Abstract
Translation of most cellular mRNAs involves cap binding by the translation initiation complex. Among this complex of proteins are cap-binding protein eIF4E and the eIF4E kinase Mnk1. Cap-dependent mRNA translation generally correlates with Mnk1 phosphorylation of eIF4E when both are bound to eIF4G. During the late phase of adenovirus (Ad) infection translation of cellular mRNA is inhibited, which correlates with displacement of Mnk1 from eIF4G by the viral 100-kDa (100K) protein and dephosphorylation of eIF4E. Here we describe the molecular mechanism for 100K protein displacement of Mnk1 from eIF4G and elucidate a structural basis for eIF4G interaction with Mnk1 and 100K proteins and Ad inhibition of cellular protein synthesis. The eIF4G-binding site is located in an N-terminal 66-amino-acid peptide of 100K which is sufficient to bind eIF4G, displace Mnk1, block eIF4E phosphorylation, and inhibit eIF4F (cap)-dependent cellular mRNA translation. Ad 100K and Mnk1 proteins possess a common eIF4G-binding motif, but 100K protein binds more strongly to eIF4G than does Mnk1. Unlike Mnk1, for which binding to eIF4G is RNA dependent, competitive binding by 100K protein is RNA independent. These data support a model whereby 100K protein blocks cellular protein synthesis by coopting eIF4G and cap-initiation complexes regardless of their association with mRNA and displacing or blocking binding by Mnk1, which occurs only on preassembled complexes, resulting in dephosphorylation of eIF4E.
Translation of most eukaryotic mRNAs involves interaction of the 5′m7GpppN (cap) structure with eukaryotic initiation factor 4E (eIF4E), the smallest subunit of the cap-initiation complex eIF4F. eIF4F consists of three polypeptides: cap-binding protein eIF4E; an ATP-dependent RNA helicase, eIF4A; and the scaffold protein eIF4G (eIF4GI and eIF4GII) (reviewed in reference 16). eIF4G also interacts with initiation factor eIF3, which in turn binds the 40S ribosomal subunit, promoting its recruitment to the 5′ end of capped mRNAs (16). Poly(A)-binding protein interacts with the N terminus of eIF4G (18, 43), potentially circularizing and promoting translation of capped and polyadenylated mRNAs (50). The C terminus of eIF4G interacts with the mitogen-activated protein kinase-interacting kinases Mnk1 and Mnk2 (Mnk2a and Mnk2b, respectively), which efficiently phosphorylate eIF4E in vivo when both eIF4E and Mnk are bound to eIF4G (3, 33, 36, 39, 49).
Phosphorylation of eIF4E at Ser209 by Mnk kinases usually correlates with stimulation of cap-dependent mRNA translation, although there are exceptions, such as the response to arsenite or anisomycin stresses (7, 11, 47). A molecular understanding of eIF4E phosphorylation in stimulating mRNA translation is lacking, and some data bring into question its importance altogether (21, 29). Recent data suggest that phosphorylation of eIF4E might decrease its affinity for capped mRNA (39), perhaps implicating eIF4E phosphorylation in release of eIF4F from the cap during the translation initiation process (38, 39). Dephosphorylation of eIF4E strongly correlates with inhibition or impairment of cap-dependent mRNA translation under certain stress conditions such as heat shock (reviewed in reference 41), nutrient deprivation, oxidative or osmotic stress (47), and infection of mammalian cells with certain viruses such as adenovirus (Ad) (17) or influenza virus (6), among others. In these cases, disassembly of the eIF4F complex (2, 3, 32, 34, 47) or displacement of Mnk1 from eIF4G (3) can explain dephosphorylation of eIF4E.
Ad infection leads to the inhibition of cap-dependent translation at the beginning of the late phase of infection, when the virus initiates DNA replication (4, 40). Ad inhibition of cellular protein synthesis correlates with a strong decrease in eIF4E phosphorylation (17, 53), but it does not involve eIF4E sequestration by the 4E-binding proteins (5, 12), in contrast to certain stress conditions (32, 34, 47). We recently established that the Ad late L4 100-kilodalton (L4 100K) protein inhibits cellular protein synthesis, consistent with its binding to eIF4G, displacement of Mnk1, and dephosphorylation of eIF4E (3). However, 100K is a large protein displaying several activities, which make it difficult to demonstrate that 100K displacement of Mnk1 from eIF4G and dephosphorylation of eIF4E are actually responsible for inhibition of host cell protein synthesis.
Ad late mRNAs are translated despite inhibition of host cell protein synthesis due to the presence of a 200-nucleotide 5′ noncoding region, known as the tripartite leader. The tripartite leader promotes translation by a novel initiation mechanism known as ribosome shunting (reviewed in references 4, 51, and 52). In ribosome shunting, 40S ribosomal subunits bind the cap structure with eIF4G but are directed by the tripartite leader to translocate to the downstream initiating AUG in a nonlinear manner, bypassing intervening RNA regions. Ribosome shunting in late Ad-infected cells is enhanced with dephosphorylation of eIF4E and inhibition of host cell protein synthesis (51, 52).
There is at present little understanding of the molecular mechanism by which 100K protein inhibits host cell protein synthesis during Ad infection, how 100K binds to eIF4G and displaces Mnk1, and whether the 100K-eIF4G interaction and dephosphorylation of eIF4E are sufficient to account for Ad inhibition of cellular protein synthesis. Here we report the molecular mechanism for Ad inhibition of host cell protein synthesis. We demonstrate that 100K and Mnk1 proteins share a binding region for eIF4G and that ectopic expression of a small (66-amino-acid) peptide of 100K protein is sufficient to block cellular mRNA translation by preventing Mnk1 binding and blocking eIF4E phosphorylation.
MATERIALS AND METHODS
Plasmids, antisera, and cells.
Plasmids pEBG, pEBG-Mnk1 (48) (kindly provided by J. A. Cooper), pcDNA-HA-eIF4GI (19) (a gift of N. Sonenberg), and pFlag-100K (3) were described previously. pFlag-CMV-2 was from Sigma, and pIRES2-EGFP was from Clontech. Plasmids pFlag-100K [1-726] (100K amino acids 1 to 726), pFlag-100K [1-345], pFlag-100K [122-805], pFlag-100K [266-805], and pFlag-100K [346-805] were generated by PCR amplification of the L4 100K sequence and cloning into pFlag-CMV-2. Plasmid pFlag-100K NES, which expresses the 100K protein mutated at its nuclear export sequence (NES), was constructed by overlap extension PCR mutagenesis (specific details for all cloning are available on request). Plasmids pEBG-100K [1-345], pEBG-100K [1-261], pEBG-100K [1-200], pEBG-100K [59-345], pEBG-100K [201-345], pEBG-100K [201-261], pEBG-100K [201-299], pEBG-100K [239-345], and pEBG-100K [280-345] were constructed by cloning into the pEBG vector. All constructs were confirmed by restriction enzyme mapping. Plasmid pEBG-100K [280-345 RRKA] was generated by PCR cloning from the pFlag-100K RRKA plasmid and inserted into the pEBG vector. pFlag-100K RRKA was constructed by overlap extension PCR mutagenesis and cloned into pFlag-CMV-2 vector. To generate the pEBG-Mnk1 RRKA plasmid, the Mnk1 sequence was amplified by PCR with primers and cloned into the pEBG vector. pFlag-Mnk1 plasmid was constructed by overlap extension PCR in three steps to add a Flag epitope at the N terminus of Mnk1 sequence. The resultant PCR product was cloned into pCMV Ad (5).
The pCMV-β-Gal plasmid was described previously (5), containing the β-galactosidase (β-Gal) sequence. Rabbit polyclonal antiserum to eIF4G was previously described (3). Other antibodies were from commercial sources and included affinity-purified rabbit polyclonal antibody to eIF4E (Cell Signaling Technology), affinity-purified anti-glutathione S-transferase (anti-GST) rabbit polyclonal antibody (Santa Cruz Biotechnology), mouse antihemagglutinin (anti-HA) monoclonal antibody (HA.11; Covance), mouse anti-Flag M2 monoclonal antibody (Sigma), rabbit anti-green fluorescent protein (anti-GFP) antibody (Molecular Probes), and rabbit anti-β-Gal antibody (Cortex Biochemicals). 293T and HeLa cells were grown in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% calf serum (HyClone). 293T cells were transfected by calcium phosphate coprecipitation. HeLa cells were transfected with Lipofectamine Plus (Invitrogen).
In vitro RNA-protein interactions.
293T cells were transfected with plasmids expressing Flag, Flag-100K [1-726], or Flag-100K [1-345] protein, and cellular lysates were prepared in NP-40 lysis buffer (0.5% NP-40, 50 mM HEPES [pH 7.0], 150 mM NaCl, 2 mM EDTA, 2 mM sodium orthovanadate, 25 mM glycerophosphate, protease inhibitor cocktail tablet [Roche]). Lysates were incubated for 3 h at 4°C with anti-Flag antisera and for 1 h at 4°C with protein G agarose (Santa Cruz Biotechnology) before being washed five times with the same lysis buffer. Capped or uncapped CR3 5′-UTR-containing RNAs were transcribed in vitro with MAXIscript (System Ambion Inc.). Equal amounts of immunocomplexes were incubated with 4 ng of RNA for 30 min at 30°C in 20 μl of binding buffer (10 mM HEPES-KOH [pH 7.2], 3 mM MgCl2, 80 mM KCl, 5% glycerol, 1 mM dithiothreitol). RNA-protein complexes were UV cross-linked on ice with a 254-nm UV light source for 10 min (1 J/cm2) and then treated with 40 μg of RNase A and 10 U of RNase T1 for 30 min at 37°C. Complexes were collected by centrifugation and resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE). Gels were fixed, and autoradiography was performed.
Immunofluorescence microscopy.
HeLa cells were transfected with plasmids expressing Flag-100K, Flag-100K [1-726], or Flag-100K [1-345] proteins and grown on coverslips. Cells were fixed with 4% paraformaldehyde for 10 min at 25°C, blocked in phosphate-buffered saline (PBS) plus 1% nonfat dry milk (NFDM) for 30 min at 25°C, and then incubated with antibodies to Flag in PBS-1% NFDM for 1 h at 37°C. Fixed cells were washed four times with PBS-1% NFDM, reacted with anti-mouse immunoglobulin G-fluorescein and F(ab′)2 fragment for 1 h at 37°C, and then washed four times with PBS-1% NFDM and stained with Hoechst 33258 (Sigma) before being mounted with Vectashield mounting medium (Vector Laboratories). Cells were visualized and photographed using a Zeiss Axiophot microscope.
Immunoprecipitation and glutathione-Sepharose 4B recovery.
293T cells were lysed in NP-40 lysis buffer and clarified by centrifugation for 10 min at 14,000 × g. Lysates were precleared for 2 h at 4°C with 30 μl of protein A or protein G agarose and then incubated overnight with the indicated antiserum (preimmune serum, anti-eIF4G, or Ezview Red anti-Flag M2 affinity gel [Sigma]). When required, protein A agarose was added and incubation was continued for 1 h at 4°C before lysates were washed five times with lysis buffer. For GST pull-downs, lysates were incubated with 30 μl of a 50% slurry of glutathione-Sepharose 4B for 1 h at 4°C and then washed five times with lysis buffer. For Mnk1-eIF4G interaction studies, cellular lysates were prepared with Triton lysis buffer (1% Triton X-100, 50 mM NaF, 10 mM HEPES [pH 7.4], 2 mM EDTA, 2 mM sodium orthovanadate, 0.1% β-mercaptoethanol, protease inhibitor cocktail tablet [Roche]) or NP-40 lysis buffer, which is the same as that above but substitutes Triton for 1% NP-40 detergent and 50 mM NaF for 150 mM NaCl.
Isoelectric focusing.
One-dimensional vertical isoelectric focusing gel electrophoresis was carried out for analysis of eIF4E phosphorylation, performed as described previously (28).
RESULTS
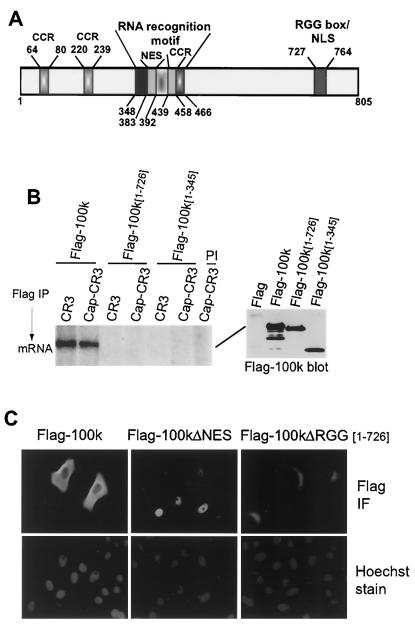
Characterization of Ad late L4 100K protein elements.
The only alteration found in the translation initiation complex during late times in Ad infection is the interaction of Ad 100K protein with eIF4G, resulting in displacement of Mnk1 from eIF4G and dephosphorylation of eIF4E (3). Studies were therefore performed to characterize the functional domains of 100K protein that are involved in translational control. As shown in Fig. 1A, an analysis of 100K structural features reveals two distinct potential RNA-binding motifs: an RNA recognition motif (RRM) located between amino acid residues 348 and 466, as previously described (14), and an RGG-rich RNA-binding motif (RGG box) located between positions 727 and 764, containing three RGG and four RGG-like repeats. Although each element has the capacity to independently bind RNA, the combination of an RGG box and other RNA-binding motifs is found in certain RNA-binding proteins such as nucleolin (10). The RGG box can also function as a nuclear localization signal (NLS), as shown for the Saccharomyces cerevisiae hnRNP protein Npl3p (42). We also identified a 10-amino-acid sequence in 100K protein, located between residues 383 and 392, which is similar to the previously described Rev-like NES (13, 30). Finally, three potential coiled-coil regions were predicted which might play a role in protein-protein interactions (e.g., eIF4G-100K binding) and whose relevance to inhibition of cellular mRNA translation was assessed as described below.
FIG. 1.
Characterization of 100K protein. (A) Predicted structural regions identified within the 100K protein are indicated by amino acid position: coiled-coil region (CCR 439-458), RRM 383-458, Rev-like NES 383-392, RGG box, and overlapping NLS 727-764. (B) 293T cells were transfected with plasmids expressing Flag, Flag-100K, or truncated versions of 100K retaining amino acid sequences as indicated: Flag-100K [1-726] (with RGG box) or Flag-100K [1-345] (without RRM and RRG box). Flag-tagged proteins were isolated by immunoprecipitation with anti-Flag antibody. Equal amounts of Flag immune complexes as shown by immunoblotting (right panel) were incubated with 4 ng of 32P-labeled, in vitro-transcribed RNAs corresponding to the capped or uncapped-CR3 5′ UTR. (see Materials and Methods for more details). RNA-protein complexes were subjected to UV cross-linking, and complexes were analyzed by denaturing SDS-10% PAGE followed by autoradiography (left panel). PI indicates immunoprecipitation with preimmune serum. Quantification in all figures was obtained by densitometry of typical results from at least three independent experiments and is described in the text. (C) HeLa cells were transfected with plasmids expressing Flag-100K, Flag-100KΔRGG [1-726], or Flag-100KΔNES and grown on coverslips. Cells were fixed-permeabilized and incubated with anti-Flag antibody, followed by incubation with mouse secondary antibody coupled to fluorescein. Nuclei were stained with Hoechst 33258 dye. Cells were visualized and photographed using a Zeiss Axiophot microscope. IF, immunofluorescence.
RNA-binding activity of 100K protein was previously demonstrated for both cellular and late Ad mRNAs (1, 35). We first mapped the RNA-binding region of 100K protein and show that RNA binding is not required for inhibition of eIF4G-Mnk1 interaction and inhibition of cellular protein synthesis. 293T cells were transfected with plasmids expressing Flag peptide or Flag-tagged 100K protein or either of two Flag-100K deletion mutants, Flag-100K [1-726] (deletion of the RGG box) or Flag-100K [1-345] (deletion of the RRM and the RGG box). Flag-100K proteins were translated from the tripartite leader and therefore immune to feedback inhibition. Flag proteins were immunoprecipitated with anti-Flag antibody, and similar amounts of 100K immune complexes were recovered (Fig. 1B, immunoblot in right panel). Immune complexes were incubated with either capped or uncapped in vitro-transcribed [32P]UTP-labeled RNAs, and RNA-protein complexes were subjected to UV cross-linking. Flag-100K/mRNA complexes were then recovered, resolved by SDS-10% PAGE, and analyzed by autoradiography. RNA binding was abolished by deletion of the C-terminal region of 100K protein containing the RGG box (Flag-100K [1-726]) or both RNA-binding motifs (Flag-100K [1-345]). In this and all other studies, data representative of at least three independent experiments are shown.
100K protein is found in both the nucleus and the cytoplasm (9). Since a cytoplasmic location is likely critical for inhibition of host cell translation, we identified the signals involved in nuclear import and export of 100K. The high arginine content within and flanking the 100K RGG box implicated this region as a component of the NLS, as described for the yeast hnRNP protein Npl3p (42). An arginine cluster occurs between amino acids 753 and 764 of 100K protein, and a potential Rev-like NES element is located between amino acids 383 and 392. The 100K sequence 383LCNLVSYLGI392 matches the consensus Rev-like NES LX(1-3)LX(2-3)LXL/I (15). Positions L383, L386, L390, and I392 were therefore changed to alanine (Flag-100KΔNES), and HeLa cells were transfected with plasmids expressing Flag-100K, Flag-100KΔRGG [1-726] (RGG box deletion), or Flag-100KΔNES proteins. Nuclei were stained with Hoechst 33258 dye, and cells were subjected to immunofluorescence analysis with anti-Flag antibody (Fig. 1C). Wild-type 100K protein localized to both the nucleus and cytoplasm in transfected cells, Flag-100KΔNES localized only to the nucleus, and Flag-100KΔRGG localized to the cytoplasm but with evident concentration about the nuclear membrane. These results demonstrate that a NES element is located between 100K positions 383 and 392 and that an NLS is located within the RGG box region.
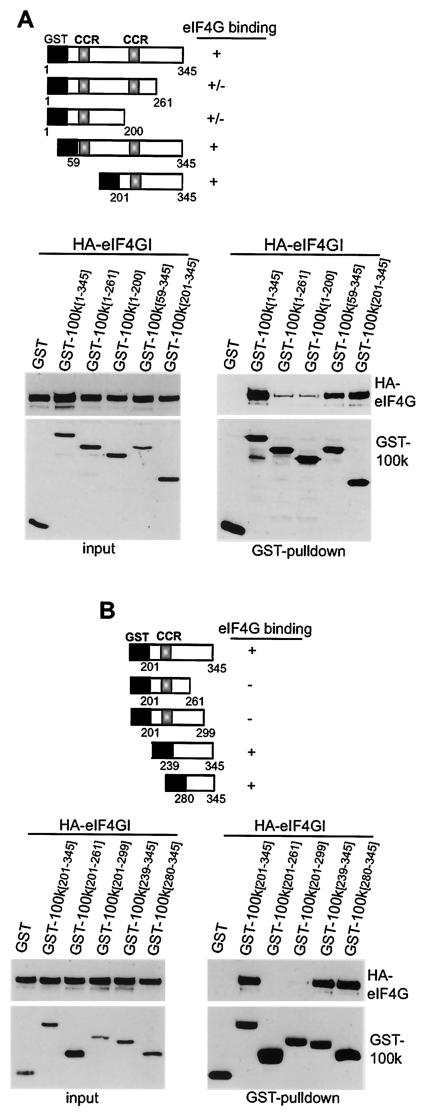
The eIF4G-binding site in 100K was next identified and mutated. 293T cells were transfected with plasmids expressing Flag-100K protein or C-terminal deletion mutants lacking either the RGG box or both the RRM element and the RGG box (Fig. 2). Three additional 100K mutants that were deleted in the N terminus, Flag-100KΔ [122-805], Flag-100KΔ [266-805], and Flag-100KΔ [346-805], were insoluble and therefore could not be investigated. Endogenous eIF4GI was immunoprecipitated from equal amounts of cell lysate, and its interaction with Flag-tagged wild-type or truncated 100K proteins was examined by immunoblot analysis. All three 100K mutants which retained the N-terminal region interacted with eIF4GI. Thus, the N terminus of 100K protein is sufficient for eIF4G binding and is located between residues 1 and 345.
FIG. 2.
Mapping of eIF4G-binding site within 100K protein. 293T cells were transfected with plasmids as indicated, and then at 36 h posttransfection equal amounts of total protein lysates were incubated with eIF4G antiserum (eIF4G IP) or preimmune serum (Preim. IP). Immune complexes were resolved by SDS-10% PAGE, and associated proteins were detected by immunoblot analysis of endogenous eIF4G (anti-eIF4G) or Flag (anti-Flag). Typical results from at least three independent experiments are shown. IgG, immunoglobulin G; CCR, coiled-coil region.
Analysis of the 100K amino acid sequence for consensus protein-protein interaction domains revealed three potential coiled-coil motifs at positions 64 to 80, 220 to 239, and 439 to 458 (Fig. 1A) (24). Since eIF4GI binds to the first 345 amino acids of 100K protein, we introduced point mutations in the first two predicted coiled-coil regions. Mutations were predicted by protein-folding algorithms to abolish these structures (unpublished observations). Studies found similar associations of wild-type and of coiled-coil mutant 100K proteins with eIF4GI, suggesting that the two coiled-coil motifs are not required for 100K-eIF4GI interaction (data not shown). We therefore developed additional GST-fused N-terminal or C-terminal deletion mutants of 100K protein (Fig. 3A), corresponding to GST-100K [1-261], GST-100K [1-200], GST-100K [59-345], and GST-100K [201-345]. 100K protein was recovered by glutathione-Sepharose chromatography, and association of 100K proteins with HA-eIF4GI was examined by immunoblot analysis (Fig. 3A, lower panels). Expression of a small region of 100K protein containing amino acids 201 to 345 was found to be sufficient to bind eIF4GI (100K [201-345]), indicating that the eIF4G-binding site maps to this region. Only a weak association was detected between HA-eIF4GI and 100K proteins that were truncated between amino acids 201 and 345 (Fig. 3A). The 100K mutant lacking the RGG motifs (Flag-100KΔRGG [1-726]) bound eIF4G strongly, as expected since it retains the N terminus of the 100K protein. However, Flag-100K deletion mutants which lack the N terminus did not bind strongly to eIF4G. The Flag-100KΔNES mutant, which is restricted to the nucleus, also failed to bind eIF4G (data not shown). Studies therefore focused on the importance of 100K peptide between positions 201 and 345. Further deletion GST-100K mutants were generated (Fig. 3B) corresponding to 100K amino acids 201 to 261, 201 to 299, 239 to 345, and 280 to 345. GST-100K protein fragments were coexpressed in 293T cells with HA-eIF4GI, GST fusion proteins were isolated by glutathione-Sepharose chromatography, and their interaction with HA-eIF4GI was determined by immunoblot analysis (Fig. 3B). Strong binding was detected between HA-eIF4GI and 100K peptides containing amino acids 239 to 345 and 280 to 345 but not 100K peptides containing amino acids 201 to 299 or 201 to 261. Thus, a small 66-amino-acid peptide of 100K protein (positions 280 to 345) is sufficient for eIF4G binding and does not require RNA binding or protein-shuttling motifs for this interaction.
FIG. 3.
Fine mapping of eIF4G-binding site within 100K protein. (A) 293T cells were cotransfected with plasmids expressing HA-eIF4GI (20) and either GST or GST-100K plasmids containing 100K peptides as shown. At 36 h posttransfection GST fusion proteins were recovered by glutathione-Sepharose chromatography and resolved by SDS-10% PAGE, and HA-eIF4GI-associated proteins were detected by immunoblot analysis. (B) 293T cells were cotransfected with plasmids as described above, and GST fusion proteins associated with HA-eIF4GI were analyzed. Typical results from at least three independent experiments are shown. CCR, coiled-coil region.
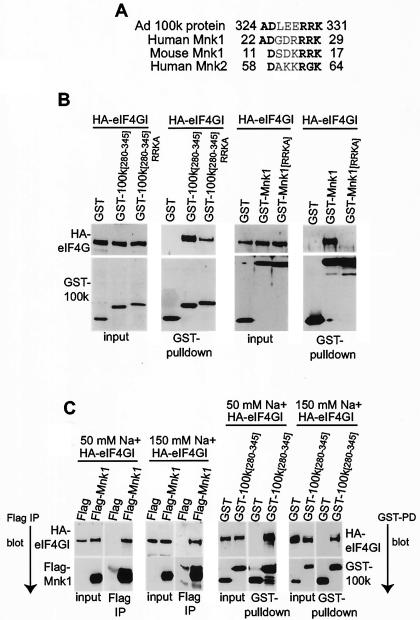
Human and mouse eIF4E kinases (Mnk1) share a basic amino acid-rich sequence with 100K protein required for binding to eIF4G.
We previously established that 100K protein interacts with the C-terminal region of eIF4G, displacing Mnk1 or blocking its binding to eIF4G (3). We therefore determined whether 100K protein and Mnk kinases share a related eIF4G-binding site. Alignment of the 100K protein region containing the eIF4G-binding site (positions 280 to 345) with either human or mouse Mnk1 showed homology in a hydrophilically charged sequence (Fig. 4A). All three proteins contain an identical basic amino acid cluster, RRK, following an aspartic acid residue (D). This homologous sequence also contains two conserved hydrophilically charged amino acids N-terminal to the RRK sequence. The homologous sequence in mouse Mnk1 (residues 11 to 17) is within the previously identified segment of 23 N-terminal amino acids shown to be required for mouse Mnk1 binding to eIF4G (49). Other studies indicate that this sequence functions as an NLS (8). Based on these data and the conclusion that a basic region is involved in eIF4G binding, the homologous sequence likely comprises the eIF4G-binding site in Mnk proteins and part of the site in Ad 100K protein. Examination of the other eIF4E kinase, Mnk2, which has also been shown to bind eIF4G (36, 37, 39), revealed that the human protein contains similar sequences (Fig. 4A). It has recently been demonstrated (37) that a basic amino acid-rich sequence between residues 58 and 64 in human Mnk2a and Mnk2b is required for binding to eIF4G. These data also support the conclusion that this sequence is the eIF4G-binding site, which is conserved in Ad 100K protein.
FIG. 4.
Human and mouse Mnk1 and Ad late 100K protein share a hydrophilically charged amino acid-rich sequence required for binding to eIF4G. (A) Alignment of homologous sequences found in human and mouse eIF4E kinase, Mnk1 proteins, and Ad 100K protein. (B) 293T cells were cotransfected with plasmids expressing HA-eIF4GI and GST or GST-100K plasmids. 100K peptides are indicated by amino acid number of retained fragments. 280-345 RRKA is a 100K mutant peptide containing AAA instead of the homologous RRK sequence. GST-Mnk1 is a wild-type clone. GST-Mnk1[RRKA] is a mouse Mnk1 mutant with AAA instead of RRK. At 36 h posttransfection cellular extracts were prepared and equal amounts of total protein were incubated with glutathione-Sepharose to recover GST fusion proteins. Complexes were resolved by SDS-10% PAGE, and the association of eIF4GI with GST proteins was detected by immunoblot analysis with HA antibody. Expression levels of input HA-eIF4GI and GST fusion proteins (first and third panels from left) were determined by immunoblotting. (C) 293T cells were transfected with plasmids expressing HA-eIF4GI and either Flag or Flag-Mnk1, or GST or GST-Mnk1. Cellular lysates were prepared with 50 mM NaF or 150 mM NaCl, respectively, at 36 h posttransfection. Flag-tagged or GST fusion proteins were isolated by immunoprecipitation with anti-Flag antibody or by glutathione-Sepharose 4B chromatography, respectively, and proteins were resolved by SDS-10% PAGE. Interaction of Flag-Mnk1 and GST-100K [280-345] with HA-eIF4GI was detected by immunoblotting with an antibody to the HA epitope. Typical results from at least three independent experiments are shown.
The RRK cluster in 100K [280-345] and mouse Mnk1 proteins was mutated to AAA to determine whether it is essential for binding to eIF4G. Protein folding algorithms predicted that this change should not disrupt the overall secondary structure of the mutant proteins (unpublished observations). Mutant GST fusion 100K proteins were expressed in 293T cells with HA-eIF4GI and purified by glutathione-Sepharose chromatography. Association of these proteins with HA-eIF4GI was examined by immunoblot analysis with HA antibody (Fig. 4B). Mutation of the RRK sequence within mouse Mnk1 abolished its interaction with eIF4GI (decreased by >90% ± 10%), indicating that this short motif is essential for eIF4GI-binding activity (Fig. 4B, fourth panel from left). However, the same mutation in the 100K peptide (amino acids 280 to 345) decreased its association with eIF4G by 50% (±20%) but did not eliminate binding (second panel from left). The persistent binding suggests that additional residues within 100K protein likely promote a stronger interaction with eIF4GI. The involvement of additional sequences in binding of 100K to eIF4GI would be expected to increase the affinity of 100K protein for eIF4GI compared to Mnk1, preventing Mnk1 association. This was tested by determining whether interaction between Mnk1 and HA-eIF4GI and that between 100K peptide and HA-eIF4GI are disrupted at similar concentrations of salt (Fig. 4C). Flag-100K peptide and Flag-Mnk1 both bound HA-eIF4GI in 50 mM NaF. However, in 150 mM NaCl (a stronger sodium dissociator than NaF), eIF4G binding by Flag-Mnk1 was abolished whereas 100K-eIF4G interaction was unaffected. These data indicate that the interaction between eIF4G and 100K protein is either stronger or more stable than that of eIF4G and Mnk1.
Binding of 100K peptide to eIF4GI is sufficient to block Mnk1 association, eIF4E phosphorylation, and non-Ad mRNA translation.
Having defined the core binding site of 100K protein for eIF4G, studies determined whether the minimal 100K peptide (amino acids 280 to 345) is sufficient to inhibit Mnk1-eIF4GI interaction. 293T cells were transfected with plasmids expressing HA-eIF4GI, Flag, or Flag-Mnk1 and either GST, GST-100K [280-345], or GST-100K [280-345 RRKA] protein. Cell extracts were prepared, and equal amounts of total protein were immunoprecipitated with anti-Flag antibody to isolate Flag or Flag-Mnk1 complexes. Complexes were resolved by SDS-10% PAGE, and the interaction of Mnk1 with HA-eIF4GI was analyzed by immunoblotting with the use of anti-HA antibody for eIF4GI, anti-Flag for Mnk1, and anti-GST for 100K protein (Fig. 5, right panels). Protein expression was consistent in the different transfections (Fig. 5, left panels). Coexpression of the minimal 100K peptide (amino acids 280 to 345, which contains the eIF4GI-binding site) and Mnk1 resulted predominantly in dissociation of Mnk1 protein from eIF4G and accumulation of eIF4G-100K peptide complexes. However, the RRKA mutant of 100K peptide, which has impaired eIF4G-binding activity, did not compete for eIF4G binding and did not displace Mnk1. Accordingly, isolation of GST-100K proteins by glutathione-Sepharose chromatography and immunoblot analysis demonstrated that the GST-100K [280-345] peptide bound strongly to HA-eIF4G, consistent with inhibition of Mnk1 binding. There was only very weak binding of the RRKA mutant of 100K peptide to eIF4G. These data indicate that binding of the minimal wild-type 100K peptide (amino acids 280 to 345) to eIF4GI is sufficient to displace Mnk1 from eIF4G or impair its binding.
FIG. 5.
Analysis of Mnk1 protein displacement from eIF4G polypeptide by either wild-type or RRKA mutant 100K [280-345] peptides. 293T cells were transfected with plasmids expressing HA-eIF4GI, Flag or Flag-Mnk1, and either GST, GST-100K [280-345], or GST-100K [280-345 RRKA]. At 36 h posttransfection, cells were lysed and Flag-tagged proteins were immunoprecipitated by Ezview Red anti-Flag M2 affinity gel and resolved by SDS-10% PAGE. Proteins associated with Flag-tagged proteins (Flag or Flag-Mnk1) were detected by immunoblot analysis using antibodies specific to the HA epitope for HA-eIF4GI and anti-GST for GST-100K [280-345] peptides (right panels). GST proteins were recovered by glutathione-Sepharose beads and resolved by SDS-10% PAGE, and associated proteins were identified by immunoblot analysis as shown. Levels of ectopically expressed proteins are shown in the left panels. Immunoblots were quantified by densitometry, and standard deviations were calculated from three independent experiments.
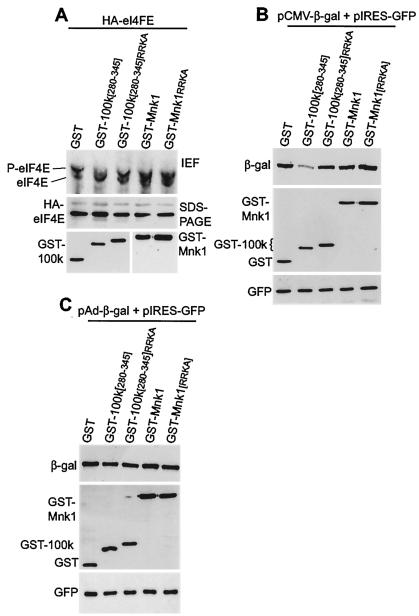
Studies next determined the ability of the minimal 100K peptide to impair eIF4E phosphorylation. 293T cells were cotransfected with plasmids at >90% transfection efficiency (data not shown), expressing HA-eIF4E and either GST, GST-100K [280-345], GST-100K [280-345 RRKA], GST-Mnk1, or GST-Mnk1[RRKA]. eIF4E phosphorylation was investigated by HA-eIF4E immunoprecipitation and one-dimensional isoelectric focusing gel electrophoresis (Fig. 6A). HA-eIF4E phosphorylation levels were normalized to the corresponding total levels of HA-eIF4E determined by HA immunoblotting, as shown in the middle panel. Cells expressing only the GST protein as a control (±10%) contained approximately 75% (±10%) phosphorylated eIF4E. Expression of wild-type 100K peptide reversed the ratio of phosphorylated to nonphosphorylated HA-eIF4E, resulting in ∼70% (±10%) being in the nonphosphorylated form. The GST-100K [280-345 RRKA] mutant peptide only slightly decreased HA-eIF4E phosphorylation. Given that 100K protein levels in Ad-infected cells are much higher than those in transfected cells (3), we can conclude that binding of the 100K peptide to eIF4G during late Ad infection is sufficient to displace Mnk1 and impair eIF4E phosphorylation. Studies were also carried out to examine the effect of overexpression of GST-Mnk1 or the GST-Mnk1[RRKA] mutant on eIF4E phosphorylation. The wild-type Mnk1 protein demonstrated slightly enhanced HA-eIF4E phosphorylation, whereas the RRKA mutant had no effect, as expected (Fig. 6A).
FIG. 6.
eIF4E phosphorylation and cap-dependent translation in cells expressing wild-type or mutant 100K peptides. (A) 293T cells were cotransfected with plasmids expressing HA-eIF4E and either GST, GST-100K [280-345], GST-100K [280-345 RRKA], mouse GST-Mnk1, or GST-Mnk1[RRKA] proteins. At 36 h posttransfection cellular extracts were prepared, HA-eIF4E was immunoprecipitated, and protein amounts were normalized by HA-eIF4E protein levels (middle panel), and then eIF4E phosphorylation was assessed by one-dimensional isoelectric focusing (IEF) gel electrophoresis (top panel). (B) 293T cells were transfected with plasmids expressing β-Gal (cap-dependent translation), GFP (EMCV IRES-dependent translation), and either GST, GST-100K [280-345], GST-100K [280-345 RRKA], GST-Mnk1, or GST-Mnk1[RRKA] proteins. At 36 h posttransfection cells were lysed, and after normalization by GFP, proteins were resolved by SDS-10% PAGE and subjected to immunoblot analysis for the indicated proteins. (C) 293T cells were transfected with plasmids expressing β-Gal (Ad tripartite leader-dependent translation), GFP (EMCV IRES-dependent translation), and either GST, GST-100K [280-345], GST-100K [280-345 RRKA], GST-Mnk1, or GST-Mnk1[RRKA]. At 36 h posttransfection, equal amounts of total protein were resolved by SDS-10% PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted as shown. Autoradiograms were quantified by densitometry of three independent experiments, and standard deviations were determined.
Many studies have demonstrated an association between eIF4E phosphorylation and increased translation rates, although the relationship remains controversial (38). In Ad-infected cells or cells transfected with the 100K protein, the only observable change in the translation apparatus is replacement of Mnk with 100K protein on eIF4G and dephosphorylation of eIF4E (3). Use of only the minimal 100K peptide to displace Mnk1 from eIF4G offered the ability to directly determine whether eIF4E dephosphorylation is sufficient to block eIF4F-dependent mRNA translation. Cells were transfected with plasmids expressing two reporter mRNAs, β-Gal (eIF4F dependent) and GFP (encephalomyocarditis virus [EMCV] internal ribosome entry site [IRES], eIF4F independent), and either GST, GST-100K [280-345], GST-100K [280-345 RRKA], mouse GST-Mnk1, or mouse GST-Mnk1[RRKA] protein. GFP levels were used to normalize protein amounts because translation of the GFP mRNA is IRES directed and not affected by expression of 100K protein (unpublished results). There was no change in reporter mRNA levels with expression of wild-type or mutant 100K peptides (data not shown). Levels of β-Gal protein were determined by immunoblotting to assess eIF4F-dependent translation. As shown in Fig. 6B, eIF4F-dependent translation (β-Gal) decreased by 70 to 80% with coexpression of the wild-type 100K peptide compared to that with GST alone, consistent with eIF4E dephosphorylation. However, when binding of the 100K peptide to eIF4GI was impaired by mutation of the RRK sequence (RRKA), eIF4F-dependent translation was not affected. Expression of wild-type or mutant Mnk1 protein did not affect eIF4F-dependent translation, as expected based on the eIF4E phosphorylation data (Fig. 6A). Importantly, tripartite leader-driven translation of β-Gal was not affected by the 100K peptide 280 to 345, in contrast to the eIF4F-dependent β-Gal reporter mRNA (Fig. 6C). We can therefore conclude that 100K peptide inhibition of eIF4E phosphorylation by displacement of Mnk1 from eIF4G is sufficient to inhibit eIF4F-dependent mRNA translation but does not alter translation directed by the Ad tripartite leader.
Mnk1 but not 100K protein interacts with the eIF4F complex in an RNA-dependent manner.
Studies were performed to determine the state of eIF4GI when targeted by 100K protein and whether it is part of translationally active cap-initiation complexes bound to mRNA, whether it is recycling between initiation events (mRNA free), or both. 293T cells were transfected with plasmids expressing GST or GST-Mnk1 proteins, equal amounts of cell lysates were treated or not treated with RNase A under conditions that abolish RNA-protein interactions (3, 23), and GST-Mnk1 protein was recovered by glutathione-Sepharose chromatography. Recovered proteins were resolved by SDS-10% PAGE and identified by immunoblotting with antibodies to GST, eIF4E, eIF4G, and eIF4A (Fig. 7A). Surprisingly, RNase treatment resulted in loss of Mnk1 from eIF4G and the eIF4F complex (eIF4E and eIF4A) (Fig. 7A). These results indicate that Mnk1 association with eIF4F is strongly dependent on mRNA association. RNA dependence for Mnk1 interaction with eIF4G has not been previously investigated. Immunoprecipitation of eIF4G (Fig. 7B) confirmed the loss of Mnk1 association after RNase treatment, whereas there was only a slight decrease in eIF4A levels and a 25 to 50% decrease in eIF4E levels after RNase treatment. The greater decrease in eIF4E than in eIF4A levels with RNase treatment might reflect eIF4G-independent binding by eIF4E to the cap structure on mRNA or perhaps a reduced stability of eIF4E/eIF4G complexes in the absence of RNA. These results indicate that Mnk1 association with eIF4G is strongly mRNA dependent and that Mnk1 likely joins the eIF4F complex following its assembly on mRNA. In contrast, 100K protein remained associated with eIF4GI despite degradation of RNA (Fig. 7C). 100K protein, therefore, interacts with eIF4G regardless of its involvement in translation initiation. It is possible that binding of eIF4G to RNA induces a conformational change required for binding by Mnk1 but not 100K and that 100K competes for the Mnk1-binding site on eIF4G during turnover and reassembly of eIF4F on mRNA.
FIG. 7.
RNA dependence for Mnk1 but not 100K interaction with eIF4F complexes. (A) 293T cells were transfected with plasmids expressing GST or GST-Mnk1 proteins, cellular lysates were prepared 36 h posttransfection, and GST fusion proteins were recovered by glutathione-Sepharose chromatography with or without prior digestion by RNase A (100 μg/ml for 2 h). Mnk1-associated proteins were resolved by SDS-10% PAGE and identified by immunoblot analysis with antibodies to eIF4G, eIF4E, eIF4A, and GST(-Mnk1) polypeptides. Original levels of proteins are shown in the right panels. (B) As in panel A but proteins were recovered by immunoprecipitation of eIF4G and subjected to immunoblot detection of eIF4G, GST(-Mnk1), eIF4A, and eIF4E. (C) Cells were transfected with Flag or Flag-100K protein and treated with RNase as described above, and immunoprecipitation of eIF4G was carried out. Associated proteins were resolved by SDS-PAGE and immunoblotted for eIF4G and Flag-100K protein. Autoradiograms were quantified by densitometry of three independent studies, and standard deviations were determined.
DISCUSSION
In this report we have demonstrated that Ad 100K protein binding to eIF4G or a 66-amino-acid peptide of 100K is sufficient to displace Mnk1 from eIF4G and impair eIF4E phosphorylation, which is linked to inhibition of cellular mRNA translation. The displacement of Mnk1 from eIF4G and dephosphorylation of eIF4E are the only alteration of the translational machinery which is observed in late Ad-infected cells. Mnk1 dissociation from eIF4G correlates with the reduction of cellular protein synthesis but without reducing translation of Ad late viral mRNAs (17, 53). Apart from this alteration, cap-initiation complexes remain intact during late Ad infection, which includes the association of eIF4G with eIF4A, eIF4E, poly(A)-binding protein, eIF3, eIF2, and 40S ribosomal subunits. In general, phosphorylation of eIF4E has been associated with enhanced cap (eIF4F)-dependent translation, and decreased eIF4E phosphorylation has been associated with decreased translation. eIF4E is phosphorylated by Mnk kinases in vivo when both are associated with eIF4G, eIF4E at the N terminus and Mnk1 at the C terminus of eIF4G (33, 36, 37, 48). We recently showed that ectopic expression of an eIF4E mutant which cannot be phosphorylated impairs eIF4F-dependent mRNA translation without affecting translation of an mRNA containing the Ad tripartite leader 5′ UTR. These results also underscore a role for eIF4E phosphorylation in promoting cellular protein synthesis (3). In addition, we demonstrated that the eIF4E kinase Mnk1 is dissociated from eIF4G during the late phase of Ad infection, which correlates with eIF4E dephosphorylation and inhibition of cellular protein synthesis (3). In this regard, studies carried out with transgenic Drosophila melanogaster which expresses in a null background a form of eIF4E that cannot be phosphorylated demonstrated reduction in viability, a smaller cell size, and developmental delays (22).
We mapped the eIF4G-binding site for 100K protein to a 66-amino-acid peptide between residues 280 and 345 (Fig. 2 and 3). There are no known protein-protein interaction motifs in this sequence, but both human and mouse Mnk1 sequences share a homology with 100K protein in this same region (Fig. 4A), which includes the region of Mnk1 thought to be essential for its binding to eIF4G (49). We demonstrated that this basic amino acid region is required for Mnk1 interaction with eIF4G, in agreement with other reports (37) (Fig. 4B), and comprises part of the 100K-binding element for eIF4G. Additional residues within the 66-amino-acid peptide of 100K protein likely contribute to its stronger binding to eIF4G (Fig. 4C).
By overexpression of either the wild-type or RRK-to-AAA mutant 66-amino-acid peptide of 100K protein, we showed that the binding of 100K protein to eIF4G is required and sufficient for dissociation of Mnk1 from eIF4G (Fig. 5). We suspect that Mnk1 displacement from eIF4G by 100K takes place by virtue of the stronger binding by 100K, substantiated by its resistance to salt dissociation (Fig. 4C). The stronger binding of 100K to eIF4G (Fig. 7) likely blocks rebinding of Mnk1 to eIF4G when complexes reassemble on mRNA. This might also reflect a requirement for an RNA-induced conformational change in eIF4G that promotes Mnk1 binding but is not necessary for 100K interaction. The association of 100K with eIF4G is responsible for inhibition of eIF4E phosphorylation (Fig. 6A). Expression of the RRKA mutant peptide that very poorly binds eIF4G (Fig. 4B) does not displace Mnk1 from eIF4G and only slightly reduces eIF4E phosphorylation (Fig. 6A). In good agreement, we observed a significant decrease in reporter eIF4F-dependent mRNA translation with overexpression of the wild-type 100K peptide but not with that of the RRKA mutant peptide (Fig. 6B). In these same studies expression of 100K peptide did not impair translation of mRNAs controlled by the Ad late tripartite leader mRNA 5′ noncoding region or the EMCV IRES (Fig. 6C). The only alterations of the initiation apparatus mediated by Ad are binding by 100K protein to eIF4G, displacement of Mnk1 from eIF4G, and dephosphorylation of eIF4E. A small peptide of 100K that binds eIF4G can recapitulate these events, and the peptide is unlikely to possess activities apart from eIF4G binding. We therefore propose that dephosphorylation of eIF4E is sufficient to impair cellular mRNA translation without affecting translation of Ad late viral mRNAs.
How does eIF4E phosphorylation affect cellular protein synthesis? Early studies proposed that phosphorylation of eIF4E increases its affinity for the cap structure at the 5′ end of nucleus-encoded mRNAs (26). Based on the crystal structure of murine eIF4E bound to 7-methyl-GDP, it was speculated that phosphorylation of eIF4E at Ser209 (the major phosphorylation site of Mnk) allows formation of a salt bridge with Lys159, which might clamp eIF4E onto the capped mRNA, increasing its affinity for the cap structure (25). However, more recent crystallographic studies (31, 45, 46) revealed that Lys159 is 19 Å away from Ser209, which is too far for formation of a salt bridge between Ser209(P) and Lys159. Instead, it was proposed that phosphorylation of eIF4E enhances its affinity for the cap structure by narrowing the size of the cap-binding entrance once the cap structure is located at the cap-binding pocket (46). Recent studies (54) suggest that phosphorylation of eIF4E at Ser209 reduces its affinity for the cap by electrostatic repulsion between the negatively charged residues of eIF4E and the cap: the phosphate group at Ser209 of eIF4E and the 5′-to-5′ phosphate chain of the cap. This prediction is consistent with recent biophysical studies (39). These studies observed that phosphorylation of eIF4E reduced its affinity for capped RNA, due to an increased rate of dissociation. Based on these data, a model was proposed for translation initiation in which eIF4E would bind the cap structure of the mRNA, which could be stabilized by its interaction with eIF4G. Association of eIF4G and eIF3 would allow loading of the 43S ribosomal complex to the 5′ end of the mRNA, although the order in which these components are assembled is not clear. Once the 48S complex forms on the mRNA (40S ribosome, eIF4F, mRNA), eIF4E would be phosphorylated by Mnk1, facilitating the release of eIF4F from the cap structure and allowing scanning. Thus, new initiation complexes could be formed on the free cap structure, facilitating a faster loading of ribosomes on the mRNA, thereby increasing the translation rate (38). An alternate mechanism proposes that phosphorylation of eIF4E might release initiation factors from existing translational complexes, allowing translation of underrepresented mRNAs (27, 39).
Once in the cytoplasm, late Ad mRNAs are preferentially loaded onto the translational machinery by the association of 100K protein with the C terminus of eIF4G (4) and possibly through its interaction with Ad mRNAs. Since translation of tripartite leader-containing mRNAs requires intact eIF4F in vitro (44), we propose that either free eIF4E or eIF4E associated with eIF4G-100K could bind the cap structure present at the 5′ end of late Ad mRNAs and stabilize the mRNA-eIF4F complex. This would allow loading of 43S ribosome complexes onto the mRNA through the interaction of eIF3 and eIF4G. As eIF4E would not be phosphorylated due to the presence of 100K protein on eIF4G instead of Mnk1, the 48S ribosomal complex would not be released from the cap structure as rapidly as that of phosphorylated eIF4E. However, the tripartite leader 5′ UTR on all late viral mRNAs directs translation by ribosome shunting (51, 52), which involves direct translocation of ribosomal complexes from the cap to shunting elements within the 5′ UTR that contain sequences complementary to the 3′ end of 18S rRNA, possibly providing a 40S ribosome docking site. A second translocation event directs the 40S ribosome to the downstream AUG. Supporting this model, we have observed considerably more eIF4E, but not other initiation factors, associated with polyribosomes in cells expressing 100K protein (Q. Xi, R. Cuesta, and R. J. Schneider, unpublished results). This suggests that phosphorylation of eIF4E could favor the release of eIF4E from the cap structure and migration of 48S ribosomal complexes into the mRNA.
Acknowledgments
We thank N. Sonenberg for the HA-eIF4GI full-length clone and J. Cooper for GST-Mnk1. We thank members of the lab for critical review of the manuscript.
This work was supported by NIH grant CA 42357 (to R.J.S.) and in part by a fellowship from Fundacion Ramon Areces, Spain (to R.C.).
REFERENCES
- 1.Adam, S. A., and G. Dreyfuss. 1987. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J. Virol. 61:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuesta, R., G. Laroia, and R. J. Schneider. 2000. Chaperone Hsp27 promotes disassembly of translation cap initiation complex during heat shock. Genes Dev. 14:1460-1470. [PMC free article] [PubMed] [Google Scholar]
- 3.Cuesta, R., Q. Xi, and R. J. Schneider. 2000. Adenovirus specific translation by selective disassembly of cap-initiation complex. EMBO J. 19:3465-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuesta, R., Q. Xi, and R. J. Schneider. 2001. Preferential translation of adenovirus mRNAs in infected cells. Cold Spring Harbor Symp. Quant. Biol. 66:259-267. [DOI] [PubMed] [Google Scholar]
- 5.Feigenblum, D., and R. J. Schneider. 1996. Cap-binding protein (eukaryotic initiation factor 4E) and 4E-inactivating protein BP-1 independently regulate cap-dependent translation. Mol. Cell. Biol. 16:5450-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigenblum, D., and R. J. Schneider. 1993. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J. Virol. 67:3027-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser, C. S., V. M. Pain, and S. J. Morley. 1999. Cellular stress in xenopus kidney cells enhances the phosphorylation of eukaryotic translation initiation factor (eIF)4E and the association of eIF4F with poly(A)-binding protein. Biochem J. 342:519-526. [PMC free article] [PubMed] [Google Scholar]
- 8.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambke, C., and W. Deppert. 1981. Late nonstructural 100,000- and 33,000-dalton proteins of adenovirus type 2. II. Immunological and protein chemical analysis. J. Virol. 40:594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghisolfi, L., A. Kharrat, G. Joseph, F. Amalric, and M. Erard. 1992. Concerted activities of the RNA recognition and the glycine-rich C-terminal domains of nucleolin are required for efficient complex formation with pre-ribosomal RNA. Eur. J. Biochem. 209:541-548. [DOI] [PubMed] [Google Scholar]
- 11.Gingras, A.-C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 12.Gingras, A. C., and N. Sonenberg. 1997. Adenovirus infection inactivates the translational inhibitors 4E-BP1 and 4E-BP2. Virology 237:182-186. [DOI] [PubMed] [Google Scholar]
- 13.Gorlich, D., and I. W. Mattaj. 1996. Nucleocytoplasmic transport. Science 271:1513-1518. [DOI] [PubMed] [Google Scholar]
- 14.Hayes, B. W., G. C. Telling, M. M. Myat, J. F. Williams, and S. J. Flint. 1990. The adenovirus L4 100-kilodalton protein is necessary for efficient translation of viral late mRNA species. J. Virol. 64:2732-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson, B. R., and A. Eleftheriou. 2000. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp. Cell Res. 256:213-224. [DOI] [PubMed] [Google Scholar]
- 16.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational regulation of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Huang, J., and R. J. Schneider. 1991. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap binding protein. Cell 65:271-280. [DOI] [PubMed] [Google Scholar]
- 18.Imataka, H., A. Gradi, and N. Sonenberg. 1998. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 17:7480-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imataka, H., H. S. Olsen, and N. Sonenberg. 1997. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J. 16:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imataka, H., and N. Sonenberg. 1997. Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Mol. Cell. Biol. 17:6940-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauf, U., C. Tschopp, and H. Gram. 2001. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 21:5500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachance, P. E., M. Miron, B. Raught, N. Sonenberg, and P. Lasko. 2002. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol. Cell. Biol. 22:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laroia, G., R. Cuesta, and R. J. Schneider. 1999. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science 284:499-502. [DOI] [PubMed] [Google Scholar]
- 24.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 25.Marcotrigiano, J., A. C. Gingras, N. Sonenberg, and S. K. Burley. 1997. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89:951-961. [DOI] [PubMed] [Google Scholar]
- 26.Minich, W. B., M. L. Balasta, D. J. Goss, and R. E. Rhoads. 1994. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. USA 91:7668-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morley, S. J. 2001. The regulation of eIF4F during cell growth and cell death. Prog. Mol. Subcell. Biol. 27:1-37. [DOI] [PubMed] [Google Scholar]
- 28.Morley, S. J., and L. McKendrick. 1997. Involvement of stress-activated protein kinase and p38/RK mitogen-activated protein kinase signaling pathways in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J. Biol. Chem. 272:17887-17893. [DOI] [PubMed] [Google Scholar]
- 29.Morley, S. J., and S. Naegele. 2002. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J. Biol. Chem. 277:32855-32859. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, R., and S. R. Wente. 1996. An RNA-export mediator with an essential nuclear export signal. Nature 383:3577-3600. [DOI] [PubMed] [Google Scholar]
- 31.Niedzwiecka, A., J. Marcotrigiano, J. Stepinski, M. Jankowska-Anyszka, A. Wyslouch-Cieszynska, M. Dadlez, A. C. Gingras, P. Mak, E. Darzynkiewicz, N. Sonenberg, S. K. Burley, and R. Stolarski. 2002. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J. Mol. Biol. 319:615-635. [DOI] [PubMed] [Google Scholar]
- 32.Patel, J., L. E. McLeod, R. G. Vries, A. Flynn, X. Wang, and C. G. Proud. 2002. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 269:3076-3085. [DOI] [PubMed] [Google Scholar]
- 33.Pyronnet, S., H. Imataka, A. C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits Mnk1 to phosphorylate eIF4E. EMBO J. 18:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyronnet, S., and N. Sonenberg. 2001. Cell-cycle-dependent translational control. Curr. Opin. Genet. Dev. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 35.Riley, D., and S. J. Flint. 1993. RNA-binding properties of a translational activator, the adenovirus L4 100-kilodalton protein. J. Virol. 67:3586-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheper, G. C., N. A. Morrice, M. Kleijn, and C. G. Proud. 2001. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol. Cell. Biol. 21:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheper, G. C., J. L. Parra, M. Wilson, B. van Kollenburg, A. C. O. Vertegaal, Z.-G. Han, and C. G. Proud. 2003. The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization. Mol. Cell. Biol. 23:5692-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheper, G. C., and C. G. Proud. 2002. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 269:5350-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheper, G. C., B. van Kollenburg, J. Hu, Y. Luo, D. J. Goss, and C. G. Proud. 2002. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Biol. Chem. 277:3303-3309. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, R. J. 2000. Adenovirus inhibition of cellular protein synthesis and preferential translation of viral mRNAs, p. 901-914. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Schneider, R. J. 2000. Translational control during heat shock, p. 581-594. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Senger, B., G. Simos, F. R. Bischoff, A. Podtelejnikov, M. Mann, and E. Hurt. 1998. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 17:2196-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarun, S. Z., S. E. Wells, and A. B. Sachs. 1997. Translation initiation factor eIF-4G mediates in vitro polyA tail dependent translation. Proc. Natl. Acad. Sci. USA. 94:9046-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas, A. M., G. C. Scheper, M. Kleijn, M. DeBoer, and H. O. Voorma. 1992. Dependence of the adenovirus tripartite leader on the p220 subunit of eukaryotic initiation factor 4F during in vitro translation. Eur. J. Biochem. 207:471-477. [DOI] [PubMed] [Google Scholar]
- 45.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, T. Ishida, T. Taniguchi, H. Hasegawa, A. Terashima, M. Sasaki, Y. Katsuya, K. Kitamura, H. Miyoshi, M. Ishikawa, and K. Miura. 2002. Crystal structures of 7-methylguanosine 5′-triphosphate (m(7)GTP)- and P(1)-7-methylguanosine-P(3)-adenosine-5′,5′-triphosphate (m(7)GpppA)-bound human full-length eukaryotic initiation factor 4E: biological importance of the C-terminal flexible region. Biochem. J. 362:539-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, M. Sasaki, T. Taniguchi, H. Miyagawa, K. Kitamura, K. Miura, and T. Ishida. 2003. Structural features of human initiation factor 4E, studied by X-ray crystal analyses and molecular dynamics simulations. J. Mol. Biol. 328:365-383. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., A. Flynn, A. J. Waskiewicz, B. L. J. Webb, R. G. Vries, I. A. Baines, J. A. Cooper, and C. G. Proud. 1998. The phosphorylation of eukaryotic initiation factor eIF-4E in response to phorbol esters, cell stresses and cytokines is mediated by distinct MAP kinase pathways. J. Biol. Chem. 273:9373-9377. [DOI] [PubMed] [Google Scholar]
- 48.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingham, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 19:1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2:135-140. [DOI] [PubMed] [Google Scholar]
- 51.Yueh, A., and R. J. Schneider. 1996. Selective translation by ribosome jumping in adenovirus infected and heat shocked cells. Genes Dev. 10:1557-1567. [DOI] [PubMed] [Google Scholar]
- 52.Yueh, A., and R. J. Schneider. 2000. Translation by ribosome shunting on adenovirus and Hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14:414-421. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Y., D. Feigenblum, and R. J. Schneider. 1994. A late adenovirus factor induces eIF-4E dephosphorylation and inhibition of cell protein synthesis. J. Virol. 68:7040-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuberek, J., A. Wyslouch-Cieszynska, A. Niedzwiecka, M. Dadlez, J. Stepinski, W. Augustyniak, A. C. Gingras, Z. Zhang, S. K. Burley, N. Sonenberg, R. Stolarski, and E. Darzynkiewicz. 2003. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA 9:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]