers a Th1- Dependent Protective Immunity. Eur J Immunol. 2004;34:1142–1149. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 63.Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf. 2006;4:178–187. doi: 10.1016/s1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 64.El-Awar N, Lee JH, Tarsitani C, Terasaki PI. HLA class I epitopes: recognition of binding sites by mAbs or eluted alloantibody confirmed with single recombinant antigens. Hum Immunol. 2007;68:170–180. doi: 10.1016/j.humimm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Pekiner FN, Aytugar E, Demirel GY, Borahan MO. HLA-A, B (Class I) and HLA-DR, DQ (Class II) Antigens in Turkish Patients with Recurrent Aphthous Ulceration and Behcet’s Disease. Med Princ Pract. 2013 doi: 10.1159/000348366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castelli EC, Gil DS, Veiga LC, de Camargo JL. Typing class I HLA-A gene using a nested PCR-RFLP procedure. Braz J Med Biol Res. 2005;38:837–842. doi: 10.1590/s0100-879x2005000600004. [DOI] [PubMed] [Google Scholar]
- 67.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, Sette A. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum Immunol. 2001;62:1200–1216. doi: 10.1016/s0198-8859(01)00319-6. [DOI] [PubMed] [Google Scholar]
- 68.Sette A, Newman M, Livingston B, McKinney D, Sidney J, Ishioka G, Tangri S, Alexander J, Fikes J, Chesnut R. Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens. 2002;59:443–451. doi: 10.1034/j.1399-0039.2002.590601.x. [DOI] [PubMed] [Google Scholar]
- 69.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Investigative ophthalmology & visual science. 2007;48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 70.Mackenzie KJ, Nowakowska DJ, Leech MD, McFarlane AJ, Wilson C, Fitch PM, O’Connor RA, Howie SE, Schwarze J, Anderton SM. Effector and central memory T helper 2 cells respond differently to peptide immunotherapy. Proc Natl Acad Sci U S A. 2014;111:E784–793. doi: 10.1073/pnas.1316178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim SK, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol. 2010;184:2825–2838. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. 2004;16:335–347. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshi NS, Cui W, Dominguez CX, Chen JH, Hand TW, Kaech SM. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol. 2011;187:4068–4076. doi: 10.4049/jimmunol.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan AA, Srivastava R, Lopes PP, Wang C, Pham TT, Cochrane J, Thai NT, Gutierrez L, BenMohamed L. Asymptomatic memory CD8 T cells: From development and regulation to consideration for human vaccines and immunotherapeutics. Hum Vaccin Immunother. 2014;10 doi: 10.4161/hv.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hufner K, Horn A, Derfuss T, Glon C, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D. Fewer latent herpes simplex virus type 1 and cytotoxic T cells occur in the ophthalmic division than in the maxillary and mandibular divisions of the human trigeminal ganglion and nerve. Journal of virology. 2009;83:3696–3703. doi: 10.1128/JVI.02464-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conrady CD, Zheng M, Stone DU, Carr DJ. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J Immunol. 2012;189:425–432. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


