Abstract
Objective
Salsalate treatment has well-known effects on improving glycemia and the objective of this study was to examine whether the mechanism of this effect is related to changes in adipose tissue.
Methods
We conducted a randomized double-blind and placebo-controlled trial in obese Hispanics (18-35 years). The intervention consisted of 4 g/day of salsalate (n=11) versus placebo (n=13) for 4 weeks. Outcome measures included glycemia, adiposity, ectopic fat, and adipose tissue gene expression and inflammation.
Results
In those receiving salsalate, plasma fasting glucose decreased by 3.4% (P<0.01), free fatty acids decreased by 42.5% (P=0.06) and adiponectin increased by 27.7% (P<0.01). Salsalate increased insulin AUC by 38% (P=0.01) and HOMA-B by 47.2% (P<0.01) while estimates of insulin sensitivity/resistance were unaffected. These metabolic improvements occurred without changes in total, abdominal, visceral, or liver fat. Plasma markers of inflammation/immune activation were unchanged following salsalate. Salsalate had no effects on adipose tissue including adipocyte size, presence of crown-like structures, or gene expression of adipokines, immune cell markers, or cytokines downstream of NF-κB with the exception of downregulation of IL-1β (P<0.01).
Conclusions
Our findings suggest that metabolic improvements in response to salsalate occurred without alterations in adiposity, ectopic fat, or adipose tissue gene expression and inflammation.
Keywords: Obesity, Salsalate, Inflammation, Adipose Tissue, Hispanics
Introduction
Obesity is often associated with chronic low-grade inflammation, which increases the risk for insulin resistance, metabolic complications, and type 2 diabetes (1-4). Evidence suggests that adipose tissue is a significant contributor to this inflammatory state (5-8). Treatment strategies have included anti-inflammatory therapies to improve metabolic health. In rodents, salicylates inhibit obesity-induced inflammation and improve insulin resistance (9, 10). Recent clinical investigations have shown that salsalate (a prodrug of salicylate) favorable affects glycemia in predominantly obese Caucasian adults with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and/or type 2 diabetes (11-14). In general, results from these studies have shown that salsalate improved glucose and lipid homeostasis (11-14). Further, salsalate has been shown to inhibit systemic inflammation and NF-κB activity in peripheral blood mononuclear cells (11) and adipose tissue (14). Collectively, these findings suggest that salsalate-induced metabolic improvements may be mediated by changes in adipose tissue, especially decreases in inflammation.
These clinical outcomes are particularly important since salsalate is an inexpensive treatment that could be used for prevention or reversal of cardiometabolic abnormalities occurring during obesity. Yet, there is limited data about the utility of salsalate to improve metabolic health in persons without type 2 diabetes (15-18) and its effects on adipose tissue inflammation are uncertain. Furthermore, treatment with salsalate has not be evaluated exclusively in Hispanics, who suffer from a greater prevalence of obesity (19) and metabolic disease risk than non-Hispanic whites (20). We therefore conducted a randomized, double-blind and placebo-controlled trial of salsalate in obese Hispanic young adults without type 2 diabetes to determine whether the known effects of salsalate on improving glycemic is 1) applicable to obese Hispanics without type 2 diabetes and 2) mediated by potential effects of salsalate on adipose tissue.
Methods
Study Design
The study was a 4-week, randomized double-blind and placebo-controlled investigation, which compared 4 g/day of salsalate (2 g twice daily) with matching placebo (Merical, Anaheim, CA, USC). Previous studies demonstrating safety, tolerability, metabolic benefits as well as the anti-inflammatory effects of salsalate were used to direct our treatment dosage and duration (11-14). Primary outcomes included effects on glycemia, insulin resistance, and markers of systemic and adipose tissue inflammation. The protocol specified stepped reductions of 500 mg/day for symptoms related to salicylate (e.g., tinnitus). Pills were counted at the end of the 4 weeks and participants were called weekly to encourage adherence and inquire about potential adverse events. Participants were instructed to maintain their current activity and dietary patterns during the study.
Participants and Screening
Participants signed an informed consent approved by the University of Southern California’s (USC) Institutional Review Board prior to undergoing study measurements or interventions. Inclusion criteria required that participants be otherwise healthy obese (body mass index [BMI] ≥30 kg/m2) Hispanic adults 18-35 years of age. Hispanic ethnicity required that both parents and grandparents be of Hispanic descent (by self-report). Participants were excluded if they had diabetes, peptic ulcer disease, history of gastrointestinal bleeding, blood clotting disorder, liver or kidney function abnormalities, asthma, allergy to non-steroidal anti-inflammatory drugs (NSAIDs), or were pregnant or lactating. Participants were excluded if they were taking any medications that could affect body composition, metabolism or inflammation (e.g., thyroid replacement, β-blockers, NSAIDs, statins). Although not included as exclusion criteria, none of the participants reported using dietary supplements (including anti-inflammatory omega-3). In the last year of the study, those enrolled had at least two of the latter; HOMA-IR ≥3.5, elevated HbA1C (5.7-6.4% or 38.8-46.4 mmol/mol), or elevated erythrocyte sedimentation rate (≥15 mm/hr). Criteria were used to increase the proportion of participants expected to have abdominal fat inflammation. Those enrolled based on these criteria were equally represented in both groups. All participants included in this study had adipose tissue biopsies at baseline and post-test.
2-Hour OGTT
A 2-hour OGTT was performed after an overnight fast at baseline and immediately following the last treatment dose. Blood samples were collected at baseline and 15, 30, 45, 60, and 120 minutes following ingestion of glucose (75 g). HOMA-IR, an index of insulin resistance, was calculated as fasting glucose (mg/dL) × insulin (μU/mL) / 205. HOMA-B, an indirect measure of β-cell function, was calculated as fasting insulin (μU/mL) × 360 / [fasting glucose (mg/dL) − 63]. QUICKI, an index for insulin sensitivity, was calculated as 1 / (log (fasting insulin (μU/mL)) + log (fasting glucose (mg/dL))) (21). Finally, the Matsuda Index, an indirect but more robust estimate of insulin sensitivity, was calculated as 10,000 / sqrt [(fasting glucose (mg/dL) × fasting insulin (μU/mL)) × (mean glucose × mean insulin during the OGTT)]. Glucose, insulin, and C-peptide area under the curve (AUC) were calculated from the OGTT data. Dietary intake and weekly activity were assessed using 24-hour diet recalls (22) and Godin-Shephard Leisure-Time Physical Activity questionnaires (23) before and after treatment. Nutrition data were analyzed using the Nutrition Data System for Research (version 2012) developed at the University of Minnesota.
Assays
Assays were performed in duplicate (except glucose and C-peptide) at the USC Metabolic Assay Core and had coefficients of variation of less than fifteen percent. Glucose was assayed on a Yellow Springs 2710 Analyzer (Yellow Springs, OH) using the glucose oxidase method. Insulin was assayed using a specific human ELISA kit from EMD Millipore (St. Charles, MO) and fasting free fatty acids (FFA) were quantified using a colorimetric kit (NEFA-HR(2)) from Wako Diagnostics (Richmond, VA). C-peptide was assayed by an automated enzyme immunoassay (Tosoh Bioscience Inc., AIA 600 II analyzer, South San Francisco, CA). Circulating cytokines and markers of immune activation, including high sensitivity interleukin-6 (hsIL-6), soluble tumor necrosis receptor-II (sTNFrII), sCD14 (24), and sCD163 (25) were measured by human ELISA from R&D Systems Inc. (Minneapolis, MN). Adipokines, including adipsin and adiponectin, were assayed using human ELISA from R&D Systems Inc. (Minneapolis, MN) and EMD Millipore (St. Charles, MO). Complete blood counts and comprehensive chemistry panels were performed by the USC Clinical Laboratory.
Adipose Tissue Biopsies
Baseline and post-test subcutaneous abdominal adipose tissue biopsies were obtained in the right anterior axillary line at the level of the umbilicus using a 6-mm Bergström side-cutting needle (Micrins Surgical, Inc; Lake Forest, IL). Adipose tissue was rapidly irrigated with iced saline and immediately transported for flow cytometry, fixed for immunohistochemistry, or flash frozen in liquid nitrogen for subsequent whole-tissue Fluidigm gene expression. To quantify immune cells in adipose tissue we used flow cytometry and gated for live cells, leukocytes (CD45+), and then monocytes and macrophages (CD45+/CD14+). The percent of monocytes and macrophages was calculated as the difference between the percent of CD45+/CD14+ cells and the percent of CD45+/CD14− isotype control cells. For immunohistochemistry, adipose tissue samples were formalin-fixed and paraffin-embedded. Four consecutive, 5-micron sections of adipose tissue were stained with hematoxylin-eosin and CD68 antibody (Leica Biosystems, Newcastle, UK) to examine crown-like structures (CLS) as a marker of adipose tissue inflammation. Since CLS were not overly abundant, a single blinded technician examined all sections for the presence or absence of macrophage CLS (6, 26). Four independent fields at 20x magnification were captured from the first mounted section on each slide for cell sizing. Adipose cell size (μ2) was obtained from each field captured using Fiji quantitative microscopy software (27). The Genoseq Core at the University of California Los Angeles performed adipose tissue gene expression assays using Fludigm DELTAgene assays. Gene expression from the post-test biopsy was compared to baseline in order to calculate fold change (FC). Assays included genes involved in adipose tissue inflammation (inflammatory cytokines and immune cell markers) as well as insulin, FFA, and lipopolysaccharide (LPS) / Toll-like receptor (TLR) signaling.
Adiposity
A DEXA scan was used to control for possible changes in body composition (e.g., percent body fat and lean tissue mass) before and after the 4-week intervention. At baseline and within one week of completing the intervention, a whole abdominal (top of liver to iliac crest) 3-Tesla magnetic resonance imaging (MRI) scan (Excite HD; GE Healthcare, Waukesha, Wisconsin) was used to measure subcutaneous abdominal adipose tissue (SAAT), visceral adipose tissue (VAT), liver fat fraction, and pancreatic fat fraction as previously reported (28). The MRI utilized the IDEAL method (GE Healthcare), which separates water and fat components using chemical-shift MRI. Fat fraction was calculated as the fat components divided by the sum of the fat and water components (28, 29). This 3-D whole abdominal MRI scan provides SAAT and VAT in liters as well as liver and pancreatic fat as the percentage of fat found within each organ tissue. A single analyst performed all MRI image post-processing, tissue segmentation, and analysis using SliceOmatic software (Tomovision, Inc., Montreal, Québec, Canada).
Statistical Analysis
Study data were managed using Research Electronic Data Capture (REDCap) tools hosted at the USC (30). Statistical analyses were performed with IBM SPSS Statistics (version 21). Data are reported as median (25th, 75th percentile) or median percent change unless otherwise noted. Paired comparisons (post-test versus baseline) and unpaired group comparisons were performed by the Mann-Whitney U Test on medians or the McNemar’s test and Fisher’s Exact test for CLS data. The effect of salsalate (Ptreatment) was examined using analysis of covariance (ANCOVA) where the dependent variable was the change from baseline in each variable of interest (e.g., plasma markers of metabolic health and adipose tissue biopsy outcomes). These models controlled for the baseline measure of each dependent variable and baseline percent body fat. Change in CLS (presence or absence) was examined using Fisher’s Exact test as well as logistic regression including the same covariates. When necessary, log transformations were performed to meet assumptions of normality. Results were considered significant when two-sided P-value was less than 0.05.
Results
Baseline Assessments
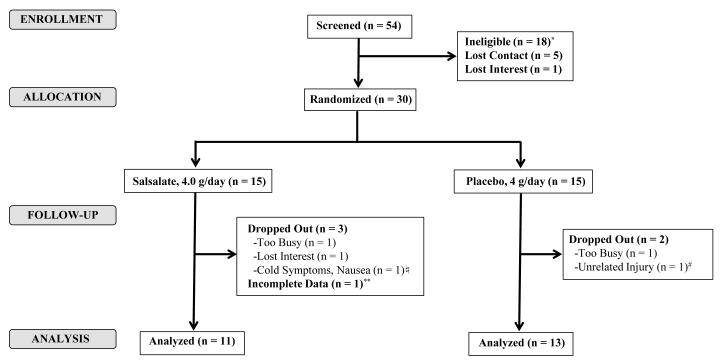
Thirty participants were randomized to the study and 25 completed the intervention (Figure 1). Table 1 lists the baseline characteristics of the 24 participants with complete paired study data. On average, participants were approximately 20 years old and 58% were male. Those in the placebo and salsalate group did not significantly differ in any parameter except fasting insulin and estimates of insulin sensitivity, resistance and secretion. The salsalate group had a higher fasting insulin [11.0(9.5, 13.5) vs. 7.2(5.0, 10.2) μU/mL, P=0.02] fasting insulin to C-peptide ratio [3.3(3.0, 3.8) vs. 2.8(2.5, 3.4), P=0.03], HOMA-IR [2.2(2.0, 2.8) vs. 1.5(1.0, 2.3), P=0.03], and lower QUICKI [0.34(0.33, 0.34) vs. 0.36(0.34, 0.34), P=0.03] and Matsuda Index [1.9(1.4, 2.1) vs. 2.7(2.0, 3.8), P=0.01].
Figure 1. Study Flow Diagram.
Of those who completed the screening visit (n = 54), 18 were deemed ineligible, 5 lost contact, and 1 lost interest. *Reasons for ineligibility after screening included smoking (n = 1), pregnancy (n = 1), abnormal routine blood chemistry results (n = 1), low BMI (n = 3), or exclusion based on HOMA-IR, HbA1C, and ESR (n = 12). Of the 30 randomized (15 salsalate / 15 placebo), only 5 dropped. Reasons for dropout included lack of time (n = 2), loss of interest (n = 1), unrelated study illness (n = 1), and unrelated study injury (n = 1). ♮Cold symptoms, including nausea, were unrelated to the intervention. #Injury involved a fractured rib that was unrelated to study involvement. **One participant did not have complete fat biopsy data and was not included in the analysis.
Table 1.
Baseline Characteristics of Participants at Study Enrollment
| Salsalate N=11 |
Placebo N=13 |
|
|---|---|---|
| General Characteristics | ||
| Age (years) | 22.0 (19.0, 27.0) | 19.0 (18.0, 25.5) |
| Sex (M/F) | 6/5 | 8/5 |
| Height (m) | 1.7 (1.6, 1.8) | 1.7 (1.6, 1.7) |
| Weight (kg) | 109.7 (106.3, 146.7) | 101.8 (95.4, 118.2) |
| Adiposity: DEXA and 3-T MRI | ||
| BMI (kg/m2) | 41.3 (37.7, 47.0) | 35.5 (33.2, 43.8) |
| Total Body Fat (%) | 44.8 (38.5, 48.8) | 40.6 (34.3, 44.3) |
| SAAT (L) | 11.0 (8.2, 11.7) | 9.1 (7.8, 10.9) |
| VAT (L) | 3.4 (2.5, 5.4) | 3.3 (2.2, 4.2) |
| Liver Fat (%) | 8.7 (6.2, 13.7) | 8.6 (3.4, 12.6) |
| Pancreatic Fat (%) | 6.9 (3.0, 11.8) | 5.3 (4.3, 6.7) |
| 2-hr OGTT | ||
| Systolic BP (mm/Hg) | 122.0 (107.0, 142.0) | 114.0 (111.5, 130.0) |
| Diastolic BP (mm/Hg) | 67.0 (63.0, 73.0) | 71.0 (68.0, 78.0) |
| Fasting Glucose (mg/dL) | 86.2 (82.9, 95.1) | 83.2 (78.7, 86.9) |
| Fasting Insulin (μU/mL) | 11.0 (9.5, 13.5) | 7.2 (5.0, 10.2)* |
| Fasting C-Peptide (ng/mL) | 3.6 (2.6, 3.9) | 2.9 (2.2, 3.3) |
| Fasting Insulin / C-peptide | 3.3 (3.0, 3.8) | 2.8 (2.5, 3.4)* |
| Fasting FFA (μM/L) | 510.7 (395.5, 519.1) | 459.2 (376.1, 502.9) |
| QUICKI | 0.34 (0.33, 0.34) | 0.36 (0.34, 0.38)* |
| HOMA-IR | 2.2 (2.0, 2.8) | 1.5 (1.0, 2.3)* |
| HOMA-B | 157.9 (146.1, 215.3) | 142.2 (85.3, 187.0) |
| Matsuda Index | 1.9 (1.4, 2.1) | 2.7 (2.0, 3.8)† |
| Glucose AUC (mg/dL × min) | 280.6 (257.9, 317.9) | 268.9 (234.6, 285.7) |
| Insulin AUC (μU/mL × min) | 114.8 (92.2, 147.8) | 88.0 (70.4, 108.5) |
| C-Peptide AUC (ng/mL × min) | 22.4 (19.3, 26.2) | 18.9 (17.9, 23.0) |
| Plasma Markers | ||
| hsIL-6 (pg/mL) | 2.6 (1.8, 3.1) | 2.4 (1.8, 3.8) |
| sTNFrII (pg/mL) × 102 | 23.8 (19.7, 27.8) | 22.8 (17.3, 26.0) |
| sCD14 (ng/mL) × 102 | 12.7 (11.6, 13.1) | 13.1 (11.5, 14.4) |
| sCD163 (ng/mL) | 47.5 (36.6, 61.5) | 41.7 (35.4, 70.3) |
| Adiponectin (ng/mL) × 102 | 63.6 (52.4, 92.6) | 64.5 (48.3, 88.0) |
| Adipsin (ng/mL) | 31.7 (27.1, 35.8) | 27.1 (24.7, 34.0) |
| Adipose Tissue Biopsies | ||
| Average Fat Cell Size (μ2) × 102 | 59.5 (44.6, 72.3) | 55.3 (51.8, 62.8) |
| CLS [Y/N, (%)] | 7/4 (64%) | 3/10 (23%) |
| Leukocytes (%) | 20.9 (8.9, 35.1) | 17.5 (9.3, 24.7) |
| Monocytes/Macrophages (%) | 29.0 (15.0, 59.1) | 34.0 (25.8, 42.5) |
Data are reported as median (25th, 75th percentile). P-value corresponds to Mann-Whitney U Test on Medians.
P<0.05 and
P≤0.01. Salsalate group: N=10 for MRI. Placebo group: N=12 for flow cytometry.
Participation and Compliance
Two participants in the placebo and three in the salsalate arm dropped out of the study. The reasons included lack of time, loss of interest, or illness / injury that was unrelated to study participation. There were four instances of tinnitus (placebo n=1 and salsalate n=3) that were graded mild to moderate, resolved within 1-2 weeks during continued treatment, and did not require a dose reduction. Changes in weight, blood pressure (BP), liver function tests, blood urea nitrogen, serum creatinine, and estimated glomerular filtration rate did not change with salsalate treatment or vary by treatment groups (data not shown). At the end of the study, there were no differences in the number of untaken pills in the placebo group compared to salsalate treatment [19.0(53.0) vs. 22.5(32.0) pills, P=0.73]. As shown in Table 2, BMI, total body fat percent, SAAT, VAT, liver, and pancreatic fat did not change within or between treatment groups. Finally, upper and lower body fat percent, lean tissue mass, total energy intake, macronutrients (protein, fat, fiber, and carbohydrates), and leisure activity did not differ between the groups at baseline or change within or between treatment groups (data not shown).
Table 2.
Variables at Baseline and After the 4-Week Intervention
| Salsalate (4 g/day) (N=11) | Placebo (4 g/day) (N=13) | Treatment | |||
|---|---|---|---|---|---|
| Baseline | Post-test | Baseline | Post-test | P-Value | |
| 2-hr OGTT | |||||
| Systolic BP (mm/Hg) | 122.0 (107.0, 142.0) | 123.0 (104.0, 131.0) | 114.0 (111.5, 130.0) | 117.0 (110.0, 125.5) | 0.69 |
| Diastolic BP (mm/Hg) | 67.0 (63.0, 73.0) | 69.0 (63.0, 75.0) | 71.0 (68.0, 78.0) | 71.0 (65.0, 73.0) | 0.14 |
| Fasting Glucose (mg/dL) | 86.2 (82.9, 95.1) | 83.3 (77.0, 87.9)† | 83.2 (78.7, 86.9) | 83.8 (82.0, 86.5) | <0.01 |
| Fasting Insulin (μ U/mL) | 11.0 (9.5, 13.5) | 14.8 (11.0, 17.3) | 7.2 (5.0, 10.2)* | 10.0 (6.4, 12.6) | 0.24 |
| Fasting C-Peptide (ng/mL) | 3.6 (2.6, 3.9) | 3.3 (2.1, 3.8) | 2.9 (2.2, 3.3) | 3.2 (2.7, 3.8)* | 0.05 |
| Fasting Insulin / C-peptide | 3.3 (3.0, 3.8) | 5.2 (3.7, 5.7)† | 2.8 (2.5, 3.4)* | 3.2 (2.4, 3.7) | <0.01 |
| Fasting FFA (μ M/L) | 510.7 (395.5, 519.1) | 293.8 (238.9, 440.7) | 459.2 (376.1, 502.9) | 491.9 (424.1, 535.8) | 0.02 |
| QUICKI | 0.34 (0.33, 0.34) | 0.32 (0.32, 0.34) | 0.36 (0.34, 0.38) | 0.34 (0.33, 0.37) | 0.56 |
| HOMA-IR | 2.2 (2.0, 2.8) | 3.2 (2.2, 3.6) | 1.5 (1.0, 2.3) | 2.1 (1.3, 2.6) | 0.43 |
| HOMA-B | 157.9 (146.1, 215.3) | 232.5 (201.2, 444.4)† | 142.2 (85.3, 187.0) | 165.1 (102.5, 233.4) | <0.01 |
| Matsuda Index | 1.9 (1.4, 2.1) | 1.4 (1.2, 2.1) | 2.7 (2.0, 3.8) | 2.2 (1.8, 3.0) | 0.40 |
| Glucose AUC (mg/dL × min) | 280.6 (257.9, 317.9) | 260.0 (254.7, 276.4) | 268.9 (234.6, 285.7) | 261.5 (240.3, 287.3) | 0.44 |
| Insulin AUC (μ U/mL × min) | 114.8 (92.2, 147.8) | 159.6 (115.9, 189.7)† | 88.0 (70.4, 108.5) | 93.2 (77.7, 118.6) | <0.01 |
| C-Peptide AUC (ng/mL × min) | 22.4 (19.3, 26.2) | 24.1 (17.5, 27.1) | 18.9 (17.9, 23.0) | 21.9 (18.9, 24.7)* | 0.16 |
| Plasma Markers | |||||
| hsIL-6 (pg/mL) | 2.6 (1.8, 3.1) | 1.7 (1.1, 3.9) | 2.4 (1.8, 3.8) | 2.3 (1.7, 3.0) | 0.54 |
| sTNFrII (pg/mL) × 102 | 23.8 (19.7, 27.8) | 22.6 (20.3, 25.8) | 22.8 (17.3, 26.0) | 23.9 (17.4, 27.7) | 0.17 |
| sCD14 (ng/mL) × 102 | 12.7 (11.6, 13.1) | 12.5 (11.3, 14.7) | 13.1 (11.5, 14.4) | 12.4 (11.0, 14.7) | 0.30 |
| sCD163 (ng/mL) | 47.5 (36.6, 61.5) | 49.8 (38.8, 51.3) | 41.7 (35.4, 70.3) | 47.7 (31.2, 71.0) | 0.37 |
| Adiponectin (ng/mL) × 102 | 63.6 (52.4, 92.6) | 81.2 (70.1, 101.7)† | 64.5 (48.3, 88.0) | 59.3 (44.3, 80.6) | <0.001 |
| Adipsin (ng/mL) | 31.7 (27.1, 35.8) | 32.6 (27.2, 34.2) | 27.1 (24.7, 34.0) | 28.0 (25.5, 33.0) | 0.99 |
| Adiposity: DEXA and 3-T MRI | |||||
| BMI (kg/m2) | 41.3 (37.7, 47.0) | 41.8 (37.7, 47.1) | 35.5 (33.2, 43.8) | 36.8 (33.4, 43.6) | 0.90 |
| Total Body Fat (%) | 44.8 (38.5, 48.8) | 45.2 (37.1, 50.0) | 40.6 (34.3, 44.3) | 39.8 (32.7, 42.3) | 0.42 |
| SAAT (L) | 11.0 (8.2, 11.7) | 9.9 (8.1, 12.6) | 9.1 (7.8, 10.9) | 8.4 (7.2, 10.3) | 0.96 |
| VAT (L) | 3.4 (2.5, 5.4) | 3.6 (2.7, 5.2) | 3.3 (2.2, 4.2) | 3.2 (1.8, 4.7) | 0.53 |
| Liver Fat (%) | 8.7 (6.2, 13.7) | 9.1 (4.1, 14.1) | 8.6 (3.4, 12.6) | 8.2 (3.6, 13.5) | 0.19 |
| Pancreatic Fat (%) | 6.9 (3.0, 11.8) | 7.8 (6.1, 9.3) | 5.3 (4.3, 6.7) | 6.2 (4.2, 7.9) | 0.94 |
| Adipose Tissue Biopsies | |||||
| Average Fat Cell Size (μ2) × 102 | 59.5 (44.6, 72.3) | 52.7 (37.2, 62.0) | 55.3 (51.8, 62.8) | 44.8 (41.3, 56.6)* | 0.90 |
| CLS [Y/N, (%)] | 7/4 (64%) | 3/8 (27%) | 3/10 (23%) | 0/13 (0%) | 0.40 |
| Leukocytes (%) | 20.9 (8.9, 35.1) | 30.7 (15.6, 41.1) | 17.5 (9.3, 24.7) | 23.9 (12.6, 28.3) | 0.14 |
| Monocytes/Macrophages (%) | 29.0 (15.0, 59.1) | 27.3 (0.0, 60.4) | 34.0 (25.8, 42.5) | 33.3 (20.4, 46.1) | 0.73 |
Data are reported as median (25th, 75th percentile). Paired P-values
(P<0.05 and
P≤0.01) correspond to Related-Samples Wilcoxon Signed Rank Test or McNemar test on proportions.
Salsalate group: N=10 for MRI. Placebo group: N=12 for flow cytometry and post-test DEXA and MRI. Treatment P-value column values are from ANCOVA, which controlled for the baseline dependent variable and body fat percent. A Fisher’s Exact test was performed on the change in crown-like structures (CLS) to determine whether treatment altered the proportion of those with CLS at post-test compared to baseline.
Fasting Measures and OGTT Results
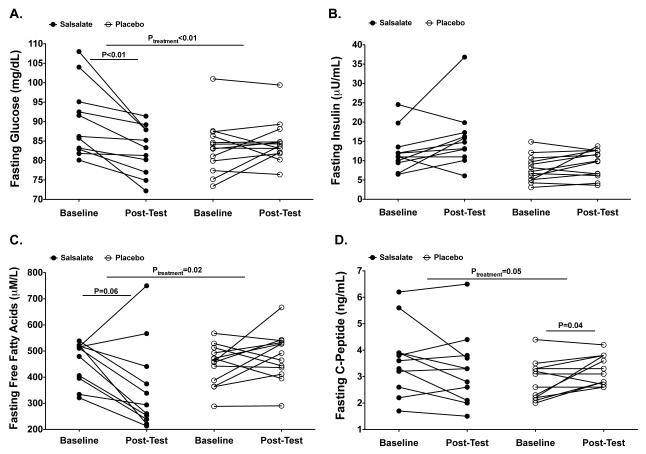
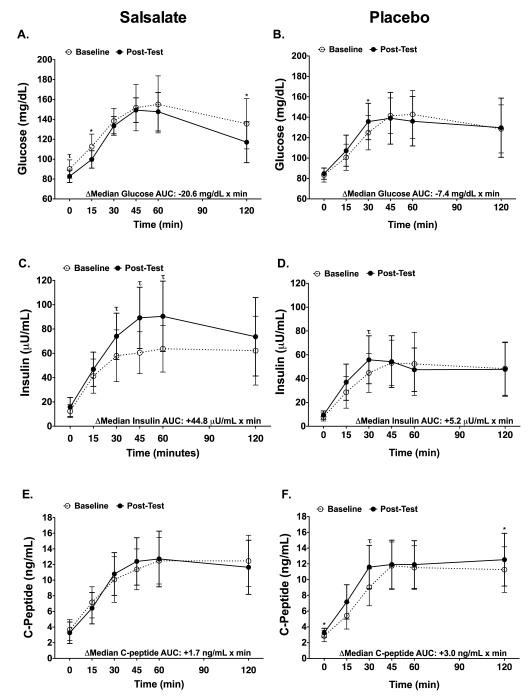
Table 2 shows outcome variables before and after the 4-week treatment intervention. There were treatment related differences in fasting blood glucose levels (Ptreatment<0.01) where the median blood glucose decreased 3.4% (P<0.01), with a median reduction of 2.9 mg/dL in the salsalate group but not placebo group (Figure 2A). Salsalate resulted in lower fasting FFA (Ptreatment=0.02), where the median blood FFA levels decreased in the salsalate (−42.5%, P=0.06, respectively) but not in the placebo group (Figure 2C). Salsalate resulted in higher insulin AUC (Ptreatment<0.01), where the median value increased in the salsalate (+38.0%, P=0.01) but not placebo group (Figure 3C-D). Although there was a trend for an increased fasting C-peptide in the placebo group (Ptreatment=0.05), salsalate treatment did not significantly alter levels of fasting insulin and fasting C-peptide (Figure 2B-C) or glucose AUC and C-peptide AUC (Figure 3A-B and E-F). Fasting insulin to C-peptide ratios were increased in the salsalate but not placebo group (+57.6%, P<0.01; Ptreatment<0.01). Treatment with salsalate did not alter estimates of insulin sensitivity or resistance (QUICKI, HOMA-IR or Matsuda Index) while a proxy for β-cell function (HOMA-B) increased in the salsalate (+47.2%, P<0.01; Ptreatment<0.01) but not placebo group.
Figure 2. Fasting Measures From the 2-hr OGTT.
Fasting glucose (A), insulin (B), C-peptide (C), and free fatty acids (D) levels from the 2-hr OGTT. Individual participant data where black circles = salsalate groups and white circles = placebo. Treatment P-values are from ANCOVA, which controlled for the baseline dependent variable and body fat percent. Within group P-values correspond to Related-Samples Wilcoxon Signed Rank Test. For visual representation, P-vales are only shown for P≤0.06.
Figure 3. Glucose, Insulin, C-Peptide During the 2-hr OGTT.
Results from the 2-hour OGTT. Mean ± standard deviation for plasma glucose (A, B), insulin (C, D), and C-Peptide (E, F) responses are shown for salsalate (left column) and placebo (right column). White circles and dashed lines = baseline. Back circles and solid lines = post-test. ΔMedian is equal to the difference between post-test and baseline AUC medians. *P<0.05, τP<0.01. P-values were obtained using ANCOVA analysis (change in salsalate vs. change placebo) with adjustment for the dependent variable at baseline as well as percent body fat. A significant treatment effect was only observed for insulin AUC (Ptreatment<0.01).
Adipokines and Markers of Inflammation
Salsalate treatment resulted in higher plasma adiponectin levels (27.7%, P<0.01; Ptreatment<0.001) with no change in the placebo group (Table 2). Circulating adipsin, cytokines, and markers of immune activation, including hsIL-6, sCD14, and sCD163, were unchanged within each group and there was no effect of salsalate treatment. Although there was a trend towards decreased sTNFrII levels in the salsalate group (−5.2%, p=0.09), there were no treatment related differences. From the fat biopsies, treatment did not alter the average fat cell size (Ptreatment=0.90) or presence of CLS (Ptreatment=0.40), yet there was a modest decrease in the average fat cell size among those in the placebo group (P=0.046). To examine adipose tissue inflammation in a more quantitative manner, the percent leukocytes and monocytes/macrophages in each fat sample were assessed using flow cytometry. Overall, there were no within group or treatment related differences in these immune cell populations. Despite systemic metabolic improvements in glycemia, FFA, and adiponectin levels, there were no within group or treatment related differences in adipose tissue gene expression for adiponectin, dipeptidyl peptidase-4 (DPP4), leptin, adipsin or any signaling pathways of interest (i.e., insulin, FFA, LPS/TLR). Further, there were no significant changes in gene expression of immune cell markers, including ACP5, CD68, CD163, MCP1, FOXP3, and LIPA within or between treatment groups (Supplemental Table 1). Of several inflammatory gene markers affected by NF-κB (e.g., IL-6, IL-8, IL-1R, TNFα, NOS, ICAM1, VCAM1), salsalate treatment was only associated with a 47% decreased IL1-β expression (P=0.002) while there was no change in the placebo group (Ptreatment=0.29).
Discussion
In this study of obese non-diabetic Hispanics, who are at increased risk for metabolic disease (20), we found that salsalate improved fasting glucose, insulin, and FFA levels as shown in prior studies in Caucasians. Previous studies suggest that these metabolic improvements are due to salsalate based suppression of inflammation, presumed to originate from abdominal fat (11, 31, 32). For this reason, we examined whether salsalate treatment had similar effects in non-diabetic obese Hispanics and whether any changes were accompanied by changes in adiposity, fat distribution, ectopic fat, adipose tissue gene expression, or attenuation of adipose tissue inflammation. Overall, we found that median blood glucose levels decreased by 2.9 mg/dL following salsalate treatment, even though ~88% of participants had baseline blood glucose levels within the normal range (≤100 mg/dL). Salsalate treatment also reduced fasting FFA by 42.5% and increased plasma adiponectin by 27.7%. Unique to this study, we show that each of these changes occurred without alterations in adipose tissue, including adiposity, fat distribution, ectopic fat, adipocyte size, and adipose tissue gene expression of adipokines and markers of adipose tissue inflammation.
Using histology, flow cytometry, and gene expression, we found that salsalate did not affect markers of adipose tissue inflammation, including the presence of CLS or the percent of immune cells (monocytes/macrophages and leukocytes) in biopsy specimens. Furthermore, we did not detect changes in expression of immune cell markers or IL-6, IL-8, IL1R, TNFα, NOS, ICAM1, and VCAM1, which is consistent with another report (14). We did observe a significant decrease in IL-1β gene expression after salsalate. Macrophage derived IL-1β stimulates IL-17 and IL-22 producing T cells (33), which are found in adipose tissue of insulin resistance individuals (7). Therefore, it is possible that salsalate exerted some of its metabolic effects through inhibition of macrophage derived IL-1β. However, given that other markers of adipose tissue inflammation were unchanged, it is unlikely that decreased IL-1β gene expression accounted for the improvements observed in fasting glucose and FFA.
Similar to previous salsalate studies, adiponectin levels significantly increased following treatment in non-diabetic obese Hispanics. Adiponectin has been shown to stimulate insulin sensitivity and modulate hepatic glucose production, fatty acid oxidation, and lipid synthesis (34-36), suggesting that metabolic improvements could relate to increases in this adiopokine. The marginal reduction in plasma sTNFrII following salsalate (P=0.09) indicates that increased adiponectin may contribute to reduced fasting glucose and FFA by decreasing systemic inflammation (35). However, we did not detect changes in other plasma markers of inflammation (hsIL-6, sTNFrII) or immune activation (sCD14, sCD163). Additionally, although adiponectin is exclusively produced by adipocytes, there were no changes in adipose tissue gene expression of adiponectin following treatment. Thus, results from our study suggest that increased circulating adiponectin levels did not result from salsalate-induced increases in adipocyte gene expression (37), but were due to either enhanced protein translation and secretion or decreased clearance by the liver or kidneys (35). These findings further indicate that salsalate does not alter adipose tissue; however, a recent study using fully differentiated human adipocytes found that salsalate increased adiponectin mRNA levels through decreased expression of 11β-hydroxysteroid dehydrogenase type 1 (37).
Since salsalate did not alter adipose tissue inflammation or adipose tissue gene expression of adiponectin, we explored whether metabolic improvements were related to changes in the liver. High-dose aspirin (a primary metabolite of salsalate) therapy has been shown to improve hepatic insulin sensitivity, glucose production, and inflammation (38). Therefore, salsalate may have decreased fasting glucose and fasting FFA through hepatic alterations or decreased rates of insulin clearance. Supporting this, in Sprague-Dawley rats, salsalate has been shown to ameliorate hepatic steatosis through decreased fetuin-A expression via the AMPK-NF-κB dependent pathway (36). Using whole abdominal MRI imaging, we found that metabolic improvements occurred without reductions in liver fat. However, it is possible that this methodology was not sensitive enough to capture small alterations in hepatocyte lipid content, which could affect metabolism. Instead, changes in liver inflammation, and not declines in hepatic fat, may contribute to metabolic improvements seen in humans treated with salsalate. For example, unmeasured changes in insulin clearance by the liver could contribute to hyperinsulinemia, decreased hepatic glucose production, increased glucose and FFA uptake by peripheral tissues, and decreased adipose tissue lipolysis. Supporting this, in the current study we found that metabolic improvements were accompanied by increased insulin concentrations during the OGTT and increased HOMA-B, which appeared to be related to decreased insulin clearance as reported previously (11, 14, 16, 18, 39).
There were several study limitations. Since race/ethnicity was based on self-report, future studies should verify these findings in a larger population with more definitive measures of ethnicity. Although participants were randomly assigned to placebo or salsalate, those in the salsalate group had somewhat higher baseline fasting insulin levels, were less insulin sensitive, and showed lower rates of insulin clearance than those in the placebo group. These differences likely resulted from the relatively small number of study participants. Notwithstanding, all findings were corrected for these baseline differences. This study examined non-diabetic obese Hispanics who had relatively normal fasting insulin levels and metabolic indices at baseline, perhaps accounting for why we did no observe improvements in insulin sensitivity or resistance (QUICKI, HOMA-IR, Matsuda Indices) following salsalate (40). Nonetheless, these findings are consistent with previous studies among those with insulin resistance and type 2 diabetes (13-15). Although there were no changes in the percent of immune cells or the proportion of those with CLS in adipose tissue, it is possible that there were changes in the proportion of pro- and anti-inflammatory macrophage subtypes that went undetected by our measures of adipose tissue inflammation. Despite these limitations, participants appeared to be adherent to all aspects of the protocol, which strengthens the treatment results. Pill counts indicated good adherence over the 4-week treatment period. The fact that there were no changes in diet, exercise, body fat percent, or ectopic fat provide further evidence that our findings can not be attributed to changes in lifestyle or adiposity. Thus, the metabolic improvements in our Hispanic participants were likely due solely to salsalate.
In conclusion, 4 weeks of salsalate treatment lowered fasting glucose and FFA levels and increased adiponectin levels among non-diabetic obese Hispanic young adults, findings that are consistent with prior observations in Caucasians (11-14). To our knowledge, this is the first study to systematically examine the metabolic effects of salsalate as they related to adiposity, ectopic fat, and various markers of adipose tissue inflammation. Similar to previous studies, we demonstrated that salsalate treatment improved metabolic outcomes and add to the existing literature by showing that these changes occurred without alterations to percent body fat, volume of abdominal adipose tissue depots, liver fat, adipose tissue gene expression of important adipokines (adiponectin, adipsin, leptin), or adipose tissue inflammation. Based on our findings, future studies should examine how salsalate affects the liver, particularly hepatic insulin sensitivity and glucose production, as well as its effects on insulin and adiponectin clearance.
Supplementary Material
Supplemental Table 1. Adipose Tissue Gene Expression
What is already known about this subject.
Salsalate favorably affects glycemia and lipid homeostasis in studies among predominantly obese Caucasian adults with impaired fasting glucose, impaired glucose tolerance, and/or type 2 diabetes.
Salsalate lowers fasting glucose in populations with relatively normal glucose levels where one study found beneficial effects in a predominantly Native American population.
Salsalate inhibits systemic inflammation possibly through decreased NF-κB activity in peripheral blood mononuclear cells and adipose tissue.
What this study adds.
Salsalate improves metabolic outcomes in obese Hispanics without type 2 diabetes, a population that suffers from a greater prevalence of obesity and metabolic disease risk.
Salsalate treatment does not alter adipose tissue, including adiposity, volume of abdominal adipose tissue depots, or liver fat.
Metabolic improvements are not accompanied by attenuation of adipose tissue inflammation or changes in adipose tissue gene expression of important adipokines (e.g., adiponectin, leptin).
Acknowledgments
TLA served as principal investigator and collected, analyzed, and interpreted data as well as prepared the manuscript. MIG (co-investigator, sponsor, and mentor), FRS (co-investigator), and EGG assisted with data analysis, data interpretation, and manuscript preparation. FRS was the study physician and performed the biopsies; EGG provided ultrasonic guidance for fat biopsies. LEG, JMR, HA, SDM, XS, and JT assisted with data collection, evaluation of statistical analysis, and manuscript preparation. SDM, XS, and JT also developed adipose tissue processing protocols for flow cytometry. The authors have nothing to disclose. No conflicts of interest existed for any of the authors.
This work was supported by the SC CTSI (NIH/NCRR/NCATS) Grant # TL1TR000132 (TLA) and the Robert C. and Veronica Atkins Foundation (MIG).
We would like to thank the USC CTU and our dedicated project assistants; Preeya Desai, Karen Elano, and Kimberly Chen, MPH. Our gratitude is especially extended to our participants for their involvement.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes M, Quintanilla C, Burrows R, Blanco E, Cifuentes M, Gahagan S. Obesity is associated with acute inflammation in a sample of adolescents. Pediatr Diabetes. 2014 doi: 10.1111/pedi.12129. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf) 2012;77:385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gokulakrishnan K, Deepa R, Mohan V, Gross MD. Soluble P-selectin and CD40L levels in subjects with prediabetes, diabetes mellitus, and metabolic syndrome--the Chennai Urban Rural Epidemiology Study. Metab Clin Exp. 2006;55:237–242. doi: 10.1016/j.metabol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, et al. Macrophage Content in Subcutaneous Adipose Tissue: Associations With Adiposity, Age, Inflammatory Markers, and Whole-Body Insulin Action in Healthy Pima Indians. Diabetes. 2008;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lê K-A, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes. 2011;60:2802–2809. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74. e1–e3. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlin S, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Walley A, Tordjman J, et al. Macrophage Gene Expression in Adipose Tissue is Associated with Insulin Sensitivity and Serum Lipid Levels Independent of Obesity. Obesity. 2013;21:E571–E576. doi: 10.1002/oby.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan M. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 10.Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 11.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2013;50:537–543. doi: 10.1007/s00592-011-0329-2. [DOI] [PubMed] [Google Scholar]
- 14.Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56:714–723. doi: 10.1007/s00125-012-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–393. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr Pract. 2012;18:826–833. doi: 10.4158/EP12064.OR. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, et al. Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study. Diabetes Care. 2014;37:1944–1950. doi: 10.2337/dc13-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014;311:806. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. pp. 3–12. [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 22.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110:1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Godin G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fitness J Canada. 2011;4:18–22. [Google Scholar]
- 24.Fernández-Real JM, Pérez del Pulgar S, Luche E, Moreno-Navarrete JM, Waget A, Serino M, et al. CD14 modulates inflammation-driven insulin resistance. Diabetes. 2011;60:2179–2186. doi: 10.2337/db10-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkner T, Sørensen LP, Nielsen AR, Fischer CP, Bibby BM, Nielsen S, et al. Soluble CD163: a biomarker linking macrophages and insulin resistance. Diabetologia. 2012;55:1856–1862. doi: 10.1007/s00125-012-2533-1. [DOI] [PubMed] [Google Scholar]
- 26.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Meth. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo-Corral CM, Alderete TL, Hu HH, Nayak K, Esplana S, Liu T, et al. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115–1121. doi: 10.1210/jc.2012-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu HH, Kim H-W, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 2010;18:841–847. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldfine AB, Fonseca V, Jablonski KA, Chen Y-DI, Tipton L, Staten MA, et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen Y-DI, Shoelson SE, et al. TINSAL-FMD (Targeting Inflammation Using Salsalate in Type 2 Diabetes–Flow-Mediated Dilation) Ancillary Study Team. Targeting inflammation using salsalate in patients with type 2 diabetes: effects on flow-mediated dilation (TINSAL-FMD) Diabetes Care. 2013;36:4132–4139. doi: 10.2337/dc13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalmas E, Venteclef N, Caer C, Poitou C, Cremer I, Aron-Winewsky J, et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63:1966–1977. doi: 10.2337/db13-1511. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki T, Yamauchi T, Okada-Iwabu M, Iwabu M. Adiponectin and its receptors: implications for obesity-associated diseases and longevity. Lancet Diabetes Endocrinol. 2014;2:8–9. doi: 10.1016/S2213-8587(13)70120-7. [DOI] [PubMed] [Google Scholar]
- 35.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: Regulation of its production and role in human diseases. Hormones. 2012;11:6–18. doi: 10.1007/BF03401534. [DOI] [PubMed] [Google Scholar]
- 36.Jung TW, Youn B-S, Choi HY, Lee SY, Hong HC, Yang SJ, et al. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013;86:960–969. doi: 10.1016/j.bcp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Nixon M, Wake DJ, Livingstone DE, Stimson RH, Esteves CL, Seckl JR, et al. Salicylate downregulates 11β-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization. Diabetes. 2012;61:790–796. doi: 10.2337/db11-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Real JM, López-Bermejo A, Ropero A-B, Piquer S, Nadal A, Bassols J, Casamitjana R, Gomis R, Arnaiz E, Pérez I, Ricart W. Salicylates increase insulin secretion in healthy obese subjects. J Clin Endocrinol Metab. 2008;93:2523–2530. doi: 10.1210/jc.2007-1212. [DOI] [PubMed] [Google Scholar]
- 40.Qu H-Q, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE. 2011;6:e21041. doi: 10.1371/journal.pone.0021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Adipose Tissue Gene Expression