Abstract
During hematopoiesis, hematopoietic stem cells constantly differentiate into granulocytes and macrophages via a distinct differentiation program that is tightly controlled by myeloid lineage-specific transcription factors. Mice with a null mutation of IFN Regulatory Factor 8 (IRF8) accumulate CD11b+Gr1+ myeloid cells that phenotypically and functionally resemble tumor-induced myeloid-derived suppressor cells (MDSCs), indicating an essential role of IRF8 in myeloid cell lineage differentiation. However, IRF8 is expressed in various types of immune cells and whether IRF8 functions intrinsically or extrinsically in regulation of myeloid cell lineage differentiation is not fully understood. Here we report an intriguing finding that although IRF8-deficient mice exhibit deregulated myeloid cell differentiation and resultant accumulation of CD11b+Gr1+ MDSCs, surprisingly, mice with IRF8 deficiency only in myeloid cells exhibit no abnormal myeloid cell lineage differentiation. Instead, mice with IRF8 deficiency only in T cells exhibited deregulated myeloid cell differentiation and MDSC accumulation. We further demonstrated that IRF8-deficient T cells exhibit elevated GM-CSF expression and secretion. Treatment of mice with GM-CSF increased MDSC accumulation, and adoptive transfer of IRF8-deficient T cells, but not GM-CSF-deficient T cells, increased MDSC accumulation in the recipient chimeric mice. Moreover, overexpression of IRF8 decreased GM-CSF expression in T cells. Our data determine that in addition to its intrinsic function as an apoptosis regulator in myeloid cells, IRF8 also acts extrinsically to represses GM-CSF expression in T cells to control myeloid cell lineage differentiation, revealing a novel mechanism that the adaptive immune component of the immune system regulates the innate immune cell myelopoiesis in vivo.
Keywords: Myeloid-derived suppressor cells, Myelopoiesis, IRF8, GM-CSF, T cells, Apoptosis
Introduction
Myeloid cells are among the most abundant and heterogeneous hematopoietic cell types that are virtually present in all mammalian tissues, where they monitor local microenvironment to maintain homeostasis (1-4). All myeloid cells originate from the pluripotent hematopoietic stem cells that undergo progressive restriction in their lineage potential to give rise to mature granulocytes and macrophages (5-8). Lineage restriction and differentiation are regulated by timely activation of specific set of lineage-specific transcription factors in concert with down-regulation of other set(s) of transcription factors that are important for alternative cell lineage potential (9, 10). Deregulation of activities of these lineage-specific transcription factors often leads to hematopoietic disorders and leukemia (11, 12). Therefore, lineage-specific transcription factors are essential for myeloid cell lineage differentiation and maturation (11, 13-15).
Several lineage-specific transcription factors have been found to be essential for regulation of myeloid cell differentiation. The ETS family transcription factor PU.1 is necessary for the earliest steps of myeloid lineage commitment, and its deficiency results in loss of monocytes and granulocytes (16-18). Krüppel-like factor-4 (KLF4) is expressed in a monocyte-restricted and stage-specific pattern during myelopoiesis and functions to promote monocyte differentiation (19). KLF4 deficiency inhibited monocyte but increased granulocyte differentiation (20). Furthermore, hematopoietic KLF4-/- chimeras generated by transplantation of KLF4-/- fetal livers cells completely lack circulating inflammatory Ly6G+ monocytes (21). In contrast, the nuclear orphan receptor NR4A1 controls later stages of monocyte development, and is required for the generation of Ly6C− monocytes but not Ly6C+ monocytes (22). The bZip transcription factor MafB is expressed specifically in the myeloid lineage of the hematopoietic system and is up-regulated successively during myeloid differentiation from multipotent progenitors to macrophages, and thus plays an essential role in early myeloid and monocytic differentiation (23). c-Maf has been observed to induce monocytic differentiation in bipotent myeloid progenitors (24), whereas, The zinc finger transcription factor Egr-1 is essential for lineage differentiation of macrophages but not granulocytes (25).
IFN regulatory factor 8 (IRF8) is a member of the IRF family that was identified in the late 1980s in the context of research into the type I interferon system. Subsequent studies over the past three decades have revealed that IRF8 is an essential transcription factor for myeloid-lineage restriction and differentiation (6-8, 26-31). IRF8 functions as either a transcriptional activator or repressor, depending on its binding partners in which it forms different heterodimeric protein complexes and on its target DNA elements (10, 19, 32). For example, IRF8 forms a protein complex with IRF1 or IRF2 and binds to the interferon-stimulated response element (ISRE; A/GNGAAANNGAAACT) to repress gene transcription. Conversely, the IRF8-PU.1 heterodimer complex specifically binds to the ETS-IRF composite element (EICE; GGAANNGAAA), or the IRF-ETS composite sequence (IECS; GAAANN[N]GGAA) to activate gene transcription (19, 27, 33, 34). In humans, a loss of function mutation of IRF8 leads to monocytic and dendritic cell immunodeficiency (35). A hallmark of IRF8 null mice is deregulated myeloid cell lineage differentiation, as characterized by lack of myeloid cell maturation, including lack of bone marrow resident macrophages, CD8α+ DCs, and plasmacytoid DCs in lymphoid organs (36, 37). This deregulated myeloid cell differentiation causes massive accumulation of heterogeneous CD11b+Gr1+ myeloid cells that phenotypically and functionally resemble myeloid-derived suppressor cells (MDSCs) (38). Therefore, IRF8 is essential for the regulation of diverse stages of myelopoiesis, namely the differentiation and lineage commitment of monocytes/macrophage versus granulocytes under physiological conditions (36, 37). However, the mechanism underlying IRF8 function in myeloid cell differentiation and MDSC accumulation is still elusive. Furthermore, IRF8 is expressed in both lymphoid and myeloid cells, but little is known about its relative functions in lymphoid and myeloid cells in regulation of myeloid cell differentiation and MDSC accumulation.
We report here a surprising finding that IRF8 expressed in myeloid cells regulates myeloid cell spontaneous apoptosis, whereas IRF8 expressed in T cells represses GM-CSF expression to suppress MDSC differentiation from myeloid progenitor cells. We proposed that IRF8 deficiency in T cells leads to increased GM-CSF expression to induce MDSC differentiation, whereas IRF8 deficiency in myeloid cells leads to decreased myeloid cell spontaneous apoptosis. Thus cooperative IRF8 functions in T cells and myeloid cells mediate myeloid cell differentiation and homeostasis in vivo.
Materials and Methods
Mice
IRF8 KO mice were as previously described (36). The IRF8 T KO mice were generated as previously described (39). The loxp-flanked Irf8 gene [B6(Cg)-Irf8tm1.1Hm/J] were generated as previously described (40). The Lyz2-cre mice [B6.129P2-Lyz2tm1(cre)Ifo/J] were obtained from the Jackson Laboratory. Use of mice was performed according to approved protocols by institutional animal use and care committee in Icahn School of Medicine at Mount Sinai and Georgia Regents University.
Antibodies
The following antibodies were purchased from BD Biosciences or Biolegend, eBiosciences, as conjugated to FITC, PE, PE-Cy5, perCP-Cy5.5 or APC: CD4 (L3T4), CD8 (53-6.7), CD3e (145-2C11), Gr-1(RB6-8C5), CD11b(M1/70), H-2kb (AF6-88.5.5.3), GM-CSF (MP1-22E9) and the respective IgG isotype controls.
Intracellular staining and flow cytometry
Cells were stimulated with anti-CD3 and anti-CD28 mAbs for 3 days and re-stimulated with PMA and ionomycin for 5h in the presence of brefeldin A before intracellular staining. Cells were fixed with IC Fixation Buffer (BD Biosciences), incubated with permeabilization buffer, and stained with PE-anti-mouse, APC-anti-mouse and FITC-anti-mouse antibodies. Flow cytometry was performed on a FACS Calibur (BD Biosciences) and LSR Fortessa (BD Biosciences).
T cell transfer models
T cell transfer was performed as previously described (Powrie et al., 1993). In brief, purified CD4+ T cells from WT and IRF8 KO mice were injected intraperitoneally into Rag1 KO recipients (5 ×105 cells per mouse in 200 μl sterile PBS per injection). Mice were weighed every week throughout the course of experiments. After 4 wks., mice were sacrificed; spleen, mesenteric lymph node and bone marrow were picked up for Flow Cytometer analysis.
IRF8 overexpression and GM-CSF expression in T cells
The retroviral vector (RV) used in this study was the MIG retroviral vector which consist of a MSCV-IRES-GFP vector. Mouse naive CD4+ T cells from C57BL/6 WT and IRF8 KO mouse spleens were transduced with empty RV control (RV-MIG) and the RV containing the IRF8 coding sequence (RV-MIG-IRF8), respectively. The cells were activated under anti-CD3 and anti-CD28 antibodies for 3 days, and then re-stimulated with PMA/ionomycin for 5h, and stained for surface CD4 and intracellular GM-CSF. Total RNA was also isolated and analyzed for GM-CSF expression by real-time RT-PCR.
Acute graft versus host disease model
All donor BM cells were isolated from C57BL/6 (H-2b) mice. T cells depletion of bone marrow was performed with auto-MACS by using anti-CD90.2 microbeads (Miltenyi Biotec). Donor CD4+ T cells (purity ∼95%) were purified from the spleens of C57BL/6 or GM-CSF-/- mice (H-2b) by using the CD4 beads (Miltenyi Biotec). The BALB/c (H-2d) hosts were irradiated with 9 Gy from [137Cs] source. One day later, the hosts were injected i.v. with 2 × 106 BM cells only or combined with 0.3 × 106 CD4+ T cells isolated from C57BL/6 or GM-CSF KO mice. The hosts were weighed every two days and monitored for survival. The H-2Kb+ myeloid cells were gated and analyzed for CD11b+Gr1+ cells by FACS in spleen and bone marrow in host at 11 days and 14 days after donor bone marrow cells transfer.
Cytokine ELISA
Supernatants from cell cultures were collected after activation under various conditions and secreted cytokines in the supernatants were measured by ELISA kits with purified coating and biotinylated detection antibodies: anti-GM-CSF (eBioscience).
RT-PCR analysis
Total RNA was isolated from cells using Trizol (Invitrogen, San Diego, CA) according to the manufacturer's instructions, and used for the first strand cDNA synthesis using the MMLV reverse transcriptase (Promega). The cDNA was then used as template for semi-quantitative and real-time PCR analysis. The sequences of primers are listed in Supplemental Table 1.
Chromatin Immunoprecipitation (ChIP)
CD4+ T cells were isolated from spleens of WT mice using negative selection method (Biolegend, San Diego, CA) and stimulated with anti-CD3 and anti-CD8 mAbs for 3 days. ChIP was performed using anti-IRF8 antibody (C19, Santa Cruz, Dallas, TX) and the protein A agarose according to the manufacturer's instructions (Millipore, Temecula, CA). Goat IgG was used as negative control for the antibody. The immunoprecipitated genomic DNA was amplified by real-time PCR using five pairs of PCR primers (supplemental table 1) covering the region approximately from -4000 to +1000 relative to the csf2 transcription initiation site in the csf2 promoter region.
Results
A key phenotype of IRF8 null mice is deregulation of myeloid cell lineage differentiation
IRF8 is a transcription factor of the IRF family. Mice with a null mutation of IRF8 exhibit two prominent phenotypes (36). The first is enhanced susceptibility to virus infections associated with impaired IFN-γ production. The second is deregulated myeloid cell lineage differentiation, characterized by splenomegaly (Fig. S1A) and massive accumulation of CD11b+Gr1+ MDSCs in BM and spleen (Fig. S1B). Therefore, IRF8 is a key transcription factor for myeloid cell lineage differentiation and is essential for the proliferation and differentiation of hematopoietic progenitor cells into mature myeloid cells (36, 37).
Myeloid cell-specific IRF8 deficiency does not ablate myeloid cell lineage differentiation
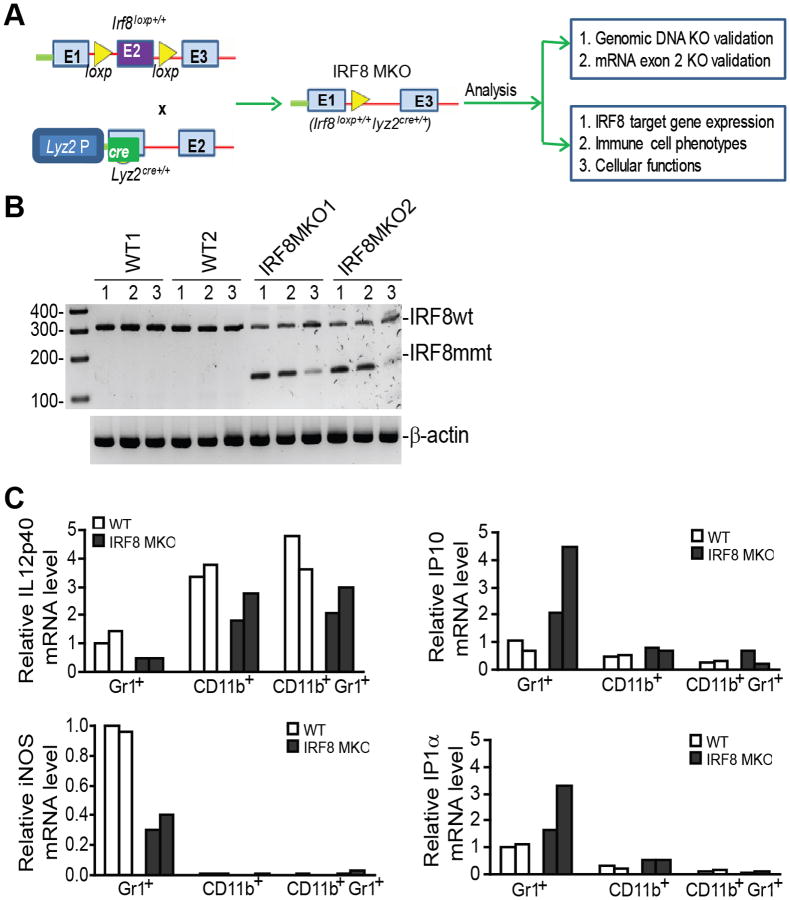
As mentioned above, IRF8-deficient mice exhibit deregulated myeloid cell lineage differentiation, resulting in accumulation of MDSCs (Fig. S1). In keeping with earlier studies (13, 19, 41, 42), this indicates that IRF8 functions in myeloid cells to regulate myeloid cell lineage differentiation. However, whether IRF8 expressed in myeloid cells regulates myeloid cell lineage differentiation is still a hypothesis to be tested. Therefore, we created mice with IRF8 deficiency only in myeloid cells by crossing mice with a loxp-flanked Irf8 gene [B6(Cg)-Irf8tm1.1Hm/J] (9, 40) with the Lyz2-cre mice [B6.129P2-Lyz2tm1(cre)Ifo/J] (Fig. 1A). Exon 2 of Irf8 in the B6(Cg)-Irf8tm1.1Hm/J mouse is flanked by loxp sites and it has been shown that deletion of exon 2 leads to depletion of IRF8 protein in cre-expressing tissues (40). We selected Irf8loxp+/+ and Lyz2cre+/+ mice based on genotypes (termed IRF8 MKO) (Fig. S2). To confirm that exon 2 is indeed deleted from these mice, we designed PCR primers that flank exon 2 of Irf8 mRNA. CD11b+, Gr1+ and CD11b+Gr1+ cells were sorted from WT and IRF8 MKO mice and treated with IFN-γ and LPS for 24h. RT-PCR analysis of IRF8 mRNA indicated that exon 2 was indeed deleted from Irf8 mRNA in IRF8 MKO mice (Fig. 1B). To determine whether the myeloid cells in IRF8 MKO mice are functionally deficient, the expression levels of IRF8 target genes in these cells were analyzed. IRF8 is a transcription activator of IL12p40 and iNOS, and is a transcriptional repressor of IP10 and IP1a (43, 44). CD11b+, Gr1+ and CD11b+Gr1+ cells were sorted from WT and IRF8 MKO mice. The cells were treated with IFN-γ and LPS overnight and then analyzed for the expression levels of these four IRF8 target genes. IL12p40 and iNOS expression levels are lower, whereas IP10 and IP1α expression levels are higher in Gr1+ cells from IRF8 MKO mice as compared to those from WT mice (Fig. 1C). IL12p40 levels were also lower in CD11b+ and CD11b+Gr1+ cells in IRF8 MKO mice as compared to WT mice (Fig. 1C). Our data thus indicate that IRF8 is functionally deficient in these myeloid cells. Therefore, we have created mice with irf8 mutation and IRF8 functional deficiency only in myeloid cells.
Figure 1. Creation of mice with IRF8 deficiency only in myeloid cells.
A. Diagram of creation and analysis of mice with IRF8 deficiency only in the myeloid cells (IRF8 MKO mice). Mice with loxp-flanked Irf8 gene [B6(Cg)-Irf8tm1.1Hm/J] (irf8loxp+/+, top left) were crossed to the Lyz2-cre mice [B6.129P2-Lyz2tm1(cre)Ifo/J](Lyz2cre+/+, left bottom). irf8loxp+/+ mice: exon 2 (E2) was flanked with loxp. E1 and E3 indicate exon 1 and exon 3 of the irf8 gene. Lyz2cre+/+ mice: cre coding sequence was inserted in Lyz2 gene exon 1. E2: Lyz2 exon 2. Lyz2 P: Lyz2 promoter. B. Myeloid cells of IRF8 MKO mice express mutant IRF8 mRNA. Gr1+ (lane 1), CD11b+ (lane 2) and CD11b+Gr1+ (lane 3) cells were sorted from WT and IRF8 MKO mice, stimulated with IFN-γ (100 U/ml) and LPS (1 μg/ml) overnight and analyzed by RT-PCR for IRF8 mRNA levels. The WT IRF8 (IRF8WT) and truncated mutant IRF8 mRNA (IRF8mmt) are indicated at the right. C. IRF8 is functionally deficient in myeloid cells of IRF8 MKO mice. Cells as shown in B were analyzed for Il12b (IL12p40), Nos2 (iNOS), Cxcr3 (IP10), and Ccl3 (MIP1a) transcripts by real-time RT-PCR using β-actin as internal controls.
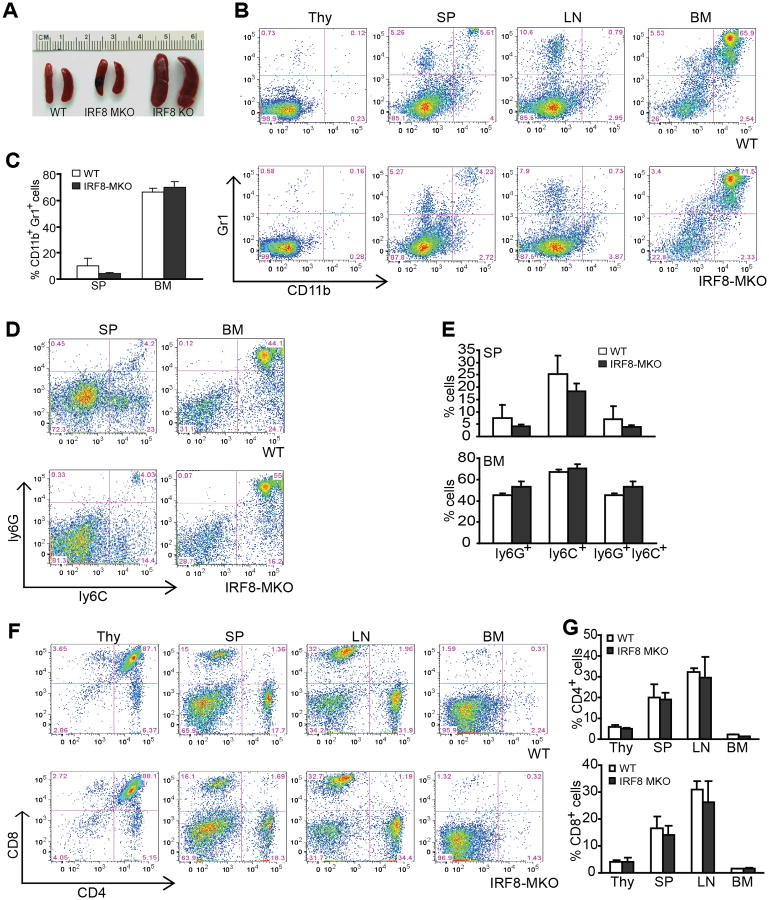
Surprisingly, analyses of IRF8 MKO mice revealed that they do not develop the splenomegaly characteristic of IRF8 KO mice (Fig. 2A). No significant differences were observed in the percentages of CD11b+, Gr1+, and CD11b+Gr1+ cells in Thy, spleen, LN and BM of WT and IRF8 MKO mice (Fig. 2B & C). In addition, the subsets of monocytic and granulocytic MDSCs (Ly6G+ and Ly6C+) also did not differ significantly between WT and IRF8 MKO cells (Fig. 2D & E). There were no significant differences between these mice in the percentages of CD4+ and CD8+ T cells (Fig. 2F & G). These data thus indicate that IRF8 expressed in myeloid cells is not sufficient enough to mediate CD11b+Gr1+ MDSC differentiation.
Figure 2. Mice with IRF8 deficiency only in myeloid cells exhibit normal myelopoiesis.
A. Spleen morphology of three month old WT, IRF8 MKO and IRF8 KO mice. B. Percentages of CD11b+Gr1+ MDSCs in thymus (Thy), spleen (SP), lymph node (LN) and bone marrow (BM) of WT and IRF8 MKO mice. Shown are representative results from one mouse of three mice. C. Quantitation of CD11b+Gr1+ in SP and BM of WT (n=3) and IRF8 MKO (n=3) mice as shown in B. D. Subsets of Gr1+ (Ly6C+ and Ly6G+) myeloid cells SP and BM. Shown are representative results from one mouse of three mice. Column: mean, bar: SD. E. Quantification of subsets of Gr1+ myeloid cells in SP and BM of WT (n=3) and IRF8MKO (n=3) mice. F. The indicated tissues were collected from WT and IRF8 MKO mice, stained with the CD4- and CD8-specific mAbs. Shown are representative images CD4+ and CD8+ T cell profiles. G. Quantification of CD4+ and CD8+ T cells in the indicated tissues from WT (n=3) and IRF8 MKO (n=3) mice. Column: mean, bar: SD.
IRF8 deficiency results in decreased spontaneous apoptosis in vivo
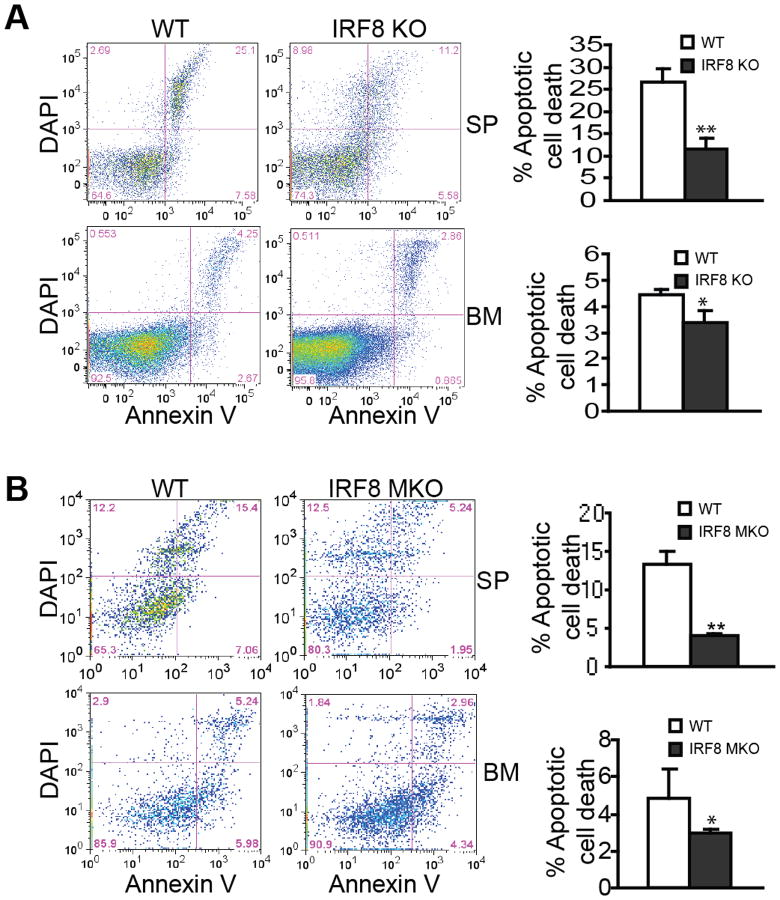
The above observation is a surprising one since IRF8-deficient myeloid cells from IRF8 MKO mice are functionally deficient (Fig. 1C) and IRF8 conventional KO mice exhibit deregulated myeloid cell lineage differentiation and resultant MDSC accumulation (36) (Fig. S1). Therefore, we further functionally examined these IRF8 MKO mice. Like T cells, myeloid cell turnover is mediated by the Fas-FasL apoptosis pathway (45). It has been shown that IRF8 regulates the expression of multiple apoptosis-regulatory genes, including Bcl-xL, Bax, FLIP and Fas, in myeloid cells (46-49), to regulate apoptosis (50, 51). Based on these observations, we then hypothesized that IRF8 deficiency at least should lead to decreased spontaneous apoptosis of MDSCs in IRF8 MKO mice. To test this hypothesis, we examined spontaneous apoptosis of MDSCs in WT and IRF8 MKO mice with IRF8 KO mice as positive controls. As expected, MDSCs from spleens and BM of IRF8 KO mice exhibited significantly reduced spontaneous apoptosis as compared to those from WT mice (Fig. 3A). Similar to what was observed in IRF8 KO mice, MDSCs from both spleens and BM of IRF8 MKO mice also exhibit significantly decreased spontaneous apoptosis (Fig. 3B). We should point out here that MDSCs from IRF8 KO and IRF8 MKO mice are all sensitive to apoptosis; only the level of spontaneous apoptosis in MDSCs of IRF8-deficient mice decreased as compared to the respective control WT mice. Therefore, we conclude that IRF8 deficiency leads to decreased MDSC spontaneous apoptosis. However, this deficiency alone apparently is not sufficient enough to cause MDSC accumulation.
Figure 3. MDSCs from IRF8 MKO mice exhibit decrease spontaneous apoptosis.
A. MDSCs from IRF8 KO mice exhibit decreased spontaneous apoptosis. Spleen and bone marrow were collected from three month-old WT (n=3) and IRF8 KO (n=3) mice. Cells were stained immediately under cold conditions with CD11b- and Gr1-specific mAbs plus Annexin V and DAPI. CD11b+Gr1+ cells were gated for Annexin V+DAPI+ cells. Shown are representative results of one of 3 pairs of mice. Apoptosis was quantified as % CD11b+Gr1+ cells that are also Annexin V+DAPI+ cells and presented at the right. Column: mean; bar: SD. ** p<0.01. B. MDSCs from IRF8 MKO mice also exhibit decreased spontaneous apoptosis. Spleen and BM cells were collected from WT (n=5) and IRF8 MKO (n=5) mice. Spontaneous apoptosis were determined as in A.
IRF8 deficiency in T cells leads to increased MDSCs
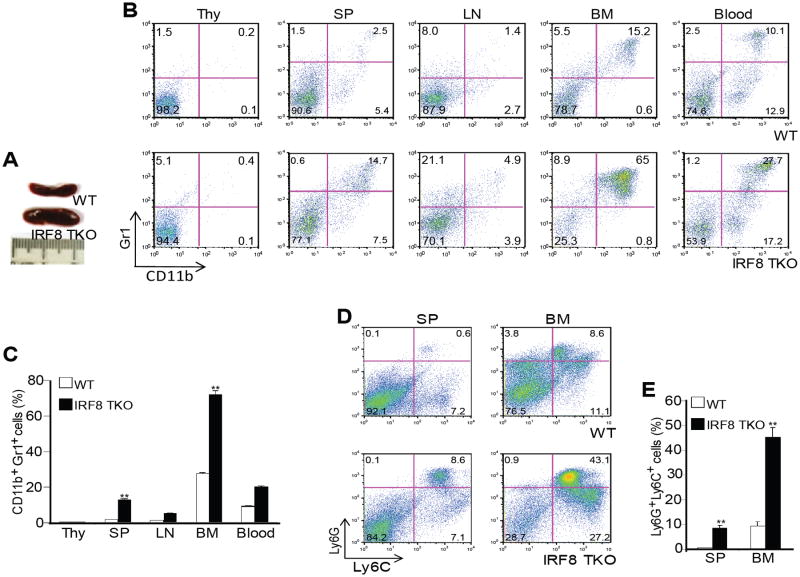
Based on the above observations, we hypothesized that IRF8 expressed in other type(s) of cells plays a role in myeloid cell differentiation. To test this hypothesis, we analyzed mice with IRF8 deficiency only in T cells (termed IRF8 TKO) (39). In IRF8 TKO mice, cre expression is controlled by lck promoter that drives T cell-specific cre expression. IRF8 TKO mice have enlarged spleens as compared to WT mice (Fig. 4A). The percentages of CD11b+Gr1+ cells (Fig. 4B & C) in spleen, BM and blood, and Ly6C+Ly6G+ (Fig. 4D & E) cells in spleen and BM were significantly higher in IRF8 TKO mice as compared to WT mice.
Figure 4. IRF8 deficiency in T cells results in accumulation of MDSCs.
A. Spleen morphology of three month-old WT and IRF8 TKO mice. B. Percentages of CD11b+Gr1+ MDSCs in thymus (Thy), spleen (SP), lymph node (LN), bone marrow (BM) and blood of WT and IRF8 TKO mice. Shown are representative results from one mouse of three mice. C. Quantitation of CD11b+Gr1+ MDSCs in the indicated tissues of WT (n=3) and IRF8 TKO (n=3) mice. Column: mean, bar: SD. ** p<0.01. D. Subsets of Gr1+ (Ly6C+ and Ly6G+) myeloid cells in SP and BM. Shown are representative results from one mouse of three mice. E. Quantification of subsets of Gr1+ myeloid cells in SP and BM of WT (n=3) and IRF8 TKO (n=3) mice as shown in D. Column: mean, bar: SD. **p<0.01.
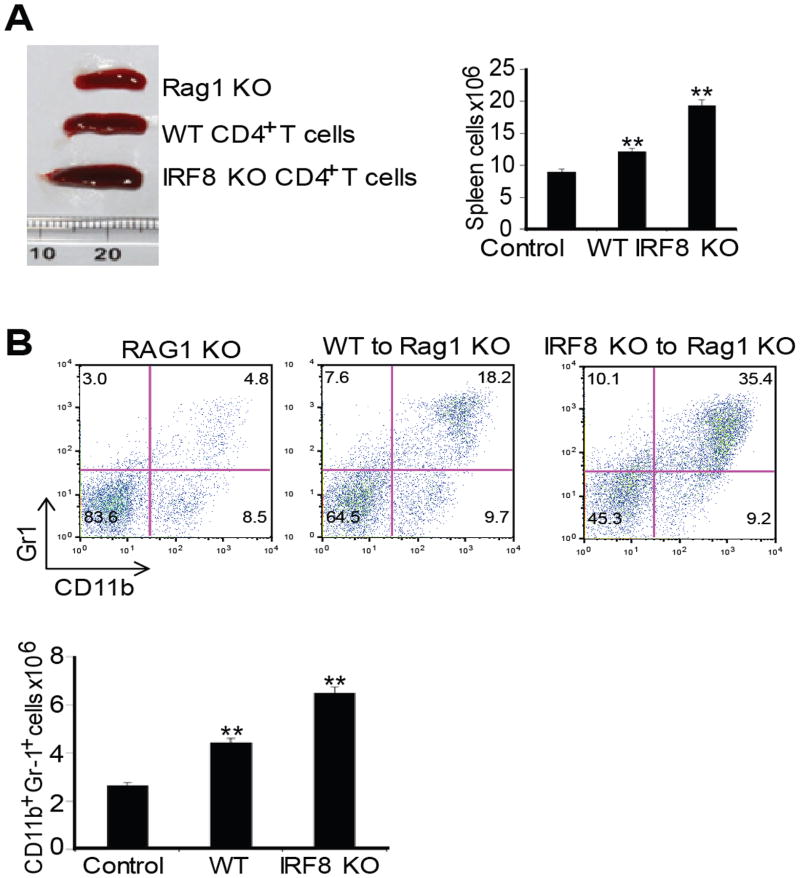
Next, we took a complementary approach to determine the role of IRF8 expressed in T cells in regulation of MDSC differentiation. We purified CD4+ T cells from WT and IRF8 TKO mice and adoptively transferred these T cells to Rag1 KO mice. The rationale is that if IRF8 deficiency in T cells regulates myeloid cell lineage differentiation, then T cells from IRF8 KO mice should increase MDSC accumulation in the Rag1 KO recipients. Indeed, adoptive transfer of IRF8-deficient T cells to Rag1 KO mice resulted in increased spleen size and spleen cell number (Fig. 5A). Analysis of CD11b+Gr1+ cells indicated that adoptive transfer of IRF8-deficient T cells significantly increased CD11b+Gr1+ MDSC accumulation in the Rag1 KO mouse spleens (Fig. 5B). Taken together, our data indicate that IRF8 expressed in T cells mediates CD11b+Gr1+ MDSC lineage differentiation in vivo.
Figure 5. IRF8-deficient T cells induce MDSC accumulation.
A. CD4+ T cells were purified from WT and IRF8 TKO mice and adoptively transferred to Rag1 KO mice. Mice were analyzed 14 days after cell transfer. Spleen morphology (left panel), and total spleen cells (right panel) are shown. B. MDSC profiles in the Rag1 KO mice, Rag1 KO mice that have received WT T cells, and Rag1 KO mice that have received IRF8 KO T cells as shown in A. Spleen cells were stained with H2Kb-, CD11b- and Gr1-specific mAbs. H-2Kb+ cells were gated and analyzed for CD11b+Gr1+ cells. Shown are representative results of one of three mice (top panel). MDSCs in each type of mice were quantified and presented at the bottom panel. Column: mean, bar: SD. ** p<0.01.
IRF8 represses GM-CSF expression in T cells to regulate myeloid cell differentiation
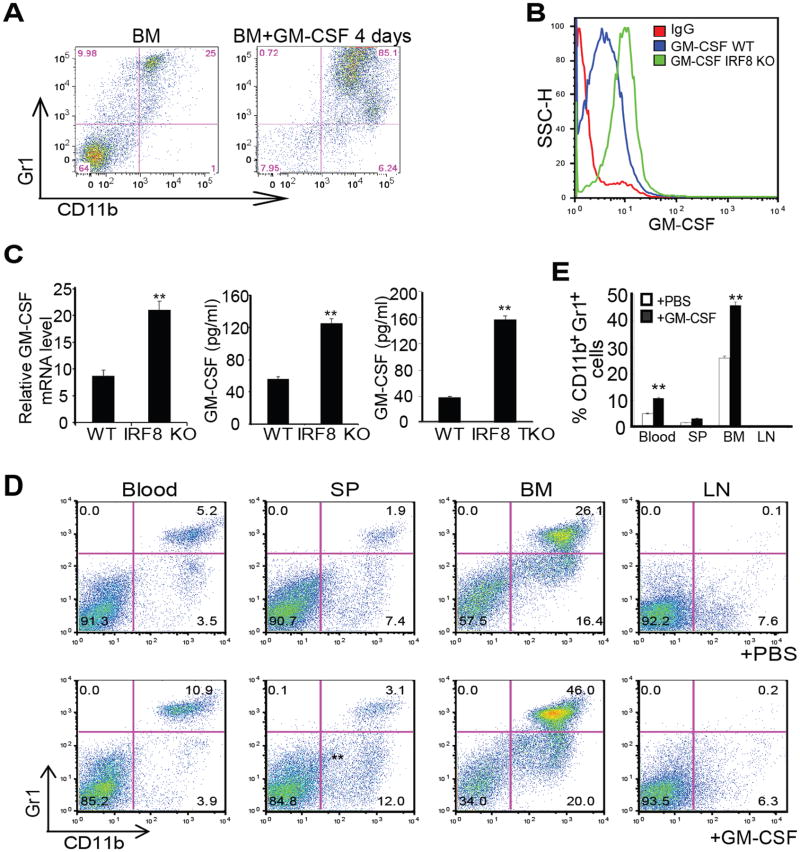
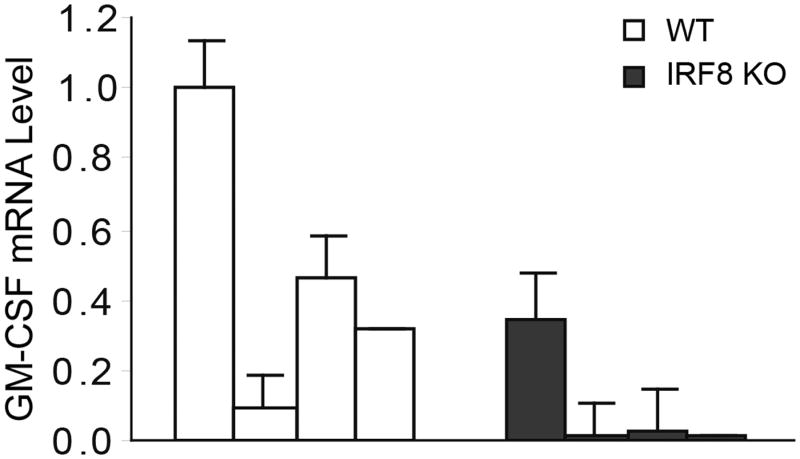
It is well documented that pro-inflammatory cytokines mediate CD11b+Gr1+ immature myeloid cell differentiation under pathological conditions (52-54). In particular, GM-CSF has been shown to induce CD11b+Gr1+ MDSC accumulation in tumor-bearing mice (53, 55-57). Recent studies have shown that tumor-secreted GM-CSF induces differentiation of MDSCs that phenotypically and functionally resembles tumor-induced MDSCs (55-57). Based on these observations, we hypothesized that IRF8 represses GM-CSF expression and IRF8 deficiency in T cells results in increased GM-CSF expression to induce deregulated myeloid cell differentiation. To test this hypothesis, we first cultured BM cells from WT mice in the presence of GM-CSF. Indeed, GM-CSF induced CD11b+Gr1+ cell differentiation from BM cells ex vivo (Fig. 6A). Next, purified CD4+ T cells from WT and IRF8 KO mice were cultured in the presence of anti-CD3 and anti-CD28 mAbs. Analysis of intracellular GM-CSF protein levels using flow cytometry revealed that GM-CSF is higher in T cells from IRF8 KO mice as compared to T cells from WT mice (Fig. 6B). Real-time RT-PCR analysis determined that GM-CSF transcript levels were also significantly higher in T cells from IRF8 KO mice than WT mice (Fig. 6C). Analysis of GM-CSF protein level in cell culture medium indicated that IRF8-deficient T cells secrete significantly more GM-CSF than WT T cells (Fig. 6C). Taken together, these observations indicate that IRF8 functions as a repressor of GM-CSF in T cells.
Figure 6. IRF8 represses GM-CSF expression in T cells to suppress MDSC accumulation.
A. GM-CSF induces BM cell differentiation to CD11b+Gr1+ MDSCs. BM cells were prepared (left panel) and cultured in vitro in the presence of GM-CSF for 4 days (right panel) and analyzed for CD11b+ and Gr1+ cells. B. Purified CD4+ T cells from WT and IRF8 KO mice were stimulated with anti-CD3 and anti-CD28 mAbs for 3 days. Cells were stained intracellularly with GM-CSF-specific mAb and analyzed by flow cytometry. C. Left panel: CD4+ T cells were purified from spleens of WT and IRF8 KO mice and stimulated with anti-CD3 and anti-CD28 mAbs for 3 days. RNAs were prepared and analyzed by real-time RT-PCR for GM-CSF mRNA level using β-actin as internal control. Right panels: CD4+ T cells were purified from spleens of WT, IRF8 KO and IRF8 TKO mice and stimulated with anti-CD3 and anti-CD28 mAbs for 3 days. The culture supernatants were analyzed by ELISA for GM-CSF protein level. Column: mean, bar: SD. ** p<0.01. D. WT mice were injected intraperitoneally with recombinant GM-CSF protein (1 μg in 100 μl PBS per mouse) every two days for three times. Myeloid cells in the indicated tissues were analyzed by flow cytometry. Shown are representative results from one mouse of three mice. E. Quantification of CD11b+Gr1+ cells as shown in D. Column: mean, bar: SD. **p<0.01.
T cell-produced GM-CSF promotes MDSC differentiation
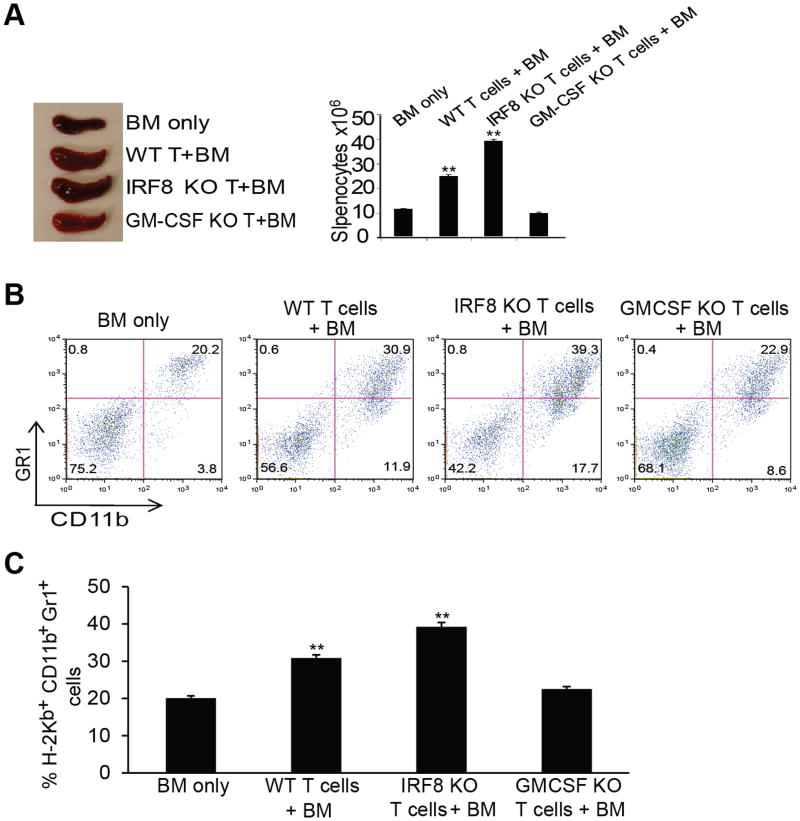
To functionally determine the role of GM-CSF in myeloid cell differentiation in vivo, recombinant GM-CSF protein was injected into WT mice. Analysis of GM-CSF-treated mice revealed that CD11b+Gr1+ MDSC increased significantly in blood, spleen and BM (Fig. 6D & E). To further determine that activated T cells can enhance myeloid cells development in vivo, we then made use of an acute graft-versus-host disease (GVHD) model to determine the function of T cell produced GM-CSF in myeloid cell differentiation. BALB/c (H-2d) recipient mice were irradiated. T cell-depleted BM cells were prepared from C57BL/6 mice (H-2Kb). CD4+ T cells were purified from WT, IRF8 KO, and GM-CSF KO mice (C57BL/6, H-2Kb). BM alone or BM in combination with the purified T cells was then adoptively transferred to the recipient mice. In this GVHD model, transferred T cells could be activated by three ways: 1) direct antigen presentation by donor antigen-presenting cells; 2) indirect antigen presentation by recipient antigen-presenting cells (cross presenting); and 3) mixed lymphocyte reaction. The rationale is that if IRF8 is a repressor of GM-CSF and activated T cell-produced GM-CSF promotes CD11b+Gr1+ cell differentiation, then more H-2Kb+ CD11b+Gr1+ cells should accumulate in the recipient H-2d mice. Analysis of the chimeric mice indicated that adoptive transfer of T cells increased spleen size and spleen cell number as compared to BM only transfer. However, both spleen sizes and number of spleen cells were greater in the recipient H-2d mice that received IRF8 KO T cells and BM as compared to recipients of WT T cells and BM (Fig. 7A). Furthermore, T cells from GM-CSF KO mice did not induce increased spleen sizes and spleen cell numbers (Fig. 7A).
Figure 7. T cell produced GM-CSF induces MDSC differentiation.
A. BALB/c mice (H-2d) were irradiated with 9 Gy from a 137Cs source, and were injected i.v. with 2×106 T cell-depleted BM cells alone, or combined with 0.3×106 CD4+ T cells isolated from C57BL/6 (H-2kb), C57BL/6-IRF8 KO, C57BL/6-GM-CSF KO mice. Mice were analyzed 14 days after cell transfer. Spleens of the indicated chimera mice are shown. Spleen cell numbers are presented at the right panel. B. The H-2kb+CD11b+ and H-2kb+Gr1+ myeloid cells in the indicated chimera mice were analyzed by flow cytometry in spleens 11 days after donor cell transfer. Shown are representative results from one mouse of three mice. C. Quantification of CD11b+ and Gr1+ cells in the indicated mice as shown in B. Column: mean, bar: SD. ** p<0.01.
Consistent with the increased spleen sizes and spleen cell numbers, the percentages of donor H-2Kb+ CD11b+Gr1+ cells were higher in the recipient H-2d mice receiving IRF8 KO T cells than those receiving WT T cells. In contrast, mice receiving GM-CSF KO T cells exhibited no differences in H-2Kb+ CD11b+Gr1+ cells as compared to control mice (Fig. 7B & C).
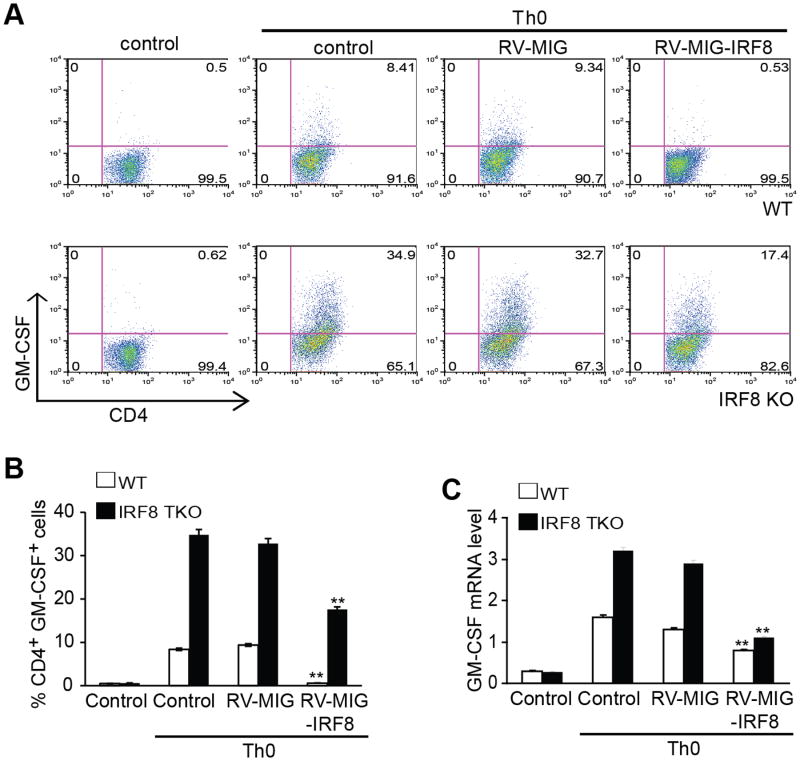
A complimentary approach was then used to further determine the function of IRF8 in repression of GM-CSF expression. Naive CD4+ T cells from C57BL/6 WT and IRF8 KO mice were transduced with retroviral vector encoding IRF8 or empty vector, and were activated with anti-CD3 and anti-CD28 antibodies. The cells were re-stimulated with PMA/ionomycin for 5h and stained for surface CD4 and intracellular GM-CSF. It is apparent that overexpression of IRF8 significantly decreases the percentage of GM-CSF-expressing cells in both WT and IRF8 KO CD4+ T cells (Fig. 8A & B). Analysis of GM-CSF mRNA level indicates that overexpression of IRF8 significantly decreased GM-CSF mRNA level (Fig. 8C).
Figure 8. Restoration of IRF8 expression decreases GM-CSF+ T cells.
Naive CD4+ T cells from C57BL/6 WT and IRF8-/- mice were transduced with retrovirus encoding IRF8 or empty vector and the cells were activated with anti-CD3 and anti-CD28 antibodies for 3 days. The cells were re-stimulated with PMA/ionomycin for 5h and stained for intracellular GM-CSF and analyzed by flow cytometry. Representative FACS dot plots gated on CD4+ T cells and the percentage of GM-CSF-producing CD4+ T cells are shown. B. Quantification of CD4+GM-CSF+ cells in three independent experiments as shown in A. Column: mean, Bar: SD. Overexpression of IRF8 significantly decreased % GM-CSF+ cells in both WT and IRF8 KO CD4+ T cells (** p<0.01). C. Total RNA was prepared from cells as shown in A and analyzed for GM-CSF mRNA levels by real-time RT-PCR using β-actin as internal control. Overexpression of IRF8 significantly decreased GM-CSF mRNA levels in both WT and IRF8 KO CD4+ T cells (** p<0.01).
To determine whether IRF8 also regulates GM-CSF expression in MDSCs, MDSCs were purified from spleen cells of WT and IRF8 KO mice. Total RNA was isolated from the purified MDSCs and analyzed for GM-CSF mRNA expression by real-time RT-PCR. It is clear that, unlike in T cells, IRF8 deficiency in MDSCs clearly does not increase GM-CSF expression in MDSCs (Fig. 9). Taken together, our data demonstrate that IRF8 expressed in T cells functions as a GM-CSF repressor to control myeloid cell lineage differentiation, and IRF8 deficiency in T cells leads to increased GM-CSF expression to deregulate myeloid cell lineage differentiation and resultant MDSC accumulation.
Figure 9. GM-CSF is not down-regulated in MDSCs from IRF8 KO mice.
CD11b+Gr1+ MDSCs were purified from spleens of WT (n=4) and IRF8 KO (n=3) mice. Total RNA was isolated from these purified MDSCs and analyzed for IRF8 expression levels by real-time RT-PCR using β-actin as the internal control. The IRF8 expression level in MDSCs from the first WT mice was arbitrarily set at 1.0. The expression levels of IRF8 in MDSCs of the rest samples were the levels relative to the IRF8 expression level in MDSCs of first WT mice.
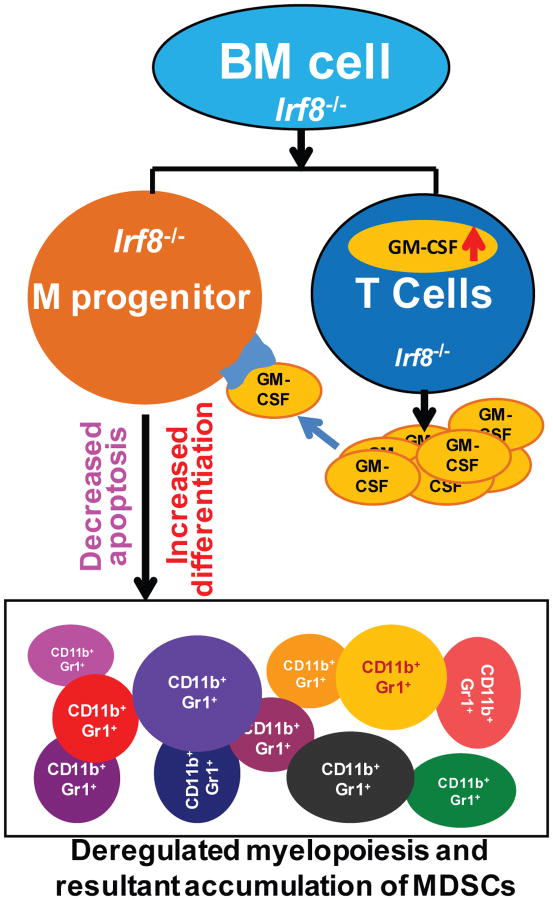
In summary, we determined that, under physiological conditions, IRF8 expressed in T cells represses GM-CSF expression to control myeloid cell lineage differentiation, whereas IRF8 expressed in myeloid cells mediates myeloid cell spontaneous apoptosis to regulate myeloid cell turnover. Loss of IRF8 function in T cells leads to increased GM-CSF expression to induce deregulated myeloid cell differentiation, which acts in concert with loss of IRF8 function in myeloid cell and resultant decreased spontaneous apoptosis to result in MDSC accumulation (Fig. 10).
Figure 10. Model of IRF8 deficiency and MDSC accumulation.
Under physiological conditions, IRF8 represses GM-CSF expression in T cells. Loss of IRF8 expression and function leads to elevated GM-CSF expression and secretion. T cell-produced GM-CSF binds to its receptor on myeloid progenitor cells to induce MDSC differentiation. On the other hand, loss of IRF8 expression or function in myeloid cells in IRF8 null mice leads to decreased spontaneous apoptosis of myeloid cells to reduce MDSC turnover. Thus, loss of IRF8 expression and function in T cells results in increased GM-CSF expression and resultant increased MDSC differentiation, which acts in concert with decreased myeloid cell spontaneous apoptosis and turnover to lead to MDSC accumulation.
Discussion
The mammalian immune system consists of an innate and an adaptive component. The innate immune system that comprises primarily myeloid cells uses a limited number of germ-line-encoded pattern-recognition receptors to detect the relatively invariant pathogen-associated molecular patterns (58). In contrast, the adaptive immune system, which is composed of T and B cells, employs antigen receptors that are not encoded in the germ line but are generated de novo. Although the adaptive immune system depends on the generation of almost unlimited repertoire of antigen receptors on T and B cells and subsequent activation and clonal expansion of T and B cells carrying the antigen-specific receptors, the innate immune system is the first line of defense that recognizes the pathogen-associated molecular patterns to deliver essential danger signals to activate the adaptive immune system (29). Thus, the innate immune cells, primarily the myeloid cells, regulate the adaptive immune response under pathophysiological conditions (4, 29, 59-61). Here, we show that T cells regulate myeloid cell differentiation through an IRF8-mediated and GM-CSF-dependent extrinsically mechanism in vivo. Our findings therefore revealed that the adaptive immune component of the immune system (i.e. T cells) regulates the innate myeloid cell lineage differentiation.
Decades ago, a seminal study with Irf8-/- mice revealed IRF8 as an essential transcription factor in regulating myeloid cell lineage differentiation (36). One of the prominent phenotypes of IRF8 null mice is the massive accumulation of CD11b+Gr1+ cells in bone marrow, spleen, blood, and peripheral lymphoid organs as a consequence of altered myelopoiesis (36). Interestingly, pathological conditions, i.e. cancer, also induce massive accumulation of CD11b+Gr1+ cells termed MDSCs (CD11b+Gr1+ in mice and CD11b+CD33+ in human) (62-64). Furthermore, glioblastoma multiforme and pancreatic cancer cells have been shown to secrete GM-CSF to induce tumor-associated macrophage and MDSC accumulation in the tumor-bearing host (55, 57, 65). Moreover, IRF8 expression is often silenced in CD11b+Gr1+ MDSCs in the tumor-bearing hosts (38, 49). These observations clearly demonstrate an inverse correlation between IRF8 expression/function and CD11b+Gr1+ MDSC accumulation under both physiological and pathological conditions. These observations thus suggest that IRF8 functions in myeloid cells mediate myeloid cell differentiation and loss of IRF8 expression or function causes deregulation of myelopoiesis and resultant MDSC accumulation. However, our present data clearly show that IRF8 expressed in myeloid cells is not sufficient enough to control myeloid cell differentiation and MDSC accumulation in vivo. Instead, we identified that IRF8 expressed in T cells mediates myeloid cell lineage differentiation. Thus, IRF8 regulates myelopoiesis through a cell-extrinsic mechanism. The relationship between IRF8 expression and GM-CSF in tumor cells is currently under study.
A previous study has convincingly demonstrated that mixed bone marrow chimeras generated from a mixture of WT and IRF8 KO bones marrows display defects only in myeloid cells differentiated from the IRF8 KO bone marrow, indicating a myeloid cell-intrinsic abnormality in IRF8-deficient myeloid progenitors (31). In this study, we did observed IRF8 intrinsic function in myeloid cells in apoptosis regulation. However, it seems that the extrinsic function of IRF8 as a regulator of GM-CSF expression plays a more dominant role in MDSC accumulation under our conditions. It is clear that IRF8 clearly represses GM-CSF expression in T cells. Analysis of the csf2 promoter region revealed 2 putative ISRE sites (Fig. S3A), however, our initial attempts have failed to detect direct IRF8 binding to the csf2 gene promoter (Fig. S3B), suggesting that IRF8 may regulate GM-CSF expression through an indirect mechanism. Further studies are needed to determine the mechanisms underlying csf2 transcription regulation by IRF8.
The lyz2 promoter used in generating the IRF8 MKO mice is the most commonly used one for myeloid cell-specific gene expression in the literature (66-70). The current expressed sequence tag (EST) data indicate that the expression level of lysozyme 2 is highest in bone marrow as compared to other tissues in mice (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Mm.45436). Therefore, it is unlikely that lyz2 promoter is not activated in bone marrow. In the literature, it is well-demonstrated that lyz2-driven Cre expression induces specific and highly efficient deletion of loxp-flanked gene segments in mature myeloid cells (66-69). However, it was also observed that lyz2-driven Cre-mediated deletion of loxp-flanked gene segments is less efficient in immature myeloid cells as compared to that in mature amyloid cells (66-68). Therefore, it is possible that IRF8 is only partially deleted in myeloid progenitor cells. On the other hand, IRF8 expression level in hematopoietic stem cells is negligible, but increases with differentiation of hematopoietic stem cells into immature myeloid cells. Differentiation of hematopoietic stem cells into the multipotent progenitor→common lymphoid progenitor→granulocyte-myeloid progenitor pathway coincided with sequential increases in IRF8 expression (9). This IRF8 expression pattern correlates at least partially with lyz2 promoter activity and lysozyme 2 expression patterns during myeloid cell lineage differentiation. It is apparent that further studies are warranted to determine the lyz2 promoter activity and irf8 deletion during myeloid cell lineage differentiation in the IRF8 MKO mice.
Although IRF8 expressed in myeloid cells is not sufficient enough to intrinsically regulate myeloid cell differentiation, the fact that IRF8 is often silenced in MDSCs in tumor-bearing mice and human cancer patients suggests that IRF8 expressed in myeloid cells should play a role in MDSC accumulation (38, 49, 71). IRF8 is a transcription factor previously shown to regulate the expression of multiple apoptosis-regulatory genes, including Bcl-xL, Bax, FLIP and Fas, in myeloid cells (46-49). Furthermore, as in T cells, it has been shown that MDSC turnover is mediated by the Fas-FasL apoptosis pathway of the host's immune cells (45). It is well-known that deregulation of Fas-mediated apoptosis in T cells leads to autoimmune lymphoproliferative syndrome with massive T cell accumulation (72), which resembles massive MDSC accumulation in IRF8 null mice. Therefore, by parallel, it is reasonable to assume that IRF8 deficiency-induced deregulation of the Fas-mediated apoptosis pathway in MDSCs might decrease MDSC sensitivity to host immune cell-mediated apoptosis. Indeed, MDSCs from IRF8 KO mice exhibit decreased spontaneous apoptosis. MDSCs from IRF8 MKO mice also exhibit decreased spontaneous apoptosis. Therefore, in addition to regulating MDSC differentiation in an extrinsic manner, IRF8 also mediates MDSC spontaneous apoptosis to control their turnover. Thus, increased differentiation induced by T cell-produced GM-CSF, acting in concert with decreased MDSC spontaneous apoptosis/turnover, leads to MDSC accumulation in IRF8-deficient host.
The molecular mechanism underlying MDSC accumulation has been subject of extensive studies (38, 46, 73). In this study, we observed that IRF8 functions extrinsically as a GM-CSF repressor in T cells. We show that IRF8-deficient T cells express elevated level of GM-CSF. Based on our observations, we propose that IRF8 is a GM-CSF repressor. Loss of IRF8 function in T cells results in increased GM-CSF expression and secretion. GM-CSF binds to its receptor on myeloid progenitor cells to increase MDSC differentiation. In the same time, loss of IRF8 function in the differentiating myeloid cells decreases their spontaneous apoptosis and turnover. GM-CSF-induced increase in MDSC differentiation, in concert with IRF8 deficiency-mediated decrease in MDSC turnover, cause massive MDSC accumulation (Fig. 10).
Supplementary Material
Acknowledgments
We thank Dr. Jeanene Pihkala in the Medical College of Georgia Flow Cytometry Core Facility for the excellent technical assistance in cell sorting.
Grant support: National Institute of Health grants CA133085, CA185909 and CA182518 (to KL), DK072201, AI091871, AI104688 (to HX), CA140622 (SIA), and in part by the Intramural Research Program of the NIH, NIAID (HCM) and NICHD (KO) and VA Merit Review Award BX001962 (to KL).
Footnotes
Conflict of interests: None
References
- 1.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 6.Milanovic M, Terszowski G, Struck D, Liesenfeld O, Carstanjen D. IFN consensus sequence binding protein (Icsbp) is critical for eosinophil development. J Immunol. 2008;181:5045–5053. doi: 10.4049/jimmunol.181.7.5045. [DOI] [PubMed] [Google Scholar]
- 7.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170:1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal H, Kaushik M, Sougrat R, Gupta M, Dey A, Verma R, Ozato K, Tailor P. Batf3 and Id2 have a synergistic effect on Irf8-directed classical CD8alpha+ dendritic cell development. J Immunol. 2013;191:5993–6001. doi: 10.4049/jimmunol.1203541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Yan M, Sun J, Jain S, Yoshimi R, Abolfath SM, Ozato K, Coleman WG, Jr, Ng AP, Metcalf D, DiRago L, Nutt SL, Morse HC., 3rd A Reporter Mouse Reveals Lineage-Specific and Heterogeneous Expression of IRF8 during Lymphoid and Myeloid Cell Differentiation. J Immunol. 2014 doi: 10.4049/jimmunol.1301939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanno Y, Levi BZ, Tamura T, Ozato K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25:770–779. doi: 10.1089/jir.2005.25.770. [DOI] [PubMed] [Google Scholar]
- 11.Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- 12.Satpathy AT, Briseno CG, Cai X, Michael DG, Chou C, Hsiung S, Bhattacharya D, Speck NA, Egawa T. Runx1 and Cbfbeta regulate the development of Flt3+ dendritic cell progenitors and restrict myeloproliferative disorder. Blood. 2014;123:2968–2977. doi: 10.1182/blood-2013-11-539643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 14.Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, Habib N, Yosef N, Chang CY, Shay T, Frampton GM, Drake AC, Leskov I, Nilsson B, Preffer F, Dombkowski D, Evans JW, Liefeld T, Smutko JS, Chen J, Friedman N, Young RA, Golub TR, Regev A, Ebert BL. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- 16.Singh H, DeKoter RP, Walsh JC. PU.1, a shared transcriptional regulator of lymphoid and myeloid cell fates. Cold Spring Harb Symp Quant Biol. 1999;64:13–20. doi: 10.1101/sqb.1999.64.13. [DOI] [PubMed] [Google Scholar]
- 17.DeKoter RP, Walsh JC, Singh H. PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 1998;17:4456–4468. doi: 10.1093/emboj/17.15.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121:1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. MafB is an inducer of monocytic differentiation. EMBO J. 2000;19:1987–1997. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde SP, Zhao J, Ashmun RA, Shapiro LH. c-Maf induces monocytic differentiation and apoptosis in bipotent myeloid progenitors. Blood. 1999;94:1578–1589. [PubMed] [Google Scholar]
- 25.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 26.Savitsky D, Tamura T, Yanai H, Taniguchi T. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother. 2010;59:489–510. doi: 10.1007/s00262-009-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 29.Ikushima H, Negishi H, Taniguchi T. The IRF Family Transcription Factors at the Interface of Innate and Adaptive Immune Responses. Cold Spring Harb Symp Quant Biol. 2013;78:105–116. doi: 10.1101/sqb.2013.78.020321. [DOI] [PubMed] [Google Scholar]
- 30.Salem S, Langlais D, Lefebvre F, Bourque G, Bigley V, Haniffa M, Casanova JL, Burk D, Berghuis A, Butler KM, Leahy TR, Hambleton S, Gros P. Functional characterization of the human dendritic cell immunodeficiency associated with the IRF8K108E mutation. Blood. 2014 doi: 10.1182/blood-2014-04-570879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura T, Ozato K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interferon Cytokine Res. 2002;22:145–152. doi: 10.1089/107999002753452755. [DOI] [PubMed] [Google Scholar]
- 33.Tamura T, Thotakura P, Tanaka TS, Ko MS, Ozato K. Identification of target genes and a unique cis element regulated by IRF-8 in developing macrophages. Blood. 2005;106:1938–1947. doi: 10.1182/blood-2005-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dror N, Alter-Koltunoff M, Azriel A, Amariglio N, Jacob-Hirsch J, Zeligson S, Morgenstern A, Tamura T, Hauser H, Rechavi G, Ozato K, Levi BZ. Identification of IRF-8 and IRF-1 target genes in activated macrophages. Mol Immunol. 2007;44:338–346. doi: 10.1016/j.molimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC, 3rd, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 37.Tailor P, Tamura T, Morse HC, 3rd, Ozato K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood. 2008;111:1942–1945. doi: 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, Liu J, Ning H, Shin MS, Gupta M, Qi CF, He JC, Lira SA, Morse HC, 3rd, Ozato K, Mayer L, Xiong H. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng J, Wang H, Shin DM, Masiuk M, Qi CF, Morse HC., 3rd IFN regulatory factor 8 restricts the size of the marginal zone and follicular B cell pools. J Immunol. 2011;186:1458–1466. doi: 10.4049/jimmunol.1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto M, Kato T, Hotta C, Nishiyama A, Kurotaki D, Yoshinari M, Takami M, Ichino M, Nakazawa M, Matsuyama T, Kamijo R, Kitagawa S, Ozato K, Tamura T. Shared and distinct functions of the transcription factors IRF4 and IRF8 in myeloid cell development. PLoS One. 2011;6:e25812. doi: 10.1371/journal.pone.0025812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol. 2007;7:105–117. doi: 10.1038/nri2024. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Kong HJ, Li H, Huang B, Yang M, Zhu C, Bogunovic M, Zheng F, Mayer L, Ozato K, Unkeless J, Xiong H. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281:10073–10080. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 44.Zhu C, Rao K, Xiong H, Gagnidze K, Li F, Horvath C, Plevy S. Activation of the murine interleukin-12 p40 promoter by functional interactions between NFAT and ICSBP. J Biol Chem. 2003;278:39372–39382. doi: 10.1074/jbc.M306441200. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–5390. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabriele L, Phung J, Fukumoto J, Segal D, Wang IM, Giannakakou P, Giese NA, Ozato K, Morse HC. Regulation of apoptosis in myeloid cells by interferon consensus sequence-binding protein. J Exp Med. 1999;190:411–421. doi: 10.1084/jem.190.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Hu X, Zimmerman M, Torres CM, Yang D, Smith SB, Liu K. Cutting edge: IRF8 regulates Bax transcription in vivo in primary myeloid cells. J Immunol. 2011;187:4426–4430. doi: 10.4049/jimmunol.1101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang D, Wang S, Brooks C, Dong Z, Schoenlein PV, Kumar V, Ouyang X, Xiong H, Lahat G, Hayes-Jordan A, Lazar A, Pollock R, Lev D, Liu K. IFN regulatory factor 8 sensitizes soft tissue sarcoma cells to death receptor-initiated apoptosis via repression of FLICE-like protein expression. Cancer Res. 2009;69:1080–1088. doi: 10.1158/0008-5472.CAN-08-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu X, Bardhan K, Paschall AV, Yang D, Waller JL, Park MA, Nayak-Kapoor A, Samuel TA, Abrams SI, Liu K. Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer. J Biol Chem. 2013;288:19103–19115. doi: 10.1074/jbc.M112.434530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiao J, Dragomir AC, Kocabayoglu P, Rahman AH, Chow A, Hashimoto D, Leboeuf M, Kraus T, Moran T, Carrasco-Avino G, Friedman SL, Merad M, Aloman C. Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils. J Immunol. 2014;192:3374–3382. doi: 10.4049/jimmunol.1300237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pathak S, Ma S, Shukla V, Lu R. A role for IRF8 in B cell anergy. J Immunol. 2013;191:6222–6230. doi: 10.4049/jimmunol.1301169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 54.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6:e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutkowski MR, Stephen TL, Conejo-Garcia JR. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell Immunol. 2012;278:21–26. doi: 10.1016/j.cellimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 61.Wermeling F, Anthony RM, Brombacher F, Ravetch JV. Acute inflammation primes myeloid effector cells for anti-inflammatory STAT6 signaling. Proc Natl Acad Sci U S A. 2013;110:13487–13491. doi: 10.1073/pnas.1312525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonda N, Simonato F, Peranzoni E, Cali B, Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner B, Trautwein C, Sackett SD, Zanovello P, Molon B, Bronte V. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38:1236–1249. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Ostrand-Rosenberg S. Looking to the future of cancer immunotherapy: many questions to answer and many therapeutic opportunities. Cancer Immunol Immunother. 2013;62:1–2. doi: 10.1007/s00262-012-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clemens RA, Lenox LE, Kambayashi T, Bezman N, Maltzman JS, Nichols KE, Koretzky GA. Loss of SLP-76 expression within myeloid cells confers resistance to neutrophil-mediated tissue damage while maintaining effective bacterial killing. J Immunol. 2007;178:4606–4614. doi: 10.4049/jimmunol.178.7.4606. [DOI] [PubMed] [Google Scholar]
- 67.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 68.Sissons JR, Peschon JJ, Schmitz F, Suen R, Gilchrist M, Aderem A. Cutting edge: microRNA regulation of macrophage fusion into multinucleated giant cells. J Immunol. 2012;189:23–27. doi: 10.4049/jimmunol.1102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalani AI, Moore CR, Luo C, Kreider BZ, Liu Y, Morse HC, 3rd, Xie P. Myeloid Cell TRAF3 Regulates Immune Responses and Inhibits Inflammation and Tumor Development in Mice. J Immunol. 2015;194:334–348. doi: 10.4049/jimmunol.1401548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gautier EL, Chow A, Spanbroek R, Marcelin G, Greter M, Jakubzick C, Bogunovic M, Leboeuf M, van Rooijen N, Habenicht AJ, Merad M, Randolph GJ. Systemic analysis of PPARgamma in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart TJ, Greeneltch KM, Reid JE, Liewehr DJ, Steinberg SM, Liu K, Abrams SI. Interferon Regulatory Factor-8 Modulates the Development of Tumor-Induced CD11bGr-1 Myeloid Cells. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 73.Hu X, Yang D, Zimmerman M, Liu F, Yang J, Kannan S, Burchert A, Szulc Z, Bielawska A, Ozato K, Bhalla K, Liu K. IRF8 regulates acid ceramidase expression to mediate apoptosis and suppresses myelogeneous leukemia. Cancer Res. 2011;71:2882–2891. doi: 10.1158/0008-5472.CAN-10-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.